Abstract
Background
The severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) epidemic has been emerged as a cardinal public health problem. Children have their own specific clinical features; notably, they seem to be escaping the severe respiratory adverse effects. The international scientific community is rapidly carrying out studies, driving to the need to reassess knowledge of the disease and therapeutic strategies.
Aim
To assess the characteristics of COVID‐19 infected children worldwide of all ages, from neonates to children and adolescents, and how they differ from their adult counterparts.
Search Strategy
An electronic search in PubMed was conducted, using combinations of the following keywords: coronavirus, SARS‐CoV‐2, COVID‐19, children. The search included all types of articles written in English between January 1, 2019 until August 15, 2020.
Results
The search identified 266 relevant articles. Children were mainly within family clusters of cases and have relatively milder clinical presentation compared with adults; children were reported to have better outcomes with a significantly lower mortality rate. Cough and fever were the most common symptoms while pneumonia was the cardinal respiratory manifestation of infected children. Laboratory results and thoracic imaging give varying results.
Conclusions
Children were mainly family cluster cases and usually presented with a mild infection, although cases presented with the multisystem inflammatory syndrome are becoming more apparent. Studies determining why the manifestations of SARS‐CoV‐2 infection are so variable may help to gain a better understanding of the disease and accelerate the development of vaccines and therapies.
Keywords: children, coronavirus, COVID‐19, SARS‐CoV‐2
Abbreviations
- ACE‐2
angiotensin converting enzyme II
- COVID‐19
2019 novel coronavirus disease
- CRP
C‐reactive protein
- EAACI
European Academy of Allergy and Clinical Immunology
- GFR
glomerular filtration rate
- ILAE
International League Against Epilepsy
- ISPAD
International Society of Pediatric and Adolescent Diabetes
- MERS‐CoV
Middle East respiratory distress syndrome coronavirus
- PCT
Procalcitonin
- PIMS‐TS
pediatric inflammatory multisystem syndrome temporally associated with SARS‐CoV‐2 infection
- RSV
respiratory syncytial virus
- SARS‐CoV
severe acute respiratory syndrome coronavirus
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus‐2
1. BACKGROUND
In early December 2019, a cluster of cases of atypical pneumonia of unknown origin appeared in Wuhan city, in the Hubei province of China. 1 , 2 The majority of patients had been exposed to the Huanan seafood and animal wet market. 1 , 2 These cases were the first signs of what was to become a pandemic which is currently causing a huge number of deaths and stressing health care systems globally to an unprecedented degree. Severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2), the underlying pathogen, belongs to the β‐coronavirus genus 3 , 4 ; and its genome sequence has considerable similarity to divers β‐coronaviruses discovered in bats. 3 , 5 Remarkably, the genome is more than 85% homologous to that of bat SARS like virus ZC45 (bat‐SL‐CoVZC45, MG772933.1). 6 Previously, four common community‐acquired human coronaviruses, namely 229E, NL63, OC43, HKU1 had been described. Two other coronaviruses led to previous epidemics infecting more than 10,000 patients, namely severe acute respiratory syndrome coronavirus (SARS‐CoV), and Middle east respiratory distress syndrome coronavirus (MERS‐CoV). 7 , 8
Symptomatic patients infected with SARS‐CoV‐2 are the main sources of transmission, but asymptomatic carriers can also transmit the disease. Respiratory droplets and person‐to‐person contact are thought to be the principal routes of transmission, 9 but fecal transmission may also play a role. 6 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 A risk of indirect transmission through fomites is also plausible. SARS‐CoV‐2 has a half‐life of 3.5 hours on cardboard, 5.6 h on stainless steel, and 6.8 h on plastic. 18 No age is exempt, but data on children are scarce compared with adults. Nonetheless, the fact that children often only have trivial symptoms which may mimic common childhood illnesses such as mild bronchiolitis or diarrhea means they may become an important source of infection transmission.
The aim of this study was to review the characteristics of SARS‐CoV‐2 infected children and discuss the differences between adult and pediatric patients with confirmed infection.
2. METHODS
2.1. Search strategy
We performed an electronic search in PubMed, using combinations of the following keywords: coronavirus, SARS‐CoV‐2, COVID‐19, children. Articles were screened by title, abstract, and full text to locate all manuscripts pertinent to children. The search included all types of articles written in English, between January 1, 2019 and August 15, 2020.
3. RESULTS
A total of 1995 articles were found, and 266 relevant scientific articles and letters were eventually included. Exclusion criteria were the following: not written in English (n = 81, 46 Chinese, 4 German, 14 French, 12 Spanish, and Russian, Hebrew, Swedish, Italian, Hungarian n = 1 each), not original research (n = 775), including letters to the editor (n = 335), reviews (n = 293), protocols/guidelines (n = 62) perspectives/expert consensus (n = 85), and data not relevant (n = 873), for example, other coronaviruses and other respiratory viruses, extraneous issues during the outbreak, such as psychological impacts, domestic violence, molecular aspects and pathophysiology of SARS‐CoV‐2 infection, pediatric surgery during the outbreak. The strategy and results are displayed in Figure 1. Table S1 summarizes the current literature on the characteristics of children infected with SARS‐CoV‐2. Table 1 summarizes the clinical presentation and outcome of infection from the literature review. In Table 2, we enumerate current knowledge of the differences between adults and children infected with SARS‐CoV‐2. Infected children are principally in family clusters, or have a history of close contact with an infected patient. 19 , 20 Furthermore, children with COVID‐19 had milder symptoms than adults. 19 , 20
Figure 1.
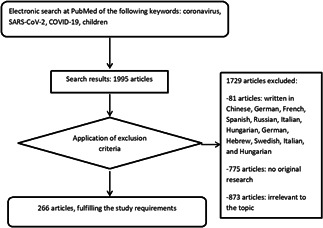
Flow chart of the search strategy. COVID‐19, 2019 novel coronavirus disease; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus‐2
Table 1.
Main clinical presentations of pediatric SARS‐CoV‐2 cases from the reviewed literature
| Clinical presentation | Studies | Number of participants (n) | Range (%) |
|---|---|---|---|
| Cough | [10, 11, 12, 14, 15, 19, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93] | 4845 | 32–75 |
| Fever | [11, 14, 15, 19, 20, 22, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 61, 62, 63, 64, 65, 67, 68, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115] | 5077 | 35–82 |
| Gastrointestinal symptoms | [11, 14, 15, 19, 20, 22, 26, 28, 31, 40, 41, 52, 53, 57, 59, 62, 65, 67, 70, 71, 72, 73, 77, 81, 83, 84, 86, 87, 88, 89, 90, 91, 92, 93, 100, 104, 106, 107, 108, 114, 116] | 4227 | Vomiting: 0–23 |
| Diarrhea: 5–37.5 | |||
| Disease severity | [19, 38, 39, 55, 57, 81, 83, 103, 117] | 2527 | Asymptomatic: 4.4–54.5 |
| Mild: 18–58% | |||
| Moderate: 19–56 | |||
| Severe: 0–3.8 | |||
| Critically ill: 0–1.9 |
Abbreviation: SARS‐CoV‐2, severe acute respiratory syndrome coronavirus‐2.
Table 2.
Summary of discrepancy aspects between adults and children COVID‐19 patients
| Feature | Adults | Children | Comment |
|---|---|---|---|
| Infection rate 118 , 119 , 120 , 121 , 122 |
|
|
|
| Incubation period 6 , 119 |
|
|
|
| Hospitalization status 123 |
|
|
|
| Fatal outcome 19 , 71 , 88 , 89 , 90 , 91 , 106 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 |
|
|
|
| Common symptoms 121 , 132 , 133 , 134 , 135 |
|
|
|
| Symptoms on admission 136 |
|
|
|
| Disease severity 34 , 126 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 |
|
|
|
| Laboratory findings 85 , 86 , 87 , 121 , 138 |
|
|
|
| Imaging pattern 2 , 11 , 22 , 23 , 24 , 26 , 28 , 29 , 30 , 33 , 94 , 95 , 122 , 138 , 146 , 147 , 148 , 149 , 150 , 151 , 152 , 153 , 154 |
|
|
|
| Treatment options 9 , 134 , 136 , 155 , 156 , 157 , 158 , 159 , 160 , 161 , 162 , 163 |
|
|
|
Abbreviations: ACE‐2, angiotensin converting enzyme II; ARDS, acute respiratory distress syndrome; CK‐MB, creatine kinase MB isoenzyme; COVID‐19, 2019 novel coronavirus disease; CT, computed tomography; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus‐2.
Characteristics of pediatric SARS‐CoV‐2 infected populations included:
Cough: Initial symptoms were fever and cough; even among neonates. 30 Cough was experienced by the majority of patients, 10 , 11 , 12 , 14 , 15 , 19 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 it was mainly dry, but was sometimes followed by productive cough in moderately severe cases. 19 There may be upper airway symptoms, such as nasal congestion, rhinitis, and sore throat. 19 Wheezing may be a feature but is generally not prominent.
Fever: One of the commonest early symptoms is fever, 11 , 14 , 15 , 19 , 20 , 21 , 22 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 61 , 62 , 63 , 64 , 65 , 67 , 68 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 occasionally low grade. In Zhejiang province, fever clinics that exclusively accept for admission patients suspected of being positive for SARS‐CoV‐2 were set up. 27
Pneumonia/CT findings: Many pediatric patients had COVID‐19 pneumonia 10 , 11 , 16 , 19 , 20 , 21 , 22 , 23 , 24 , 26 , 28 , 29 , 30 , 31 , 34 , 38 , 40 , 42 , 43 , 44 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 56 , 57 , 60 , 61 , 64 , 66 , 68 , 70 , 71 , 72 , 74 , 75 , 76 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 90 , 91 , 92 , 94 , 95 , 99 , 100 , 103 , 155 and thoracic computerized tomography (CT) scan showed multifocal or nodular consolidation with ground glass opacities. 11 , 15 , 16 , 19 , 22 , 23 , 26 , 27 , 28 , 29 , 30 , 33 , 34 , 39 , 42 , 43 , 44 , 47 , 48 , 49 , 50 , 52 , 53 , 54 , 60 , 61 , 64 , 66 , 72 , 74 , 78 , 79 , 80 , 81 , 83 , 84 , 85 , 86 , 91 , 95 , 100 , 103 , 107 , 164 , 165 More adult patients had all five lobes affected 33 , 166 ; the manifestations of the COVID‐19 pneumonia are diverse and our understanding of the spectrum of disease is changing rapidly. Radiologists must therefore have a high index of suspicion. The role of follow up CT scans in children is currently unclear. Chest CT is a particularly sensitive diagnostic instrument to detect pneumonia; the sensitivity relevant to COVID‐19 is estimated to be 97.5%. 149
Gastrointestinal symptoms: It is currently reported that SARS‐CoV‐2, despite being predominantly a respiratory virus, causes gastrointestinal symptoms in approximately 10% of infected children, 134 , 167 including abdominal pain, diarrhea, and vomiting. 11 , 14 , 15 , 19 , 20 , 22 , 26 , 28 , 31 , 40 , 41 , 52 , 53 , 57 , 59 , 62 , 65 , 67 , 70 , 71 , 72 , 73 , 77 , 81 , 83 , 84 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 100 , 104 , 106 , 107 , 108 , 114 , 116
Laboratory results: Complete blood counts may be normal, 11 , 14 , 15 , 16 , 19 , 22 , 26 , 29 , 32 , 39 , 40 , 44 , 48 , 49 , 50 , 54 , 61 , 79 , 93 , 102 , 165 , 168 or show either decreased 14 , 16 , 19 , 25 , 26 , 29 , 31 , 39 , 40 , 44 , 49 , 55 , 61 , 70 , 93 , 100 , 108 or elevated white blood cell counts. 11 , 14 , 20 , 22 , 26 , 44 , 47 , 50 , 54 , 60 , 61 , 93 , 99 Lymphocyte counts may be increased, 15 , 19 , 24 , 26 , 29 , 44 , 60 , 78 , 79 , 82 , 84 , 93 , 96 , 103 , 169 or decreased. 14 , 19 , 22 , 23 , 25 , 26 , 27 , 28 , 31 , 34 , 39 , 44 , 49 , 55 , 70 , 79 , 82 , 84 , 88 , 99 , 100 , 103 , 113 , 116 Increased C‐reactive protein (CRP), 15 , 19 , 22 , 25 , 26 , 28 , 29 , 31 , 40 , 41 , 44 , 47 , 49 , 50 , 56 , 73 , 74 , 77 , 83 , 84 , 88 , 93 , 101 , 103 , 104 procalcitonin (PCT) 15 , 22 , 26 , 31 , 44 , 49 , 50 , 56 , 73 , 77 , 84 , 88 , 93 , 99 and lactate dehydrogenase 14 , 15 , 22 , 25 , 47 , 81 , 83 , 84 , 93 have been reported. CRP is estimated to rise in 10%–20% of cases, in one study, the maximum value was 35 mg/L. 10 One group reported that leukocytopenia and CRP levels above 10 mg/L, were pointers to underlying pneumonia. 34 Serum ferritin was markedly higher in severe adult cases compared with moderately affected patients. 170 High serum levels of liver enzymes, muscle enzymes and myoglobin, and raised d‐dimers are found in severely affected patients. 157 In addition, in critically ill cases; a cytokine storm is a feature, characterized by increased serum proinflammatory and anti‐inflammatory cytokines. 14 , 22 , 56 , 73 , 77 , 84 , 88 , 93 , 156 , 171 , 172 In a study of 157 children, moderate cases had higher interleukin 10 levels compared with mild cases. 81 Another original article reporting eight severe or critically ill children, ranged from 2 months to 15 years, reported elevated IL‐6 (2/8), IL‐10 (5/8), and IFN‐γ (2/8). 22 IL‐6 and IL‐10 levels were significantly elevated in two critically ill children, of whom one was suffering from acute lymphocytic leukemia. These patients had a prolonged course of the disease duration (more than 20 days). A paper written in Chinese described a case of a severely ill infant with increased IL‐6 levels. 171 Abnormal blood examinations may prompt pediatricians to screen for SARS‐CoV‐2. 26
Co‐infections: Children not only vulnerable to SARS‐CoV‐2 infection, but can also be coinfected with numerous respiratory viral and bacterial pathogens. In a single‐center study of 50 children with COVID‐19 from Wuhan, there was documented co‐infection in 14% of children, n = 6 (12%) Mycoplasma infection and one (2%) with respiratory syncytial virus (RSV). 103 In a multicenter Italian study of 168 children with laboratory‐confirmed SARS‐CoV‐2, a viral co‐infection was demonstrated in 5.9% (n = 3 with RSV, n = 3 rhinovirus, n = 2 Epstein–Barr virus, n = 1 influenza A virus, n = 1 non‐SARS coronavirus, and a bacterial co‐infection with Streptococcus pneumoniae was also reported. 41 In another study from Wuhan, 74 children with COVID‐19 were included and 19 of the 34 were also coinfected with other common respiratory pathogens, comprising mycoplasma pneumoniae (n = 11), mycoplasma pneumoniae and RSV (n = 2), mycoplasma pneumoniae and Epstein–Barr virus (n = 2), cytomegalovirus (n = 2), cytomegalovirus and Ebstein–Barr virus (n = 1), and mycoplasma pneumoniae and influenza A (n = 1). 44 In another Chinese study 37.3% of children were coinfected with mycoplasma pneumoniae, however, the infection was diagnosed using an antibody test. 53 In a study of 36 infants with SARS‐CoV‐2 infection 62.86% had at least one other pathogen detected. 84 In a French study of 192 children, among them 157 confirmed and 35 suspected SARS‐CoV‐2 cases, co‐infection was documented in 8.3%, including 10 children with febrile urinary tract infection and 1 child with parvovirus B‐19, 5 children had bacterial co‐infection, Bordetella pertussis (n = 1), methicillin‐susceptible Staphylococcus aureus (n = 1), methicillin‐resistant Staphylococcus aureus (n = 1), Proteus mirabilis (n = 1), and Fusobacterium necrophorum (n = 1). 89 In a multinational European study of 582 confirmed SARS‐CoV‐2 children, additional viruses were detected in 5%, comprising enterovirus or rhinovirus (n = 18), influenza virus (n = 5), parainfluenza virus (n = 3), adenovirus (n = 3), RSV (n = 2), bocavirus (n = 2), human metapneumovirus (n = 1), and other coronaviruses (NL63, HKU1, and OC43, n = 1 each). 90
Perinatal/neonatal transmission: A neonate could become positive for SARS‐CoV‐2 as a result of close contact with the infected mother postnatally or antenatally via vertical transmission. The evidence on intrauterine transmission and early positive neonatal testing is scarce. This is an important issue in perinatology, as SARS‐CoV‐2 may have adverse outcomes for the fetus and the newborn. 173 , 174 The risk of vertical transmission remains unknown as currently published reports are inconsistent. 30 , 37 , 59 , 68 , 94 , 99 , 102 , 103 , 104 , 105 , 106 , 173 , 175 , 176 , 177 , 178 , 179 , 180 , 181 , 182 , 183 , 184 , 185 , 186 , 187 There is potential for nosocomial transmission of SARS‐CoV‐2 because many neonatal infections are asymptomatic, or of insidious onset or present with mild and nonspecific clinical features. There is insufficient data to determine if cesarean section could prevent transmission from a pregnant SARS‐CoV‐2 positive patient compared with vaginal delivery. 162
Comorbidities: One study demonstrated a higher risk of hospitalization in children with a history of arrhythmia in a series of 20 children with COVID‐19. Seven had an underlying condition; specifically one had sinus tachycardia, two had a history of atrial septal defect surgery, one had an atrial arrhythmia, one had first‐degree atrioventricular block, one had an incomplete right bundle‐branch block, and one had a previous history of epilepsy as a consequence of previous viral encephalitis. 26 The International League Against Epilepsy (ILAE) and Epilepsy Foundation recommend that children with Dravet syndrome, those taking steroids, everolimus, and other immunosuppressants should be regarded as a group at high risk for complications if they have been infected with SARS‐CoV‐2. 188 In another study two out of eight children with COVID‐19 required invasive mechanical ventilation, of whom one was suffering from acute lymphocytic leukemia. 22 In a study of 171 children with COVID‐19, only three children required intensive care support and invasive mechanical ventilation. All three children had an underlying medical condition; one had hydronephrosis, one had leukemia and was receiving maintenance chemotherapy, and one had intussusception. 34 This last baby died 4 weeks after admission from multiorgan failure. 34 In an Italian pediatric study from a hepatology and liver transplantation center, three of ten children became positive for SARS‐CoV‐2, did not develop pneumonia and survived. 189 In the CONFIDENCE study, 100 Italian children positive for SARS‐CoV‐2 were enrolled between March 3 and March 27 from 17 pediatric emergency departments, nine needed respiratory support of whom six had an underlying condition. 38 In a Spanish study, 16 children with chronic renal disease, including renal dysplasia, nephrotic syndrome, uropathy, IgA nephropathy, scarring nephropathy, vasculitis, and cortical necrosis, were positive for SARS‐CoV‐2 and none of the patients required oxygen therapy or Intensive Care Unit admission, indicating a similar clinical course as healthy peers. 116 In a French study of 27 children hospitalized in a pediatric intensive and high‐dependency care unit in Paris, 5 children had a fatal outcome, among them 3 previously well children, one was on chemotherapy for refractory relapse of acute lymphoblastic leukemia, and one had a history of epilepsy and major neonatal encephalopathy. 128 In the U.S. and Canadian study of 48 children admitted to intensive care units, more than 80% had underlying conditions, the most frequent was characterized as medically complex and defined as a long‐term dependence on technological support related to either developmental delay or genetic disease. 125 The International Society of Pediatric and Adolescent Diabetes (ISPAD) stresses the importance of adherence to standard diabetes care, 190 although no cases of COVID‐19 in children or young adults with diabetes have been published. The European Academy of Allergy and Clinical Immunology (EAACI) published a statement dealing with the issue of managing childhood allergies and immunodeficiencies in the ongoing pandemic. 191 There were six recommendations; ensure optimal control of allergy symptoms and asthma; avoid over‐diagnosis COVID‐19 as seasonal allergies may present with flu‐like symptoms; continue treating atopic children according to guidelines; be flexible in revising any pre‐existing recommendations according to new evidence; and have a high index of suspicion in patients with immunodeficiency. 191 In a review of how SARS‐CoV‐2 affects immunocompromised adults and children, cancer was most frequently linked with a more severe clinical course, but overall patients prescribed immunosuppression appeared to have a favorable outcome, as compared with a general population and a better outcome compared with those with other comorbidities. This may be by preventing an over‐exuberant inflammatory response (https://www.rcpch.ac.uk/resources/guidance-paediatric-multisystem-inflammatory-syndrome-temporally-associated-covid-19). 192 , 193 Of 18 children with renal disease prescribed immunosuppressive medication in 16 pediatric nephrology centers in 11 countries who developed SARS‐CoV2 infection, none needed admission in intensive care and generally all were only mildly affected. 194 A study of all children with malignancy, included hematological malignancies and solid tumors, who were infected with SARS‐CoV‐2 showed similar disease severity as the general pediatric population, with a mild‐moderate clinical course. Only 2 out of 15 received oxygen therapy, and all had favorable outcomes. 195 A web‐based study of 404 children with autoinflammatory diseases, among whom 375 were on colchicine and 48 were receiving biologic drugs found only 7 confirmed and mild cases of COVID‐19, all having a favorable outcome. No infected child was on biologic treatment. 196 In a retrospective study of 182 children, 43 were suffering from allergies, including allergic rhinitis (n = 28), drug allergy (n = 3), atopic dermatitis (n = 3), allergic rhinitis and drug allergy (n = 5), allergic rhinitis and atopic dermatitis (n = 1), allergic rhinitis and food allergy (n = 1), allergic rhinitis and food allergy and drug allergy (n = 1), asthma and urticaria and drug allergy (n = 1); there were no differences in COVID‐19 clinical features and disease course between atopic and non‐atopic children. 91 Data in cystic fibrosis are scarce and few patients, mainly adults, had confirmed COVID‐19, without obvious impact on cystic fibrosis disease severity. 197 , 198 , 199 Interestingly, despite the frequency of these conditions, there are very few reports of SARS‐CoV‐2 in children with asthma, bronchopulmonary dysplasia, or chronic suppurative lung disease such as cystic fibrosis.
Risk factors for disease severity: Very little is known about this important subject. Radiologic involvement of more than three lung segments carried a higher risk of development of a severe form of the disease. 47 In addition, the elevation of IL‐6, total bilirubin and d‐dimer are associated with worse disease. 47 In a review of the Chinese experience, risk factors for severity were increased respiratory rate, clouding of consciousness, elevated levels of lactic acid, bilateral or multilobular lesions on imaging, pleural effusion, or rapid progression. 200 Other risk groups were patients below 3 months of age or those with co‐existing conditions, such as congenital heart disease, bronchopulmonary dysplasia, severe malnutrition, and immunodeficiency. 200 The majority of infected children were within family clusters; so infection risk will probably be higher for those living in low‐income families, in crowded housing, and with blue‐collar parents. 201 Sadly, physical distancing is harder to implement in those with adverse social determinants and might further impact the risk of COVID‐19. 202 In a study of 48 children admitted to the intensive care unit 83% had underlying conditions including obesity (n = 7). 125 Three adolescents with Covid‐19 and septic shock were also all obese. 203 Among 50 children, 9 children with severe disease had significantly higher CRP and PCT on admission, elevated peak IL‐6, ferritin, and d‐dimer levels and obesity was again significantly related to the need for mechanical ventilation in patients 2 years or older. 88 Obesity is a known significant risk factor for severe disease in adults. 204 The Centers for Disease Control and Prevention COVID‐19 Response Team suggests a high index of suspicion and follow up for disease course, especially among infants and children with pre‐existing comorbidities. 123 In a study from New York City of 67 hospitalized and critically ill children, admission to intensive care unit was significantly associated with higher CRP, PCT, pro‐B type natriuretic peptide, and blood urea and with lower platelet counts; the only clinical symptom significantly related with intensive care admission was shortness of breath. 58 Notably, 23.1% of patients required intensive care had a history of epilepsy (n = 3). 58 In a multicenter study involving 82 institutions across 25 European countries, using univariable analysis, significant factors for requiring intensive care were being younger than 1 month, male sex, pre‐existing medical conditions, pyrexia, lower respiratory tract infection, imaging findings suggestive of pneumonia or acute respiratory distress syndrome, and viral co‐infection. 90 Moreover, in a study from nine New York City teaching hospitals, 70 critically ill children admitted to the intensive care unit and 90% of patients who developed acute respiratory distress syndrome initially presented with dyspnea, but also had significantly lower platelet counts compared with those without acute respiratory distress syndrome. 131 In the morbidity and mortality weekly report during the period March 1 to July 25 hospitalization rates were higher among Hispanic and black children, eight times and five times, respectively, who also had a higher prevalence of underlying conditions, 45.7% and 29.8%, respectively, compared with their white peers, 14,9%. 205 Gastrointestinal symptoms were reported in 42% of hospitalized children. 205
Epidemiology: The epidemiology data on pediatric SARS‐CoV‐2 is incomplete, but some themes are emerging. In particular, there are lower rates of infection in children compared with adults, similar to those reported with other coronaviruses, such as SARS and MERS. 206 Children with COVID‐19 are mostly part of a family cluster or have a history of a close contact with a confirmed case.
4. DISCUSSION
Most studies have shown children infected with COVID‐19 have a mild clinical course, analogous with SARS, characterized by relatively nonspecific symptoms such as dry cough, sore throat, rhinorrhea, nasal congestion, fatigue, myalgia, and sneezing, although emerging data about less frequent symptoms are discussed below. This is a much milder disease than that seen in COVID‐19 adults 19 , 21 , 24 , 127 and understanding the reasons for this may open up potential new avenues for treatment. Understanding the pathogenesis of the novel coronavirus SARS‐CoV‐2 will hopefully enable future vaccine development and targeted therapeutics.
SARS‐CoV‐2 can be transmitted by a patient or an asymptomatic carrier and is an extremely infectious disease. There are probably large numbers of asymptomatic or nonspecifically mildly unwell individuals in the community 25 ; asymptomatic carriers can lead to human‐to‐human transmission and are a covert source of infection keeping the virus is circulating within local communities. Children of all ages are susceptible to SARS‐CoV‐2, however, symptoms mimic nonspecific childhood infections, and missing the diagnosis means they may transmit the infection. However, anecdotally, a child with COVID‐19 did not transmit the virus despite interacting with peers in three schools while symptomatic. 35 Young children were prone to have silent infections with normal CT scans even at the time of hospitalization, 25 , 155 which suggests widespread testing of even relatively well children will be needed to contain outbreaks. However, preliminary findings from population‐based and school studies suggest that children may be less commonly infected or transmit the disease to others, although the current evidence is not definitive. 207
The issue of testing children for acute infection and antibodies postinfection is complex. Testing kits in at least some settings may be in short supply, the technical requirements are high, and there may be false positive and negative tests. Serological tests, detect the presence of antibodies against SARS‐CoV‐2. A positive test result is evidence of only a recent exposure and there may also be false positives and negatives. Given these complexities current community preventive strategies, such as physical distancing, use of personal protection measures such as wearing a mask, hand and surface hygiene must be encouraged. The evidence base for much of this in children is not definitive, but there would seem to be no negative consequences of adopting them.
Although most children have mainly respiratory symptoms, there are some who present with gastrointestinal symptoms. Surprisingly, studies reported SARS‐CoV‐2 detection in stools samples in mild patients and prolonged virus RNA shedding in stools at 10 days 11 , 67 , 72 and even longer. 10 , 12 , 13 , 14 , 15 , 16 , 42 , 44 , 55 , 56 , 67 , 72 , 108 These studies mandate careful infection control procedures for gastrointestinal as well as respiratory samples. The fecal‐oral route may constitute more risk of transmission in infected children compared with adults. 207 Whether it is valuable to test stool samples for viral clearance as a routine merits further study, but feces should certainly be considered a potential source of infection and handled with appropriate precautions.
The role of investigations other than swabs to confirm the diagnosis and limit the spread of infection are controversial, especially given the paucity of data in children. Most are mildly affected and require no other testing. The value of blood tests in the management of more severe cases is uncertain, given the results are highly variable between cases, and it is not known if any can be used reliably prognostically or to predict complications such as secondary bacterial pneumonia. The role of imaging is unclear; as adults, mild cases do not need imaging. 208 Chest radiography may be normal or show only nonspecific changes. 30 Chest CT may show more characteristic abnormalities, such as ground‐glass opacities and multi‐focal consolidation. 25 However, the role of CT scanning in pediatric CoVID‐19 requires further study.
Levels of many cytokines have been found to be increased in severe, critically ill and fatal cases. 14 , 22 , 156 , 166 , 171 Of note, there are emerging reports of a rare systemic inflammatory condition in children, resembling typical or atypical Kawasaki disease, presently named Pediatric Inflammatory Multisystem Syndrome temporally associated with SARS‐CoV‐2 infection (PIMS‐TS), toxic shock syndromes, bacterial sepsis and macrophage activation syndromes (https://www.rcpch.ac.uk/resources/guidance-paediatric-multisystem-inflammatory-syndrome-temporally-associated-covid-19) 77 , 106 , 107 , 111 , 114 , 203 , 209 , 210 , 211 , 212 , 213 , 214 , 215 , 216 , 217 , 218 , 219 , 220 , 221 , 222 , 223 , 224 , 225 , 226 , 227 , 228 , 229 of whom some but not all test positive for COVID‐19. The clinical characteristics of PIMS‐TS include cardiac disease sometimes including coronary artery aneurysms, the need for intensive care and inotrope, and additional treatments. There is insufficient data on more specific treatments such as intravenous immunoglobulin. 209 , 210 , 211 The identification of PIMS‐TS during the with SARS‐CoV‐2 pandemic is poorly understood and underscores the need for vigilance to detect new manifestations of the disease.
There are no trials of antiviral therapies in children. Management is supportive, including supplemental oxygen, nutritional support, and maintenance fluid and electrolyte balance. 137 , 157 Systemic steroids are not indicated.
There is a compelling need to understand why the natural history of SARS‐CoV‐2 is milder in children. This may be related to alterations in the pediatric immune system leading to a qualitatively distinct response to the virus. 137 Developmental differences in the location, quantity, and activation status of the viral receptors are regularly revealed as potent causes of the age‐associated variations in incidence. 230 , 231 One hypothesis about the frequency of mild cases among children is linked to changes in the expression of angiotensin‐converting enzyme II (ACE‐2) receptor to which SARS‐CoV‐2 binds 137 ; there is lower nasal epithelial ACE‐2 expression in children compared with adults. 231
Most patients described were adults who usually have pneumonia and abnormal CT chest imaging. 2 , 149 , 150 Elderly males with comorbidities do worse. 134 , 136 Those adults doing badly had a high prevalence of diabetes, obesity, hypertension, cardiovascular, and chronic airway disease. 9 Children suffering from other medical conditions, such as congenital heart, and chronic respiratory diseases may be vulnerable to COVID‐19. 6 One pediatric study indicates an increased risk of hospitalization in children with a history of arrhythmia. 26 However it is noteworthy that children with pre‐existing diseases do not seem to figure prominently in pediatric series.
A major limitation of the current study methodology is that for resource reasons we could not include any foreign language papers and unsurprisingly, many studies are published solely in Chinese, the pandemic having originated in China.
4.1. Future work
We need more data on how COVID‐19 affects children with underlying medical conditions. As with so many diseases, there is a need for randomized controlled trials of treatment in children. The development of a safe and effective vaccine is of paramount importance. We need to be alert to novel manifestations of the disease in children, and understand the pathophysiology and how best to treat the emerging multi‐system inflammatory complications discussed above.
5. CONCLUSION
SARS‐CoV‐2 infection affects all ages. In contrast to adults, children mostly have a mild form of the disease, as was the case in SARS, but nonetheless, they may transmit the disease. Identification of effective treatment strategies, and above all, a vaccine, is imperative. This literature review has highlighted the paucity of information about the interaction of the disease with underlying medical conditions in children and the lack of evidence with regard to investigation and treatment.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Supporting information
Supporting information.
Perikleous E, Tsalkidis A, Bush A, Paraskakis E. Coronavirus global pandemic: An overview of current findings among pediatric patients. Pediatric Pulmonology. 2020;55:3252‐3267. 10.1002/ppul.25087
REFERENCES
- 1. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020;382(13):1199‐1207. 10.1056/NEJMoa2001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang C, Wang Y, Li X, et al. Clinical features of patients with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727‐733. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization . Laboratory testing for 2019 novel coronavirus (2019‐nCoV) in suspected human cases; 2020. https://www.who.int/docs/default-source/coronaviruse/20200114-interim-laboratoryguidanceversion.pdf?sfvrsn=6967c39b_4&download=true
- 5. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270‐273. 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. She J, Liu L, Liu W COVID‐19 epidemic: disease characteristics in children. J Med Virol. 2020;92(7):747‐754. 10.1002/jmv.25807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organization . SARS (severe acute respiratory syndrome); 2019. https://www.who.int/ith/diseases/sars/en/
- 8. Azhar EI, Hui DSC, Memish ZA, Drosten C, Zumla A The middle east respiratory syndrome (MERS). Infect Dis Clin North Am. 2019;33(4):891‐905. 10.1016/j.idc.2019.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Health Commission of People's Republic of China. Diagnosis and treatment of pneumonia caused by novel coronavirus (trial version 4); https://www.nhc.gov.cn/xcs/zhengcwj/202001/4294563ed35b43209b31739bd0785e67/fles/7a9309111267475a99d4_30696_2c8bf_78.pdf. Accessed January 28, 2020.
- 10. Jiehao C, Jin X, Daojiong L, et al. A Case Series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin Infect Dis. 2020;71:1547‐1551. 10.1093/cid/ciaa198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang T, Cui X, Zhao X, et al. Detectable SARS‐CoV‐2 viral RNA in feces of three children during recovery period of COVID‐19 pneumonia. J Med Virol. 2020;92(7):909‐914. 10.1002/jmv.25795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lin J, Duan J, Tan T, Fu Z, Dai J The isolation period should be longer: lesson from a child infected with SARS‐CoV‐2 in Chongqing, China. Pediatr Pulmonol. 2020;55(6):E6‐E9. 10.1002/ppul.24763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tang A, Tong Z, Wang H, et al. Detection of novel coronavirus by RT‐PCR in stool specimen from asymptomatic child, China. Emerg Infect Dis. 2020;26(6):1337‐1339. 10.3201/eid2606.200301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu Y, Li X, Zhu B, et al. Characteristics of pediatric SARS‐CoV‐2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26(4):502‐505. 10.1038/s41591-020-0817-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xing YH, Ni W, Wu Q, et al. Prolonged viral shedding in feces of pediatric patients with coronavirus disease 2019. J Microbiol Immunol Infect. 2020;53(3):473‐480. 10.1016/j.jmii.2020.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Su L, Ma X, Yu H, et al. The different clinical characteristics of corona virus disease cases between children and their families in China—the character of children with COVID‐19. Emerg Microbes Infect. 2020;9(1):707‐713. 10.1080/22221751.2020.1744483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yuan C, Zhu H, Yang Y, et al. Viral loads in throat and anal swabs in children infected with SARS‐CoV‐2. Emerg Microbes Infect. 2020;9(1):1233‐1237. 10.1080/22221751.2020.1771219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS‐CoV‐2 as compared with SARS‐CoV‐1. N Engl J Med. 2020;382(16):1564‐1567. 10.1056/NEJMc2004973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID‐19 among children in China. Pediatrics. 2020;145(6):e20200702. 10.1542/peds.2020-0702 [DOI] [PubMed] [Google Scholar]
- 20. Ji LN, Chao S, Wang YJ, et al. Clinical features of pediatric patients with COVID‐19: a report of two family cluster cases. World J Pediatr. 2020;16(3):267‐270. 10.1007/s12519-020-00356-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lou XX, Shi CX, Zhou CC, Tian YS Three children who recovered from novel coronavirus 2019 pneumonia. J Paediatr Child Health. 2020;56(4):650‐651. 10.1111/jpc.14871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sun D, Li H, Lu XX, et al. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center's observational study. World J Pediatr. 2020;16(3):251‐259. 10.1007/s12519-020-00354-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li Y, Guo F, Cao Y, Li L, Guo Y Insight into COVID‐2019 for pediatricians. Pediatr Pulmonol. 2020;55(5):E1‐E4. 10.1002/ppul.24734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cui Y, Tian M, Huang D, et al. A 55‐day‐old female Infant infected with COVID 19: presenting with pneumonia, liver injury, and heart damage. J Infect Dis. 2020;221(11):1775‐1781. 10.1093/infdis/jiaa113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hu Z, Song C, Xu C, et al. Clinical characteristics of 24 asymptomatic infections with COVID‐19 screened among close contacts in Nanjing, China. Sci China: Life Sci. 2020;63(5):706‐711. 10.1007/s11427-020-1661-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xia W, Shao J, Guo Y, Peng X, Li Z, Hu D. Clinical and CT features in pediatric patients with COVID‐19 infection: different points from adults. Pediatr Pulmonol. 2020;55(5):1169‐1174. 10.1002/ppul.24718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu XW, Wu XX, Jiang XG, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS‐Cov‐2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. 10.1136/bmj.m606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zheng F, Liao C, Fan Q, et al. Clinical characteristics of children with coronavirus disease 2019 in Hubei, China. Curr Med Sci. 2020;40(2):275‐280. 10.1007/s11596-020-2172-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu H, Liu F, Li J, Zhang T, Wang D, Lan W Clinical and CT imaging features of the COVID‐19 pneumonia: focus on pregnant women and children. J Infect. 2020;80(5):e7‐e13. 10.1016/j.jinf.2020.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhu H, Wang L, Fang C, et al. Clinical analysis of 10 neonates born to mothers with 2019‐nCoV pneumonia. Transl Pediatr. 2020;9(1):51‐60. 10.21037/tp.2020.02.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID‐19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020;20(6):689‐696. 10.1016/S1473-3099(20)30198-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Le HT, Nguyen LV, Tran DM, et al. The first infant case of COVID‐19 acquired from a secondary transmission in Vietnam. Lancet Child Adolesc Health. 2020;4(5):405‐406. 10.1016/S2352-4642(20)30091-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu M, Song Z, Xiao K High‐resolution computed tomography manifestations of 5 pediatric patients with 2019 novel coronavirus. J Comput Assist Tomogr. 2020;44(3):311‐313. 10.1097/RCT.0000000000001023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lu X, Zhang L, Du H, et al. SARS‐CoV‐2 infection in children. N Engl J Med. 2020;382(17):1663‐1665. https://doi.org/10.1056/NEJMc2005073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Danis K, Epaulard O, Bénet T, et al. Cluster of coronavirus disease 2019 (Covid‐19) in the French Alps, 2020. Clin Infect Dis. 2020;71(15):825‐832. 10.1093/cid/ciaa424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Canarutto D, Priolo A, Russo G, Pitea M, Vigone MC, Barera G. COVID‐19 infection in a paucisymptomatic infant: raising the index of suspicion in epidemic settings. Pediatr Pulmonol. 2020;55(6):E4‐E5. 10.1002/ppul.24754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Alzamora MC, Paredes T, Caceres D, Webb CM, Valdez LM, La Rosa M. Severe COVID‐19 during pregnancy and possible vertical transmission. Am J Perinatol. 2020;37(8):861‐865. 10.1055/s-0040-1710050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Parri N, Lenge M, Buonsenso D, Coronavirus Infection in Pediatric Emergency Departments (CONFIDENCE) Research Group . Children with Covid‐19 in pediatric emergency departments in Italy. N Engl J Med. 2020;383(2):187‐190. 10.1056/NEJMc2007617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Song W, Li J, Zou N, Guan W, Pan J, Xu W Clinical features of pediatric patients with coronavirus disease (COVID‐19). J Clin Virol. 2020;127:104377. 10.1016/j.jcv.2020.104377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Musolino AM, Supino MC, Buonsenso D, et al. Lung ultrasound in children with COVID‐19: preliminary findings. Ultrasound Med Biol. 2020;46(8):2094‐2098. 10.1016/j.ultrasmedbio.2020.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Garazzino S, Montagnani C, Donà D, et al. Multicentre Italian study of SARS‐CoV‐2 infection in children and adolescents, preliminary data as at 10 April 2020. Multicenter Study Euro Surveill. 2020;25(18):2000600. 10.2807/1560-7917.ES.2020.25.18.2000600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mao L, Xu J, Xu Z, et al. A child with household transmitted COVID‐19. BMC Infect Dis. 2020;20(1):329. 10.1186/s12879-020-05056-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhong Z, Xie X, Huang W, et al. Findings and clinical features of coronavirus disease 2019 in children. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2020;45(3):236‐242. 10.11817/j.issn.1672-7347.2020.200206 [DOI] [PubMed] [Google Scholar]
- 44. Wu Q, Xing Y, Shi L, et al. Coinfection and other clinical characteristics of COVID‐19 in children. Pediatrics. 2020;146(1):e20200961. 10.1542/peds.2020-0961 [DOI] [PubMed] [Google Scholar]
- 45. Kumar K, Prakash A, Gangasagara S, et al. Presence of viral RNA of SARS‐CoV‐2 in conjunctival swab specimens of COVID‐19 patients. Indian J Ophthalmol. 2020;68(6):1015‐1017. 10.4103/ijo.IJO_1287_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wehl G, Laible M, Rauchenzauner M. Co‐infection of SARS CoV‐2 and influenza A in a pediatric patient in Germany. Klin Padiatr. 2020;232(4):217‐218. 10.1055/a-1163-7385 [DOI] [PubMed] [Google Scholar]
- 47. Wang Y, Zhu F, Wang C, et al. Children hospitalized with severe COVID‐19 in Wuhan. Pediatr Infect Dis J. 2020;39(7):e91‐e94. 10.1097/INF.0000000000002739 [DOI] [PubMed] [Google Scholar]
- 48. Chen M, Fan P, Liu Z, et al. A SARS‐CoV‐2 familial cluster infection reveals asymptomatic transmission to children. J Infect Public Health. 2020;13(6):883‐886. 10.1016/j.jiph.2020.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Korkmaz MF, Türe E, Dorum BA, Kılıç ZB. The epidemiological and clinical characteristics of 81 children with COVID‐19 in a pandemic hospital in Turkey: an observational cohort study. J Korean Med Sci. 2020;35(25):e236. 10.3346/jkms.2020.35.e236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jiang S, Liu P, Xiong G, et al. Coinfection of SARS‐CoV‐2 and multiple respiratory pathogens in children. Clin Chem Lab Med. 2020;58(7):1160‐1161. 10.1515/cclm-2020-0434 [DOI] [PubMed] [Google Scholar]
- 51. See KC, Liew SM, Ng DCE, et al. COVID‐19: four paediatric cases in Malaysia. Int J Infect Dis. 2020;94:125‐127. 10.1016/j.ijid.2020.03.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shen Q, Guo W, Guo T, et al. Novel coronavirus infection in children outside of Wuhan, China. Pediatr Pulmonol. 2020;55(6):1424‐1429. 10.1002/ppul.24762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Peng H, Gao P, Xu Q, et al. Coronavirus disease 2019 in children: characteristics, antimicrobial treatment, and outcomes. J Clin Virol. 2020;128:104425. 10.1016/j.jcv.2020.104425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tan YP, Tan BY, Pan J, Wu J, Zeng SZ, Wei HY Epidemiologic and clinical characteristics of 10 children with coronavirus disease 2019 in Changsha, China. J Clin Virol. 2020;127:104353. 10.1016/j.jcv.2020.104353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Du W, Yu J, Liu X, Chen H, Lin L, Li Q Persistence of SARS‐CoV‐2 virus RNA in feces: a case series of children. J Infect Public Health. 2020;13(7):926‐931. 10.1016/j.jiph.2020.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Liu P, Cai J, Jia R, et al. Dynamic surveillance of SARS‐CoV‐2 shedding and neutralizing antibody in children with COVID‐19. Emerg Microbes Infect. 2020;9(1):1254‐1258. 10.1080/22221751.2020.1772677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Denina M, Scolfaro C, Silvestro E, et al. Lung ultrasound in children with COVID‐19. Pediatrics. 2020;146(1):e20201157. 10.1542/peds.2020-1157 [DOI] [PubMed] [Google Scholar]
- 58. Chao JY, Derespina KR, Herold BC, et al. Clinical characteristics and outcomes of hospitalized and critically Ill Children and adolescents with coronavirus disease 2019 at a tertiary care medical center in New York city. J Pediatr. 2020;223:14‐19.e2. 10.1016/j.jpeds.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Salvatori G, De Rose DU, Concato C, et al. Managing COVID‐19‐positive maternal‐infant dyads: an Italian experience. Breastfeed Med. 2020;15(5):347‐348. 10.1089/bfm.2020.0095 [DOI] [PubMed] [Google Scholar]
- 60. Li C, Luo F, Wu B A 3‐month‐old child with COVID‐19: a case report. Medicine (Baltimore). 2020;99(23):e20661. 10.1097/MD.0000000000020661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bai K, Liu W, Liu C, et al. Clinical analysis of 25 COVID‐19 infections in children. Pediatr Infect Dis J. 2020;39(7):e100‐e103. 10.1097/INF.0000000000002740 [DOI] [PubMed] [Google Scholar]
- 62. Dodi I, Castellone E, Pappalardo M, et al. SARS‐CoV‐2 infection in children in Parma. Acta Biomed. 2020;91(2):214‐215. 10.23750/abm.v91i2.9563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Genovese G, Colonna C, Marzano AV. Varicella‐like exanthem associated with COVID‐19 in an 8‐year‐old girl: a diagnostic clue? Pediatr Dermatol. 2020;37(3):435‐436. 10.1111/pde.14201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Qiu L, Jiao R, Zhang A, et al. A Case of critically ill infant of coronavirus disease 2019 with persistent reduction of T lymphocytes. Pediatr Infect Dis J. 2020;39(7):e87‐e90. 10.1097/INF.0000000000002720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhao W, Wang Y, Tang Y, et al. Characteristics of children with reactivation of SARS‐CoV‐2 infection after hospital discharge. Clin Pediatr (Phila). 2020;59(9‐10):929‐932. 10.1177/0009922820928057 [DOI] [PubMed] [Google Scholar]
- 66. Shi B, Xia Z, Xiao S, Huang C, Zhou X, Xu H. Severe pneumonia due to SARS‐CoV‐2 and respiratory syncytial virus infection: a case report. Clin Pediatr (Phila). 2020;59(8):823‐826. 10.1177/00099228209200 [DOI] [PubMed] [Google Scholar]
- 67. De Ioris MA, Scarselli A, Ciofi Degli Atti ML, et al. Dynamic viral severe acute respiratory syndrome coronavirus 2 RNA shedding in children: preliminary data and clinical consideration from a Italian regional center. J Pediatric Infect Dis Soc. 2020;9(3):366‐369. 10.1093/jpids/piaa065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lorenz N, Treptow A, Schmidt S, et al. Neonatal early‐onset infection with SARS‐CoV‐2 in a newborn presenting with encephalitic symptoms. Pediatr Infect Dis J. 2020;39(8):e212. 10.1097/INF.0000000000002735 [DOI] [PubMed] [Google Scholar]
- 69. Russell MR, Halnon NJ, Alejos JC, Salem MM, Reardon LC COVID‐19 in a pediatric heart transplant recipient: emergence of donor‐specific antibodies. J Heart Lung Transplant. 2020;39(7):732‐733. 10.1016/j.healun.2020.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Parri N, Magistà AM, Marchetti F, et al. Characteristic of COVID‐19 infection in pediatric patients: early findings from two Italian Pediatric Research Networks. Eur J Pediatr. 2020;179(8):1315‐1323. 10.1007/s00431-020-03683-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. de Ceano‐Vivas M, Martín‐Espín I, Del Rosal T, et al. SARS‐CoV‐2 infection in ambulatory and hospitalised Spanish children. Arch Dis Child. 2020;105(8):808‐809. 10.1136/archdischild-2020-319366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sun D, Zhu F, Wang C, et al. Children infected with SARS‐CoV‐2 from family clusters. Front Pediatr. 2020;8:386. 10.3389/fped.2020.00386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Patel PA, Chandrakasan S, Mickells GE, Yildirim I, Kao CM, Bennett CM. Severe pediatric COVID‐19 presenting with respiratory failure and severe thrombocytopenia. Pediatrics. 2020;146(1):e20201437. 10.1542/peds.2020-1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Li B, Shen J, Li L, Yu C. Radiographic and clinical features of children with coronavirus disease (COVID‐19) pneumonia. Indian Pediatr. 2020;57(5):423‐426. 10.1007/s13312-020-1816-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wardell H, Campbell JI, VanderPluym C, Dixit A. SARS‐CoV‐2 infection in febrile neonates. J Pediatric Infect Dis Soc. 2020;39(6):469‐477. 10.1093/jpids/piaa084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kakuya F, Okubo H, Fujiyasu H, Wakabayashi I, Syouji M, Kinebuchi T. The first pediatric patients with coronavirus disease 2019 (COVID‐19) in Japan; the risk of co‐infection with other respiratory viruses. Jpn J Infect Dis. 2020;73:377‐380. 10.7883/yoken.JJID.2020.181 [DOI] [PubMed] [Google Scholar]
- 77. Ng KF, Kothari T, Bandi S, et al. COVID‐19 multisystem inflammatory syndrome in three teenagers with confirmed SARS‐CoV‐2 infection. J Med Virol. 2020:jmv.26206. 10.1002/jmv.26206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Li Y, Cao J, Zhang X, et al. Imaging characteristics of COVID‐19 pneumonia in preschool children: a retrospective study. BMC Pediatr. 2020;20(1):227. 10.1186/s12887-020-02140-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lu Y, Wen H, Rong D, Zhou Z, Liu H. Clinical characteristics and radiological features of children infected with the 2019 novel coronavirus. Clin Radiol. 2020;75(7):520‐525. 10.1016/j.crad.2020.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zhu L, Wang J, Huang R, et al. Clinical characteristics of a case series of children with coronavirus disease 2019. Pediatr Pulmonol. 2020;55(6):1430‐1432. 10.1002/ppul.24767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wu H, Zhu H, Yuan C, et al. Clinical and immune features of hospitalized pediatric patients with coronavirus disease 2019 (COVID‐19) in Wuhan. China. JAMA Netw Open. 2020;3(6):e2010895. 10.1001/jamanetworkopen.2020.10895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zheng G, Wang B, Zhang H, et al. Clinical characteristics of acute respiratory syndrome with SARS‐CoV‐2 infection in children in South China. Pediatr Pulmonol. 2020;55:2419‐2426. 10.1002/ppul.24921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zhang C, Gu J, Chen Q, et al. Clinical and epidemiological characteristics of pediatric SARS‐CoV‐2 infections in China: a multicenter case series. PLOS Med. 2020;17(6):e1003130. 10.1371/journal.pmed.1003130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sun D, Chen X, Li H, et al. SARS‐CoV‐2 infection in infants under 1 year of age in Wuhan City, China. World J Pediatr. 2020;16(3):260‐266. 10.1007/s12519-020-00368-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Dong X, Cao Y, Lu X, et al. Eleven faces of coronavirus disease 2019. Allergy. 2020;75(7):1699‐1709. 10.1111/all.14289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Chen J, Zhang ZZ, Chen YK, et al. The clinical and immunological features of pediatric COVID‐19 patients in China. Genes Dis. 2020. 10.1016/j.gendis.2020.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. García‐Salido A, Leoz‐Gordillo I, Martínez de Azagra‐Garde A, et al. Children in critical care due to severe acute respiratory syndrome coronavirus 2 infection: experience in a Spanish hospital. Pediatr Crit Care Med. 2020;21(8):e576‐e580. 10.1097/PCC.0000000000002475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zachariah P, Johnson CL, Halabi KC, et al. Epidemiology, clinical features, and disease severity in patients with coronavirus disease 2019 (COVID‐19) in a Children's hospital in New York city, New York. JAMA Pediatr. 2020:e202430. 10.1001/jamapediatrics.2020.2430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Gaborieau L, Delestrain C, Bensaid P, et al. Epidemiology and clinical presentation of children hospitalized with SARS‐CoV‐2 infection in suburbs of Paris. J Clin Med. 2020;9(7):2227. 10.3390/jcm9072227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Götzinger F, Santiago‐García B, Noguera‐Julián A, et al. COVID‐19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020;4(9):653‐661. 10.1016/S2352-4642(20)30177-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Du H, Dong X, Zhang J, et al. Clinical characteristics of 182 pediatric COVID‐19 patients with different severities and allergic status. Allergy. 2020:all.14452. 10.1111/all.14452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Foster CE, Moulton EA, Munoz FM, et al. Coronavirus disease 2019 in Children Cared for at Texas Children's Hospital: initial clinical characteristics and outcomes. J Pediatric Infect Dis Soc. 2020;9(3):373‐377. 10.1093/jpids/piaa072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Liu X, Tang J, Xie R, et al. Clinical and epidemiological features of 46 children under 1 year old with coronavirus disease 2019 (COVID‐19) in Wuhan, China: a descriptive study. J Infect Dis. 2020;222:1293‐1297. 10.1093/infdis/jiaa472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wang S, Guo L, Chen L, et al. A case report of neonatal COVID‐19 infection in China. Clin Infect Dis. 2020;71(15):853‐857. 10.1093/cid/ciaa225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Park JY, Han MS, Park KU, Kim JY, Choi EH. First pediatric case of coronavirus disease 2019 in Korea. J Korean Med Sci. 2020;35(11):e124. 10.3346/jkms.2020.35.e124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Liu Y, Yang Y, Zhang C, et al. Clinical and biochemical indexes from 2019‐nCoV infected patients linked to viral loads and lung injury. Sci China: Life Sci. 2020;63(3):364‐374. 10.1007/s11427-020-1643-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Chan JFW, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person‐to‐person transmission: a study of a family cluster. Lancet. 2020;395(10223):514‐523. 10.1016/S0140-6736(20)30154-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kamali Aghdam M, Jafari N, Eftekhari K. Novel coronavirus in a 15‐day‐old neonate with clinical signs of sepsis, a case report. Infect Dis (Lond). 2020;52(6):427‐429. 10.1080/23744235.2020.1747634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Zeng L, Xia S, Yuan W, et al. Neonatal early‐onset infection with SARS‐CoV‐2 in 33 neonates born to mothers with COVID‐19 in Wuhan, China. JAMA Pediatr. 2020;174(7):722‐725. 10.1001/jamapediatrics.2020.0878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Liu W, Zhang Q, Chen J, et al. Detection of Covid‐19 in children in early January 2020 in Wuhan, China. N Engl J Med. 2020;382(14):1370‐1371. 10.1056/NEJMc2003717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Jones VG, Mills M, Suarez D, et al. COVID‐19 and Kawasaki disease: novel virus and novel case. Hosp Pediatr. 2020;10(6):537‐540. 10.1542/hpeds.2020-0123 [DOI] [PubMed] [Google Scholar]
- 102. Dong L, Tian J, He S, et al. Possible vertical transmission of SARS‐CoV‐2 from an infected mother to her newborn. JAMA. 2020;323(18):1846‐1848. 10.1001/jama.2020.4621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID‐19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395(10226):809‐815. 10.1016/S0140-6736(20)30360-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Yan J, Guo J, Fan C, et al. Coronavirus disease 2019 (COVID‐19) in pregnant women: a report based on 116 cases. Am J Obstet Gynecol. 2020;223(1):111.e1‐111.e14. 10.1016/j.ajog.2020.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Lu D, Sang L, Du S, Li T, Chang Y, Yang XA. Asymptomatic COVID‐19 infection in late pregnancy indicated no vertical transmission. J Med Virol. 2020;92:1660‐1664. 10.1002/jmv.25927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Yang Z, Y Liu Y. Vertical transmission of severe acute respiratory syndrome coronavirus 2: a systematic review. Am J Perinatol. 2020;37(10):1055‐1060. 10.1055/s-0040-1712161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Toubiana J, Poirault C, Corsia A, et al. Kawasaki‐like multisystem inflammatory syndrome in children during the covid‐19 pandemic in Paris, France: prospective observational study. BMJ. 2020;369:m2094. 10.1136/bmj.m2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Wolf GK, Glueck T, Huebner J, et al. Clinical and epidemiological features of a family cluster of symptomatic and asymptomatic severe acute respiratory syndrome coronavirus 2 infection. J Pediatric Infect Dis Soc. 2020;9(3):362‐365. 10.1093/jpids/piaa060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Scheier E, Guri A, Balla U Lung ultrasound cannot be used to screen for Covid‐19 in children. Eur Rev Med Pharmacol Sci. 2020;24(9):4623‐4624. 10.26355/eurrev_202005_21145 [DOI] [PubMed] [Google Scholar]
- 110. Akcabelen YM, Koca Yozgat A, Parlakay AN, Yarali N COVID‐19 in a child with severe aplastic anemia. Pediatr Blood Cancer. 2020;67(8):e28443. 10.1002/pbc.28443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Dasgupta K, Finch SE. A case of pediatric multisystem inflammatory syndrome temporally associated with COVID‐19 in South Dakota. S D Med. 2020;73(6):246‐251. [PubMed] [Google Scholar]
- 112. Del Barba P, Canarutto D, Sala E, et al. COVID‐19 cardiac involvement in a 38‐day old infant. Pediatr Pulmonol. 2020;55(8):1879‐1881. 10.1002/ppul.24895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Feld L, Belfer J, Kabra R, et al. A case series of the 2019 novel coronavirus (SARS‐CoV‐2) in 3 febrile infants in New York. Pediatrics. 2020;146(1):e20201056. 10.1542/peds.2020-1056 [DOI] [PubMed] [Google Scholar]
- 114. Tullie L, Ford K, Bisharat M, et al. Gastrointestinal features in children with COVID‐19: an observation of varied presentation in eight children. Lancet Child Adolesc Health. 2020;4(7):e19‐e20. 10.1016/S2352-4642(20)30165-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Gefen AM, Palumbo N, Nathan SK, Singer PS, Castellanos‐Reyes LJ, Sethna CB. Pediatric COVID‐19‐associated rhabdomyolysis: a case report. Pediatr Nephrol. 2020;35(8):1517‐1520. 10.1007/s00467-020-04617-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Melgosa M, Madrid A, Alvárez O, et al. SARS‐CoV‐2 infection in Spanish children with chronic kidney pathologies. Pediatr Nephrol. 2020;35(8):1521‐1524. 10.1007/s00467-020-04597-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Kam K, Yung CF, Maiwald M, et al. Clinical utility of buccal swabs for severe acute respiratory syndrome coronavirus 2 detection in coronavirus disease 2019‐infected children. J Pediatric Infect Dis Soc. 2020;9(3):370‐372. 10.1093/jpids/piaa068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Wang Z, Ma W, Zheng X, Wu G, Zhang R. Household transmission of SARS‐CoV‐2. J Infect. 2020;81(1):179‐182. 10.1016/j.jinf.2020.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Feng W, Zong W, Wang F, Ju S. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2): a review. Version 2. Mol Cancer. 2020;19(1):100. 10.1186/s12943-020-01218-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Wu Z, McGoogan JM Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239. 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 121. Han Y, Feng Z, Sun L, et al. A comparative‐descriptive analysis of clinical characteristics in 2019‐Coronavirus‐infected children and adults. J Med Virol. 2020;92:1596‐1602. 10.1002/jmv.25835 [DOI] [PubMed] [Google Scholar]
- 122. Lai CC, Liu YH, Wang CY, et al. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2): facts and myths. J Microbiol Immunol Infect. 2020;53(3):404‐412. 10.1016/j.jmii.2020.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. CDC COVID‐19 Response Team . Severe outcomes among patients with coronavirus disease 2019 (COVID‐19)—United States, February 12‐March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343‐346. 10.15585/mmwr.mm6912e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Du Y, Tu L, Zhu P, et al. Clinical features of 85 fatal cases of COVID‐19 from Wuhan. A retrospective observational study. Am J Respir Crit Care Med. 2020;201(11):1372‐1379. 10.1164/rccm.202003-0543OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Shekerdemian LS, Mahmood NR, Wolfe KK, et al. Characteristics and outcomes of children with coronavirus disease 2019 (COVID‐19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr. 2020;174:868. 10.1001/jamapediatrics.2020.1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Sinha IP, Harwood R, Semple MG, et al. COVID‐19 infection in children. Lancet Respir Med. 2020;8(5):446‐447. 10.1016/S2213-2600(20)30152-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Oualha M, Bendavid M, Berteloot L, et al. Severe and fatal forms of COVID‐19 in children. Arch Pediatr. 2020;27(5):235‐238. 10.1016/j.arcped.2020.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Craver R, Huber S, Sandomirsky M. Fatal eosinophilic myocarditis in a healthy 17‐year‐old male with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2c). Fetal Pediatr Pathol. 2020;39(3):263‐268. 10.1080/15513815.2020.1761491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Yildirim AI, Karaagac AT. COVID‐19 in a young girl with restrictive cardiomyopathy and chronic lung disease. Indian Pediatr. 2020;57(6):577‐578. 10.1007/s13312-020-1863-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Derespina KR, Kaushik S, Plichta A, et al. Clinical manifestations and outcomes of critically ill children and adolescents with COVID‐19 in New York city. J Pediatr. 2020. 10.1016/j.jpeds.2020.07.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Jeng MJ. Coronavirus disease 2019 in children: current status. J Chin Med Assoc. 2020;83(6):527‐533. 10.1097/JCMA.0000000000000323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Kelvin AA, Halperin S. COVID‐19 in children: the link in the transmission chain. Lancet Infect Dis. 2020;20(6):633‐634. 10.1016/S1473-3099(20)30236-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Goldman RD. Coronavirus disease 2019 in children: surprising findings in the midst of a global pandemic. Can Fam Physician. 2020;66(5):332‐334. [PMC free article] [PubMed] [Google Scholar]
- 135. De Sanctis V, Ruggiero L, Soliman AT, Daar S, Di Maio S, Kattamis C. Coronavirus disease 2019 (COVID‐19) in adolescents: an update on current clinical and diagnostic characteristics. Acta Biomed. 2020;91(2):184‐194. 10.23750/abm.v91i2.9543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):1‐11. 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Brodin P. Why is COVID‐19 so mild in children? Acta Paediatr. 2020;109(6):1082‐1083. 10.1111/apa.15271 [DOI] [PubMed] [Google Scholar]
- 138. Ludvigsson JF. Systematic review of COVID‐19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109(6):1088‐1095. 10.1111/apa.15270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Sankar J, Dhochak N, Kabra SK, Lodha R. COVID‐19 in children: clinical approach and management. Indian J Pediatr. 2020;87(6):433‐442. 10.1007/s12098-020-03292-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Devulapalli CS COVID‐19—a mild disease in children. Tidsskr Nor Laegeforen. 2020;140(6), 10.4045/tidsskr.20.0231 [DOI] [PubMed] [Google Scholar]
- 141. Castagnoli R, Votto M, Licari A, et al. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection in children and adolescents: a systematic review. JAMA Pediatr. 2020;174:882. 10.1001/jamapediatrics.2020.1467 [DOI] [PubMed] [Google Scholar]
- 142. Dhochak N, Singhal T, Kabra SK, Lodha R. Pathophysiology of COVID‐19: why children fare better than adults? Review. Indian J Pediatr. 2020;87(7):537‐546. 10.1007/s12098-020-03322-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Zimmermann P, Curtis N. COVID‐19 in children, pregnancy and neonates: a review of epidemiologic and clinical features. Review. Pediatr Infect Dis J. 2020;39(6):469‐477. 10.1097/INF.0000000000002700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Giwa AL, Desai A, Duca A. Novel 2019 coronavirus SARS‐CoV‐2 (COVID‐19): an updated overview for emergency clinicians. Emerg Med Pract. 2020;22(5):1‐28. [PubMed] [Google Scholar]
- 145. Pavone P, Ceccarelli M, Taibi R, La Rocca G, Nunnari G. Outbreak of COVID‐19 infection in children: fear and serenity. Eur Rev Med Pharmacol Sci. 2020;24(8):4572‐4575. 10.26355/eurrev_202004_21043 [DOI] [PubMed] [Google Scholar]
- 146. Duan YN, Zhu YQ, Tang LL, Qin J. CT features of novel coronavirus pneumonia (COVID‐19) in children. Eur Radiol. 2020;30(8):4427‐4433. 10.1007/s00330-020-06860-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Pan Y, Guan H, Zhou S, et al. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019‐nCoV): a study of 63 patients in Wuhan, China. Eur Radiol. 2020;30(6):3306‐3309. 10.1007/s00330-020-06731-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Pan F, Ye T, Sun P, et al. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID‐19) pneumonia. Radiology. 2020;295(3):715‐721. 10.1148/radiol.2020200370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Chung M, Bernheim A, Mei X, et al. CT imaging features of 2019 novel coronavirus (2019‐nCoV). Radiology. 2020;295(1):202‐207. 10.1148/radiol.2020200230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT‐PCR testing in coronavirus disease 2019 (COVID‐19) in China: a report of 1014 cases. Radiology. 2020;296(2):E32‐E40. 10.1148/radiol.2020200642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Rodrigues JCL, Hare SS, Edey A, et al. An update on COVID‐19 for the radiologist—a British Society of Thoracic Imaging statement. Clin Radiol. 2020;75(5):323‐325. 10.1016/j.crad.2020.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Xu YH, Dong JH, An WM, et al. Clinical and computed tomographic imaging features of novel coronavirus pneumonia caused by SARS‐CoV‐2. J Infect. 2020;80(4):394‐400. 10.1016/j.jinf.2020.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Khan EA. COVID‐19 in children: epidemiology, presentation, diagnosis and management. J Pak Med Assoc. 2020;70(Suppl 3)(5):S108‐S112. 10.5455/JPMA.25 [DOI] [PubMed] [Google Scholar]
- 155. Shen K, Yang Y, Wang T, et al. Diagnosis, treatment, and prevention of 2019 novel coronavirus infection in children: experts' consensus statement. World J Pediatr. 2020;16(3):223‐231. 10.1007/s12519-020-00343-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sinha I. Management of Children admitted to Hospital with COVID‐19 infection (Version 2). Guidance for the clinical management of children admitted to hospital with suspected COVID‐19. British Paediatric Respiratory Society. 2020. https://www.rcpch.ac.uk/sites/default/files/2020‐03/bprs_management_of_children_admitted_to_hospital_with_covid19_‐_20200319.pdf
- 157. Chen ZM, Fu JF, Shu Q, et al. Diagnosis and treatment recommendations for pediatric respiratory infection caused by the 2019 novel coronavirus. World J Pediatr. 2020;16(3):240‐246. 10.1007/s12519-020-00345-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Barlow A, Landolf KM, Barlow B, et al. Review of emerging pharmacotherapy for the treatment of coronavirus disease 2019. Pharmacotherapy. 2020;40(5):416‐437. 10.1002/phar.2398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Cao B, Wang Y, Wen D, et al. A trial of Lopinavir–Ritonavir in adults hospitalized with severe Covid‐19. N Engl J Med. 2020;382(19):1787‐1799. 10.1056/NEJMoa2001282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Chiotos K, Hayes M, Kimberlin DW, et al. Multicenter initial guidance on use of antivirals for children with COVID‐19/SARS‐CoV‐2. J Pediatric Infect Dis Soc. 2020. 10.1093/jpids/piaa045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Shen KL, Yang YH, Jiang RM, et al. Updated diagnosis, treatment and prevention of COVID‐19 in children: experts' consensus statement (condensed version of the second edition). World J Pediatr. 2020;16(3):232‐239. 10.1007/s12519-020-00362-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. De Rose DU, Piersigilli F, Ronchetti MP, et al. Novel coronavirus disease (COVID‐19) in newborns and infants: what we know so far. Version 2. Ital J Pediatr. 2020;46(1):56. 10.1186/s13052-020-0820-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Jean SS, Lee PI, Hsueh PR. Treatment options for COVID‐19: the reality and challenges. J Microbiol Immunol Infect. 2020;53(3):436‐443. 10.1016/j.jmii.2020.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Luo W, Xiong Z, Tang H, Zhou H. A family outbreak of coronavirus disease 2019. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2020;45(3):275‐279. 10.11817/j.issn.1672-7347.2020.200099 [DOI] [PubMed] [Google Scholar]
- 165. Lan L, Xu D, Xia C, Wang S, Yu M, Xu H. Early CT findings of coronavirus disease 2019 (COVID‐19) in asymptomatic children: a single‐center experience. Korean J Radiol. 2020;21(7):919‐924. 10.3348/kjr.2020.0231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475‐481. 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Matthai J, Shanmugam N, Sobhan P, Indian Society of Pediatric Gastroenterology, Hepatology and Nutrition; Pediatric Gastroenterology Chapter of Indian Academy of Pediatrics. Coronavirus disease (COVID‐19) and the gastrointestinal system in children. Indian Pediatr. 2020;57(6):533‐535. 10.1007/s13312-020-1851-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Pan X, Chen D, Xia Y, et al. Asymptomatic cases in a family cluster with SARS‐CoV‐2 infection. Lancet Infect Dis. 2020;20(4):410‐411. 10.1016/S1473-3099(20)30114-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Wu P, Liang L, Chen CB, Nie SQ. A child confirmed COVID‐19 with only symptoms of conjunctivitis and eyelid dermatitis. Graefes Arch Clin Exp Ophthalmol. 2020;258(7):1565‐1566. 10.1007/s00417-020-04708-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Chen G, Wu D, Guo W, et al. Clinical and immunologic features in severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620‐2629. 10.1172/JCI137244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Henry BM, Lippi G, Plebani M. Laboratory abnormalities in children with novel coronavirus disease 2019. Clin Chem Lab Med. 2020;58(7):1135‐1138. 10.1515/cclm-2020-0272 [DOI] [PubMed] [Google Scholar]
- 172. Cook J, Harman K, Zoica B, Verma A, D'Silva P, Gupta A. Horizontal transmission of severe acute respiratory syndrome coronavirus 2 to a premature infant: multiple organ injury and association with markers of inflammation. Lancet Child Adolesc Health. 2020;4(7):548‐551. 10.1016/S2352-4642(20)30166-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Buonsenso D, Costa S, Sanguinetti M, et al. Neonatal late onset infection with severe acute respiratory syndrome coronavirus 2. Am J Perinatol. 2020;37(8):869‐872. 10.1055/s-0040-1710541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Shah PS, Diambomba Y, Acharya G, Morris SK, Bitnun A. Classification system and case definition for SARS‐CoV‐2 infection in pregnant women, fetuses, and neonates. Acta Obstet Gynecol Scand. 2020;99(5):565‐568. 10.1111/aogs.13870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Hu X, Gao J, Luo X, et al. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) vertical transmission in neonates born to mothers with coronavirus disease 2019 (COVID‐19) pneumonia. Obstet Gynecol. 2020;136(1):65‐67. 10.1097/AOG.0000000000003926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Kirtsman M, Diambomba Y, Poutanen SM, et al. Probable congenital SARS‐CoV‐2 infection in a neonate born to a woman with active SARS‐CoV‐2 infection. CMAJ. 2020;192(24):E647‐E650. 10.1503/cmaj.200821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Xiong Y, Zhang Q, Zhao L, Shao J, Zhu W. Clinical and imaging features of COVID‐19 in a neonate. Chest. 2020;158(1):e5‐e7. 10.1016/j.chest.2020.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Wu YT, Liu J, Xu JJ, et al. Neonatal outcome in 29 pregnant women with COVID‐19: a retrospective study in Wuhan, China. PLOS Med. 2020;17(7):e1003195. 10.1371/journal.pmed.1003195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Song R, Han B, Song M, et al. Clinical and epidemiological features of COVID‐19 family clusters in Beijing, China. J Infect. 2020;81(2):e26‐e30. 10.1016/j.jinf.2020.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Ma H, Hu J, Tian J, et al. A single‐center, retrospective study of COVID‐19 features in children: a descriptive investigation. BMC Med. 2020;18(1):123. 10.1186/s12916-020-01596-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Sieni E, Pegoraro F, Casini T, et al. Favourable outcome of Coronavirus‐19 in a 1‐year‐old girl with acute myeloid leukaemia and severe treatment‐induced immunosuppression. Br J Haematol. 2020;189(6):e222‐e224. 10.1111/bjh.16781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Nathan N, Prevost B, Corvol H. Atypical presentation of COVID‐19 in young infants. Lancet. 2020;395(10235):1481. 10.1016/S0140-6736(20)30980-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Riphagen S, Gomez X, Gonzalez‐Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID‐19 pandemic. Lancet 2020. 2020;395(10237):1607‐1608. 10.1016/S0140-6736(20)31094-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Cao Q, Chen YC, Chen CL, Chiu CH. SARS‐CoV‐2 infection in children: transmission dynamics and clinical characteristics. J Formos Med Assoc. 2020;119(3):670‐673. 10.1016/j.jfma.2020.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Peng Z, Wang J, Mo Y, et al. Unlikely SARS‐CoV‐2 vertical transmission from mother to child: a case report. J Infect Public Health. 2020;13(5):818‐820. 10.1016/j.jiph.2020.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Yang P, Wang X, Liu P, et al. Clinical characteristics and risk assessment of newborns born to mothers with COVID‐19. J Clin Virol. 2020;127:104356. 10.1016/j.jcv.2020.104356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Li Y, Zhao R, Zheng S, et al. Lack of vertical transmission of severe acute respiratory syndrome coronavirus 2, China. Emerg Infect Dis. 2020;26(6):1335‐1336. 10.3201/eid2606.200287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Panda PK, Sharawat IK. COVID‐19 (SARS‐CoV‐2 infection) and children: pediatric neurologist's perspective. Indian J Pediatr. 2020;87(7):556‐557. 10.1007/s12098-020-03326-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. D'Antiga L. Coronaviruses and immunosuppressed patients: the facts during the third epidemic. Liver Transpl. 2020;26(6):832‐834. 10.1002/lt.25756 [DOI] [PubMed] [Google Scholar]
- 190. International Society of Pediatric and Adolescent Diabetes (ISPAD) . Summary of recommendations regarding COVID‐19 in children with diabetes: keep calm and mind your diabetes care and public health advice. Pediatr Diabetes. 2020;21(3):413‐414. 10.1111/pedi.13013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Brough HA, Kalayci O, Sediva A, et al. Managing childhood allergies and immunodeficiencies during respiratory virus epidemics—the 2020 COVID‐19 pandemic. Pediatr Allergy Immunol. 2020;31:442‐448. 10.1111/pai.13262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Minotti C, Tirelli F, Barbieri E, Giaquinto C, Donà D. How is immunosuppressive status affecting children and adults in SARS‐CoV‐2 infection? A systematic review. J Infect. 2020;81(1):e61‐e66. 10.1016/j.jinf.2020.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Ferrari A, Zecca M, Rizzari C, et al. Children with cancer in the time of COVID‐19: an 8‐week report from the six pediatric onco‐hematology centers in Lombardia, Italy. Pediatr Blood Cancer. 2020;67(8):e28410. 10.1002/pbc.28410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Marlais M, Wlodkowski T, Vivarelli M, et al. The severity of COVID‐19 in children on immunosuppressive medication. Lancet Child Adolesc Health. 2020;4(7):e17‐e18. 10.1016/S2352-4642(20)30145-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. de Rojas T, Pérez‐Martínez A, Cela E, et al. COVID‐19 infection in children and adolescents with cancer in Madrid. Pediatr Blood Cancer. 2020;67(7):e28397. 10.1002/pbc.28397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Haslak F, Yildiz M, Adrovic A, et al. Management of childhood‐onset autoinflammatory diseases during the COVID‐19 pandemic. Rheumatol Int. 2020;40(9):1423‐1431. 10.1007/s00296-020-04645-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Colombo C, Burgel PR, Gartner S, et al. Impact of COVID‐19 on people with cystic fibrosis. Lancet Respir Med. 2020;8(5):e35‐e36. 10.1016/S2213-2600(20)30177-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Blanchon S, Fernandez C, Guerin S, Crisinel PA, Rochat I. COVID‐19: a message of hope from a young girl with severe cystic fibrosis. Pediatr Pulmonol. 2020;55(7):1546‐1547. 10.1002/ppul.24812 [DOI] [PubMed] [Google Scholar]
- 199. Poli P, Timpano S, Goffredo M, Padoan R, Badolato R. Asymptomatic case of Covid‐19 in an infant with cystic fibrosis. J Cyst Fibros. 2020;19(3):e18. 10.1016/j.jcf.2020.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Ye Q, Wang B, Mao J, et al. Epidemiological analysis of COVID‐19 and practical experience from China. J Med Virol. 2020;92(7):755‐769. 10.1002/jmv.25813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Pathak EB, Salemi JL, Sobers N, Menard J, Hambleton IR. COVID‐19 in children in the United States: intensive care admissions, estimated total infected, and projected numbers of severe pediatric cases in 2020. J Public Health Manag Pract. 2020;26(4):325‐333. 10.1097/PHH.0000000000001190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Abrams EM, Szefler SJ. COVID‐19 and the impact of social determinants of health. Lancet Respir Med. 2020;8(7):659‐661. 10.1016/S2213-2600(20)30234-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Dallan C, Romano F, Siebert J, Politi S, Lacroix L, Sahyoun C. Septic shock presentation in adolescents with COVID‐19. Lancet Child Adolesc Health. 2020;4(7):e21‐e23. 10.1016/S2352-4642(20)30164-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Garg S, Kim L, Whitaker M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory‐confirmed coronavirus disease 2019—COVID‐NET, 14 states, March 1‐30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):458‐464. 10.15585/mmwr.mm6915e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Kim L, Whitaker M, O'Halloran A, et al. Hospitalization rates and characteristics of children aged < 18 years hospitalized with laboratory‐confirmed COVID‐19—COVID‐NET, 14 states, March 1‐July 25, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(32):1081‐1088. 10.15585/mmwr.mm6932e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Zimmermann P, Curtis N. Coronavirus infections in children including COVID‐19: an overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. Pediatr Infect Dis J. 2020;39(5):355‐368. 10.1097/INF.0000000000002660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Li X, Xu W, Dozier M, He Y, Kirolos A, Theodoratou E. The role of children in transmission of SARS‐CoV‐2: a rapid review. J Glob Health. 2020;10(1):011101. 10.7189/jogh.10.011101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Rubin GD, Ryerson CJ, Haramati LB, et al. The role of chest imaging in patient management during the COVID‐19 pandemic: a multinational consensus statement from the Fleischner society. Chest. 2020;158(1):106‐116. 10.1016/j.chest.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe kawasaki‐like disease at the Italian epicentre of the SARS‐CoV‐2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771‐1778. 10.1016/S0140-6736(20)31103-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Ouldali N, Pouletty M, Mariani P, et al. Emergence of Kawasaki disease related to SARS‐CoV‐2 infection in an epicentre of the French COVID‐19 epidemic: a time‐series analysis. Lancet Child Adolesc Health. 2020;S2352‐4642(20):30175‐30179. 10.1016/S2352-4642(20)30175-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Chiotos K, Bassiri H, Behrens EM, et al. Multisystem inflammatory syndrome in children during the coronavirus 2019 pandemic: a case series. J Pediatric Infect Dis Soc. 2020;9(3):393‐398. 10.1093/jpids/piaa069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Belot A, Antona D, Renolleau S, et al. SARS‐CoV‐2‐related paediatric inflammatory multisystem syndrome, an epidemiological study, France, 1 March to 17 May 2020. Euro Surveill. 2020;25(22):2001010. 10.2807/1560-7917.ES.2020.25.22.2001010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Latimer G, Corriveau C, DeBiasi RL, et al. Cardiac dysfunction and thrombocytopenia‐associated multiple organ failure inflammation phenotype in a severe paediatric case of COVID‐19. Lancet Child Adolesc Health. 2020;4(7):552‐554. 10.1016/S2352-4642(20)30163-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Regev T, Antebi M, Eytan D, et al. Pediatric inflammatory multisystem syndrome with central nervous system involvement and hypocomplementemia following SARS‐COV‐2 infection. Pediatr Infect Dis J. 2020;39(8):e206‐e207. 10.1097/INF.0000000000002804 [DOI] [PubMed] [Google Scholar]
- 215. Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383(4):334‐346. 10.1056/NEJMoa2021680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Dufort EM, Koumans EH, Chow EJ, et al. Multisystem inflammatory syndrome in children in New York state. N Engl J Med. 2020;383(4):347‐358. 10.1056/NEJMoa2021756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Riollano‐Cruz M, Akkoyun E, Briceno‐Brito E, et al. Multisystem inflammatory syndrome in children (MIS‐C) related to COVID‐19: a New York city experience. J Med Virol. 2020:jmv.26224. 10.1002/jmv.26224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Nguyen DC, Haydar H, Pace ER, Zhang XS, Dobbs KR. Pediatric case of severe COVID‐19 with shock and multisystem inflammation. Cureus. 2020;12(6):e8915. 10.7759/cureus.8915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Licciardi F, Pruccoli G, Denina M, et al. SARS‐CoV‐2‐induced Kawasaki‐like hyperinflammatory syndrome: a novel COVID phenotype in children. Pediatrics. 2020;146(2):e20201711. 10.1542/peds.2020-1711 [DOI] [PubMed] [Google Scholar]
- 220. Greene AG, Saleh M, Roseman E, Sinert R. Toxic shock‐like syndrome and COVID‐19: a case report of multisystem inflammatory syndrome in children (MIS‐C). Am J Emerg Med. 2020;S0735‐6757(20):30492‐30497. 10.1016/j.ajem.2020.05.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS‐CoV‐2. JAMA. 2020;324:259. 10.1001/jama.2020.10369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Diorio C, Henrickson SE, Vella LA, et al. Multisystem inflammatory syndrome in children and COVID‐19 are distinct presentations of SARS‐CoV‐2. J Clin Invest. 2020. 10.1172/JCI140970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Kest H, Kaushik A, DeBruin W, Colletti M, Goldberg D Multisystem inflammatory syndrome in children (MIS‐C) associated with 2019 novel coronavirus (SARS‐CoV‐2) infection. Case Rep Pediatr. 2020;2020:8875987−4, 10.1155/2020/8875987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Cabrero‐Hernández M, García‐Salido A, Leoz‐Gordillo I, et al. Severe SARS‐CoV‐2 infection in children with suspected acute abdomen: a case series from a tertiary hospital in Spain. Pediatr Infect Dis J. 2020;39(8):e195‐e198. 10.1097/INF.0000000000002777 [DOI] [PubMed] [Google Scholar]
- 225. Klocperk A, Parackova Z, Dissou J, et al. Case report: systemic inflammatory response and fast recovery in a pediatric patient with COVID‐19. Front Immunol. 2020;11:1665. 10.3389/fimmu.2020.01665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Gupta A, Gill A, Sharma M, Garg M. Multi‐system inflammatory syndrome in a child mimicking Kawasaki disease. J Trop Pediatr. 2020. 10.1093/tropej/fmaa060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Heidemann SM, Tilford B, Bauerfeld C, et al. Three cases of pediatric multisystem inflammatory syndrome associated with COVID‐19 due to SARS‐CoV‐2. Am J Case Rep. 2020;21:e925779. 10.12659/AJCR.925779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Pouletty M, Borocco C, Ouldali N, et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS‐CoV‐2 mimicking Kawasaki disease (Kawa‐COVID‐19): a multicentre cohort. Ann Rheum Dis. 2020;79(8):999‐1006. 10.1136/annrheumdis-2020-217960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Cazzaniga M, Baselli LA, Cimaz R, Guez SS, Pinzani R, Dellepiane RM. SARS‐COV‐2 infection and Kawasaki disease: case report of a hitherto unrecognized association. Front Pediatr. 2020;8:398. 10.3389/fped.2020.00398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Lee PI, Hu YL, Chen PY, Huang YC, Hsueh PR. Are children less susceptible to COVID‐19? J Microbiol Immunol Infect. 2020. 10.1016/j.jmii.2020.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Bunyavanich S, Do A, Vicencio A. Nasal gene expression of angiotensin‐converting enzyme 2 in children and adults. JAMA. 2020;323(23):2427‐2429. 10.1001/jama.2020.8707 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.


