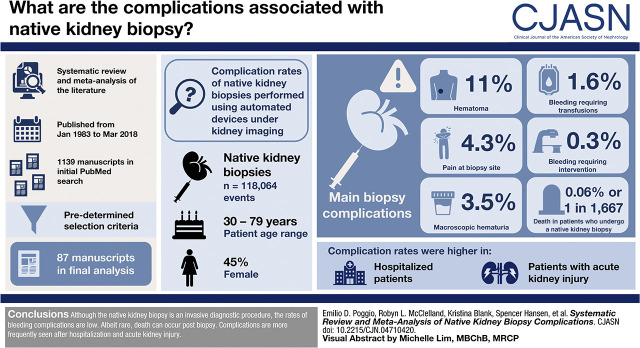
Visual Abstract
Keywords: kidney biopsy, chronic kidney disease, acute renal failure
Abstract
Background and objectives
Native kidney biopsies are commonly performed in the diagnosis of acute kidney diseases and CKD. Because of the invasive nature of the procedure, bleeding-related complications are not uncommon. The National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases–sponsored Kidney Precision Medicine Project requires that all participants undergo a kidney biopsy; therefore, the objective of this analysis was to study complication rates of native kidney biopsies performed using automated devices under kidney imaging.
Design, setting, participants, & measurements
This is a systematic review and meta-analysis of the literature published from January 1983 to March 2018. The initial PubMed search yielded 1139 manuscripts. Using predetermined selection criteria, 87 manuscripts were included in the final analysis. A random effects meta-analysis for proportions was used to obtain combined estimates of complication rates. Freeman–Tukey double-arcsine transformations were used to stabilize variance as complications were rare.
Results
A total of 118,064 biopsies were included in this study. Patient age ranged from 30 to 79 years, and 45% of patients were women. On the basis of our meta-analysis, pain at the site of biopsy is estimated to occur in 4.3% of biopsied patients, hematomas are estimated to occur in 11%, macroscopic hematuria is estimated to occur in 3.5%, bleeding requiring blood transfusions is estimated to occur in 1.6%, and interventions to stop bleeding are estimated to occur in only 0.3%. Death attributed to native kidney biopsy was a rare event, occurring only in an estimated 0.06% of all biopsies but only 0.03% of outpatient biopsies. Complication rates were higher in hospitalized patients and in those with acute kidney disease. The reported complications varied on the basis of study type and geographic location.
Conclusions
Although the native kidney biopsy is an invasive diagnostic procedure, the rates of bleeding complications are low. Albeit rare, death can occur postbiopsy. Complications are more frequently seen after kidney biopsies of hospitalized patients with AKI.
Introduction
The native kidney biopsy was introduced into clinical practice in the 1950s, but the technique has evolved over time. Since the late 1980s, kidney biopsies have been done with the assistance of automated biopsy devices and imaging of the kidneys, mostly ultrasonography. This evolution of the procedure has therefore changed the type and severity of postbiopsy complications. The primary complications of native kidney biopsies are related to hemorrhagic events that can manifest in the form of pain, hematuria, perinephric bleeding that is self-contained as a hematoma, or active bleeding requiring red blood cell transfusions or interventions to control the bleed. Albeit rare, the most serious adverse event is death.
The medical literature on complications related to native kidney biopsies is vast and dates back more than a half century, but it is of limited quality due to study heterogeneity, variability in the definition of complications, and reporting bias. Most studies described single-center experiences from different regions of the world. From the numerous available publications, there has been one meta-analysis and systematic review of 34 studies (9474 biopsies) that focused on bleeding complications after biopsies that were performed under kidney imaging with an automated biopsy device (1).
In the Kidney Precision Medicine Project (KPMP), protocol kidney biopsies will be performed for research purposes. The overarching goal of the KPMP is to conceptually change the paradigms of CKD and acute kidney disease by integrating deep molecular phenotyping of kidney tissue with patient characteristics and disease outcomes. Native kidney biopsies from such patients will undergo regional and single-cell interrogation with a variety of techniques, including RNA sequencing, proteomics, and metabolomics. The current meta-analysis was undertaken to obtain an estimate of percutaneous native kidney biopsy complications in order to provide patients in the KPMP with accurate risk information during the informed consent process. We did an intentional and detailed review of the literature describing the risks and complications associated with native kidney biopsies. The KPMP Kidney Biopsy Working Group expanded upon the prior meta-analysis by adding relevant publications from June 2011 to 2017 (1). The focus of this investigation was again on complication rates of native kidney biopsies performed using automated devices in conjunction with kidney imaging for acute kidney diseases and CKD.
Materials and Methods
Search Strategy and Review Process
Our initial literature search captured articles published from January 1983 to March 2018 and used MEDLINE, Embase, and the Cochrane Library; it was restricted to publications in English. The following medical subject headings terms were used to identify potential papers: kidney, biopsy/kidney, biopsy/fine needle, biopsy/adverse effects, and biopsy/complication. Each medical subject heading term was then combined with “biopsy” and “kidney.”
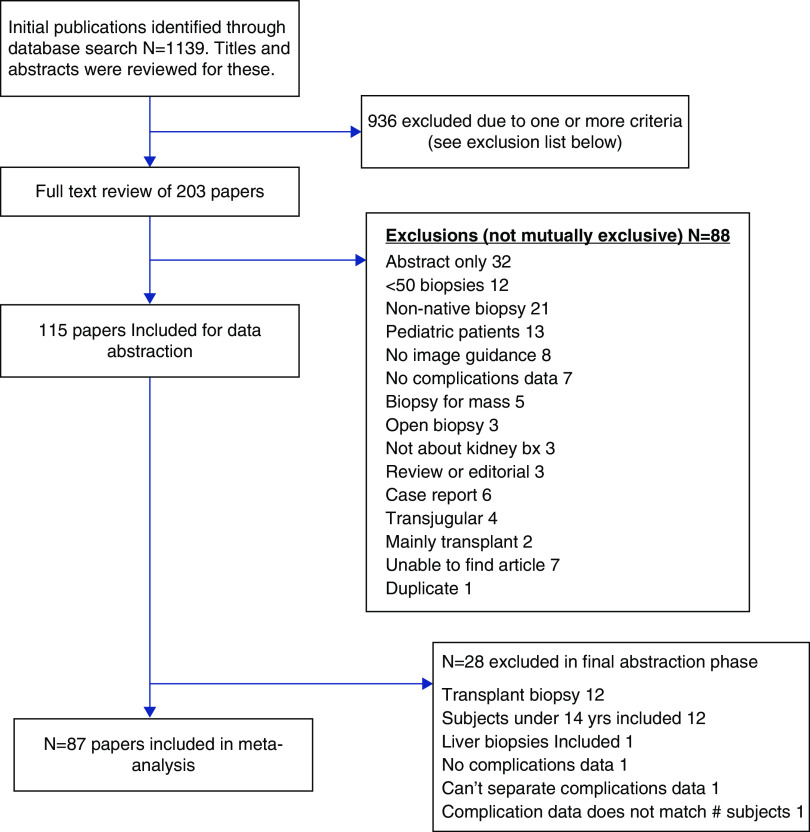
This search strategy identified 1139 potential papers. The review of these papers was conducted in three phases. In the first phase, the papers were randomly divided among 16 reviewers. The title and abstract for each paper were evaluated by a single reviewer. Papers were eliminated on the basis of one or more of the following criteria: abstract only (no accompanying paper); <50 biopsies; non-native biopsies included and unable to be excluded; pediatric patients included and unable to be excluded; no image guidance; no complication data provided; biopsy for kidney mass; open kidney biopsy; nonkidney biopsy; review or editorial; patient report; and use of a transjugular approach. In the first round of review, 936 papers were eliminated, leaving 203 papers for full-text review. In the full-text review, the 203 papers were again randomly divided and evaluated by a single reviewer. The entire paper was assessed, and the reasons for exclusion (same as the first round) were recorded in detail for each paper. In this phase, 88 additional papers were excluded.
For full data abstraction, the remaining 115 papers were randomly assigned to two reviewers. The reviewers entered general descriptive data from the paper (e.g., country of origin, number of patients, number of biopsies, number of sites, study design, average age, percent women), the procedures (e.g., needle gauge, average number of passes, duration of monitoring), and the complications reported from our prespecified list of complications (pain, hematomas, macroscopic hematuria, need for transfusion, need for interventions to stop bleeding, and death). All extracted data elements (n=46) were then compared between the two reviewers by an independent third reviewer. Of 115 papers, 90 had at least one data element for which the two reviewers disagreed. There were a total of 185 disagreements overall, of a possible 5290 comparisons. The disagreements were sent back as queries to the original two reviewers who then discussed and resolved via consensus. This protocol was not registered online.
Statistical Methods
We conducted a meta-analysis of proportions on the basis of a random effects model (2). This model divides the heterogeneity into two components: the between-study variance due to the true variation among different studies, and the within-study variance due to sampling error. The between-study variance is denoted by τ2. We tested the null hypothesis H0:τ2 = 0 using Cochran Q and a chi-squared test to determine P values. Heterogeneity was quantified by the I2 statistic, which is the percentage of total variation across studies that is due to heterogeneity rather than chance (3). We estimated the random effects model using the restricted maximum likelihood (4) for all complications except death. Because of the number of zero proportions, we used the Freeman–Tukey double-arcsine transformation (5) to avoid bias and stabilize the variance for the estimated effect sizes (6). We used back transformation (7) to find the estimated proportion for the total effect estimate. Because of the rarity of death, the random effects model was unable to provide a stable estimate for the true proportion of death. Thus, we used a β-binomial model to model the number of deaths using a binomial distribution and the underlying proportion of deaths with a β-distribution (8). We did not report any heterogeneity statistics for this approach as it was not comparable with the other analyses. This is because we do not calculate a value for τ2 in this approach. Outlier studies were identified on the basis of visual inspection of forest plots and absolute residuals more than two. Influential studies were identified on the basis of leave-one-out analysis. We conducted subgroup analysis for all complications except death. We assumed common between-study variance for subgroups and used an omnibus test to examine if there was a significant difference between subgroup estimates. All analyses were conducted using R version 3.6.1 with the Meta, Metafor, and Forestplot packages.
Results
After extensive review of the English literature and application of the selection process described in Figure 1, 87 papers were used for this meta-analysis. These studies were published between 1983 and March 2018 and included 182,546 kidney biopsies. The largest study comprised 118,064 biopsies, and the smallest had 50 biopsies. Most of these investigations described clinical cohorts, but seven were randomized controlled trials. The average age of the patients included in each study ranged from 30 to 79 years, and 45% were women. The details of the reported studies are given in Supplemental Table 1.
Figure 1.
This flow chart describes the number of papers reviewed at each of the three rounds of review. At each stage, papers were excluded from further review on the basis of one or more of the exclusion criteria. The final meta-analysis was conducted on the basis of data from 87 papers. bx, biopsy.
The biopsy complications of interest were pain, kidney hematoma, macroscopic hematuria, red blood cell transfusion, need for surgical/radiologic intervention to control bleeding from the kidney, and death. Not all of these domains were specifically examined in each investigation. There was significant heterogeneity between studies in the various domains (Table 1). Heterogeneity for all of the complication domains is visually depicted through forest plots of the proportion of events found in each study contributing to that domain (Supplemental Figures 1–6).
Table 1.
Summary of kidney biopsy complications
| Complication Domain | All Studies | Influential Studies Excluded | ||||
|---|---|---|---|---|---|---|
| Proportion | 95% Confidence Interval | I2, % | Proportion | 95% Confidence Interval | I2, % | |
| Pain | 0.043 | 0.02 to 0.07 | 94 | |||
| Hematoma | 0.11 | 0.07 to 0.15 | 99 | 0.088 | 0.06 to 0.12 | 98 |
| Hematuria | 0.035 | 0.03 to 0.04 | 99 | |||
| Transfusion | 0.016 | 0.01 to 0.02 | 99 | 0.014 | 0.01 to 0.02 | 88 |
| Intervention | 0.003 | 0.00 to 0.01 | 73 | |||
| Death | 0.0006 | 0.00 to 0.00 | 0.0003 | 0.00 to 0.00 | ||
The proportion of patients who experienced one or more of these biopsy complications is summarized in Table 1. For each complication domain except death, a more detailed examination of occurrence stratified by geographical region, biopsy vintage, and biopsy needle gauge is given in Tables 2 and 3. The overall incidence of complications was low, especially for the serious adverse events of interventions to stop bleeding and death. These interventions and red blood cell transfusions occurred significantly less frequently in Asia than the United States or Europe, and Europe had a lower incidence of macroscopic hematuria than the United States and Asia. There were more pain events when a smaller needle (18 versus 16 gauge) was used, but this analysis included <1500 biopsies. There was also a numerical trend toward more hematomas and transfusions with the smaller needle, but statistical significance was not reached.
Table 2.
Pain, hematoma, and macroscopic hematuria complications stratified by region, year, and needle gauge
| Subgroup | Papers, n | Pain or Hematoma, n | Biopsies, n | Estimate | 95% Confidence Interval | I2, % | Modifier Test: P Value |
|---|---|---|---|---|---|---|---|
| Pain | |||||||
| America | 3 | 10 | 1440 | 0.0110 | [0.00 to 0.06] | 76.5 | |
| Asia | 7 | 118 | 1485 | 0.0596 | [0.02 to 0.11] | 94.6 | |
| Europe | 8 | 66 | 1488 | 0.0455 | [0.02 to 0.09] | 88.3 | 0.24 |
| Pre-2000 | 6 | 57 | 763 | 0.0728 | [0.03 to 0.13] | 84.1 | |
| 2000–2009 | 6 | 115 | 1938 | 0.0427 | [0.01 to 0.09] | 96.2 | |
| 2010–2018 | 6 | 22 | 1712 | 0.0212 | [0.00 to 0.06] | 85.3 | 0.21 |
| 16 Gauge | 3 | 13 | 612 | 0.0230 | [0.00 to 0.08] | 85.6 | |
| 18 Gauge | 3 | 106 | 812 | 0.1274 | [0.06 to 0.22] | 93.7 | 0.02 |
| Overall | 18 | 194 | 4413 | 0.0429 | [0.02 to 0.07] | 93.8 | |
| Hematoma | |||||||
| America | 15 | 428 | 5012 | 0.0947 | [0.03 to 0.18] | 95.7 | |
| Asia | 19 | 1136 | 6658 | 0.1319 | [0.07 to 0.22] | 99.3 | |
| Europe | 26 | 877 | 15,989 | 0.0924 | [0.04 to 0.16] | 98.6 | 0.67 |
| Pre-2000 | 12 | 257 | 2053 | 0.1249 | [0.04 to 0.24] | 97.4 | |
| 2000–2009 | 16 | 765 | 5639 | 0.1060 | [0.04 to 0.20] | 99.3 | |
| 2010–2018 | 34 | 1419 | 19,967 | 0.0980 | [0.05 to 0.16] | 98.9 | 0.88 |
| 16 Gauge | 23 | 420 | 8423 | 0.0574 | [0.02 to 0.11] | 95.9 | |
| 18 Gauge | 9 | 534 | 1728 | 0.1614 | [0.07 to 0.29] | 99.1 | 0.06 |
| Overall | 62 | 2441 | 27,659 | 0.1050 | [0.07 to 0.15] | 98.9 | |
| Macroscopic hematuria | |||||||
| America | 14 | 15,466 | 122,779 | 0.0481 | [0.03 to 0.07] | 97.3 | |
| Asia | 25 | 280 | 7321 | 0.0397 | [0.03 to 0.05] | 84.4 | |
| Europe | 25 | 722 | 27,511 | 0.0244 | [0.02 to 0.04] | 93.5 | 0.05 |
| Pre-2000 | 14 | 138 | 2389 | 0.0518 | [0.03 to 0.07] | 43.5 | |
| 2000–2009 | 16 | 449 | 9543 | 0.0318 | [0.02 to 0.05] | 94.1 | |
| 2010–2018 | 34 | 15,881 | 145,679 | 0.0305 | [0.02 to 0.04] | 99.3 | 0.10 |
| 16 Gauge | 22 | 232 | 8614 | 0.0249 | [0.01 to 0.04] | 88.9 | |
| 18 Gauge | 9 | 78 | 1659 | 0.0351 | [0.02 to 0.06] | 68.5 | 0.37 |
| Overall | 64 | 16,468 | 157,611 | 0.0347 | [0.03 to 0.04] | 98.8 |
Table 3.
Transfusion and surgical/radiologic intervention complications stratified by region, year, and needle gauge
| Subgroup | Papers, n | Transfusion or Intervention, n | Biopsies, n | Estimate | 95% Confidence Interval | I2, % | Modifier Test: P Value |
|---|---|---|---|---|---|---|---|
| Transfusion | |||||||
| America | 15 | 31,029 | 123,864 | 0.0460 | [0.03 to 0.07] | 99.5 | |
| Asia | 23 | 195 | 22,141 | 0.0075 | [0.00 to 0.02] | 85.9 | |
| Europe | 21 | 187 | 16,800 | 0.0103 | [0.00 to 0.02] | 65.0 | <0.001 |
| Pre-2000 | 7 | 33 | 1231 | 0.0172 | [0.00 to 0.04] | 69.0 | |
| 2000–2009 | 17 | 119 | 6759 | 0.0108 | [0.00 to 0.02] | 81.3 | |
| 2010–2018 | 35 | 31,259 | 154,815 | 0.0187 | [0.01 to 0.03] | 99.8 | 0.49 |
| 16 Gauge | 21 | 219 | 10,711 | 0.0574 | [0.02 to 0.11] | 95.9 | |
| 18 Gauge | 9 | 31 | 2777 | 0.1614 | [0.07 to 0.29] | 99.1 | 0.06 |
| Overall | 59 | 31,411 | 162,805 | 0.0160 | [0.01 to 0.02] | 99.8 | |
| Surgical/radiologic intervention | |||||||
| America | 19 | 216 | 124,630 | 0.0047 | [0.00 to 0.01] | 80.3 | |
| Asia | 23 | 43 | 21,897 | 0.0006 | [0.00 to 0.00] | 59.5 | |
| Europe | 24 | 74 | 17,467 | 0.0052 | [0.00 to 0.01] | 62.8 | 0.04 |
| Pre-2000 | 9 | 9 | 1645 | 0.0033 | [0.00 to 0.01] | 0.0 | |
| 2000–2009 | 17 | 34 | 6654 | 0.0029 | [0.00 to 0.01] | 35.2 | |
| 2010–2018 | 40 | 290 | 155,695 | 0.0036 | [0.00 to 0.01] | 77.6 | 0.80 |
| 16 Gauge | 24 | 55 | 10,799 | 0.0024 | [0.00 to 0.01] | 39.8 | |
| 18 Gauge | 11 | 12 | 2994 | 0.0005 | [0.00 to 0.00] | 23.7 | 0.28 |
| Overall | 66 | 333 | 163,994 | 0.0033 | [0.00 to 0.01] | 72.8 |
The most serious complication, death, was highly influenced by one study (8). This study investigated over 100,000 patients and recorded 2125 deaths. All of the other studies together reported only 15 deaths in 42,066 biopsies. Unlike any of the other studies, the investigation of Al Turk et al. (8) interrogated a nationwide inpatient database to identify patients who had a kidney biopsy at some point during their hospitalization. Deaths occurred during the hospitalizations and could not necessarily be attributed to the kidney biopsy. Excluding the study of Al Turk et al. (8) decreased the meta-analyzed estimated proportions of death from 0.0006 to 0.0003. Similarly, the need for blood transfusion postbiopsy was influenced by the study by Al Turk et al. (8), but removing that study did not change the proportion of transfusions needed or study heterogeneity much (Table 1).
Another common complication of kidney biopsy was perinephric hematoma. Two studies were identified as influential for hematoma occurrence, each finding hematomas in over 80% of the cohort (9,10). Excluding these studies only decreased the proportion of hematomas from 11% to 8.8% (from one in nine to one in 11) and had little effect on study heterogeneity (Table 1). In both of these studies, kidney imaging was done postbiopsy to prospectively assess for hematomas as opposed to waiting for a clinical indication to do postbiopsy imaging. Most hematomas were small (<2 cm). Several other studies reported relatively high hematoma rates (>30%), and postbiopsy imaging was also done routinely in these studies.
Discussion
This analysis was done to obtain an estimate of percutaneous native kidney biopsy complications in order to provide patients undergoing research biopsies for the KPMP with accurate risk information during the informed consent process. We determined the occurrence of adverse events using six biopsy complication domains of importance to patients and clinicians. The most severe adverse event was death, with an incidence of 0.008% (one in 12,500), followed by an intervention to stop bleeding with an incidence of 0.3% (one in 333). The need for a red blood cell transfusion was 1.6% (one in 62.5). Gross hematuria developed in 3.5% of patients (one in 29), and pain developed in 4.3% of patients (one in 23). The incidence of perinephric hematoma was 11% (one in nine).
These risk estimates were on the basis of available data largely from retrospective reports of patient series for biopsies performed for clinical indications. As such, the overall data quality was modest, and the studies were not large. Although the ranges of patients and kidney biopsies assessed were wide, the median number of patients per study was 210. There were no studies that were both prospective and designed specifically to identify complication rates. Additionally, many of the studies did not assess the full range of biopsy complication domains considered important for the KPMP. Although several biopsy complications were readily quantified, such as death, interventions to stop bleeding, red blood cell transfusions, and presence of macroscopic hematuria, the postbiopsy observation period was highly variable; therefore, events could have been missed, and rules for attribution to the biopsy procedure were not in place. Pain and hematoma were more difficult to assess. Pain is subjective, and no uniform pain assessment standard was applied in the few studies that reported pain. Similarly, there was no uniform approach to the identification or measurement of perinephric hematomas. These issues produced significant heterogeneity between studies, at least in part due to reporting bias. Because of this heterogeneity, we suggest that it is reasonable to use the upper limit of the confidence intervals provided for each complication domain (Table 1) to provide patients with the most conservative estimate of risk.
The most frequent complication of the percutaneous native kidney biopsy seems to be a postbiopsy perinephric hematoma. Although the overall incidence of hematoma was 11%, this was derived from a mixture of studies that routinely imaged the kidney after biopsy to look for bleeding and studies that only imaged the kidney if there was a clinical indication, such as pain or a fall in hemoglobin. We speculate that if hematomas are specifically sought by imaging the kidney postbiopsy, they will be found often. However, many hematomas will be small and of arguable clinical significance. In many of the reviewed papers, the size of the hematoma was not reported, so size of a clinically relevant hematoma is unclear.
A particularly difficult complication to assess was pain related to the kidney biopsy. Only 18 papers attempted to quantify pain, and only 194 pain events were reported in nearly 4400 biopsies. No standard method of assessing pain was used across studies, and an accepted amount of pain after an uncomplicated kidney biopsy has not been determined. Therefore, the pain domain is the least accurately evaluated complication. The development of a standardized pain assessment is needed.
Death and need for red blood cell transfusion were highly influenced by one study that interrogated the US Nationwide Inpatient Sample database between 2008 and 2012 (9). All included patients (n=118,064) were identified by the International Classification of Disease code for percutaneous native kidney biopsy. In general, these patients may have been sicker than typical patients having elective outpatient diagnostic kidney biopsies. For example, only 27% of these patients had a diagnosis of GN on the basis of administrative codes. Notably, two thirds of the patients had AKI, and 15% had a pathologic diagnosis of acute tubular necrosis. Administrative codes were also used to identify complications. Mortality in this cohort was 1.8%, but it was twice as high (2%) in patients admitted to the hospital nonelectively compared with electively (0.99%). Red blood cell transfusions were administered to a quarter of the patients. These complication rates are greater than those reported in other studies of native kidney biopsy. The findings may be explained by the acuity of illness for many of the hospitalized patients, including the presence of comorbidities such as coagulopathies or BP instability, and inability to accurately attribute complications to the biopsy itself as opposed to other conditions occurring during hospitalization. These results are similar to those from a recent investigation that examined native kidney biopsy complications in patients with acute kidney disease that was mainly AKI (11). Mortality was 3% in this cohort, but none of the deaths were directly attributed to the kidney biopsy. Red blood cell transfusions were required in 8% of patients, and 2% needed an intervention to stop bleeding; these adverse events were biopsy complications. These higher complication rates may more accurately reflect risk of performing native kidney biopsies in patients with AKI in the KPMP who are often hospitalized with significant comorbidities, as opposed to those undergoing elective, outpatient kidney biopsies.
Difficulty arises when analyzing the mortality end point due to the rarity of the event. The paper by Al Turk et al. (8), which has a much higher death rate then all of the other studies where death was reported, caused issues in the initial analysis (9). Furthermore, with many studies reporting zero deaths, the preferred analysis that uses random effects could not be used. Instead, we fit a β-binomial model, a method that has been shown to be useful in the setting of meta-analysis of proportions for very rare outcomes (8).
Since performing this systematic analysis, four additional investigations of complications in adults undergoing a native kidney biopsy have been published (12–15). Death was examined in three studies and occurred in one of 17,125 biopsies, less than the one in 1667 we found in our meta-analysis (12,14,15). The need for blood transfusion postbiopsy was variable. The rate was below 0.5% in an all-outpatient cohort (12), but it was 4.3% in a mixed outpatient-inpatient cohort and 5.7% in an all-inpatient cohort (13,15). Importantly, the mixed cohort observed a 57% transfusion rate among inpatients who needed an urgent kidney biopsy (15). The all-inpatient cohort data were obtained from the Nationwide Inpatient Sample database using diagnostic codes and included 35,183 biopsies (13). Most biopsies (70%) were done for AKI, and 28% of the patients had diabetes. The meta-analysis found an overall need for blood transfusion in 1.6% of patients, but when stratified by region, transfusions were needed in 4.6% of patients from America, perhaps reflecting a large number of inpatient biopsies. This estimate may be more relevant when discussing biopsy complications with potential research subjects who are inpatients. Finally, the need for angiography or surgical intervention to control bleeding was 0.6% or less in all four studies, a bit higher than the meta-analysis rate of 0.3%. A meta-analysis of 23 investigations of kidney biopsy complications in pediatric patients also demonstrated a low incidence of major bleeding events (16). Blood transfusions were required in 0.6% of patients, and an intervention to control bleeding was needed in 1.2% of patients.
Relevant to the underlying question of whether an extra research core of kidney tissue can be safely obtained during native kidney biopsy, the Transformative Research in Diabetic Neproplathy (TRIDENT) study recently reported its initial biopsy experience (17). The TRIDENT is examining the molecular pathology of diabetic kidney disease. In the first 160 biopsies, 11 patients (7%) had complications, including three patients who needed a blood transfusion, three patients who had gross hematuria, and seven patients who had large (>5-cm) hematomas. Importantly, no patient required an invasive procedure to control bleeding, and there were no deaths.
This analysis did not find an advantage of using an 18-gauge biopsy needle over a 16-gauge needle for any of the complication domains; however, we cannot exclude the possibility that the 18-gauge needles were used for a specific indication in these observational studies. Nonetheless, this suggests that a 16-gauge biopsy needle may be safely used to comfortably obtain enough tissue for histologic diagnosis and research purposes.
This large meta-analysis of all published literature related to native kidney biopsies is limited to some extent by the heterogeneity of the available literature, but its strength relies in the comprehensive approach taken by the KPMP to evaluate all complication domains that are clinically relevant. By systematically reviewing and evaluating all reported complications, especially from recent single-center experiences in the United States and abroad, the presented estimates most likely reflect current practice by minimizing single-center biases.
In conclusion, this meta-analysis has considered the best available data to guide clinicians and patients to make an informed decision regarding the safety of a kidney biopsy. Overall, the data suggest the percutaneous native kidney biopsy, when done for diagnostic and prognostic purposes, is usually very safe and, by extension, is expected to be correspondingly safe in the setting of biopsies being done electively for research purposes, such as the KPMP. However, patients who are hospitalized may be at higher risk for complications than patients undergoing an elective outpatient biopsy.
Disclosures
S. Bansal reports other from Home Therapy Institutes, Osprey Medical, and UpToDate outside the submitted work. K. Kiryluk reports employment by Columbia University and grants from the National Institutes of Health (the National Institute of Diabetes and Digestive and Kidney Diseases, the National Center for Advancing Translational Sciences, the National Human Genome Research Institute) during the conduct of the study and reports other from Goldfinch Bio and nonfinancial support from AstraZeneca outside the submitted work. G.M. McMahon reports receiving nonfinancial support from GSK. P.M. Palevsky reports receiving personal fees from Baxter and grants from BioPorto and Dascena outside the submitted work. S. Parikh reports receiving grant U01: IN4687813OSU from the National Institute of Diabetes and Digestive and Kidney Diseases/the National Institutes of Health during the conduct of the study. E.D. Poggio reports receiving consulting fees from CareDx. S.E. Rosas reports attending one scientific advisory board each for Bayer HealthCare Pharmaceuticals Inc. and Reata in 2019, for which she was compensated. She has received grant support from Bayer HealthCare Pharmaceuticals Inc. and is about to start a study with MedImmune Limited, a wholly-owned subsidiary of AstraZeneca AB; both are clinical trials related to diabetic nephropathy. B.H. Rovin reports receiving personal fees from AstraZeneca, Aurinia, Bristol Myers Squibb, Callidatis, Chemocentryx, EMD Serono, Janssen, Morphosys, Novartis, Omeros, and Retrophin; nonfinancial support from the Lupus Foundation of America; and grants from the National Institutes of Health outside the submitted work. K. Tuttle reports receiving personal fees from AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Gilead, and Novo Nordisk and grants and personal fees from Goldfinch Bio outside the submitted work. A. Vijayan reports receiving personal fees from Boeringher Ingelheim, NxStage, and Sanofi Aventis outside the submitted work. All remaining authors have nothing to disclose.
Funding
The KPMP is funded by National Institute of Diabetes and Digestive and Kidney Diseases grants UH3DK114861, UH3DK114866, UH3DK114870, UH3DK114908, UH3DK114915, UH3DK114926, UH3DK114907, UH3DK114920, UH3DK114923, UH3DK114933, and UH3DK114937.
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.04710420/-/DCSupplemental.
Supplemental Figure 1. Overall pain forest plot.
Supplemental Figure 2. Overall hematoma forest plot.
Supplemental Figure 3. Overall macroscopic hematuria forest plot.
Supplemental Figure 4. Overall erythrocyte transfusion forest plot.
Supplemental Figure 5. Overall surgical/IR intervention forest plot.
Supplemental Figure 6. Overall death forest plot.
Supplemental Material. References.
Supplemental Table 1. Study characteristics.
References
- 1.Corapi KM, Chen JL, Balk EM, Gordon CE: Bleeding complications of native kidney biopsy: A systematic review and meta-analysis. Am J Kidney Dis 60: 62–73, 2012. [DOI] [PubMed] [Google Scholar]
- 2.DerSimonian R, Laird N: Meta-analysis in clinical trials. Control Clin Trials 7: 177–188, 1986. [DOI] [PubMed] [Google Scholar]
- 3.Higgins JP, Thompson SG, Deeks JJ, Altman DG: Measuring inconsistency in meta-analyses. BMJ 327: 557–560, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raudenbush S, Bryk A: Empirical Bayes meta-analysis. J Educ Behav Stat 10: 75–98, 1985 [Google Scholar]
- 5.Freeman M, Tukey J: Transformations related to the angular and the square root. Ann Math Stat 21: 607–611, 1950 [Google Scholar]
- 6.Ma Y, Chu H, Mazumdar M: Meta-analysis of proportions of rare events-A comparison of exact likelihood methods with robust variance estimation. Commun Stat Simul Comput 45: 3036–3052, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller J: The inverse of the Freeman-Tukey double arcsine transformation. Am Stat 32: 138, 1978 [Google Scholar]
- 8.Al Turk AA, Estiverne C, Agrawal PR, Michaud JM: Trends and outcomes of the use of percutaneous native kidney biopsy in the United States: 5-Year data analysis of the nationwide inpatient sample. Clin Kidney J 11: 330–336, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishikawa E, Nomura S, Hamaguchi T, Obe T, Inoue-Kiyohara M, Oosugi K, Katayama K, Ito M: Ultrasonography as a predictor of overt bleeding after renal biopsy. Clin Exp Nephrol 13: 325–331, 2009. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka K, Kitagawa M, Onishi A, Yamanari T, Ogawa-Akiyama A, Mise K, Inoue T, Morinaga H, Uchida HA, Sugiyama H, Wada J: Arterial stiffness is an independent risk factor for anemia after percutaneous native kidney biopsy. Kidney Blood Press Res 42: 284–293, 2017. [DOI] [PubMed] [Google Scholar]
- 11.Moledina DG, Luciano RL, Kukova L, Chan L, Saha A, Nadkarni G, Alfano S, Wilson FP, Perazella MA, Parikh CR: Kidney biopsy-related complications in hospitalized patients with acute kidney disease. Clin J Am Soc Nephrol 13: 1633–1640, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aaltonen S, Finne P, Honkanen E: Outpatient kidney biopsy: A single center experience and review of literature. Nephron 144: 14–20, 2020. [DOI] [PubMed] [Google Scholar]
- 13.Charu V, O’Shaughnessy MM, Chertow GM, Kambham N: Percutaneous kidney biopsy and the utilization of blood transfusion and renal angiography among hospitalized adults. Kidney Int Rep 4: 1435–1445, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawaguchi T, Nagasawsa T, Tsuruya K, Miura K, Katsuno T, Morikawa T, Ishikawa E, Ogura M, Matsumura H, Kurayama R, Matsumoto S, Marui Y, Hara S, Maruyama S, Narita I, Okada H, Ubara Y; Committee of Practical Guide for Kidney Biopsy 2019 : A nationwide survey on clinical practice patterns and bleeding complications of percutaneous native kidney biopsy in Japan [published correction appears in Clin Exp Nephrol 24: 389–401, 2020]. Clin Exp Nephrol 24: 389–401, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palsson R, Short SAP, Kibbelaar ZA, Amodu A, Stillman IE, Rennke HG, McMahon GM, Waikar SS: Bleeding complications after percutaneous native kidney biopsy: Results from the boston kidney biopsy cohort. Kidney Int Rep 5: 511–518, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varnell CD Jr., Stone HK, Welge JA: Bleeding complications after pediatric kidney biopsy: A systematic review and meta-analysis. Clin J Am Soc Nephrol 14: 57–65, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hogan JJ, Owen JG, Blady SJ, Almaani S, Avasare RS, Bansal S, Lenz O, Luciano RL, Parikh SV, Ross MJ, Sharma D, Szerlip H, Wadhwani S, Townsend RR, Palmer MB, Susztak K, Mottl AK; TRIDENT Study Investigators : The feasibility and safety of obtaining research kidney biopsy cores in patients with diabetes: An interim analysis of the TRIDENT study. Clin J Am Soc Nephrol 15: 1024–1026, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.