Abstract
Obesity is known to increase breast cancer incidence and mortality, but the underlying mechanisms remain unsolved. Recent studies demonstrate that adipose fatty acid binding protein (FABP4) promotes obesity-associated breast cancer development, thus suggesting FABP4 as a novel player linking obesity and breast cancer risk.
The Rising Incidence of Breast Cancer and Obesity
Breast cancer is the most frequent cancer diagnosed among women and there has been a progressive rise in breast cancer incidence in the past few decades. Despite improvements in early detection and treatment, breast cancer annually claims the lives of around half a million women worldwide mainly due to metastasis. Even for women with early-stage breast cancer, around 40% of patients have micrometastatic tumor cells after surgery [1]. Current therapies are frequently insufficient to completely eradicate residual dormant cancer cells and triggers promoting the exit of dormancy of tumor cells remain poorly defined. Therefore, uncovering novel mechanisms for breast cancer development and recurrence and identifying new targets for breast cancer prevention and treatment represent critically important foci in the field of breast cancer research.
Obesity has risen at an alarming rate in recent decades due to a sedentary lifestyle in modern society and excess intake of dietary calories, including overconsumption of high-fat diets (HFDs) [2]. Given the prevalence of obesity and the rising incidence of breast cancer, there is an urgent need to identify biological mediators underlying obesity-associated breast cancer.
Association of Obesity and Breast Cancer
Multiple risk factors contribute to breast cancer development, including aging, genetic background, and family and reproductive history, while obesity is associated with increased risk of developing breast cancer for postmenopausal women and increased mortality from breast cancer in women of all ages [3]. Excess energy in obese subjects is stored as lipids in cells and can negatively affect cell metabolism and homeostasis. Compared with lean adipose tissue, obese adipose tissue contains crown-like structures formed by hypertrophic adipocytes surrounded by macrophages. The interaction between macrophages and dying adipocytes promotes obesity-associated chronic inflammation and further pathological alterations [4]. As an endocrine organ, adipose tissue produces multiple mediators (e.g., adipokines, cytokines, chemokines, hormones) to maintain metabolic balance. Dysregulation of these mediators leads to obesity-associated diseases, including many types of cancer [5].
Obesity is strongly associated with an increased risk of postmenopausal hormone receptor-positive breast cancer, and an increased level of circulating estrogens has been proposed as a driver of obesity-associated breast cancer development [6]. However, the estrogen hypothesis does not fully explain the complex obesity/breast cancer association. For example, obesity is directly associated with poor outcome among women diagnosed with triple-negative breast cancer, which is hormone independent and therefore not estrogen responsive [3]. Several non-estrogenic molecular mechanisms have been proposed to explain the obesity/breast cancer association, including obesity-related inflammatory cytokines [e.g., tumor necrosis factor (TNF)α, interleukin (IL)-6], insulin and insulin-like growth factors (IGFs), leptin, and adiponectin dysregulation.
Although supported by experimental data from animal models, these mechanisms have not been consistently confirmed in human studies. Here we explore adipose FABP4 as a possible obesity-related mediator promoting obesity-associated breast cancer development and progression.
A Novel Hypothesis: FABP4 as Driver of Obesity-Associated Breast Cancer
To facilitate the absorption and utilization of water-insoluble dietary long-chain fatty acids (FAs), an evolutionarily conserved protein family known as the FABPs is expressed in various tissues. The FABP family comprises at least nine members each with a distinct tissue expression pattern, such as adipose FABP (A-FABP/FABP4). FABPs function to solubilize various FAs and coordinate their trafficking and responses inside cells. Depending on the cellular and tissue environment, individual FABPs exhibit unique biological functions by regulating different metabolic and inflammatory signaling pathways [7]. FABP4 has attracted the most attention in the field of obesity due to its high expression in adipose tissue and biological functions in macrophages and adipocytes [8]. In macrophages FABP4 modulates inflammatory responses through activation of the nuclear factor kappa B (NF-κB) and c-Jun N-terminal kinase (JNK) pathways. In adipocytes FABP4 promotes lipolysis and inhibits lipogenesis through interactions with hormone-sensitive lipase (HSL) and peroxisome proliferator-activated receptor gamma (PPARγ). Compared with lean individuals, FABP4 is upregulated in the adipose tissue and increasingly released into the circulation of obese patients. It is well established that circulating serum FABP4 is positively associated with body mass index and exerts hormonal regulations in obesity-associated diseases [9,10]. Recent studies identified a critical role for FABP4 in protumor macrophages and in enhancing obesity-associated breast cancer development in mouse models and human studies [11,12]. Thus, we hypothesize that FABP4 plays a central role in the regulation of metabolism, especially in obese individuals, and represents a link between obesity and breast cancer risk.
Potential Mechanisms for FABP4 in Breast Cancer Development
FABP4 could promote breast cancer development and progression via several potential pathways (Figure 1).
Figure 1. Fatty Acid Binding Protein 4 (FABP4) Promotes Breast Cancer Development.
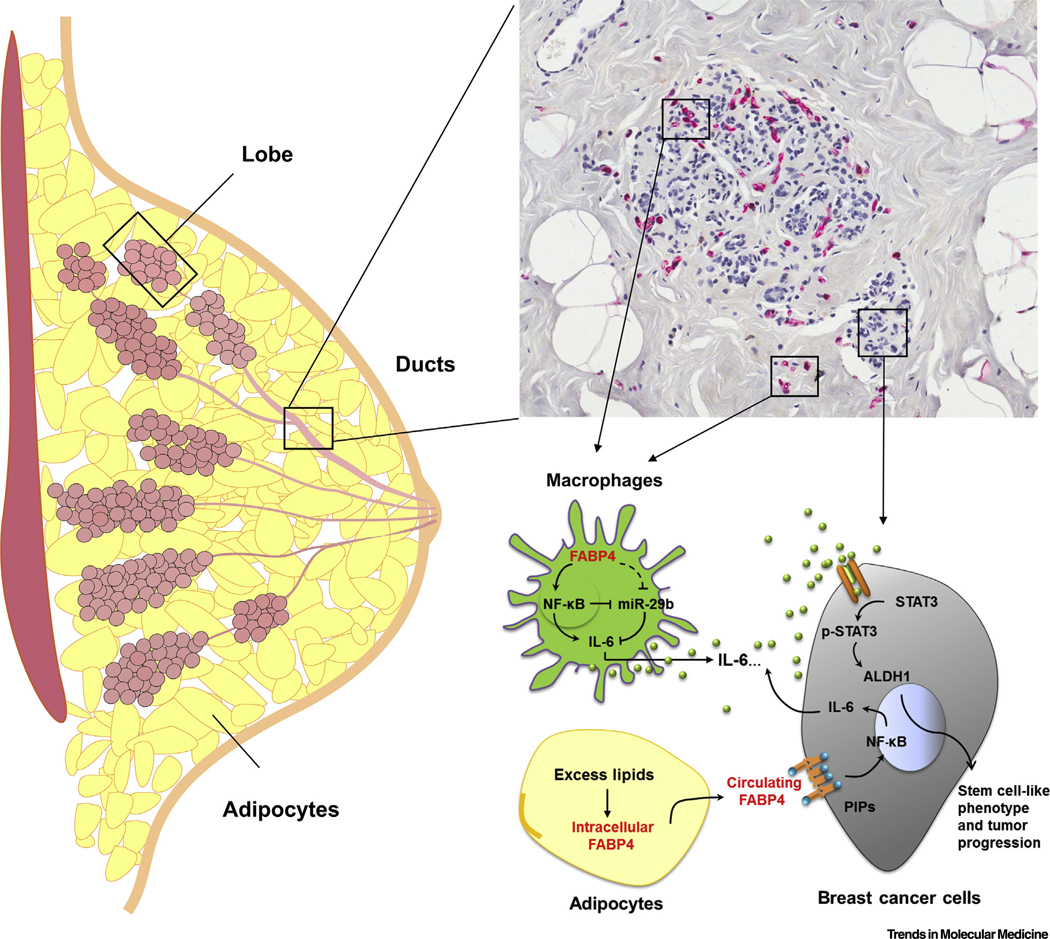
Breast cancer cells originate from either lobules or ducts. FABP4 (red staining) is mainly expressed in tumor stroma, particularly in tumor-associated macrophages (green), inducing protumor interleukin (IL)-6 production via the nuclear factor kappa B (NF-κB)/miR-29b pathway in macrophages. Circulating FABP4 released by adipocytes (yellow) during obesity enhances IL-6/STAT3/ALDH1 activity in breast/mammary tumor cells (gray), leading to an enhanced stem cell-like phenotype and tumor progression. Abbreviations: ALDH1, aldehyde dehydrogenase 1; PIPs, phosphatidylinositol phosphates; STAT3, signal transducer and activator of transcription 3.
Intracellular FABP4
Intracellular FABP4 enhances protumor macrophage function. Macrophages are very heterogeneous in tumor stroma, some with antitumor activity while others exhibit the opposite effect [11]. Interestingly, FABP4 is highly expressed in a small subset of tumor associated macrophages (TAMs) of the CD11b+F4/80+MHCII−Ly6C−CD11c− phenotype. The FABP4-positive TAM subset accumulates in the late stage of mammary tumors to promote tumor growth. Mechanistically, intracellular expression of FABP4 enhances the activation of NF-κB, which negatively regulates miR-29b expression, and reduced binding of miR-29b to the IL-6 3′untranslated region (UTR) region leads to protumor IL-6 signaling in macrophages. Moreover, inhibition of FABP4 either by genetic ablation or by chemical inhibitors in macrophages suppresses mammary tumor growth. Thus, intracellular FABP4 serves as a functional marker for protumor TAMs [11].
Circulating FABP4
Circulating FABP4 mediates expansion of cancer cells with a stem cell-like phenotype. Circulating levels of FABP4 are markedly increased in obese subjects due to increased release from expanded adipose tissue [10]. Elevated FABP4 interacts with cell membrane phospholipids, particular phosphatidylinositol phosphates (PIPs), initiating oncogenic signaling events, including signal transducer and activator of transcription 3 (STAT3) activation, which further enhances the expression of aldehyde dehydrogenase 1 (ALDH1), a hallmark of breast cancer stem cells [13]. In HFD-induced obese mouse models, FABP4 deficiency uncouples obesity and mammary tumor development in various mouse breast cancer models, further demonstrating that FABP4 is a new link underlying obesity-associated breast tumor development [12].
Circulating FABP4 can also promote tumor progression through facilitating exergonic FA transport. It is commonly believed that serum albumins are the primary carrier for circulating free FA (FFA). With obesity, adipocyte death or lipolysis induces pathological elevation of FFA. Emerging evidence suggests that FABP4 is able to bind FFA and provide energy for rapid ovarian tumor growth and metastasis [14]. However, it remains to be clarified whether FABP4 adopts new lipid cargo in circulation or whether it is secreted with the lipid cargo it carries to potential target cells.
Moreover, FABP4 has been shown to promote tumor progression using other mechanisms. For example, FABP4 inhibits tumor suppressor genes by enhancing aberrant DNA methylation in myeloid-derived leukemia cells, linking obesity to epigenetic alterations and leukemia tumor progression [15]. FABP4 also enhances new blood vessel formation in tumor stroma. Taking these findings together, FABP4 strengthens interactions among tumor stromal macrophages, adipocytes, and tumor cells and connects obesity-associated adipokines to tumor-promoting signaling, thus representing a mechanism by which obesity could promote obesity-associated cancer development and progression.
Concluding Remarks
Approximately 20–25% of newly diagnosed breast cancers are ductal carcinoma in situ (DCIS), a noninvasive early-stage breast cancer. While it is believed that most DCIS will never progress to invasive cancers, few factors reliably predict a more aggressive course and progression to overtly invasive disease. Since macrophages are critical in orchestrating invasive breast cancer progression, and FABP4 is a functional marker for protumor TAMs, intracellular FABP4 might prove to be a key molecular sensor regulating protumor macrophage differentiation and function, thus leading to progression of DCIS to invasive disease. Thus, targeting of FABP4 could be exploited as a therapeutic target for breast cancer treatment. Moreover, breast cancer cells escaping from the primary lesion can remain dormant in vivo for many years. It remains unknown what triggers breast cancer awakening in vivo. Circulating FABP4 enhances tumor stem cell-like phenotype via IL-6/STAT3/ALDH1-mediated activity, suggesting that circulating FABP4 released by host adipose tissue might trigger the exit from tumor dormancy. Thus, dynamic monitoring of intracellular and circulating levels of FABP4 could provide a new biomarker for clinical evaluation of breast cancer progression. Finally, targeting FABP4 with either small molecular inhibitors or specific antibodies has been shown to be feasible in other disease settings [9]. Considering the persistent risk of breast cancer recurrence after current therapeutic strategies, inhibition of FABP4 activity might offer a novel strategy for the treatment of obesity-associated breast cancer.
Acknowledgments
This research was supported by National Institutes of Health grants R01CA180986, R01CA177679, and R01AI137324. We thank Dr Claudia Willmes for the insightful suggestions during preparation of this article.
References
- 1.Richman J and Dowsett M (2019) Beyond 5 years: enduring risk of recurrence in oestrogen receptor-positive breast cancer. Nat. Rev. Clin. Oncol 16, 296–311 [DOI] [PubMed] [Google Scholar]
- 2.Hu S et al. (2018) Dietary fat, but not protein or carbohydrate, regulates energy intake and causes adiposity in mice. Cell Metab. 28, 415–431 [DOI] [PubMed] [Google Scholar]
- 3.Picon-Ruiz M et al. (2017) Obesity and adverse breast cancer risk and outcome: mechanistic insights and strategies for intervention. CA Cancer J. Clin. 67, 378–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee MJ et al. (2010) Adipose tissue remodeling in pathophysiology of obesity. Curr. Opin. Clin. Nutr. Metab. Care 13, 371–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khandekar MJ et al. (2011) Molecular mechanisms of cancer development in obesity. Nat. Rev. Cancer 11, 886–895 [DOI] [PubMed] [Google Scholar]
- 6.Li B et al. (2019) A-FABP and oestrogens are independently involved in the development of breast cancer. Adipocyte 8, 379–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Storch J and Corsico B (2008) The emerging functions and mechanisms of mammalian fatty acid-binding proteins. Annu. Rev. Nutr 28, 73–95 [DOI] [PubMed] [Google Scholar]
- 8.Hotamisligil GS and Bernlohr DA (2015) Metabolic functions of FABPs – mechanisms and therapeutic implications. Nat. Rev. Endocrinol 11, 592–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prentice KJ et al. (2019) Adipokine FABP4 integrates energy stores and counterregulatory metabolic responses. J. Lipid Res. 60, 734–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao H et al. (2013) Adipocyte lipid chaperone AP2 is a secreted adipokine regulating hepatic glucose production. Cell Metab. 17, 768–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hao J et al. (2018) Expression of adipocyte/macrophage fatty acid-binding protein in tumor-associated macrophages promotes breast cancer progression. Cancer Res. 78, 2343–2355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hao J et al. (2018) Circulating adipose fatty acid binding protein is a new link underlying obesity-associated breast/mammary tumor development. Cell Metab. 28, 689–705.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ginestier C et al. (2007) ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 1, 555–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nieman KM et al. (2011) Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med. 17, 1498–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan F et al. (2016) Fatty acid-binding protein FABP4 mechanistically links obesity with aggressive AML by enhancing aberrant DNA methylation in AML cells. Leukemia 31, 1434–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]


