Visual Abstract
Keywords: cinacalcet, calcimimetic, vitamin D, pharmacoepidemiology, dialysis, phosphate binders, hemodialysis, mineral metabolism
Abstract
Background and objectives
With multiple medications indicated for mineral metabolism, dialysis providers can apply various strategies to achieve target phosphate and parathyroid hormone (PTH) levels. We describe common prescribing patterns and practice variation in mineral metabolism treatment strategies over the last decade.
Design, setting, participants, & measurements
In a cohort of adults initiating hemodialysis at Dialysis Clinic, Inc. facilities, we assessed prescriptions of vitamin D sterols, phosphate binders, and cinacalcet longitudinally. To identify the influence of secular trends in clinical practice, we stratified the cohort by dialysis initiation year (2006–2008, 2009–2011, and 2012–2015). To measure practice variation, we estimated the median odds ratio for prescribing different mineral metabolism treatment strategies at 12 months post–dialysis initiation across facilities using mixed effects multinomial logistic regression. Sensitivity analyses evaluated strategies used after detection of first elevated PTH.
Results
Among 23,549 incident patients on hemodialysis, there was a decline in vitamin D sterol–based strategies and a corresponding increase in strategies without PTH-modifying agents (i.e., phosphate binders alone or no mineral metabolism medications) and cinacalcet-containing treatment strategies between 2006 and 2015. The proportion with active vitamin D sterol–based strategies at dialysis initiation decreased across cohorts: 15% (2006–2008) to 5% (2012–2015). The proportion with active vitamin D sterol–based strategies after 18 months of dialysis decreased across cohorts: 52% (2006–2008) to 34% (2012–2015). The odds of using individual strategies compared with reference (active vitamin D sterol with phosphate binder) varied from 1.5- to two-fold across facilities in 2006–2008 and 2009–2011 cohorts, and increased to two- to three-fold in the 2012–2015 cohort. Findings were similar in sensitivity analyses starting from first elevated PTH measurement.
Conclusions
Over time, mineral metabolism management involved less use of vitamin D sterol–based strategies, greater use of both more conservative and cinacalcet-containing strategies, and increased practice variation, suggesting growing equipoise.
Introduction
Mineral metabolism is commonly disordered in patients with kidney failure on hemodialysis and associates with adverse outcomes (1,2). Routinely, kidney care providers manage mineral metabolism. Most management strategies are characterized by the use of three classes of mineral metabolism agents alone or in combination, including: (1) gastrointestinal phosphate binders, (2) active vitamin D sterols, and (3) calcimimetics (3–5). The best pharmacologic management strategies are unknown, with few mineral metabolism practices supported by randomized controlled clinical trials of clinical, as opposed to biochemical, outcomes (4,5). In this vacuum, guidelines are vague and provide suggestions on how to treat, with a focus on levels of control of biochemical factors, such as serum phosphate and parathyroid hormone (PTH). Even less guidance is provided on agent selection across classes or combination use (6–9). Hence, we hypothesize that practice could be driven heavily by providers’ preferences and beliefs, resulting in substantial practice variation.
Over the past decade, additional events have influenced mineral metabolism management. First, cinacalcet was introduced into the market in 2004 as the first-in-class calcimimetic, and it rapidly diffused into practice, broadening therapeutic choices (10,11). Second, guideline-based biochemical targets were effectively relaxed in the United States in 2009 with the first publication of the Kidney Disease Improving Global Outcomes (KDIGO) Guideline for CKD Mineral and Bone Disorder (CKD-MBD), supplanting the prior 2003 Kidney Disease Quality Outcome Initiative Guidelines on the topic (7,8). Third, dialysis payment was transformed in 2011 with the implementation of a bundled dialysis payment structure, which included vitamin D sterols but not phosphate binders or cinacalcet in the bundled payment (12). Finally, the Evaluation of Hydrochloride Therapy to Lower Cardiovascular Events (EVOLVE) study was published in 2012 with inconclusive results regarding the effectiveness of cinacalcet added to standard of care for reducing cardiovascular disease (13).
In this study, our three objectives were to: (1) describe recent use of common combinations of mineral metabolism medications (treatment strategies); (2) investigate changes in treatment strategies over time from dialysis incidence and characterize secular trends over the period from 2006 to 2015; and (3) characterize practice variation in treatment strategies. To achieve these objectives, we conducted an observational study using electronic health record (EHR) data from patients with kidney failure incident to in-center hemodialysis between 2006 and 2015.
Materials and Methods
Study Population and Data Sources
Our cohort comprised adult patients (≥18 years of age) initiating in-center hemodialysis in the United States between January 1, 2006 through October 2, 2015, at facilities affiliated with Dialysis Clinic, Inc. (DCI), a moderately sized, not-for-profit dialysis organization; included participants were those who were alive and observed on in-center hemodialysis at 90 days. DCI EHR data were linked to the United States Renal Data System (USRDS) Standard Analysis Files to ascertain covariates and insurance coverage (14). Over follow-up, patients were terminally censored for death, kidney transplantation, or transfer out of DCI, and interval censored for periods where there was an absence of hemodialysis treatments at a DCI facility for ≥30 days. The remaining participants were administratively censored on December 31, 2015. Analyses that evaluated practice variation focused on the subset of individuals at 12 months postincidence who had at least one hemodialysis treatment in the 30 days before their 12-month vintage date. This study was approved by DCI, USRDS, and the Duke University Institutional Review Board.
Key Variable Ascertainment and Definitions
We identified prescriptions for three medication classes: active vitamin D sterols (intravenous and oral); phosphate binders; and cinacalcet, the only available calcimimetic during the study period. These were identified using generic product identifier codes identified in the DCI EHR medication list (see Supplemental Methods). We validated the DCI EHR medication list as an accurate source for medication ascertainment (see Supplemental Methods). Prescriptions for each medication were evaluated longitudinally in continuous time using EHR start and stop dates. For vitamin D sterols and cinacalcet, missing stop dates were imputed as the end of follow-up or the day before the start date of another medication in the same class. For phosphate binders, which are often used in combination, missing stop dates were imputed as the end of follow-up or the day before a new prescription for the same agent at an alternate dose. To develop treatment strategies, we combined medications into all available permutations across classes. Infrequent combinations were combined with other groups to create the following mutually exclusive mineral metabolism treatment strategies: (1) no mineral metabolism medications; (2) phosphate binder alone; (3) active vitamin D sterol alone; (4) active vitamin D sterol with phosphate binder; and (5) cinacalcet-containing strategy, indicating cinacalcet alone or with any other mineral metabolism medication (see Supplemental Methods). Although mineral metabolism derangements can also be treated by parathyroidectomy, it was infrequent in this incident cohort (n=86), and parathyroidectomy was not considered further.
Covariates include variables as reported in the Centers for Medicare and Medicaid Services (CMS) Form 2728 (age, sex, race, ethnicity, primary cause of kidney failure, and functional status), insurance status from USRDS Standard Analysis Files and Part D prescription coverage files, and clinical parameters from the DCI EHR. EHR variables include intact PTH, serum phosphate, albumin-corrected serum calcium, serum albumin, single-pool Kt/V, prescribed dialysis time, and dialysate calcium concentration. We ascertained comorbidities and estimated a comorbidity index for each patient using comorbidity data from the CMS Form 2728, with weights defined by Liu et al. (15) according to conditions available on the Form 2728.
Statistical Analyses
Using longitudinal graphs and descriptive statistics, we described the evolution of mineral metabolism treatment strategies over follow-up, beginning with the incident dialysis date. Longitudinal graphs (e.g., Sankey plots) were created from a random 100-person subset of the study population. Because of known secular trends in treatment related to guideline changes and key clinical trials, we stratified these visualizations by dialysis initiation year as 2006–2008, 2009–2011, and 2012–2015. These time windows were designed to correspond with publication of the 2009 KDIGO CKD-MBD Clinical Practice Guidelines and the 2012 landmark EVOLVE clinical trial (8,13). Because not all patients on hemodialysis had an indication for mineral metabolism treatment, we conducted sensitivity analyses among patients starting from first observed PTH >300, 400, 500, and 600 pg/ml, where present.
To test observed time since dialysis initiation and incident cohort year trends observed graphically, we constructed a mixed-effects, multinomial model of each treatment strategy compared with a reference strategy of active vitamin D sterols and phosphate binder. The model included fixed effects for time since hemodialysis initiation (3, 6, 12, and 24 months), incident cohort year (2006–2008, 2009–2011, and 2012–2015), and their interaction. The model also included random effects for patients and facilities.
To investigate practice variation in mineral metabolism treatment, we constructed a mixed-effects, multinomial logistic regression of the probability of each mineral metabolism treatment strategy compared with the reference strategy of active vitamin D sterol and phosphate binder at the 12-months postincidence time point. The model was adjusted for case mix (i.e., age, sex, comorbidity index, and insurance status) and included an anonymized facility identifier as a random intercept for the odds of use of each strategy. We calculated best linear unbiased predictors for the random effects, and interpreted each as a measure of the shift in odds of using a particular strategy at a certain facility and reflecting a specific dialysis practice pattern. Intraclass correlation coefficients were estimated using the equation 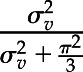 , where
, where  is the variance of the random effect for the facility (16).
is the variance of the random effect for the facility (16).
To aid interpretation, we also estimated median odds ratios (median ORs), which can be interpreted as the median of the relative increase in odds for use of a specific strategy for individuals with otherwise similar traits from two randomly chosen clusters, where the one with the higher odds was placed in the numerator (17). The prevalence of missing covariate data was low (2%); therefore, analyses were conducted using a complete case approach. All analyses were conducted using SAS version 9.4.
Results
Study Population
A total of 31,111 patients initiated hemodialysis at DCI facilities in the time period and 23,549 remained active on hemodialysis at 90 days, comprising the full source population for analyses of trends. Cohort characteristics according to incident cohort years 2006–2008 (n=7586), 2009–2011 (n=7286), and 2012–2015 (n=8677) are provided in Table 1. Across these three cohorts, comorbidity burden, functional status, presence of diabetes, and causes of kidney failure were similar. Median PTH and the proportion of patients using lower dialysate calcium baths (<2.5 mEq/L) appeared higher in later years.
Table 1.
Baseline characteristics of the 90-d study population by cohort year and overall
| Baseline Characteristics | Cohort Year | Total (n=23,549) | ||
|---|---|---|---|---|
| 2006–2008 (n=7586) | 2009–2011 (n=7286) | 2012–2015 (n=8677) | ||
| Age (yr), median (IQR) | 63 (52–74) | 63 (53–74) | 64 (54–74) | 64 (53–74) |
| Race, n (%) | ||||
| White | 4580 (60) | 4532 (62) | 5235 (60) | 14,347 (61) |
| Black | 2681 (35) | 2440 (34) | 3001 (35) | 8122 (35) |
| Other | 325 (4) | 314 (4) | 441 (5) | 1080 (5) |
| Sex, n (%) | ||||
| Male | 4195 (55) | 4201 (58) | 4982 (57) | 13,378 (57) |
| Female | 3391 (45) | 3085 (42) | 3695 (43) | 10,171 (43) |
| Hispanic ethnicity, n (%) | 423 (6) | 478 (7) | 513 (6) | 1414 (6) |
| Insurance status (90 d), n (%) | ||||
| Total subsidy | 335 (4) | 332 (5) | 600 (7) | 1267 (5) |
| Moderate subsidy | 1571 (21) | 1532 (21) | 1137 (13) | 4240 (18) |
| Small or no subsidy | 5049 (67) | 4756 (65) | 6096 (70) | 15,901 (68) |
| Other/unknown | 615 (8) | 654 (9) | 834 (10) | 2103 (9) |
| Primary cause of kidney failure, n (%) | ||||
| Diabetes | 3591 (50) | 3358 (48) | 4142 (50) | 11,091 (49) |
| Hypertension | 2099 (29) | 2035 (29) | 2463 (29) | 6597 (29) |
| GN | 668 (9) | 614 (9) | 694 (8) | 1976 (9) |
| Other cause | 890 (12) | 938 (14) | 1076 (13) | 2904 (13) |
| Comorbidity index, median (IQR) | 3 (1–5) | 3 (1–5) | 3 (1–5) | 3 (1–5) |
| Atherosclerotic heart disease, n (%) | 1801 (24) | 1672 (23) | 1757 (20) | 5230 (22) |
| Congestive heart failure, n (%) | 2581 (34) | 2321 (32) | 2766 (32) | 7668 (33) |
| Cerebrovascular accident/transient, n (%) | 897 (12) | 845 (12) | 910 (11) | 2652 (11) |
| Peripheral vascular disease, n (%) | 1143 (15) | 1016 (14) | 1187 (14) | 3346 (14) |
| Other cardiac, n (%) | 1601 (21) | 1627 (22) | 2196 (25) | 5424 (23) |
| Chronic obstructive pulmonary disease, n (%) | 817 (11) | 786 (11) | 1104 (13) | 2707 (12) |
| Cancer, n (%) | 656 (9) | 656 (9) | 786 (9) | 2098 (9) |
| Diabetes mellitus, n (%) | 3911 (52) | 3808 (52) | 4690 (54) | 12,409 (53) |
| Inability to ambulate, n (%) | 537 (7) | 501 (7) | 671 (8) | 1709 (7) |
| Parathyroid hormone at 90 d (pg/ml), median (IQR) | 181 (101–303) | 242 (135–416) | 310 (165–507) | 239 (130–426) |
| <150 pg/ml, n (%) | 2937 (41) | 2018 (28) | 1883 (22) | 6838 (30) |
| 150–299 pg/ml, n (%) | 2423 (34) | 2319 (32) | 2283 (27) | 7025 (31) |
| 300–599 pg/ml, n (%) | 1480 (21) | 2192 (31) | 3019 (35) | 6691 (29) |
| ≥600 pg/ml, n (%) | 363 (5) | 622 (8) | 1416 (17) | 2401 (11) |
| Parathyroid hormone at 12 mo, n (%) | ||||
| <150 pg/ml | 1834 (32) | 1286 (23) | 981 (18) | 4101 (24) |
| 150–299 pg/ml | 2207 (38) | 1857 (33) | 1603 (30) | 5667 (34) |
| 300–599 pg/ml | 1479 (26) | 2103 (37) | 2014 (38) | 5596 (33) |
| ≥600 pg/ml | 239 (4) | 420 (7) | 761 (14) | 1420 (9) |
| Phosphate at 90 d (mg/dl), median (IQR) | 5.1 (4.2–6.3) | 5.2 (4.3–6.3) | 5.1 (4.3–6.2) | 5.1 (4.3–6.2) |
| Albumin-corrected calcium at 90 d (mg/dl), median (IQR) | 9.3 (8.9–9.7) | 9.3 (8.9–9.7) | 9.3 (8.9–9.7) | 9.3 (8.9–9.7) |
| Albumin at 90 d (g/dl), median (IQR) | 3.6 (3.3–3.9) | 3.6 (3.3–3.9) | 3.7 (3.4–4.0) | 3.6 (3.3–3.9) |
| Dialysis time at 90 d (min/wk), median (IQR) | 630 (585–720) | 630 (585–720) | 675 (630–720) | 630 (630–720) |
| Kt/V at 90 d, median (IQR) | 1.5 (1.4–1.7) | 1.5 (1.4–1.7) | 1.5 (1.4–1.7) | 1.5 (1.4–1.7) |
| Dialysate calcium at 90 d, n (%)a | ||||
| <2.00 mEq/L | 98 (1) | 87 (1) | 60 (1) | 245 (1) |
| 2.00–2.24 mEq/L | 624 (8) | 919 (13) | 1214 (14) | 2757 (12) |
| 2.25–2.44 mEq/L | 78 (1) | 94 (1) | 320 (4) | 492 (2) |
| 2.45–2.94 mEq/L | 5851 (78) | 5665 (78) | 6595 (76) | 18,111 (77) |
| ≥3.00 mEq/L | 884 (12) | 510 (7) | 469 (6) | 1863 (8) |
IQR, interquartile range.
Common dialysate calcium values are 2.0, 2.25, 2.5, 3.0, and 3.5 mEq/L.
An additional 6660 patients were censored between 90 days and 12 months, and 1259 patients who had not had an in-center hemodialysis treatment within 30 days of the 12-month time point were also censored for models of practice variation. The characteristics of the 15,630 patients included in the practice variation sample compared with those not included and the overall population are depicted in Supplemental Table 1. Individuals in this 12-month analytic cohort are generally similar, but median age was 2 years younger, and they were more likely to be Black (38% for those included versus 29% for those excluded). A full diagram of cohort flow and exclusion for the 90-day and 12-month cohorts is provided in Supplemental Figure 1.
Description of Mineral Metabolism Treatment Strategies
Within the 90-day cohort, a total of 623,334 person-months of follow-up were available. Figure 1 shows transitions from predominantly no mineral metabolism medications or phosphate binders alone at the start of dialysis to active vitamin D sterol (yellow and green bar segments) or cinacalcet-containing regimens (teal bar segments) over time. Overall, the visuals suggest that many strategies are stable, particularly by 12 months. Similar patterns were seen examining cohorts in which baseline was established as the first PTH >300 pg/ml (Supplemental Figure 2).
Figure 1.

Patients transitioned from predominantly no mineral metabolism medications or phosphate binders alone at the start of dialysis to active vitamin D sterols or cinacalcet-containing strategies over time. Longitudinal plots of mineral metabolism treatment strategies by month for a random sample of n=100 drawn from each set of incident years. Individuals are re-sorted by strategies at each time point to clearly demonstrate transitions between strategies. From the bottom of time 0, these strategies are as follows: blue indicates no mineral metabolism medications, orange indicates phosphate binders alone, teal indicates cinacalcet-containing agents, light green indicates vitamin D sterols and phosphate binders, and yellow indicates vitamin D sterols alone. Transition to unobserved (lavender) or censored (light brown) are depicted at the bottom of each subsequent time point. Only individuals who survive and remain on hemodialysis until day 90 after the incident hemodialysis date are included.
Mineral Metabolism Strategy Use over Time from the Incident Hemodialysis Date
Figure 2, A and B, depicts the percentage of participants who were using different strategies at distinct time points post–dialysis initiation. At dialysis initiation, most (65%–73%) cohort members had no active prescriptions for mineral metabolism medications, whereas 19%–22% had prescriptions for phosphate binders alone (Figure 2, A and B, Supplemental Tables 2 and 3). Among those who remained in the sample, the most common treatment strategy from 3 to 12 months after dialysis initiation was phosphate binders alone in all study cohorts. For the incident 2006–2008 cohort, the most common treatment strategy by 18 months was active vitamin D sterol with phosphate binders. For the 2009–2011 and 2012–2015 cohorts, the most common treatment strategy by 18 months continued to be phosphate binders alone. These patterns were more evident when censored individuals were removed from the analysis (Figure 2B, Supplemental Table 3).
Figure 2.
Later cohorts had more use of phosphate binder alone and cinacalcet-containing strategies and less use of active vitamin D sterols over time after dialysis initiation. Each bar plot represents the proportion of patients on a given strategy. Bars repeat to represent different time points from the hemodialysis incident date. Sets of bars repeat for each incident cohort, including 2006–2008, 2009–2011, and 2012–2015. (A) Demonstrates the full cohort over time, including terminally censored and unobserved individuals. (B) Removes censoring to demonstrate the percentage of patients using each treatment strategy among those that remain in the sample.
Statistical models confirmed these patterns. Compared with the 2006–2008 cohort, patients in the 2009–2011 and 2012–2015 cohorts were more likely to receive no mineral metabolism medications at dialysis initiation and were less likely to receive active vitamin D sterol–based regimens (active vitamin D sterols and phosphate binders or active vitamin D sterols alone; Figure 3, Supplemental Table 4 for model estimates). In all incident cohorts, use of active vitamin D sterols with phosphate binders and use of cinacalcet-containing strategies became more common over time from the incident hemodialysis date. However, use of active vitamin D sterol–based regimens, including both active vitamin D sterols alone or with phosphate binders, declined in later cohorts, whereas use of cinacalcet-containing strategies increased modestly across cohorts. We also observed more frequent use of phosphate binders alone across cohorts (Figure 3).
Figure 3.
Later cohorts had more use of no mineral metabolism medications at dialysis initiation and were less likely to receive active vitamin D sterol-based strategies. Proportions are based on a mixed-effects, multinomial model, including time from hemodialysis initiation, incident cohort year, interaction between time from hemodialysis initiation and incident cohort year, and random effects for patients and facilities. Time is depicted in months from the incident hemodialysis date, with proportions indicated for each incident cohort: 2006–2008 (blue), 2009–2011 (red), and 2012–2015 (green).
Mineral Metabolism Strategy Use over Time from the First Elevated PTH Measurement
At 3 months after first PTH measurement >300 pg/ml in all incident cohorts, the most common strategy was active vitamin D sterols with phosphate binder or phosphate binder alone (Figure 4, A and B, Supplemental Tables 5 and 6). However, there was a shift in the latest cohort in which the phosphate binder alone strategy exceeded active vitamin D sterols with phosphate binder over the 2-year observation period. Similar to the overall cohort, cinacalcet-containing strategies were increasingly used with time in all years. This was particularly evident when proportions were based on uncensored individuals (Figure 4B, Supplemental Table 6). Additional results starting from PTH >400, 500, and 600 pg/ml showed similar trends (Supplemental Table 7). For instance, 61% of patients received strategies with active vitamin D sterols at 12 months after PTH >300 pg/ml in 2006–2008, but only 37% in 2012–2015. When indexed from first PTH >600 pg/ml, 54% were on a strategy with active vitamin D sterols and 29% on a cinacalcet-containing strategy by 12 months in 2006–2008, but this decreased to 34% on a strategy with active vitamin D sterols with still 28% on cinacalcet-containing strategies in 2012–2015.
Figure 4.
From the first elevated PTH, the phosphate binders alone strategy became more common and vitamin D sterol-based strategies became less common in later cohorts. Each bar plot represents the proportion of patients on a given strategy. Bars repeat to represent different time points from the date of first PTH >300 pg/ml. Sets of bars repeat for each cohort defined by the year of first PTH >300 pg/ml, including 2006–2008, 2009–2011, and 2012–2015. (A) Demonstrates the full cohort over time, including terminally censored and unobserved individuals. (B) Removes censoring to demonstrate the percentage of patients using each treatment strategy among those that remain in the sample.
Facility Variation in Mineral Metabolism Strategies
Including adjustment for case-mix variables, we identified an increase in variance of random effects and the corresponding intraclass correlation coefficients and median ORs across facilities in later, compared with earlier, cohorts for both strategies without PTH-modifying agents (i.e., no mineral metabolism medications and phosphate binder alone) and cinacalcet-containing strategies. For instance, the median ORs across facilities for use of an alternate strategy compared with the reference strategy ranged from approximately 1.5- to two-fold in 2006–2008 and 2009–2011 cohorts to approximately two- to three-fold in the 2012–2015 cohort (Supplemental Table 8, Table 2). A similar increase in variance of random effects was seen in sensitivity analyses among those with PTH >300 pg/ml (data not shown). Results are consistent with increasing practice variation and equipoise in mineral metabolism treatment over time.
Table 2.
Interfacility variation in mineral metabolism treatment strategies presented as median odds ratio (95% confidence interval)
| Treatment Strategies | Median Odds Ratio (95% Confidence Interval) by Cohort Year | |||
|---|---|---|---|---|
| All | 2006–2008 | 2009–2011 | 2012–2015 | |
| Vitamin D alone | 1.8 (1.6 to 2.0) | 2.0 (1.8 to 2.5) | 1.5 (1.3 to 1.9) | 1.9 (1.6 to 2.8) |
| No mineral metabolism medication | 2.0 (1.8 to 2.3) | 1.7 (1.5 to 2.0) | 1.8 (1.6 to 2.2) | 3.2 (2.7 to 4.0) |
| Cinacalcet-containing | 1.9 (1.7 to 2.1) | 1.7 (1.5 to 2.1) | 2.0 (1.8 to 2.5) | 2.6 (2.2 to 3.2) |
| Phosphate binder alone | 1.9 (1.8 to 2.1) | 1.6 (1.5 to 1.8) | 1.9 (1.7 to 2.1) | 3.5 (3.0 to 4.2) |
| Number of groups (facilities) | 359 | 253 | 257 | 265 |
| Number of patients | 15,591 | 5399 | 5225 | 4967 |
The reference strategy is vitamin D sterol and phosphate binder. Model is adjusted for age (linear and quadratic term), comorbidity, sex, race (Black, White, and other), and insurance status. The median odds ratio for a specified strategy describes the effect of the facility on receiving that strategy, rather than the reference strategy, for two otherwise similar patients. A median odds ratio near one implies facility variation plays a minor role in the probability of receiving the specified strategy; larger values indicate greater discrepancies across facilities, after controlling for patient characteristics. A median odds ratio of 1.8 implies a median increase of 80% in the odds of using that strategy (relative to the reference strategy) when a patient is switched from a facility with lower odds to one with greater odds, holding all other predictors constant.
Discussion
Mineral metabolism management changed substantially over a 10-year time period in a national sample of incident patients on hemodialysis. While most patients began hemodialysis either without any dedicated medications for mineral metabolism or using phosphate binders alone, vitamin D sterols or cinacalcet-containing strategies were increasingly used over time. In later cohort years, particularly 2012–2015, more patients either stayed on strategies without PTH-modifying agents longer or used cinacalcet-containing therapies earlier, with declining use of vitamin D sterol–based regimens compared with earlier periods. The use of any PTH-modifying agent (vitamin D–based or cinacalcet-containing agents) was less common over time, even when indexed to specific PTH thresholds. Practice variation in strategies across facilities also increased over time, suggesting increasing equipoise in the field.
Other investigators have also demonstrated a decrease in use and dose of vitamin D sterols over a similar time frame, particularly after the introduction of the bundled prospective payment system (PPS) in 2011 (12,18). Both phosphate binders alone and cinacalcet remained outside of the PPS through the end of this study, and—although it is possible that these financial factors may have encouraged these prescribing patterns—some of these trends preceded the PPS in our data, becoming evident early in the 2009–2011 cohort. Practice changes may have been driven by changes in guideline targets for PTH and phosphate, or growing concerns about vascular calcification with vitamin D sterol use. PTH targets were effectively relaxed in 2009 in the United States, with the prior upper bound of 300 pg/ml rising to approximately 600 pg/ml. Serum phosphate targets were also less stringently defined in the 2009 guidelines (8). The generally weak quality of evidence underlying each recommendation was also more clearly presented, encouraging judgment and individualization over standardization. Practice changes may also have been driven by introduction of the Quality Incentive Program hypercalcemia performance measure in 2014, corresponding with prescribing less vitamin D sterol–based strategies and more cinacalcet-containing strategies in the 2012–2015 cohort. Our results suggest policy and guideline changes affected mineral metabolism treatment strategies in the United States and resulted in greater practice variation in the field. In fact, policy changes since 2015, such as addition of calcimimetics into the bundled PPS, may further affect practice and practice variation.
Facility variation is common in hemodialysis care and has been reported in anemia management, dialysis access, timing of dialysis initiation, and kidney transplant referrals (19–22). Our findings are generally consistent with results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) that demonstrated substantial facility variation in cinacalcet use for mineral metabolism management (23). However, we extend these findings to discuss the full constellation of approaches taken to mineral metabolism. Providers have choices about when to start therapy, how intensively to treat, and what combinations of medications to choose. By evaluating all classes of mineral metabolism drugs in concert, and evaluating them over a common time axis, we can draw more complete inferences about an integrated “style” in the approach to mineral metabolism, including both drug selection and rapidity of initiation. Unlike prior studies, we evaluated for practice variation and found significant variation in mineral metabolism strategies across facilities after case-mix adjustment. Although variations described in our study and others could be interpreted as highlighting either nonstandard care or care individually tailored to different patient populations, it may simply reflect the uncertainty about best practices and the dearth of randomized controlled trials. Given the range of strategies observed when patients had PTH >300 pg/ml, comparative effectiveness research studies are urgently needed to identify the optimal approach to mineral metabolism management.
Cinacalcet use was generally low in our study, particularly in the earlier cohorts. In contrast, prior studies have demonstrated that approximately 20%–30% of US patients on hemodialysis used cinacalcet during this period (11,18,23). However, these estimates apply to prevalent patients on hemodialysis. Cinacalcet is known to be used more frequently among patients who have spent a longer duration on dialysis (11,23). Milder secondary hyperparathyroidism around the time of dialysis initiation in our incident cohort likely accounts for the lower frequency of cinacalcet that we observed. Similar to our study, other studies using USRDS data have also found low use of cinacalcet proximate to dialysis initiation (18). In our sensitivity analyses, cinacalcet use was significantly higher, up to about 30%, 12 months after the first PTH >600 pg/ml.
Although prescribing patterns for mineral metabolism can begin in the predialysis period, most patients in our cohort received no mineral metabolism medications at initiation. A strength of our study is our incident design that allows us to evaluate how treatment evolves over time from dialysis initiation, without extrapolating from cross-sectional data. Further, our evaluation of mineral metabolism treatment strategies early on, as disease progresses, can best distinguish providers’ initial therapeutic intent. For instance, it is possible to identify providers or facilities that target PTH lowering more aggressively, such as with early cinacalcet use, versus more conservatively, such as delaying the start of vitamin D sterols or cinacalcet. Patterns in prevalent data may reflect providers’ initial preferences to a certain extent, but also reflect patient-level factors such as treatment resistance or intolerance of initial therapies.
Additional strengths include our comprehensive prescription data obtained from DCI’s EHR that allowed us to include individuals who may not have been enrolled in Medicare Parts A, B, and D. In this regard, our data are more broadly generalizable to the hemodialysis population compared with studies ascertaining medications exclusively from Medicare. Our cohort’s characteristics approximate USRDS data with a median age just >65 years in USRDS versus 64 years in our data, 27% Black race in USRDS versus 34% in our data, and the similar findings of 58% men and about half with kidney failure due to diabetes in both our data and USRDS (14). Mineral metabolism prescriptions (e.g., cinacalcet, phosphate binders) are also comparable with reported national use from the broader US hemodialysis population in the DOPPS Practice Monitor (www.dopps.org/dpm) (24), supporting generalizability. It is possible, however, that care within DCI may differ from other dialysis facilities due to structural features such as EHR pathways or pharmacy formularies, which we could not investigate. DCI did not have specific protocols relating to mineral metabolism management during the study’s observation period, and prescribing these agents was left to the provider’s discretion.
Our study also had limitations. We did not have detailed data on patient preferences or social circumstances that may have influenced treatment strategies. Our models of practice variation incorporated basic clinical characteristics, such as demographics and insurance, to account for differences in major case-mix factors that could affect treatment choices, but could not fully account for all clinical factors. We noted some additional differences, such as longer dialysis treatment time in later cohort years. Extending dialysis time could be another strategy used to manage mineral metabolism derangements that we were not able to incorporate into our strategies. Other features of mineral metabolism management, such as dialysate calcium or dietary phosphate restriction, also could not be fully assessed.
In summary, our data demonstrate secular changes in mineral metabolism treatment over the past decade, accompanied by growing practice variation. These changes may not be simply attributable to payment-model changes in hemodialysis, but also to changes in guidelines and the nephrology community’s beliefs about the relative risks and benefits of intensively managing mineral metabolism. The growing equipoise is an opportunity for natural experiments and randomized controlled trials to build the evidence base that is needed in nephrology for stronger, evidence-based recommendations in this important area in hemodialysis care.
Disclosures
R. Hall reports receiving consulting fees from United Health Group for consulting on a CKD telehealth program, outside the submitted work. J.J. Scialla has received modest research support for clinical event activities related to trials sponsored by Eli Lilly, GlaxoSmithKline, and Sanofi, and has received consulting fees from Tricida. D.E. Weiner reports receiving support paid to his institution for his role as medical director of clinical research at DCI, outside the submitted work. All remaining authors have nothing to disclose.
Funding
The Comparative Effectiveness Studies in Dialysis Patients Group was supported by National Institute of Diabetes and Digestive and Kidney Diseases grant R01DK111952. Additional support was provided in part by National Institute on Aging award number K76AG059930 (to R. Hall), National Center for Advancing Translational Sciences award number UL1TR002553, and the ASN Foundation for Kidney Research (to R. Hall). P.L. Ephraim reports receiving grants from Johns Hopkins University, during the conduct of the study.
Supplementary Material
Acknowledgments
The authors are grateful to the staff and patients of DCI.
Neither the sponsors nor DCI had a deciding role in the study design, analysis, interpretation of the data, writing of the report, or the decision to submit the report for publication. The manuscript reflects the interpretation and opinions of the authors and is not expressly endorsed by the National Institutes of Health, the National Institute of Diabetes and Digestive and Kidney Diseases, National Institute on Aging, or DCI.
The data reported here have been supplied by the USRDS. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the US Government.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.04350420/-/DCSupplemental.
Supplemental Table 1. Characteristics of individuals not included and included in the 12-month cohort used in facility variation models compared with the overall sample.
Supplemental Table 2. Cross-sectional distributions of treatment strategies by months from incidence and incident cohort year (reported as percentage of incident year starters).
Supplemental Table 3. Cross-sectional distributions of treatment strategies by months from incidence and incident cohort year (reported as percentage of observed individuals at each time point).
Supplemental Table 4. Mixed effects multinomial (nominal) logit models for active mineral metabolism treatment strategies based on cohort and days since incident hemodialysis compared with the vitamin D and phosphate binder reference strategy.
Supplemental Table 5. Cross-sectional distributions of treatment strategies by months from PTH >300 pg/ml and incident cohort year (reported as percentage of incident year starters).
Supplemental Table 6. Cross-sectional distributions of treatment strategies by months from PTH >300 pg/ml and incident cohort year (reported as percentage of observed individuals at each time point).
Supplemental Table 7. Cross-sectional distributions of treatment strategies at 0 and 12 months by months from PTH >300, >400, >500, and >600 pg/ml) and incident cohort year (reported as percentage of observed individuals at each time point).
Supplemental Table 8. Variation in mineral metabolism treatment strategies across facilities described by random effects (RE), intraclass correlation coefficient (ICC), and median odds ratio (MOR).
Supplemental Figure 1. Flow of patients into cohort and sub-analyses reflecting inclusion and exclusion criteria.
Supplemental Figure 2. Sankey plots depicting mineral metabolism treatment strategies from parathyroid hormone (PTH) >300 pg/ml (HyperPTH) by year of eligible PTH: (a) 2006–2008; (b) 2009–2011; (c) 2012–2015.
References
- 1.Palmer SC, Hayen A, Macaskill P, Pellegrini F, Craig JC, Elder GJ, Strippoli GF: Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: A systematic review and meta-analysis. JAMA 305: 1119–1127, 2011. [DOI] [PubMed] [Google Scholar]
- 2.Lunyera J, Scialla JJ: Update on chronic kidney disease mineral and bone disorder in cardiovascular disease. Semin Nephrol 38: 542–558, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palmer SC, Teixeira-Pinto A, Saglimbene V, Craig JC, Macaskill P, Tonelli M, de Berardis G, Ruospo M, Strippoli GF: Association of drug effects on serum parathyroid hormone, phosphorus, and calcium levels with mortality in CKD: A meta-analysis. Am J Kidney Dis 66: 962–971, 2015. [DOI] [PubMed] [Google Scholar]
- 4.Scialla JJ: Evidence basis for integrated management of mineral metabolism in patients with end-stage renal disease. Curr Opin Nephrol Hypertens 27: 258–267, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Block GA: Therapeutic interventions for chronic kidney disease-mineral and bone disorders: Focus on mortality. Curr Opin Nephrol Hypertens 20: 376–381, 2011. [DOI] [PubMed] [Google Scholar]
- 6.Isakova T, Nickolas TL, Denburg M, Yarlagadda S, Weiner DE, Gutiérrez OM, Bansal V, Rosas SE, Nigwekar S, Yee J, Kramer H: KDOQI US commentary on the 2017 KDIGO clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Am J Kidney Dis 70: 737–751, 2017. [DOI] [PubMed] [Google Scholar]
- 7.Uhlig K, Berns JS, Kestenbaum B, Kumar R, Leonard MB, Martin KJ, Sprague SM, Goldfarb S: KDOQI US commentary on the 2009 KDIGO clinical practice guideline for the diagnosis, evaluation, and treatment of CKD-mineral and bone disorder (CKD-MBD). Am J Kidney Dis 55: 773–799, 2010. [DOI] [PubMed] [Google Scholar]
- 8.Kidney Disease Improving Global Outcomes (KDIGO) CKD-MBD Work Group : KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Available at: https://kdigo.org/wp-content/uploads/2017/02/KDIGO-2009-CKD-MBD-Guideline-English.pdf. Accessed September 12, 2020 [DOI] [PubMed]
- 9.Kidney Disease Improving Global Outcomes CKD-MBD Update Work Group : KDIGO 2017. Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Available at: https://kdigo.org/wp-content/uploads/2017/02/2017-KDIGO-CKD-MBD-GL-Update.pdf. Accessed September 12, 2020 [DOI] [PMC free article] [PubMed]
- 10.Block GA, Martin KJ, de Francisco AL, Turner SA, Avram MM, Suranyi MG, Hercz G, Cunningham J, Abu-Alfa AK, Messa P, Coyne DW, Locatelli F, Cohen RM, Evenepoel P, Moe SM, Fournier A, Braun J, McCary LC, Zani VJ, Olson KA, Drüeke TB, Goodman WG: Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med 350: 1516–1525, 2004. [DOI] [PubMed] [Google Scholar]
- 11.St Peter WL, Li Q, Liu J, Persky M, Nieman K, Arko C, Block GA: Cinacalcet use patterns and effect on laboratory values and other medications in a large dialysis organization, 2004 through 2006. Clin J Am Soc Nephrol 4: 354–360, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiner D, Watnick S: The ESRD quality incentive program-Can we bridge the chasm? J Am Soc Nephrol 28: 1697–1706, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chertow GM, Block GA, Correa-Rotter R, Drüeke TB, Floege J, Goodman WG, Herzog CA, Kubo Y, London GM, Mahaffey KW, Mix TC, Moe SM, Trotman ML, Wheeler DC, Parfrey PS; EVOLVE Trial Investigators : Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med 367: 2482–2494, 2012. [DOI] [PubMed] [Google Scholar]
- 14.United States Renal Data System (USRDS) : 2019 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2019. Available at: https://www.usrds.org/annual-data-report/. Accessed September 8, 2020
- 15.Liu J, Huang Z, Gilbertson DT, Foley RN, Collins AJ: An improved comorbidity index for outcome analyses among dialysis patients. Kidney Int 77: 141–151, 2010. [DOI] [PubMed] [Google Scholar]
- 16.Hedeker D: A mixed-effects multinomial logistic regression model. Stat Med 22: 1433–1446, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Merlo J, Chaix B, Ohlsson H, Beckman A, Johnell K, Hjerpe P, Råstam L, Larsen K: A brief conceptual tutorial of multilevel analysis in social epidemiology: Using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Community Health 60: 290–297, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spoendlin J, Schneeweiss S, Tsacogianis T, Paik JM, Fischer MA, Kim SC, Desai RJ: Association of medicare’s bundled payment reform with changes in use of vitamin D among patients receiving maintenance hemodialysis: An interrupted time-series analysis. Am J Kidney Dis 72: 178–187, 2018. [DOI] [PubMed] [Google Scholar]
- 19.Scialla JJ, Liu J, Crews DC, Guo H, Bandeen-Roche K, Ephraim PL, Tangri N, Sozio SM, Shafi T, Miskulin DC, Michels WM, Jaar BG, Wu AW, Powe NR, Boulware LE; DEcIDE Network Patient Outcomes in End Stage Renal Disease Study Investigators : An instrumental variable approach finds no associated harm or benefit with early dialysis initiation in the United States [published correction appears in Kidney Int 89: 957, 2016]. Kidney Int 86: 798–809, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenwood RN, Ronco C, Gastaldon F, Brendolan A, Homel P, Usvyat L, Bruno L, Carter M, Levin NW: Erythropoeitin dose variation in different facilities in different countries and its relationship to drug resistance. Kidney Int Suppl [87]: S78–S86, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Clark EG, Akbari A, Hiebert B, Hiremath S, Komenda P, Lok CE, Moist LM, Schachter ME, Tangri N, Sood MM: Geographic and facility variation in initial use of non-tunneled catheters for incident maintenance hemodialysis patients. BMC Nephrol 17: 20, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paul S, Plantinga LC, Pastan SO, Gander JC, Mohan S, Patzer RE: Standardized transplantation referral ratio to assess performance of transplant referral among dialysis facilities. Clin J Am Soc Nephrol 13: 282–289, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuller DS, Xing S, Belozeroff V, Yehoshua A, Morgenstern H, Robinson BM, Rubin RJ, Bhatt N, Pisoni RL: Variability in cinacalcet prescription across US hemodialysis facilities. Clin J Am Soc Nephrol 14: 241–249, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arbor Research Collaborative for Health: The Dialysis Outcomes and Practice Patterns Study (DOPPS) Practice Monitor. Available at: https://www.dopps.org/dpm/. Accessed June 16, 2020
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






