Abstract
Background
Emerging evidence implicates dysfunctional platelet responses in thrombotic complications in COVID‐19 patients. Platelets are important players in inflammation‐induced thrombosis. In particular, procoagulant platelets support thrombin generation and mediate thromboinflammation.
Objectives
To examine if procoagulant platelet formation is altered in COVID‐19 patients and if procoagulant platelets contribute to pulmonary thrombosis.
Patients/Methods
Healthy donors and COVID‐19 patients were recruited from the University of Utah Hospital System. Platelets were isolated and procoagulant platelet formation measured by annexin V binding as well as mitochondrial function were examined. We utilized mice lacking the ability to form procoagulant platelets (CypDplt−/−) to examine the role of procoagulant platelets in pulmonary thrombosis.
Results and Conclusions
We observed that platelets isolated from COVID‐19 patients had a reduced ability to become procoagulant compared to those from matched healthy donors, as evidenced by reduced mitochondrial depolarization and phosphatidylserine exposure following dual stimulation with thrombin and convulxin. To understand what impact reduced procoagulant platelet responses might have in vivo, we subjected mice with a platelet‐specific deletion of cyclophilin D, which are deficient in procoagulant platelet formation, to a model of pulmonary microvascular thrombosis. Mice with platelets lacking cyclophilin D died significantly faster from pulmonary microvascular thrombosis compared to littermate wild‐type controls. These results suggest dysregulated procoagulant platelet responses may contribute to thrombotic complications during SARS‐CoV‐2 infection.
Keywords: COVID‐19, infections, mitochondria, platelet activation, thrombosis
Essentials
-
•
Procoagulant platelet formation regulates thrombin generation and thromboinflammation.
-
•
COVID‐19 patients have increased thrombosis and inflammation, but the contributions of procoagulant platelets to the pathophysiology of COVID‐19 is unknown.
-
•
We observed less procoagulant platelet formation in COVID‐19 patients due, in part, to mitochondrial dysfunction.
-
•
Mice lacking the ability to form procoagulant platelets surprisingly had increased mortality in a pulmonary thrombosis model.
Alt-text: Unlabelled Box
1. INTRODUCTION
Thrombotic complications are common in patients infected with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2).1., 2., 3. In particular, intravascular pulmonary thrombosis is believed to contribute to acute respiratory distress syndrome (ARDS) and mortality in COVID‐19 patients.4 Previously, we demonstrated that neutrophil extracellular traps (NETs) and platelets accumulate in the lung microvasculature of COVID‐19 patients.5 Furthermore, platelets from COVID‐19 patients were hyperresponsive to agonist stimulation and interacted more with leukocytes at baseline, indicating crosstalk between platelets and leukocytes during SARS‐CoV‐2 infection.6
Upon strong activation by classical agonists, a subpopulation of platelets becomes procoagulant. These procoagulant platelets have distinct properties, including the ability to support thrombin generation, an increased tendency to interact with leukocytes, and a reduced capacity to aggregate.7 Recently, we and others described a critical role for procoagulant platelets in the formation of detrimental platelet‐‐neutrophil aggregates during reperfusion injury.8., 9. Interestingly, in septic patients,10 as well as patients infected with human immunodeficiency virus (HIV)11 and dengue,12 elevated levels of procoagulant platelets have been reported. Whether procoagulant platelet responses are altered in COVID‐19 patients is currently unknown.
In this study, we report our observation that procoagulant platelet responses are reduced in COVID‐19 patients, compared to healthy donors. Additionally, mice with impaired procoagulant platelet responses died faster in a model of pulmonary microvascular thrombosis. These results warrant further investigation into the role of procoagulant platelets in the pathophysiology of COVID‐19.
2. METHODS
2.1. Study design
Eleven patients with acute SARS‐CoV‐2 infection were recruited from 17 to 20 March 17 2020 at the University of Utah Health Sciences Center in Salt Lake City. SARS‐CoV‐2 infection was confirmed molecularly by reverse transcription polymerase chain reaction (RT‐PCR), in accordance with current standards (Table 1 ).13 All COVID‐19 patients were identified under study protocols approved by the Institutional Review Board (IRB) of the University of Utah (IRB#: 00102638, 00093575). Eleven healthy, age‐ and gender‐matched donors were enrolled under a separate IRB protocol (IRB#: 0051506). Each study participant or their legal authorized representative gave written informed consent for study enrollment in accordance with the Declaration of Helsinki. Enrollment criteria for COVID‐19 patients included age >18 years, respiratory symptoms (cough, shortness of breath) or fever, hospital admission, positive SARS‐CoV‐2 testing, and written informed consent. The standard of care at the University of Utah does not include screening ultrasound, and no clinically diagnosed thrombotic events occurred in our patient population. From each COVID‐19 patient or healthy donor, we collected ~60 mL of anticoagulant citrate dextrose (ACD)‐anticoagulated whole blood via venipuncture for assays described below.
Table 1.
Clinical characteristics of COVID‐19 patients
| Healthy donors (n = 11) | Hospitalized COVID‐19 patients (n = 11) | Reference rangea | |
|---|---|---|---|
| Age (mean, ±SD) | 55.6 (±15.8) | 60.7 (±11.5) | — |
| Male (%) | 45.5% | 45.8% | — |
| Hispanic/Latino (%) | 9% | 54.5% | — |
| Diabetes (%) | 0% | 45.5% | — |
| Hypertension (%) | 0% | 54.5% | — |
| SOFA score (median [range]) | — | 4 [0‐9] | — |
| ARDS (%) | — | 63.6% | — |
| Mechanical ventilation (%) | — | 18.2% | — |
| Survival to date (%) | — | 91% | — |
| Platelet count (K/µL, mean ± SD) | — | 225.8 ± 43.8 | 159‐439 K/µL |
| MPV (fL, mean ± SD) | — | 10.20 ± 0.84 | 8.6‐12.3 fL |
| Aspirin (%) | 0.0% | 0.0% | |
| Hydroxychloroquine (%) | — | 9% | |
| Remdesivir (%) | — | 18.2% | |
| Convalescent plasma (%) | — | 9% |
Abbreviations: ARDS, acute respiratory distress syndrome; MPV, mean platelet volume; SD, standard deviation; SOFA, sequential organ failure assessment.
Reference range from ARUP Laboratories as of 26 August 2020.
2.2. Flow cytometry
To detect phosphatidylserine positive (PS+) platelets, washed platelets were resuspended in Medium 199 (12‐117 F; Lonza) containing calcium and stimulated for 15 minutes with thrombin (1.0 U/mL, final, Thermo Fisher) and convulxin (250 ng/mL, final, Santa Cruz) in the presence of fluorescein isothiocyanate (FITC)‐labelled annexin V and anti‐human CD41 Allophycocyanin (APC). In some experiments, platelets were stimulated with A23187 (50 µmol/L, final, Sigma Aldrich) in the presence of FITC‐labelled Annexin V and anti‐human CD41 APC. To measure mitochondrial membrane potential and mitochondrial reactive oxygen species, washed platelets were resuspended in M199 in the presence of mitotracker green (200 nmol/L, final) and tetramethylrhodamine methyl ester (TMRM; 60 nmol/L, final) or MitoSox Red (200 nmol/L, final) for 40 minutes at 37°C in the presence or absence of thrombin and convulxin. Samples were immediately run on a Beckman Coulter Cytoflex. TMRM and MitoSox Red median fluorescence intensity (MFI) were normalized to MitoTracker Green MFI. For TMRM, subsequently the fold change relative to baseline was calculated.
2.3. Pulmonary thrombosis model
All animal experiments complied with the regulatory standards of the University of Utah. Pulmonary thrombosis experiments were performed as described previously in mice with a platelet specific deficiency in CypD (CypDplt−/−) and their littermate controls (CypDplt+/+).14 Mice lacking CypD are unable to form procoagulant platelets. A mixture of collagen (0.4 mg/kg; Chronolog) and epinephrine (30 mg/kg; Sigma‐Aldrich) in 100 µL of phosphate buffered saline (PBS) was administered through retro‐orbital injection. Time until cessation of respiration (time needed to the onset of respiratory arrest that lasted at least 2‐3 minutes) was recorded.
2.4. Statistical analyses
For all analyses, continuous variables were assessed for normality with skewness and kurtosis tests. Summary statistics were used to describe the study cohort and clinical variables are expressed as the mean ± standard deviation or as a number and percentage (%). Parametric two‐tailed t‐tests or analysis of variance were used for continuous variables. Statistical analyses were performed by using GraphPad Prism (version 7, San Diego, CA). A two‐tailed P‐value < .05 was considered statistically significant.
3. RESULTS AND DISCUSSION
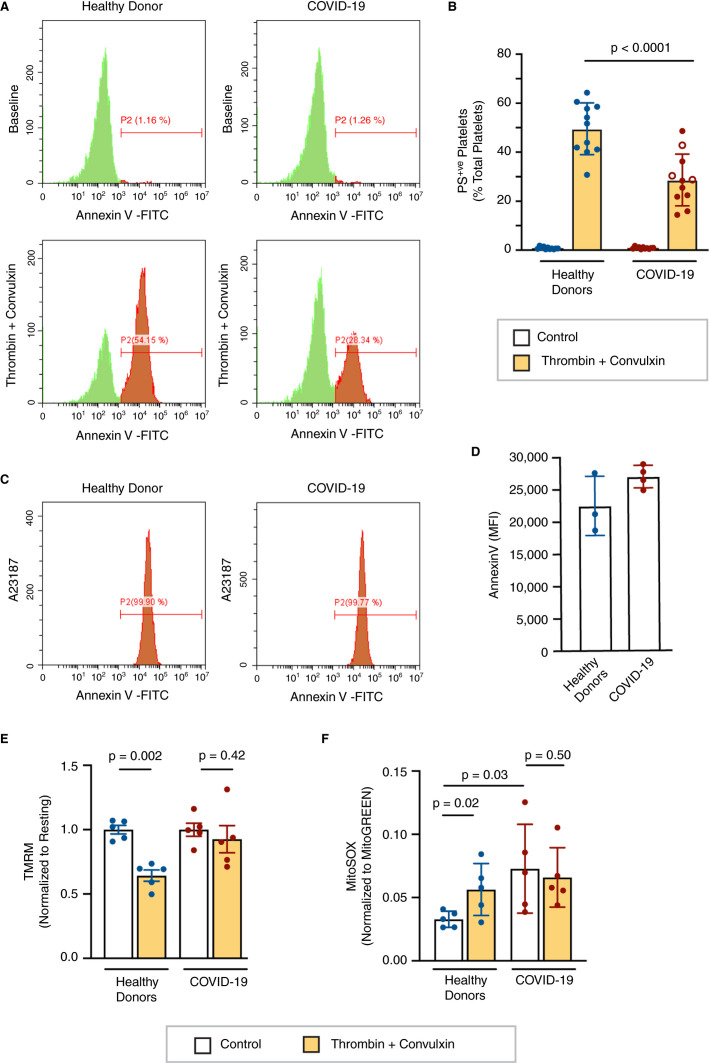
Pulmonary thrombotic events significantly contribute to COVID‐19 pathology.4., 15., 16. As platelets are key players in thrombosis under systemic inflammatory settings, we investigated procoagulant platelet responses in COVID‐19 patients. Unlike dengue‐12 or HIV‐infected patients,11 COVID‐19 patients had similar levels of annexin V positive, procoagulant platelets at baseline compared to healthy donors (Figure 1A‐B ). To assess procoagulant platelet formation, platelets from COVID‐19 patients or healthy donors were stimulated with thrombin and the glycoprotein VI (GPVI) agonist convulxin, and phosphatidylserine (PS) exposure was assessed using annexin V binding. Interestingly, dual agonist stimulation induced significantly less PS exposure in COVID‐19 patients compared to healthy donors (Figure 1A‐B). Procoagulant platelet responses were similarly reduced in non–intensive care unit (ICU) and ICU patients (P = .7, Figure 1B) and did not correlate with disease severity (data not shown). In contrast, platelets from healthy donors and COVID‐19 patients stimulated with the Ca2+ ionophore A23187 displayed similar levels of PS exposure (Figure 1C‐D). The latter result suggests that reduced procoagulant platelet responses observed in COVID‐19 patients are likely not due to defective or impaired PS scramblase activity.17., 18.
FIGURE 1.
Altered mitochondrial function reduces procoagulant platelet formation. Platelets were isolated from healthy donors and COVID‐19 patients. A, Phosphatidylserine (PS) exposure was assessed by flow cytometry using annexin V binding under resting conditions and after dual agonist stimulation (1 U/mL thrombin + 250 ng/mL convulxin). Representative flow plots are shown. B, The percentage of platelets with high PS exposure was quantified (n = 11). Open circles represent non–intensive care unit (ICU) patients, closed circles represent ICU patients. C, Platelet PS exposure in response to 50 µmol/L A23187 was measured by annexin V binding. Representative flow plots are shown. D, Mean fluorescence intensity (MFI) of annexin V staining after A23187 treatment (n = 3‐4). E, Platelet mitochondrial membrane potential was measured by tetramethylrhodamine methyl ester staining analyzed by flow cytometry under resting conditions and after dual agonist stimulation (1 U/mL thrombin + 250 ng/mL convulxin; n = 5). F, Mitochondrial reactive oxygen species was measured by MitoSOX staining analyzed by flow cytometry under resting conditions and after dual agonist stimulation (1 U/mL thrombin + 250 ng/mL convulxin; n = 5)
Formation of the mitochondrial permeability transition pore is a critical event in the generation of procoagulant platelets19., 20. and results in a decrease in mitochondrial membrane potential and the subsequent release of mitochondrial Ca2+ initiating PS exposure. To investigate whether dysfunction of the mitochondrial permeability transition pore contributes to our observations in COVID‐19 platelets, we measured changes in mitochondrial membrane potential following dual agonist stimulation by TMRM staining. As expected, dual agonist stimulation induced a significant decrease in mitochondrial membrane potential compared to resting platelets from healthy donors. Consistent with the lack of PS exposure, we did not observe a significant loss of mitochondrial membrane potential upon dual agonist stimulation of platelets from COVID‐19 patients (Figure 1E). These results indicate dysregulated procoagulant platelet responses in COVID‐19 patients are due to defects upstream or at the point of the mitochondrial permeability transition pore.
Because PS exposure is dependent on mitochondrial function18., 21. and alterations in mitochondrial function are known to alter procoagulant platelet formation,22 we next assessed the level of mitochondrial reactive oxygen species (ROS), a marker of mitochondrial function. Platelets from COVID‐19 patients exhibited higher mitochondrial ROS levels at baseline compared to those from healthy donors (Figure 1F), consistent with mitochondrial dysfunction in platelets during SARS‐CoV‐2 infection. Furthermore, dual agonist stimulation of platelets from COVID‐19 patients did not elicit further increases in mitochondrial ROS generation (Figure 1F). This aligns with a defect in mitochondrial permeability transition, as opening of the mitochondrial permeability transition pore is known to result in increased mitochondrial ROS.22
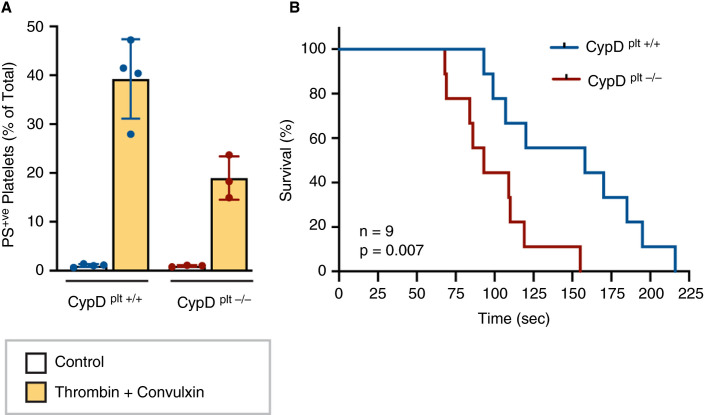
Pulmonary microvascular thrombosis is a common finding in COVID‐19 patients. To investigate the contribution of reduced procoagulant platelets to this phenomenon, we subjected mice lacking the ability to form procoagulant platelets (CypDplt−/−, Figure 2A ) to a model of pulmonary microvascular thrombosis, in which platelets are the primary driver of vascular occlusion.23 To induce pulmonary thrombosis, collagen and epinephrine are injected intravenously and the time until breathing cessation is recorded. We observed that mice lacking procoagulant platelet responses died significantly faster after induction of pulmonary thrombosis, relative to littermate wild‐type controls in which procoagulant platelet formation was intact (Figure 2B).
FIGURE 2.
Mice deficient in procoagulant platelet formation die faster in a pulmonary thrombosis model. A, Platelets were isolated from wild‐type (CypDplt+/+, n = 4) and platelet specific cyclophilin D knockout mice (CypDplt−/−, n = 3) and stimulated with 1 U/mL thrombin + 250 ng/mL convulxin and annexin V staining was measured. B, Pulmonary thrombosis was induced in anesthetized mice by intravenous injection of a mixture of collagen (0.4 mg/kg; Chronolog) and epinephrine (30 mg/kg; Sigma‐Aldrich) in 100 µL of phosphate buffered saline. Time to death was monitored. Shown is the Kaplan–Meier survival curve (n = 9 per genotype).
While classically recognized for their prothrombotic properties, several studies have indicated an important role for procoagulant platelets in the regulation of thrombotic events.20., 24., 25. Indeed, while procoagulant platelets have an excellent ability to bind coagulation factors, they also have a reduced ability to aggregate.26 In particular, CypD‐dependent procoagulant platelet formation initiates a negative‐feedback mechanism that limits platelet aggregation and subsequent thrombosis.24 The reduced ability to form procoagulant platelets in COVID‐19 patients could therefore potentially result in hyper‐aggregating platelets, as previously described.6 Consistent with this hypothesis, cyclosporin, a potent inhibitor of CypD and procoagulant platelet formation, has been reported to increase the risk of thromboembolic events in renal transplant patients.27., 28. Our current findings support these observations, as mice lacking the ability to form procoagulant platelets through CypD deletion died faster in a pulmonary thrombosis model. Interestingly, also in a model of venous thromboembolism, CypD‐deficient animals developed larger thrombi and in a more rapid manner.29
Mitochondrial depolarization through CypD and the mitochondrial permeability transition pore are necessary for the formation of procoagulant platelets.19., 20. However, only minor depolarization of platelets from COVID‐19 patients was observed upon dual agonist stimulation, coinciding with a lack of PS expression. Ingenuity Pathway Analysis on RNA‐seq performed on platelets from COVID‐19 patient demonstrated significant enrichment of genes involved in mitochondrial dysfunction.6 It is unclear why COVID‐19 induces significant alterations in gene expression related to mitochondrial function. Viral infections are known to induce mitochondrial dysfunction12 and our results suggest SARS‐CoV‐2 infection alters platelet gene expression, although the mechanism remains unknown at this time. As platelets and megakaryocytes do not express ACE2, the receptor necessary for SARS‐CoV‐2 infection30 and we have not detected SARS‐CoV‐2 virus within platelets from COVID‐19 patients,6 alterations in the platelet transcriptome may be due to systemic changes related to the infection.
Finally, our study has several limitations. First, only a limited number of patients were included in this study and therefore our results will need to be validated in larger COVID‐19 cohorts. Second, the small sample size in our study precluded correlation analysis with thrombotic biomarkers or thrombotic events due to limited statistical power and the lack of screening for asymptomatic thrombotic events in COVID‐19 patients at our institution. Last, our animal studies were performed in the absence of a viral challenge, and in a microvascular thrombosis model primarily driven by platelets. This limits translation of our murine findings to clinical COVID‐19 pathophysiology.
While we recognize the limitations of our clinical observations and animal models of thrombosis, these results warrant further investigation into the role of dysregulated procoagulant platelet responses and thrombotic events observed in COVID‐19 patients.
CONFLICTS OF INTEREST
Frederik Denorme, Bhanu Kanth Manne, Irina Portier, Aaron Petrey, Elizabeth Middleton, Benjamin Kile, and Robert Campbell have nothing to disclose. Matthew Rondina serves on the Scientific Advisory Board for Acticor Biotech SAS.
AUTHOR CONTRIBUTIONS
FD, BKM, IP, ACP, EAM, and RAC designed and performed experiments; FD, BKM, and RAC analyzed results and made the figures; FD, BKM, IP, ACP, EAM, BTK, MTR, and RAC wrote the paper. All authors reviewed and critically edited the manuscript.
ACKNOWLEDGMENTS
The authors wish to acknowledge figure preparation expertise from Diana Lim and assistance with participant recruitment from Antoinette Blair, Macy Barrios, Amber Plante, Jordan Greer, Amy DeNardo, Amanda Bailey, and Lindsey Waddoups.
National Heart, Lung, and Blood InstituteR01HL135265R01HL142804
Fonds voor Wetenschappelijk Onderzoek Vlaanderen12U7818N
National Institute on AgingK01AG059892R01AG048022R56AG059877
U.S. Department of Veterans AffairsI01 CX001696
Footnotes
Manuscript handled by: Katsue Suzuki‐Inoue
Final decision: Katsue Suzuki‐Inoue, 08 September 2020
Funding informationThis work was supported by grants from the NIH (R01HL135265 to ACP; K01AG059892 to RAC, R01HL142804, R01AG048022, R56AG059877, and R01HL130541 to MTR), the University of Utah Triple I Program (EAM). This work was also supported in part by Merit Review Award Number I01 CX001696 to MTR from the United States Department of Veterans Affairs Clinical Sciences R&D (CSRD) This material is the result of work supported with resources and the use of facilities at the George E. Wahlen VA Medical Center, Salt Lake City, Utah. The contents do not represent the views of the US Department of Veterans Affairs or the United States Government. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The work was also supported by the Flow Cytometry Core at the University of Utah. FD is a postdoctoral fellow of Fonds voor Wetenschappelijk Onderzoek Vlaanderen (12U7818N).
REFERENCES
- 1.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lax S.F., Skok K., Zechner P., et al. Pulmonary arterial thrombosis in COVID‐19 with fatal outcome: results from a prospective, single‐center, clinicopathologic case series. Ann Intern Med. 2020;173:350–361. doi: 10.7326/M20-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Middleton E.A., He X.‐.Y., Denorme F., et al. Neutrophil extracellular traps (NETs) contribute to immunothrombosis in COVID‐19 acute respiratory distress syndrome. Blood. 2020;136:1169–1179. doi: 10.1182/blood.2020007008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manne B.K., Denorme F., Middleton E.A., et al. Platelet gene expression and function in COVID‐19 patients. Blood. 2020;136:1317–1329. doi: 10.1182/blood.2020007214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agbani E.O., Poole A.W. Procoagulant platelets: generation, function, and therapeutic targeting in thrombosis. Blood. 2017;130:2171–2179. doi: 10.1182/blood-2017-05-787259. [DOI] [PubMed] [Google Scholar]
- 8.Yuan Y., Alwis I., Wu M.C.L., et al. Neutrophil macroaggregates promote widespread pulmonary thrombosis after gut ischemia. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aam5861. [DOI] [PubMed] [Google Scholar]
- 9.Denorme F., Manne B.K., Portier I., et al. Platelet necrosis mediates ischemic stroke outcome in mice. Blood. 2020;135:429–440. doi: 10.1182/blood.2019002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma R., Xie R., Yu C., et al. Phosphatidylserine‐mediated platelet clearance by endothelium decreases platelet aggregates and procoagulant activity in sepsis. Sci Rep. 2017;7:1–14. doi: 10.1038/s41598-017-04773-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holme P.A., Müller F., Solum N.O., Brosstad F., Frøland S.S., Aukrust P. Enhanced activation of platelets with abnormal release of RANTES in human immunodeficiency virus type 1 infection. FASEB. 1998;12:79–89. doi: 10.1096/fasebj.12.1.79. [DOI] [PubMed] [Google Scholar]
- 12.Hottz E.D., Oliveira M.F., Nunes P.C.G., et al. Dengue induces platelet activation, mitochondrial dysfunction and cell death through mechanisms that involve DC‐SIGN and caspases. J Thromb Haemost. 2013;11:951–962. doi: 10.1111/jth.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corman V.M., Landt O., Kaiser M., et al. Detection of 2019 novel coronavirus (2019‐nCoV) by real‐time RT‐PCR. Eurosurveillance. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rowley J.W., Chappaz S., Corduan A., et al. Dicer1‐mediated miRNA processing shapes the mRNA profile and function of murine platelets. Blood. 2016;127:1743–1751. doi: 10.1182/blood-2015-07-661371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wichmann D., Sperhake J.‐.P., Lütgehetmann M., et al. Autopsy findings and venous thromboembolism in patients with COVID‐19. Ann Intern Med. 2020;173:269–277. doi: 10.7326/L20-1206. [DOI] [PubMed] [Google Scholar]
- 16.McGonagle D., O'Donnell J.S., Sharif K., Emery P., Bridgewood C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID‐19 pneumonia. Lancet Rheumatology. 2020;2(7):e437–e445. doi: 10.1016/S2665-9913(20)30121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choo H.‐.J., Saafir T.B., Mkumba L., Wagner M.B., Jobe S.M. Mitochondrial calcium and reactive oxygen species regulate agonist‐initiated platelet phosphatidylserine exposure. ATVB. 2012;32:2946–2955. doi: 10.1161/ATVBAHA.112.300433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang H., Kim A., David T., et al. TMEM16F forms a Ca2+‐activated cation channel required for lipid scrambling in platelets during blood coagulation. Cell. 2012;151:111–122. doi: 10.1016/j.cell.2012.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Remenyi G., Szasz R., Friese P., Dale G.L. Role of mitochondrial permeability transition pore in coated‐platelet formation. ATVB. 2005;25:467–471. doi: 10.1161/01.ATV.0000152726.49229.bf. [DOI] [PubMed] [Google Scholar]
- 20.Jobe S.M., Wilson K.M., Leo L., et al. Critical role for the mitochondrial permeability transition pore and cyclophilin D in platelet activation and thrombosis. Blood. 2008;111:1257–1265. doi: 10.1182/blood-2007-05-092684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujii T., Sakata A., Nishimura S., Eto K., Nagata S. TMEM16F is required for phosphatidylserine exposure and microparticle release in activated mouse platelets. Proc Natl Acad Sci USA. 2015;112:12800–12805. doi: 10.1073/pnas.1516594112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choo H.‐.J., Kholmukhamedov A., Zhou C., Jobe S. Inner mitochondrial membrane disruption links apoptotic and agonist‐initiated phosphatidylserine externalization in platelets. ATVB. 2017;37:1503–1512. doi: 10.1161/ATVBAHA.117.309473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Momi S., Falcinelli E., Giannini S., et al. Loss of matrix metalloproteinase 2 in platelets reduces arterial thrombosis in vivo. J Exp Med. 2009;206:2365–2379. doi: 10.1084/jem.20090687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu F., Gamez G., Myers D.R., Clemmons W., Lam W.A., Jobe S.M. Mitochondrially mediated integrin αIIbβ3 protein inactivation limits thrombus growth. J Biol Chem. 2013;288:30672–30681. doi: 10.1074/jbc.M113.472688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whyte C.S., Swieringa F., Mastenbroek T.G., et al. Plasminogen associates with phosphatidylserine‐exposing platelets and contributes to thrombus lysis under flow. Blood. 2015;125:2568–2578. doi: 10.1182/blood-2014-09-599480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heemskerk J.W.M., Mattheij N.J.A., Cosemans J.M.E.M. Platelet‐based coagulation: different populations, different functions. J Thromb Haemost. 2013;11:2–16. doi: 10.1111/jth.12045. [DOI] [PubMed] [Google Scholar]
- 27.Oates J.A., Wood A.J.J., Kahan B.D. Cyclosporine. N Engl J Med. 1989;321:1725–1738. doi: 10.1056/NEJM198912213212507. [DOI] [PubMed] [Google Scholar]
- 28.Brunkwall J., Bergqvist D., Bergentz S.E., Bornmyr S., Husberg B. Postoperative deep venous thrombosis after renal transplantation. Effects of cyclosporine. Transplant J. 1987;43:647–649. doi: 10.1097/00007890-198705000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Skaer C., Kholmukhamedov A., Liu F., Jobe S.M. Platelet cyclophilin D‐dependent events limit venous thrombotic occlusion and platelet accretion. Blood. 2017;130(1):454. [Google Scholar]
- 30.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS‐CoV‐2 by full‐length human ACE2. Science. 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]