Abstract
Opioid addiction and overdose are at record levels in the United States. This is driven, in part, by their widespread prescription for the treatment of pain, which also increased opportunity for diversion by sensation-seeking users. Despite considerable research on the neurobiology of addiction, treatment options for opioid abuse remain limited. Mood disorders, particularly depression, are often comorbid with both pain disorders and opioid abuse. The endogenous opioid system, a complex neuromodulatory system, sits at the neurobiological convergence point of these three comorbid disease states. We review evidence for dysregulation of the endogenous opioid system as a mechanism for the development of opioid addiction and/or mood disorder. Specifically, individual differences in opioid system function may underlie differences in vulnerability to opioid addiction and mood disorders. We also review novel research, which promises to provide more detailed understanding of individual differences in endogenous opioid neurobiology and its contribution to opioid addiction susceptibility.
Keywords: affect, endogenous opioid, depression, opiate, pain
INTRODUCTION
Opiate drugs have been a part of the human experience since the Neolithic period (Merlin 2003), with cultivation of the opium poppy and religious, medicinal, and recreational use of its resin by nearly every culture throughout history (Brownstein 1993, Schiff 2002). However, many of the root causes of the current opiate crisis are uniquely modern. Beginning with the isolation of morphine from opium in 1804 (Schmitz 1985), the synthesis of thousands of opioid compounds throughout the twentieth century has led to an enormous field of drugs with diverse pharmacological characteristics. In the late nineteenth and early twentieth centuries, there was no regulation of morphine and other opiates. For example, the Sears catalog sold heroin and morphine preparations by mail order (Inciardi 1986). This led to widespread dependence and addiction, triggering massive regulation of narcotics and a sentiment of opiophobia in the medical community, which persisted through most of the twentieth century (Morgan 1985).
In the last decades of the twentieth century, the pendulum of public and medical opinion on the medical use of opioids began to swing in the opposite direction. Multiple factors led to a massive increase in prescription and consumption of opioids in the United States. These included the wide array of available pharmaceuticals, complaints of undertreatment of pain (Max 1990), the widespread and inaccurate belief that use of opiates for pain rarely culminates in addiction (Porter & Jick 1980), the successful but fraudulent marketing of OxyContin as having ‘greatly reduced addiction potential’ (Van Zee 2009), and the American Pain Society’s 1995 recommendation to record pain as “the fifth vital sign” and eliminate it using opioid analgesics (Campbell 1996) This increase in the number of people exposed to opioids, often through prescriptions, coupled with the increase in very potent opioid drugs, has led to a new wave of opioid use disorder (OUD), which has recently been characterized as a national public health emergency (Jones et al. 2018).
We should note, however, that only a small percentage of those taking opioids for chronic pain convert to addiction (Vowles et al. 2015). Moreover, treatment for pain is by no means the only path to OUD. Temperamental and personality traits have been implicated in the propensity to seek drugs in general and opioids in particular (Amirabadi et al. 2015, Milivojevic et al. 2012). Although the majority of heroin addicts report beginning with a prescription drug (Am. Soc. Addict. Med. 2016), it is unclear whether these were legitimately prescribed to them, and prescription opioids are often diverted by others who are not suffering from physical pain (Ford et al. 2019). It is critical, therefore, to consider the heterogeneity of paths to opioid addiction as we develop strategies for fundamental research into the treatment and prevention of this devastating disorder.
Historically, our understanding of the mechanisms of opiate drug action lagged behind the proliferation of pharmaceutical compounds. However, thanks to advances in molecular biology and neuroscience, there has been an explosion of knowledge since we last reviewed the opioid field for this journal (Akil et al. 1984). This includes cloning and crystal structure of the opioid receptors, elucidation of structure and function of their peptide ligands, and elaboration of the functional neuroanatomy of the opioid system. While many gaps remain in our understanding of this complex system, the knowledge that has been gained provides insights into the underlying biology of addiction and associated disorders. However, this understanding has not yet translated into meaningful strategies for treatment or prevention of OUD. This lack of success suggests that some critical factors are not being considered. We suggest that these factors involve unique biological and psychological characteristics of individuals exposed to opioids that impact propensity to transition from use to misuse and addiction. We also suggest that these differences result in different biological responses to the drugs, and these distinct consequences require different treatment strategies. Thus, OUD demands a precision medicine approach, which strives to tailor treatment based on unique features of each patient’s disease process, particularly individual differences in opioid system biology.
In this review, we summarize the current literature regarding the biology of the endogenous opioid system, in particular the highly interdependent nature of its roles in pain, depression, and addiction. We then examine how this knowledge informs the etiology and treatment of OUD, as well as discussing promising areas of research that can move the field toward a precision medicine approach.
THE ENDOGENOUS OPIOID SYSTEM
Molecular Elements of the Opioid System
The opioid system is a highly diverse peptide neurotransmitter system, composed of three main neuropeptide families, the β-endorphins derived from pro-opiomelanocortin, the enkephalins derived from proenkephalin and prodynorphin (PDYN) precursors, and the dynorphins derived from PDYN. Each family contains multiple member peptides with diverse binding characteristics (Mansour et al. 1995) (Figure 1a).
Figure 1.
(a) The three canonical opioid receptors demonstrate relatively similar degrees of affinity for various cleavage products of the endogenous opioid propeptides. The kappa opioid receptor (KOR) displays the highest degree of ligand specificity, with high degrees of affinity for prodynorphin (PDYN) peptides and very low affinity for β-endorphin or Leu/Met-Enk. Note that despite extremely low affinity for Leu/Met-Enk, the KOR shows high degrees of affinity for other proenkephalin (PENK) cleavage products. The biological relevance of this remains inconclusive, as the degree to which these peptides are produced from proenkephalin (PENK) and the conditions under which they are released in the brain remain understudied but raise the possibility that KOR-expressing regions are responsive to PENK-producing cells. Likewise, all three receptors show comparable binding affinity for PDYN peptides, indicating that cells expressing any opioid receptor type are likely to respond to PDYN release. Binding affinities data (indicated in nanomolar concentrations) in panel a are from Mansour et al. (1995). (b) There are high degrees of overlapping expression between the three canonical opioid receptors in a variety of brain regions established to be relevant to pain, mood/affect, and reward/motivation. When considered with the relative nonselectivity between receptors and signal peptides (as illustrated in panel a), it becomes apparent that any alteration in the volume and/or specific make-up of opioid peptide release is capable of complex, difficult-to-anticipate effects on a wide variety of neurobiological and behavioral outputs, especially regarding the modulation of pain, affect, and reward, often concurrently. Figure adapted from Mansour et al. (1988).
The system also includes three canonical receptors, the mu, delta, and kappa opioid receptors. A fourth receptor-peptide pair, nociceptin and its receptor, the nociceptin receptor or opioid-receptor-like 1, is also part of the opioid system. However, this pair was only recently discovered in comparison to the other three (Meunier et al. 1995, Mollereau et al. 1994). Their role in pain, affect, and addiction are still under investigation and will not be reviewed, but note that contemporary research indicates a role in pain and motivation (Di Cesare Mannelli et al. 2015, Kiguchi et al. 2016, Parker et al. 2019).
All three canonical receptors are members of the G protein–coupled receptor family, coupling to inhibitory Gi/oα subunits. The importance of the opioid system is underscored by its evolutionary age, appearing in essentially modern form in all jawed vertebrates and thus already established in a common ancestor over 450 million years ago (Dreborg et al. 2008). Furthermore, opioid receptors are among the most widely expressed receptors in the brain, as they are found throughout the neocortex, hippocampus, striatum, thalamus, hypothalamus, substantia nigra, and several nuclei of the brainstem and have widespread expression in the spinal cord and peripheral neurons.
The peptides have historically been portrayed to exhibit selectivity for particular receptors; however, it is important to recognize that the opioid peptides are fairly promiscuous, particularly the dynorphins. There is not a 1:1 correspondence between a signal peptide and receptor; members of all three peptide families are capable of activating the three receptors to varying degrees (Figure 1a). The opioid receptors also show a high degree of overlap in their regional expression patterns, particularly in regions relevant to pain, affect, and reward (Mansour et al. 1988) (Figure 1b). This results in a system whereby all the opioid receptor types within a brain region can be simultaneously activated by different peptides or even by the same peptide.
In addition to G protein–dependent signaling pathways, the opioid receptors signal via a wide assortment of second- and third-messenger systems (Al-Hasani & Bruchas 2011) and exhibit ligand-directed signaling (Pradhan et al. 2012). Importantly, although biased signaling is mainly studied with regard to divergent responses to exogenous drugs, it appears to be an intrinsic feature of the natural function of the system, enabling nuanced receptor responses to different signal peptide variants. For example, three PDYN products, dynorphin A, dynorphin B, and α-neoendorphin, display highly divergent capacities to desensitize and internalize the kappa receptor despite similar binding affinity, receptor occupancy, and intrinsic efficacy to engage G protein activity (Chen et al. 2007).
An additional layer of complexity is the existence of splice variants of the three opioid receptors (Pasternak & Pan 2013, Piltonen et al. 2019, Wei et al. 2000), which show altered affinity for the peptide ligands (and exogenous opioids) and potentially different biases for G protein–dependent versus –independent signaling (Margolis et al. 2017, Pasternak 2004). The activities of the opioid receptors are also influenced by positive and negative allosteric modulators (Livingston & Traynor 2018), which include other endogenous neurotransmitters/neurohormones (Kathmann et al. 2006, Meguro et al. 2018). Thus, opioid signaling can be tuned and refined based on concurrent activity of other systems.
The Regulation and Dysregulation of the Endogenous Opioid System
The endogenous opioid system interfaces with a number of other key neurotransmitter systems, including the endocannabinoid system, the serotonergic system, the oxytocin and vasopressin systems, and the glucocorticoid axis (Bicknell 1985, Diniz et al. 2018, Pfeiffer & Herz 1984, Robledo et al. 2008, Tao & Auerbach 2005, Watson et al. 1982, Young et al. 1986). This interplay between the opioid system and other pathways warrants a separate review, and we have included here only a handful of examples. For a more comprehensive look at the neurobiology of the endogenous opioid system, we refer the reader to Gutstein & Akil (2005).
The opioid system is a highly integrated system, which depends on fine-tuned balance, with many mechanisms for compensation and regulation (e.g., Cawley et al. 2016). In most cases, this complexity is highly adaptive, permitting fine-tuned control over the circuitry modulated by the opioid system. But it also makes the system vulnerable to disturbances, and given its broad influence, this can lead to significant and lasting maladaptive outcomes. For example, the various enzymes that convert opioid precursors to their final products function at different rates, leading to a different mix of neuropeptides being released as a function of neural activity (Bronstein et al. 1990). Exposure to chronic morphine inhibits activity of neurons that synthesize the highly potent β-endorphin(1–31). Reduced release allows for its conversion to the less potent form, β-endorphin(1–27). Upon withdrawal from chronic morphine, the endogenous system suffers from not only downregulated receptor function but also a shift toward a less potent opioid neurotransmitter signal, likely contributing to negative affect and increased sensitivity to pain.
Many factors are known to dysregulate the opioid system, ranging from genetic differences to perturbations from environmental sources such as the experience of childhood adversity (Lutz et al. 2018), chronic pain (Llorca-Torralba et al. 2019), migraines (Jassar et al. 2019), or alterations resulting from exposure to opioid drugs (Trujillo et al. 1995), alcohol (Shibasaki et al. 2013), and other addictive drugs (Mongi-Bragato et al. 2018; Turchan et al. 1998, 2002). Dysregulation of the opioid system has in turn been linked to neuropsychiatric diseases, including depression (Lutz & Kieffer 2013, Pecina et al. 2019), personality disorders (Bandelow & Wedekind 2015, Prossin et al. 2010), post-traumatic stress disorder (PTSD), Alzheimer’s disease (Torres-Berrio & Nava-Mesa 2019), and anxiety (Bruchas et al. 2010, Sher 1998).
While the breadth and complexity of this system result in widespread involvement in a variety of areas, we limit the scope of the remaining overview to the role of the opioid system in the overlapping disorders of addiction and affective disorders, particularly depression. However, these disorders do not occur in a vacuum. The opioid system sits at the point of convergence between many systems, including addiction, stress, affect/mood, feeding, sexual behavior, immune function, and others, and influences them all simultaneously. Thus, dysregulations in the endogenous opioid system could manifest as disturbances in any of these.
THE ROLE OF THE ENDOGENOUS OPIOID SYSTEM IN AFFECTIVE REGULATION
Endogenous Opioids and Negative Affect
The role of the endogenous opioid system in the mechanisms directly underlying addiction have been well studied. By contrast, the role of this system in the regulation of affect and this contribution to the development and maintenance of addictive states, including OUD, are understudied. In the human clinical population, mood disorders, particularly depression, are often comorbid with opioid use/misuse (Goesling et al. 2015). This relationship is bidirectional, with OUD and depression being risk factors for the emergence of the other (Manchikanti et al. 2007, Scherrer et al. 2014). Pain is often (though not always) a mediating factor in this relationship. Opioid use can also trigger opioid-induced hyperalgesia, which in turn can precipitate or worsen depression.
The opioid system is, among other functions, a regulator of the emotional circuitry of the brain, responsible for moment-to-moment fine tuning of affective state as well as emotional responses to both positive and negative experiences (Drolet et al. 2001, Kennedy et al. 2006, Koepp et al. 2009, Ribeiro et al. 2005, Tejeda & Bonci 2019). This regulation is constantly being refined by changes in peptide tone, composition of the peptide milieu (which specific combinations of peptides are released), and the level and composition of receptor expression, as described above.
A Balancing Act
Different subsystems within the opioid system mediate distinct components of the affective response. For example, the dynorphin/kappa subsystem has been shown to mediate the aversive affective component of pain (Cahill et al. 2014, Liu et al. 2019, Massaly et al. 2019). In contrast, the mu opioid receptor in affective circuits acts to induce or increase hedonic pleasure and to blunt aversive emotional responses (Inui & Shimura 2017, Pecina & Berridge 2005). Thus, it can be broadly hypothesized that the basal functional profile of kappa versus mu receptor systems may mediate trait-level affective valence and intensity of responses to emotionally relevant stimuli, and this functional profile might be tunable through various mechanisms. It is worth noting, however, that viewing the mu and kappa systems as always mutually antagonistic is simplistic, as it appears these systems can also work together to mediate hedonic responses in some brain regions such as the nucleus accumbens shell (Castro & Berridge 2014). The complex relationship between kappa and mu receptor biology, where kappa receptor agonism is sometimes functionally antimu and sometimes not, is likely mediated by nuances in receptor expression profiles in particular brain areas, functional selectivity of particular combinations of peptide transmitters and receptor variants, and the temporal dynamics of activation (acute versus chronic) (Emery & Eitan 2019).
The opioid system has long been described like a seesaw, dynorphin/kappa on one side and mu-delta/β-endorphin-enkephalin on the other, in balance. Imbalance toward the mu side leads to increased vulnerability to addiction, while imbalance in favor of kappa signaling drives negative affect and mood disorder. However, hypotheses rooted in such a model have not been borne out consistently in the literature. In place of the seesaw model, the opioid system can instead be imagined as a carnival plate-spinner; a complex act balancing synthesis, processing, and release of peptides and the expression profile of receptors (and splice variants), as well as a balance between functional receptors, expressed but desensitized receptors, internalized receptors in reserve, and internalized receptors tagged for degradation. Unlike the seesaw model, where balance can be restored by readjusting either side of a mutually opponent system, even a slight nudge toward imbalance anywhere can set the whole system off-balance, much like our plate-spinner when a single plate among many begins to wobble. The relatively slow-responding regulatory biology of the opioid system leaves the system prone to overcorrection, leading to swings from one state of imbalance to another. If the opioid system is chronically disturbed (e.g., via drug abuse), it may be difficult for the body to reestablish homeostasis, requiring a restabilizing influence from without.
A Vicious Cycle Between Addiction and Mood
Repeated use of opioids creates a vicious cycle whereby the resulting affective dysregulation can be relieved by opiate drugs, driving the individual to continue seeking and taking opiates to restore allostatic balance to the now-compromised system. This interpretation aligns with the addiction model offered by Koob (2015), whereby continued use of addictive drugs is driven largely by attempts to ameliorate the negative physiological and psychological consequences of drug withdrawal. This vicious cycle may manifest not only as relapse to drug taking but also through any of the behaviors impacted by endogenous opioid imbalance, such as a mood disorder, which in turn may reactivate drug seeking.
Depression represents an excellent example of the close interplay between addiction, mood, and the opioid system. Half of patients with depression report that their symptoms are not adequately managed by available interventions, and about 35% are completely resistant to interventions (Akil et al. 2018). The neurocircuitry of depression contains many regions with high degrees of opioid expression, including the hippocampus, amygdala, nucleus accumbens, prefrontal cortex, and hypothalamus (Akil et al. 2018, Mansour et al. 1988). The opioid system has also been functionally implicated in mood disorders (Lutz & Kieffer 2013). While conventional antidepressants target the monoamine systems, there is a growing appreciation of the contribution of the opioid system and interest in targeting it for novel antidepressants, and these efforts, while preliminary, have been promising.
One example of an effective, novel antidepressant is tianeptine, which has recently been demonstrated to be a highly selective mu receptor agonist (Gassaway et al. 2014). Activity at the mu receptor was demonstrated to be necessary for the antidepressant effect of tianeptine, though the exact mechanism behind this effect is elusive (Samuels et al. 2017). Another example is ketamine, an NMDA receptor antagonist, which demonstrates rapid and long-lasting antidepressant effects (Zarate et al. 2006) and has recently been approved for treatment-resistant depression. Data suggest that activity at the opioid receptors is partially responsible (Williams et al. 2018). Interactions between the opioid and NMDA systems also play a role in pain (Trujillo & Akil 1991, 1994), hinting at another mechanism where pain and depression interact.
The Interface with Chronic Pain
The use of prescription opioids for pain is regarded as a gateway mechanism (Am. Soc. Addict. Med. 2016, Compton et al. 2016, Miech et al. 2015) where pain puts individuals into initial contact with opioid medications, and chronic use of opioid medications can develop into dependence and addiction. However, the relationship may be more complex. The relationship between pain and depression implies an underlying biological dysregulation of the endogenous opioid system. Thus, pain, particularly chronic pain, or depression may indicate vulnerability to opioid abuse resulting from endogenous opioid dysregulation, even without previously identified genetic propensity or environmental risk.
MULTIPLE PATHS TO ADDICTION, MULTIPLE PATHS TO TREATMENT
Evidence for Genetic Factors
The complex and delicate balance of the endogenous opioid system provides for several paths to the functional end state of addiction (Figure 2). One such pathway is genetic propensity, which largely delineates the starting state of an individual’s opioid system. Indeed, a genetic component to addiction vulnerability has been well known for a long time (Merikangas et al. 1998) and leveraged by researchers for decades by employing rodent models with differing addiction vulnerability (Berrettini et al. 1994, Flagel et al. 2016, Murphy et al. 2001, Shoaib et al. 1995).
Figure 2.
Genes and environment interact to provide multiple distinct behavioral and biological pathways that converge on the same behavioral output of opioid abuse/addiction. For example, a genetic propensity toward novelty- or sensation-seeking behavior would provide one pathway to opioid use and abuse, while attempts at self-medicating depression resulting from endogenous opioid system dysregulation represent another pathway, which is likely very different in its etiology and trajectory. The opioids themselves are likely to be the dominant factor in altering brain structure and function while the drug is being used, largely masking individual differences when examined during this period. However, the long-term consequences and associated vulnerabilities are not likely to be identical between the groups following cessation of drug use. For instance, individuals who arrived at chronic opioid use via these different pathways may display sensitivity to different triggers of relapse. This then would necessitate different strategies to support abstinence and prevent relapse dependent upon individual differences.
In humans, twin studies have revealed that about 50% of the susceptibility to opiate addiction is heritable (Kendler et al. 2003, Tsuang et al. 1998), comparable to the heritability estimates for alcohol abuse and nicotine addiction (Sharp & Chen 2019, Verhulst et al. 2015). A large fraction of the heritability is due to factors shared across addiction broadly, though a fair proportion is unique to opiates specifically (Tsuang et al. 2001). Addiction is highly polygenic (Hall et al. 2013), though a short list of genes accounts for a relatively large amount of the variance (Reed et al. 2014). However, at present, genome-wide association studies (GWASs) of opioid addiction are of considerably smaller scale than those targeting psychiatric disorders such as depression or schizophrenia. Thus, there remain several unanswered questions regarding the genetics of addiction.
In humans, well over one hundred single nucleotide polymorphisms (SNPs) in the mu opioid receptor alone have been characterized (Ikeda et al. 2005). The most frequent and best-studied SNP in the mu opioid receptor, A118G, has been strongly linked with increased susceptibility to addiction, both to opioids and to other addictive substances (Deb et al. 2010). This mutation also alters responsiveness of the receptor to endogenous opioid peptides (Bond et al. 1998). A homologous mutation in a mouse model exhibits altered reward sensitivity and addiction-like behaviors (Mague et al. 2009, Zhang et al. 2015). These results highlight the importance of genetically mediated initial system state to the development of opioid addiction.
The Role of Temperament
As noted above, a major factor that modulates the initial vulnerability to OUD is temperament. Human studies show that some individuals are prone to externalizing behavior such as aggression, impulsivity, sensation seeking, and psychopathic behavior, and these tendencies are strong predisposing factors to substance abuse in general and OUD in particular (Amirabadi et al. 2015, Cloninger 1987, Milivojevic et al. 2012, Zuckerman & Kuhlman 2000). Others are prone to internalizing behavior and are more likely to exhibit anxiety, depression, and other mood disorders, and following stress they become vulnerable to substance use and likely account for overlap between mood disorders and OUD (Khan et al. 2005).
We have developed an animal model to capture these temperamental tendencies (Stead et al. 2006). At one extreme, rats that are externalizers and respond strongly to novelty [bred high responders (bHRs)] are highly prone to seeking drugs of abuse and becoming addicted to them (Flagel et al. 2016). On the other extreme are internalizers [bred low responders (bLRs)] that are highly prone to anxiety-like (Stead et al. 2006), depressive-like (Stedenfeld et al. 2011), and PTSD-like behaviors (Prater et al. 2017). These lines of animals capture two distinct paths to substance abuse: sensation seeking (bHRs) and negative affect (bLRs). Importantly, we have shown that these tendencies are highly genetically rooted. Indeed, only seven genetic loci account for two-thirds of the genetic variance and one-third of total variance of novelty-induced locomotor behavior in these animals (Zhou et al. 2019). It should be noted that novelty-induced locomotion has also been genetically linked to opiate seeking in various mouse strains (Ambrosio et al. 1995). We know a great deal about neural differences that emerged in these animals through selective breeding. Of relevance here is that the endogenous opioid system is clearly different, including differences in the relative balance between mu and kappa opioid receptors (Turner et al. 2019). This then provides a valuable genetic model for analyzing gene by environment interactions that lead to differential vulnerability to opioid use, addiction, and relapse.
Evidence for Environmental Factors
Another variable that influences susceptibility—or resilience—to addiction is that of the environment. While genetic makeup provides the boundary conditions determining the range in which the opioid system can be tuned, environmental influences are the mechanisms that are responsible for the tuning within those boundary conditions. Environmental conditions can be broadly positive or negative, enhancing resilience or vulnerability, respectively. Enrichment of the physical environment provides a protective effect, reducing opioid self-administration (Hofford et al. 2017) and blunting conditioned place preference (CPP) to heroin (El Rawas et al. 2009). Environmental enrichment also aids in the establishment and maintenance of drug abstinence in dependent animals (Peck et al. 2015) and protects against cue-induced reinstatement of extinguished heroin seeking (Galaj et al. 2016).
The role of social environment per se on opioid addiction–like behaviors is distinct from enrichment of the physical environment. Social enrichment has been shown to blunt morphine CPP (Kennedy et al. 2012) and opioid self-administration (Bozarth et al. 1989). Additionally, when given a choice between heroin self-administration and social interaction with a conspecific within the drug-paired context, rats demonstrate a strong preference for social interaction. Furthermore, social interaction blocks incubation of heroin craving following forced abstinence in an opiate-dependent animal (Venniro et al. 2016). Conversely, social isolation increases the locomotor-activating effects of morphine (Coudereau et al. 1996). Paradoxically, social isolation blunts morphine CPP (Coudereau et al. 1997) and physical dependence on morphine (Adler et al. 1975). This is likely mediated through alteration of endogenous opioid function, reducing expression of mu opioid receptors throughout the brain, including areas responsible for the rewarding effects of morphine (Van den Berg et al. 1999).
The quality of social interaction also matters. Mice housed with drug-naïve conspecifics show reduced CPP, sensitization, analgesic tolerance, and opioid-induced hyperalgesia following morphine compared to mice housed with morphine-exposed conspecifics, despite an otherwise-similar social environment (Bates et al. 2014, 2016; Cole et al. 2013; Hodgson et al. 2010), implying alterations in endogenous opioid function. This effect is mediated in part by reduced positive physical interaction, particularly social grooming (Bates et al. 2017).
The effect of social interaction upon addiction liability is not independent of its role on opioid neurobiology vis-à-vis mood and/or pain. The influence of the endogenous opioid system on social bonding in mammals, as well as influencing human feelings of social connection, is well established (Inagaki 2018, Panksepp et al. 1994, Resendez et al. 2016) and is perturbed by the use of opiate drugs (Ragen et al. 2015, Rubin & Bridges 1984, Wang et al. 2018). Thus, the social network has a large impact on the biology mediating desire for, and response to, opioid drugs both directly and through intermediary states of pain (physical and emotional) and depression. Indeed, anecdotal clinical reports indicate that social isolation, abandonment, and despair increase opiate seeking, while social support provides a strong ameliorating effect (Rosenthal 2009).
The Role of the Specific Opioid Drugs
Another facet of environment is the specific opioid used. Different opioids demonstrate different capabilities to foster sensitization, opioid-induced hyperalgesia, and rate/degree of tolerance at initially equianalgesic doses (Barwatt et al. 2013; Emery et al. 2015, 2016, 2017a, 2017b). Thus, the specific opioid drugs used/misused should be considered as another component that contributes to vulnerability to opiate addiction and/or outcomes influenced by opiate use, such as depression. This is particularly important when reporting adverse clinical effects and epidemiological statistics, where often categories such as opioids are not further subdivided.
Recent findings suggest that opioid drugs may not only drive the endogenous system to superphysiological levels but evoke signaling responses that are qualitatively different from those evoked by endogenous opioids (Stoeber et al. 2018). This is thought to be due partially to the fact that exogenous opioid drugs are membrane permeable, enabling them to activate intracellular opioid receptors. The degree to which endogenous peptides cross the cell membrane remains controversial (Ganapathy & Miyauchi 2005, Marinova et al. 2005); however, such membrane penetration is likely to have very different kinetics compared to small-molecule opioids. Thus, the use of opioid drugs may cause a unique physiological state that the opioid system was not designed to encounter often or at all (i.e., significant and sustained activation of cell-interior opioid receptors). These variables (genetic predisposition, environmental factors, and their interplay with the nature of the drug itself) should not be considered independently of each other, as they interact to influence the probability of addiction and severity.
In spite of the scale of the current epidemic, the treatment options for OUD are very limited. In a recent report by the US National Academies (US Natl. Acad. Sci. Eng. Med. 2019), only three drugs were listed as currently approved by the US Food and Drug Administration and effective for OUD treatment—methadone, buprenorphine, and naltrexone, all of which target the endogenous opioid system (Hedegaard et al. 2018). Significant challenges are associated with each, including lack of compliance with naltrexone because of negative affect and limited access to methadone and buprenorphine, as they require special clinics and/or licensing to dispense them. The social stigma associated with replacement therapy is also a major barrier to treatment, reducing compliance and contributing to relapse. Finding new strategies for treatment that lead to greater compliance and better long-term success is urgent. This, in our view, cannot be successful without taking into account the multiple paths that have led to the OUD and tailoring treatments accordingly. A precision medicine approach should be grounded in a better understanding of the stable and genetically mediated temperamental features of the individual, coupled with the history and current status of that individual, beyond medical history and including their affective state, the chronicity and psychological features of their pain, and the availability of social support.
At one extreme may be the patient with a propensity for internalizing behavior who is presenting with a pain condition. Given that the opioid system mediates affective state, it can be assumed that in a depressed patient, this system is in a state of imbalance. Since pain itself triggers negative affect, a pain patient with a history or current incidence of depression may be at particular risk for opiate abuse, where the opiate temporarily relieves their affective misery while simultaneously amplifying the underlying dysregulation of their endogenous opioid biology.
The other extreme includes people who have temperamental tendencies for sensation-seeking and antisocial behavior, who have a history of abusing drugs, and who might be seeking the rewarding aspect of opioids. Here again, the use of opioids will trigger adaptations that could lead to an OUD but with distinctly different neurobiological sequelae, likely requiring different approaches to treatment.
WHERE DO WE GO FROM HERE?
In order to move toward more strategies for precision treatments of OUD, as well as better approaches to prevention by considering the patient prior to pain treatment, there are several gaps that need to be addressed in the opioid field.
Genetics and genomics. While OUD appears highly heritable, the size of GWASs aimed at understanding the contribution of genetic variants is remarkably small. Other psychiatric disorders, including schizophrenia, bipolar disorder, and major depressive disorders, have profited from very large samples of subjects allowing identification of contributing genetic loci (Bergen & Petryshen 2012, Liu et al. 2011). While they explain a limited percentage of the variance, they have been valuable in pointing to unexpected genes and loci of interest that can be studied in animal models. In addition, the relationship to personality traits and temperament merits further exploration at the genetic level. There are recent studies that have focused on features such as impulsivity and neuroticism (de Wit 2009, Terracciano et al. 2008), both of which are clearly relevant to the two paths to OUD that we have described above. Finally, the pharmacogenomics associated with various opioid drugs is worthy of further exploration, as it might inform treatment of patients to modulate pain while minimizing OUD.
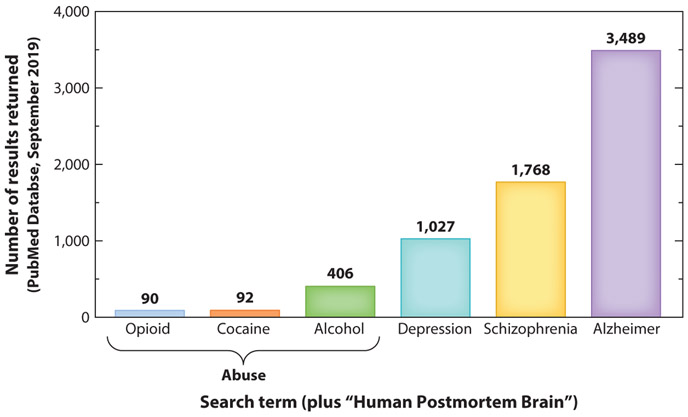
The impact of opioids on the human brain. While animal studies on the neurobiology of opioids have been extensive, we know rather little about the impact of OUD on the human brain. A recent literature search of “human postmortem brain” combined with various drugs of abuse was severely underrepresented compared to other disorders (Figure 3). While the reasons for this are complex, one possibility is the assumption that these consequences are more easily, reliably, and cheaply studied in animal models. However, animal studies likely fail to capture the heterogeneity of human addiction and comorbidity with other disorders that might interact with and amplify the effects of opioids. Thus, genetics, gene expression, and anatomical studies of human postmortem tissue from opioid addicts, healthy controls, and individuals with a history of depression or pain are likely to be highly informative. As importantly, this will likely uncover novel players that might serve as biomarkers or novel targets for the treatment of these diseases.
Reverse and forward translation. Results from human studies, whether genetic or postmortem, should be reverse translated to animal models that more completely represent the complex gene–environment interaction leading to human OUD. Such models will result in much more reliable preclinical findings and, hopefully, more successful translation.
Refinement of our understanding of endogenous opioid and other interacting systems. In particular, understanding the dynamic regulation and interplay of opioid system elements in the context of known circuits and in behaving animals is a critical backdrop to understanding the impact of various conditions such as pain and treatments such as exogenous opioids. Given the overlapping interactions between multiple opioid peptides and receptors anatomically and functionally, this may prove critical in understanding the changes elicited by various conditions, including pain and addiction. There has been recent progress in our ability to detect the real-time release kinetics of opioid peptides in response to stimuli and their effect on downstream circuit function. This includes the refinement of fast-scan cyclic voltammetry to detect enkephalins and dynorphins with high spatiotemporal precision in vivo (Calhoun et al. 2019). Another exciting approach to tackle this problem is the refinement of microdialysis liquid chromatography/mass spectrometry to identify release of specific peptides. Coupled with optogenetic stimulation, this allows examination of specific cell populations on motivated behavior while simultaneously monitoring which specific opioid peptides are responsible for such effects (Al-Hasani et al. 2018).
Figure 3.
Despite a growing appreciation of addiction as a neurobiological disease, human postmortem studies of the neuropathology of addiction currently lag behind postmortem studies of other psychopathologies. This limits our understanding and appreciation of individual differences in addiction pathology. Furthermore, this limits the ability to determine the degree to which genetic and neurobiological factors are universal as opposed to substance specific.
The combination of the above approaches across levels of analyses and between animals and humans is essential in developing the prevention and treatment strategies that recognize the great heterogeneity of this drug epidemic.
CONCLUDING REMARKS
In summary, opioid addiction, depression, and pain are highly comorbid diseases. They are likely all manifestations of biological dysfunctions sharing a common factor: the endogenous opioid system. The endogenous opioid system is itself regulated by a complex dance between genetics and environmental factors. The latter includes the individual’s physical and social context, past and current emotional state, pain state, and exposure to drugs. While there are multiple genetic and environmental paths leading to a common state we term opioid addiction, the heterogeneity of the causes implies a heterogeneity of consequences both biological and psychological. They therefore require different treatments. Deeper and more systematic exploration of these variables will prove vital to developing better treatment and prevention strategies not only for addiction but for all the related neuropsychiatric ailments involving the endogenous opioid system.
ACKNOWLEDGMENTS
The authors would like to thank A. Parsegian and C.A. Turner for their helpful discussions regarding this manuscript. This work was supported by the National Institute on Drug Abuse U01DA043098, the National Institutes of Health R01MH104261, the Office of Naval Research N00014–12-1–0366 and 00014–19-1–2149, the Hope for Depression Research Foundation, and the Pritzker Neuropsychiatric Research Consortium.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Adler MW, Bendotti C, Ghezzi D, Samanin R, Valzelli L. 1975. Dependence to morphine in differentially housed rats. Psychopharmacologia 41:15–18 [DOI] [PubMed] [Google Scholar]
- Akil H, Gordon J, Hen R, Javitch J, Mayberg H, et al. 2018. Treatment resistant depression: a multi-scale, systems biology approach. Neurosci. Biobehav. Rev 84:272–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akil H, Watson SJ, Young E, Lewis ME, Khachaturian H, Walker JM. 1984. Endogenous opioids: biology and function. Annu. Rev. Neurosci 7:223–55 [DOI] [PubMed] [Google Scholar]
- Al-Hasani R, Bruchas MR. 2011. Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology 115:1363–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hasani R, Wong J-MT, Mabrouk OS, McCall JG, Schmitz GP, et al. 2018. In vivo detection of optically-evoked opioid peptide release. eLife 7:e36520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Am. Soc. Addict. Med. 2016. Opioid addiction 2016 facts & figures Fact Sheet, Am. Soc. Addict. Med, Chevy Chase, MD [Google Scholar]
- Ambrosio E, Goldberg SR, Elmer GI. 1995. Behavior genetic investigation of the relationship between spontaneous locomotor activity and the acquisition of morphine self-administration behavior. Behav. Pharmacol 6:229–37 [PubMed] [Google Scholar]
- Amirabadi B, Nikbakht M, Nokani M, Alibeygi N, Safari H. 2015. Role of temperament, personality traits and onset age of smoking in predicting opiate dependence. Int. J. High Risk Behav. Addict 4:e24585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandelow B, Wedekind D. 2015. Possible role of a dysregulation of the endogenous opioid system in antisocial personality disorder. Hum. Psychopharmacol 30:393–415 [DOI] [PubMed] [Google Scholar]
- Barwatt JW, Hofford RS, Emery MA, Bates ML, Wellman PJ, Eitan S. 2013. Differential effects of methadone and buprenorphine on the response of D2/D3 dopamine receptors in adolescent mice. Drug Alcohol Depend. 132:420–26 [DOI] [PubMed] [Google Scholar]
- Bates ML, Emery MA, Wellman PJ, Eitan S. 2014. Social housing conditions influence morphine dependence and the extinction of morphine place preference in adolescent mice. Drug Alcohol Depend. 142:283–89 [DOI] [PubMed] [Google Scholar]
- Bates ML, Emery MA, Wellman PJ, Eitan S. 2016. Social environment alters opioid-induced hyperalgesia and antinociceptive tolerance in adolescent mice. Eur. J. Pain 20:998–1009 [DOI] [PubMed] [Google Scholar]
- Bates MLS, Emery MA, Wellman PJ, Eitan S. 2017. Inhibiting social support from massage-like stroking increases morphine dependence. Behav. Pharmacol 28:642–47 [DOI] [PubMed] [Google Scholar]
- Bergen SE, Petryshen TL. 2012. Genome-wide association studies of schizophrenia: Does bigger lead to better results? Curr. Opin. Psychiatry 25:76–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrettini WH, Alexander R, Ferraro TN, Vogel WH. 1994. A study of oral morphine preference in inbred mouse strains. Psychiatr. Genet 4:81–86 [DOI] [PubMed] [Google Scholar]
- Bicknell RJ. 1985. Endogenous opioid peptides and hypothalamic neuroendocrine neurones. J. Endocrinol 107:437–46 [DOI] [PubMed] [Google Scholar]
- Bond C, LaForge KS, Tian M, Melia D, Zhang S, et al. 1998. Single-nucleotide polymorphism in the human mu opioid receptor gene alters β-endorphin binding and activity: possible implications for opiate addiction. PNAS 95:9608–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozarth MA, Murray A, Wise RA. 1989. Influence of housing conditions on the acquisition of intravenous heroin and cocaine self-administration in rats. Pharmacol. Biochem. Behav 33:903–7 [DOI] [PubMed] [Google Scholar]
- Bronstein DM, Przewlocki R, Akil H. 1990. Effects of morphine treatment on pro-opiomelanocortin systems in rat brain. Brain Res. 519:102–11 [DOI] [PubMed] [Google Scholar]
- Brownstein MJ. 1993. A brief history of opiates, opioid peptides, and opioid receptors. PNAS 90:5391–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Lemos JC, Chavkin C. 2010. CRF1-R activation of the dynorphin/kappa opioid system in the mouse basolateral amygdala mediates anxiety-like behavior. PLOS ONE 4:e8528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill CM, Taylor AM, Cook C, Ong E, Moron JA, Evans CJ. 2014. Does the kappa opioid receptor system contribute to pain aversion? Front. Pharmacol 5:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun SE, Meunier CJ, Lee CA, McCarty GS, Sombers LA. 2019. Characterization of a multiple-scan-rate voltammetric waveform for real-time detection of met-enkephalin. ACS Chem. Neurosci 10:2022–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JN. 1996. APS 1995 Presidential address. J. Pain 5:85–88 [Google Scholar]
- Castro DC, Berridge KC. 2014. Opioid hedonic hotspot in nucleus accumbens shell: mu, delta, and kappa maps for enhancement of sweetness “liking” and “wanting.” J. Neurosci 34:4239–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawley NX, Li Z, Loh YP. 2016. 60 years of POMC: biosynthesis, trafficking, and secretion of pro-opiomelanocortin-derived peptides. J. Mol. Endocrinol 56:T77–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Chen C, Liu-Chen LY. 2007. Dynorphin peptides differentially regulate the human κ opioid receptor. Life Sci. 80:1439–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloninger CR. 1987. A systematic method for clinical description and classification of personality variants. A proposal. Arch. Gen. Psychiatry 44:573–88 [DOI] [PubMed] [Google Scholar]
- Cole SL, Hofford RS, Evert DJ, Wellman PJ, Eitan S. 2013. Social influences on morphine conditioned place preference in adolescent mice. Addict. Biol 18:274–85 [DOI] [PubMed] [Google Scholar]
- Compton WM, Jones CM, Baldwin GT. 2016. Relationship between nonmedical prescription-opioid use and heroin use. N. Engl. J. Med 374:154–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coudereau JP, Debray M, Monier C, Bourre JM, Frances H. 1996. Effect of isolation on morphine-induced running and changes in body temperature. Prog. Neuropsychopharmacol. Biol. Psychiatry 20:827–38 [DOI] [PubMed] [Google Scholar]
- Coudereau JP, Debray M, Monier C, Bourre JM, Frances H. 1997. Isolation impairs place preference conditioning to morphine but not aversive learning in mice. Psychopharmacology 130:117–23 [DOI] [PubMed] [Google Scholar]
- de Wit H 2009. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict. Biol 14:22–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deb I, Chakraborty J, Gangopadhyay PK, Choudhury SR, Das S. 2010. Single-nucleotide polymorphism (A118G) in exon 1 of OPRM1 gene causes alteration in downstream signaling by mu-opioid receptor and may contribute to the genetic risk for addiction. J. Neurochem 112:486–96 [DOI] [PubMed] [Google Scholar]
- Di Cesare Mannelli L, Micheli L, Ghelardini C. 2015. Nociceptin/orphanin FQ receptor and pain: feasibility of the fourth opioid family member. Eur. J. Pharmacol 766:151–54 [DOI] [PubMed] [Google Scholar]
- Diniz DA, Petrocchi JA, Navarro LC, Souza TC, Castor M, et al. 2018. Serotonin induces peripheral antinociception via the opioidergic system. Biomed. Pharmacother 97:1434–37 [DOI] [PubMed] [Google Scholar]
- Dreborg S, Sundström G, Larsson TA, Larhammar D. 2008. Evolution of vertebrate opioid receptors. PNAS 105:15487–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drolet G, Dumont ÉC, Gosselin I, Kinkead R, Laforest S, Trottier J-F. 2001. Role of endogenous opioid system in the regulation of the stress response. Prog. Neuropsychopharmacol. Biol. Psychiatry 25:729–41 [DOI] [PubMed] [Google Scholar]
- El Rawas R, Thiriet N, Lardeux V, Jaber M, Solinas M. 2009. Environmental enrichment decreases the rewarding but not the activating effects of heroin. Psychopharmacology 203:561–70 [DOI] [PubMed] [Google Scholar]
- Emery MA, Bates ML, Wellman PJ, Eitan S. 2015. Differential effects of oxycodone, hydrocodone, and morphine on the responses of D2/D3 dopamine receptors. Behav. Brain Res 284:37–41 [DOI] [PubMed] [Google Scholar]
- Emery MA, Bates ML, Wellman PJ, Eitan S. 2016. Differential effects of oxycodone, hydrocodone, and morphine on activation levels of signaling molecules. Pain Med. 17:908–14 [DOI] [PubMed] [Google Scholar]
- Emery MA, Bates MLS, Wellman PJ, Eitan S. 2017a. Hydrocodone, but neither morphine nor oxycodone, is effective in suppressing burn-induced mechanical allodynia in the uninjured foot contralateral to the burn. J. Burn Care Res 38:319–26 [DOI] [PubMed] [Google Scholar]
- Emery MA, Bates MLS, Wellman PJ, Eitan S. 2017b. Hydrocodone is more effective than morphine or oxycodone in suppressing the development of burn-induced mechanical allodynia. Pain Med. 18:2170–80 [DOI] [PubMed] [Google Scholar]
- Emery MA, Eitan S. 2019. Members of the same pharmacological family are not alike: different opioids, different consequences, hope for the opioid crisis? Prog. Neuropsychopharmacol. Biol. Psychiatry 92:428–49 [DOI] [PubMed] [Google Scholar]
- Flagel SB, Chaudhury S, Waselus M, Kelly R, Sewani S, et al. 2016. Genetic background and epigenetic modifications in the core of the nucleus accumbens predict addiction-like behavior in a rat model. PNAS 113:E2861–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JA, Pomykacz C, Szalewski A, Esteban McCabe S, Schepis TS. 2019. Friends and relatives as sources of prescription opioids for misuse among young adults: the significance of physician source and race/ethnic differences. Subst. Abus In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaj E, Manuszak M, Ranaldi R. 2016. Environmental enrichment as a potential intervention for heroin seeking. Drug Alcohol Depend. 163:195–201 [DOI] [PubMed] [Google Scholar]
- Ganapathy V, Miyauchi S. 2005. Transport systems for opioid peptides in mammalian tissues. AAPS J. 7:E852–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassaway MM, Rives ML, Kruegel AC, Javitch JA, Sames D. 2014. The atypical antidepressant and neurorestorative agent tianeptine is a μ-opioid receptor agonist. Transl. Psychiatry 4:e411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goesling J, Henry MJ, Moser SE, Rastogi M, Hassett AL, et al. 2015. Symptoms of depression are associated with opioid use regardless of pain severity and physical functioning among treatment-seeking patients with chronic pain. J. Pain 16:844–51 [DOI] [PubMed] [Google Scholar]
- Gutstein HB, Akil H. 2005. Opioid analgesics In Goodman & Gilman’s The Pharmacological Basis of Therapeutics, ed. Brunton LL, Lazo JS, Parker K, pp. 547–90. New York: McGraw-Hill [Google Scholar]
- Hall FS, Drgonova J, Jain S, Uhl GR. 2013. Implications of genome wide association studies for addiction: Are our a priori assumptions all wrong? Pharmacol. Ther 140:267–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedegaard H, Minino AM, Warner M. 2018. Drug overdose deaths in the United States, 1999–2017 NCHS Data Brief 329, Natl. Cent. Health Stat, Hyattsville, MD: [PubMed] [Google Scholar]
- Hodgson SR, Hofford RS, Roberts KW, Wellman PJ, Eitan S. 2010. Socially induced morphine pseudosensitization in adolescent mice. Behav. Pharmacol 21:112–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofford RS, Chow JJ, Beckmann JS, Bardo MT. 2017. Effects of environmental enrichment on self-administration of the short-acting opioid remifentanil in male rats. Psychopharmacology 234:3499–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Ide S, Han W, Hayashida M, Uhl GR, Sora I. 2005. How individual sensitivity to opiates can be predicted by gene analyses. Trends Pharmacol. Sci 26:311–17 [DOI] [PubMed] [Google Scholar]
- Inagaki TK. 2018. Opioids and social connection. Curr. Dir. Psychol. Sci 27:85–90 [Google Scholar]
- Inciardi JA. 1986. The War on Drugs: Heroin, Cocaine, Crime, and Public Policy. Palo Alto, CA: Mayfield Publishing [Google Scholar]
- Inui T, Shimura T. 2017. Activation of mu-opioid receptors in the ventral pallidum decreases the negative hedonic evaluation of a conditioned aversive taste in rats. Behav. Brain Res 320:391–99 [DOI] [PubMed] [Google Scholar]
- Jassar H, Nascimento TD, Kaciroti N, DosSantos MF, Danciu T, et al. 2019. Impact of chronic migraine attacks and their severity on the endogenous μ-opioid neurotransmission in the limbic system. NeuroImage Clin. 23:101905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MR, Viswanath O, Peck J, Kaye AD, Gill JS, Simopoulos TT. 2018. A brief history of the opioid epidemic and strategies for pain medicine. Pain Ther. 7:13–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathmann M, Flau K, Redmer A, Trankle C, Schlicker E. 2006. Cannabidiol is an allosteric modulator at mu- and delta-opioid receptors. Naunyn-Schmiedeberg’s Arch. Pharmacol 372:354–61 [DOI] [PubMed] [Google Scholar]
- Kendler KS, Jacobson KC, Prescott CA, Neale MC. 2003. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. Am. J. Psychiatry 160:687–95 [DOI] [PubMed] [Google Scholar]
- Kennedy BC, Panksepp JB, Runckel PA, Lahvis GP. 2012. Social influences on morphine-conditioned place preference in adolescent BALB/cJ and C57BL/6J mice. Psychopharmacology 219:923–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy SE, Koeppe RA, Young EA, Zubieta JK. 2006. Dysregulation of endogenous opioid emotion regulation circuitry in major depression in women. Arch. Gen. Psychiatry 63:1199–208 [DOI] [PubMed] [Google Scholar]
- Khan AA, Jacobson KC, Gardner CO, Prescott CA, Kendler KS. 2005. Personality and comorbidity of common psychiatric disorders. Br. J. Psychiatry 186:190–96 [DOI] [PubMed] [Google Scholar]
- Kiguchi N, Ding H, Ko MC. 2016. Central N/OFQ-NOP receptor system in pain modulation. Adv. Pharmacol 75:217–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepp MJ, Hammers A, Lawrence AD, Asselin MC, Grasby PM, Bench CJ. 2009. Evidence for endogenous opioid release in the amygdala during positive emotion. Neuroimage 44:252–56 [DOI] [PubMed] [Google Scholar]
- Koob GF. 2015. The dark side of emotion: the addiction perspective. Eur. J. Pharmacol 753:73–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SS, Pickens S, Burma NE, Ibarra-Lecue I, Yang H, et al. 2019. Kappa opioid receptors drive a tonic aversive component of chronic pain. J. Neurosci 39:4162–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Blackwood DH, Caesar S, de Geus EJC, Farmer A, et al. 2011. Meta-analysis of genome-wide association data of bipolar disorder and major depressive disorder. Mol. Psychiatry 16:2–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston KE, Traynor JR. 2018. Allostery at opioid receptors: modulation with small molecule ligands. Br. J. Pharmacol 175:2846–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorca-Torralba M, Pilar-Cuellar F, Bravo L, Bruzos-Cidon C, Torrecilla M, et al. 2019. Opioid activity in the locus coeruleus is modulated by chronic neuropathic pain. Mol. Neurobiol 56:4135–50 [DOI] [PubMed] [Google Scholar]
- Lutz PE, Gross JA, Dhir SK, Maussion G, Yang J, et al. 2018. Epigenetic regulation of the kappa opioid receptor by child abuse. Biol. Psychiatry 84:751–61 [DOI] [PubMed] [Google Scholar]
- Lutz PE, Kieffer BL. 2013. Opioid receptors: distinct roles in mood disorders. Trends Neurosci. 36:195–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mague SD, Isiegas C, Huang P, Liu-Chen LY, Lerman C, Blendy JA. 2009. Mouse model of OPRM1 (A118G) polymorphism has sex-specific effects on drug-mediated behavior. PNAS 106:10847–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchikanti L, Giordano J, Boswell MV, Fellows B, Manchukonda R, Pampati V. 2007. Psychological factors as predictors of opioid abuse and illicit drug use in chronic pain patients. J. Opioid Manag 3:89–100 [DOI] [PubMed] [Google Scholar]
- Mansour A, Hoversten MT, Taylor LP, Watson SJ, Akil H. 1995. The cloned μ, δ and κ receptors and their endogenous ligands: evidence for two opioid peptide recognition cores. Brain Res. 700:89–98 [DOI] [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. 1988. Anatomy of CNS opioid receptors. Trends Neurosci. 11:308–14 [DOI] [PubMed] [Google Scholar]
- Margolis EB, Fujita W, Devi LA, Fields HL. 2017. Two delta opioid receptor subtypes are functional in single ventral tegmental area neurons, and can interact with the mu opioid receptor. Neuropharmacology 123:420–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinova Z, Vukojević V, Surcheva S, Yakovleva T, Cebers G, et al. 2005. Translocation of dynorphin neuropeptides across the plasma membrane: a putative mechanism of signal transmission. J. Biol. Chem 280:26360–70 [DOI] [PubMed] [Google Scholar]
- Massaly N, Copits BA, Wilson-Poe AR, Hipolito L, Markovic T, et al. 2019. Pain-induced negative affect is mediated via recruitment of the nucleus accumbens kappa opioid system. Neuron 102:564–73.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max MB. 1990. Improving outcomes of analgesic treatment: Is education enough? Ann. Intern. Med 113:885–89 [DOI] [PubMed] [Google Scholar]
- Meguro Y, Miyano K, Hirayama S, Yoshida Y, Ishibashi N, et al. 2018. Neuropeptide oxytocin enhances μ opioid receptor signaling as a positive allosteric modulator. J. Pharmacol. Sci 137:67–75 [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Stolar M, Stevens DE, Goulet J, Preisig MA, et al. 1998. Familial transmission of substance use disorders. Arch. Gen. Psychiatry 55:973–79 [DOI] [PubMed] [Google Scholar]
- Merlin MD. 2003. Archaeological evidence for the tradition of psychoactive plant use in the Old World. Econ. Bot 57:295–323 [Google Scholar]
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, et al. 1995. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature 377:532–35 [DOI] [PubMed] [Google Scholar]
- Miech R, Johnston L, O’Malley PM, Keyes KM, Heard K. 2015. Prescription opioids in adolescence and future opioid misuse. Pediatrics 136:e1169–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milivojevic D, Milovanovic SD, Jovanovic M, Svrakic DM, Svrakic NM, et al. 2012. Temperament and character modify risk of drug addiction and influence choice of drugs. Am. J. Addict 21:462–67 [DOI] [PubMed] [Google Scholar]
- Mollereau C, Parmentier M, Mailleux P, Butour JL, Moisand C, et al. 1994. ORL1, a novel member of the opioid receptor family: cloning, functional expression and localization. FEBS Lett. 341:33–38 [DOI] [PubMed] [Google Scholar]
- Mongi-Bragato B, Avalos MP, Guzman AS, Bollati FA, Cancela LM. 2018. Enkephalin as a pivotal player in neuroadaptations related to psychostimulant addiction. Front. Psychiatry 9:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JP. 1985. American opiophobia: customary underutilization of opioid analgesics. Adv. Alcohol Subst. Abuse 5:163–73 [DOI] [PubMed] [Google Scholar]
- Murphy NP, Lam HA, Maidment NT. 2001. A comparison of morphine-induced locomotor activity and mesolimbic dopamine release in C57BL6, 129Sv and DBA2 mice. J. Neurochem 79:626–35 [DOI] [PubMed] [Google Scholar]
- Panksepp J, Nelson E, Siviy S. 1994. Brain opioids and mother-infant social motivation. Acta Paediatr. Suppl 397:40–46 [DOI] [PubMed] [Google Scholar]
- Parker KE, Pedersen CE, Gomez AM, Spangler SM, Walicki MC, et al. 2019. A paranigral VTA nociceptin circuit that constrains motivation for reward. Cell 178:653–71.e19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak GW. 2004. Multiple opiate receptors: déjà vu all over again. Neuropharmacology 47:312–23 [DOI] [PubMed] [Google Scholar]
- Pasternak GW, Pan Y-X. 2013. Mu opioids and their receptors: evolution of a concept. Pharmacol. Rev 65:1257–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecina M, Karp JF, Mathew S, Todtenkopf MS, Ehrich EW, Zubieta JK. 2019. Endogenous opioid system dysregulation in depression: implications for new therapeutic approaches. Mol. Psychiatry 24:576–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecina S, Berridge KC. 2005. Hedonic hot spot in nucleus accumbens shell: Where do μ-opioids cause increased hedonic impact of sweetness? J. Neurosci 25:11777–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck JA, Galaj E, Eshak S, Newman KL, Ranaldi R. 2015. Environmental enrichment induces early heroin abstinence in an animal conflict model. Pharmacol. Biochem. Behav 138:20–25 [DOI] [PubMed] [Google Scholar]
- Pfeiffer A, Herz A. 1984. Endocrine actions of opioids. Horm. Metab. Res 16:386–97 [DOI] [PubMed] [Google Scholar]
- Piltonen M, Parisien M, Grégoire S, Chabot-Doré A-J, Jafarnejad SM, et al. 2019. Alternative splicing of the delta-opioid receptor gene suggests existence of new functional isoforms. Mol. Neurobiol 56:2855–69 [DOI] [PubMed] [Google Scholar]
- Porter J, Jick H. 1980. Addiction rare in patients treated with narcotics. N. Engl. J. Med 302:123. [DOI] [PubMed] [Google Scholar]
- Pradhan AA, Smith ML, Kieffer BL, Evans CJ. 2012. Ligand-directed signalling within the opioid receptor family. Br. J. Pharmacol 167:960–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prater KE, Aurbach EL, Larcinese HK, Smith TN, Turner CA, et al. 2017. Selectively bred rats provide a unique model of vulnerability to PTSD-like behavior and respond differentially to FGF2 augmentation early in life. Neuropsychopharmacology 42:1706–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prossin AR, Love TM, Koeppe RA, Zubieta JK, Silk KR. 2010. Dysregulation of regional endogenous opioid function in borderline personality disorder. Am. J. Psychiatry 167:925–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragen BJ, Maninger N, Mendoza SP, Bales KL. 2015. The effects of morphine, naloxone, and κ opioid manipulation on endocrine functioning and social behavior in monogamous titi monkeys (Callicebus cupreus). Neuroscience 287:32–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed B, Butelman ER, Yuferov V, Randesi M, Kreek MJ. 2014. Genetics of opiate addiction. Curr. Psychiatry Rep 16:504. [DOI] [PubMed] [Google Scholar]
- Resendez SL, Keyes PC, Day JJ, Hambro C, Austin CJ, et al. 2016. Dopamine and opioid systems interact within the nucleus accumbens to maintain monogamous pair bonds. eLife 5:e15325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro SC, Kennedy SE, Smith YR, Stohler CS, Zubieta JK. 2005. Interface of physical and emotional stress regulation through the endogenous opioid system and μ-opioid receptors. Prog. Neuropsychopharmacol. Biol. Psychiatry 29:1264–80 [DOI] [PubMed] [Google Scholar]
- Robledo P, Berrendero F, Ozaita A, Maldonado R. 2008. Advances in the field of cannabinoid–opioid cross-talk. Addict. Biol 13:213–24 [DOI] [PubMed] [Google Scholar]
- Rosenthal SM. 2009. Social pain and opioid use. CMAJ 181:827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin BS, Bridges RS. 1984. Disruption of ongoing maternal responsiveness in rats by central administration of morphine sulfate. Brain Res. 307:91–97 [DOI] [PubMed] [Google Scholar]
- Samuels BA, Nautiyal KM, Kruegel AC, Levinstein MR, Magalong VM, et al. 2017. The behavioral effects of the antidepressant tianeptine require the mu-opioid receptor. Neuropsychopharmacology 42:2052–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer JF, Svrakic DM, Freedland KE, Chrusciel T, Balasubramanian S, et al. 2014. Prescription opioid analgesics increase the risk of depression. J. Gen. Intern. Med 29:491–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff PL Jr. 2002. Opium and its alkaloids. Am. J. Pharm. Educ 66:186–94 [Google Scholar]
- Schmitz R 1985. Friedrich Wilhelm Serturner and the discovery of morphine. Pharm. Hist 27:61–74 [PubMed] [Google Scholar]
- Sharp BM, Chen H. 2019. Neurogenetic determinants and mechanisms of addiction to nicotine and smoked tobacco. Eur. J. Neurosci 50:2164–79 [DOI] [PubMed] [Google Scholar]
- Sher L 1998. The role of the endogenous opioid system in the pathogenesis of anxiety disorders. Med. Hypotheses 50:473–74 [DOI] [PubMed] [Google Scholar]
- Shibasaki M, Watanabe K, Takeda K, Itoh T, Tsuyuki T, et al. 2013. Effect of chronic ethanol treatment on μ-opioid receptor function, interacting proteins and morphine-induced place preference. Psychopharmacology 228:207–15 [DOI] [PubMed] [Google Scholar]
- Shoaib M, Spanagel R, Stohr T, Shippenberg TS. 1995. Strain differences in the rewarding and dopamine-releasing effects of morphine in rats. Psychopharmacology 117:240–47 [DOI] [PubMed] [Google Scholar]
- Stead JDH, Clinton S, Neal C, Schneider J, Jama A, et al. 2006. Selective breeding for divergence in novelty-seeking traits: heritability and enrichment in spontaneous anxiety-related behaviors. Behav. Genet 36:697–712 [DOI] [PubMed] [Google Scholar]
- Stedenfeld KA, Clinton SM, Kerman IA, Akil H, Watson SJ, Sved AF. 2011. Novelty-seeking behavior predicts vulnerability in a rodent model of depression. Physiol. Behav 103:210–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeber M, Jullié D, Lobingier BT, Laeremans T, Steyaert J, et al. 2018. A genetically encoded biosensor reveals location bias of opioid drug action. Neuron 98:963–76.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R, Auerbach SB. 2005. μ-Opioids disinhibit and κ-opioids inhibit serotonin efflux in the dorsal raphe nucleus. Brain Res. 1049:70–79 [DOI] [PubMed] [Google Scholar]
- Tejeda HA, Bonci A. 2019. Dynorphin/kappa-opioid receptor control of dopamine dynamics: implications for negative affective states and psychiatric disorders. Brain Res. 1713:91–101 [DOI] [PubMed] [Google Scholar]
- Terracciano A, Löckenhoff CE, Crum RM, Bienvenu OJ, Costa PT Jr. 2008. Five-Factor Model personality profiles of drug users. BMC Psychiatry 8:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Berrio A, Nava-Mesa MO. 2019. The opioid system in stress-induced memory disorders: from basic mechanisms to clinical implications in post-traumatic stress disorder and Alzheimer’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 88:327–38 [DOI] [PubMed] [Google Scholar]
- Trujillo KA, Akil H. 1991. Inhibition of morphine tolerance and dependence by the NMDA receptor antagonist MK-801. Science 251:85–87 [DOI] [PubMed] [Google Scholar]
- Trujillo KA, Akil H. 1994. Inhibition of opiate tolerance by non-competitive N-methyl-d-aspartate receptor antagonists. Brain Res. 633:178–88 [DOI] [PubMed] [Google Scholar]
- Trujillo KA, Bronstein DM, Sanchez IO, Akil H. 1995. Effects of chronic opiate and opioid antagonist treatment on striatal opioid-peptides. Brain Res. 698:69–78 [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Bar JL, Harley RM, Lyons MJ. 2001. The Harvard Twin Study of Substance Abuse: what we have learned. Harv. Rev. Psychiatry 9:267–79 [PubMed] [Google Scholar]
- Tsuang MT, Lyons MJ, Meyer JM, Doyle T, Eisen SA, et al. 1998. Co-occurrence of abuse of different drugs in men: the role of drug-specific and shared vulnerabilities. Arch. Gen. Psychiatry 55:967–72 [DOI] [PubMed] [Google Scholar]
- Turchan J, Maj M, Przewlocka B, Przewlocki R. 2002. Effect of cocaine and amphetamine on biosynthesis of proenkephalin and prodynorphin in some regions of the rat limbic system. Pol. J. Pharmacol 54:367–72 [PubMed] [Google Scholar]
- Turchan J, Przewlocka B, Lason W, Przewlocki R. 1998. Effects of repeated psychostimulant administration on the prodynorphin system activity and kappa opioid receptor density in the rat brain. Neuroscience 85:1051–59 [DOI] [PubMed] [Google Scholar]
- Turner CA, Hagenauer MH, Aurbach EL, Maras PM, Fournier CL, et al. 2019. Effects of early-life FGF2 on ultrasonic vocalizations (USVs) and the mu-opioid receptor in male Sprague-Dawley rats selectively-bred for differences in their response to novelty. Brain Res. 1715:106–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Natl. Acad. Sci. Eng. Med. 2019. Medications for opioid use disorder save lives Rep., US Natl. Acad. Sci. Eng. Med, Washington, DC [Google Scholar]
- Van den Berg CL, Kitchen I, Gerrits MA, Spruijt BM, Van Ree JM. 1999. Morphine treatment during juvenile isolation increases social activity and opioid peptides release in the adult rat. Brain Res. 830:16–23 [DOI] [PubMed] [Google Scholar]
- Van Zee A 2009. The promotion and marketing of oxycontin: commercial triumph, public health tragedy. Am. J. Public Health 99:221–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro M, Zhang M, Shaham Y, Caprioli D. 2016. Incubation of methamphetamine but not heroin craving after voluntary abstinence in male and female rats. Neuropsychopharmacology 42:1126–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhulst B, Neale MC, Kendler KS. 2015. The heritability of alcohol use disorders: a meta-analysis of twin and adoption studies. Psychol. Med 45:1061–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vowles KE, McEntee ML, Julnes PS, Frohe T, Ney JP, van der Goes DN. 2015. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain 156:569–76 [DOI] [PubMed] [Google Scholar]
- Wang A-L, Lowen SB, Elman I, Shi Z, Fairchild VP, et al. 2018. Sustained opioid antagonism modulates striatal sensitivity to baby schema in opioid use disorder. J. Subst. Abuse Treat 85:70–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson SJ, Akil H, Fischli W, Goldstein A, Zimmerman E, et al. 1982. Dynorphin and vasopressin: common localization in magnocellular neurons. Science 216:85–87 [DOI] [PubMed] [Google Scholar]
- Wei L-N, Hu X, Bi J, Loh H. 2000. Post-transcriptional regulation of mouse κ-opioid receptor expression. Mol. Pharmacol 57:401–8 [PubMed] [Google Scholar]
- Williams NR, Heifets BD, Blasey C, Sudheimer K, Pannu J, et al. 2018. Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am. J. Psychiatry 175:1205–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EA, Lewis J, Akil H. 1986. The preferential release of beta-endorphin from the anterior pituitary lobe by corticotropin releasing factor (CRF). Peptides 7:603–7 [DOI] [PubMed] [Google Scholar]
- Zarate CA Jr., Singh JB, Carlson PJ, Brutsche NE, Ameli R, et al. 2006. A randomized trial of an N-methyl-d-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry 63:856–64 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Picetti R, Butelman ER, Ho A, Blendy JA, Kreek MJ. 2015. Mouse model of the OPRM1 (A118G) polymorphism: differential heroin self-administration behavior compared with wild-type mice. Neuropsychopharmacology 40:1091–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Blandino P, Yuan Q, Shen P-H, Hodgkinson CA, et al. 2019. Exploratory locomotion, a predictor of addiction vulnerability, is oligogenic in rats selected for this phenotype. PNAS 116:13107–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M, Kuhlman DM. 2000. Personality and risk-taking: common biosocial factors. J. Pers 68:999–1029 [DOI] [PubMed] [Google Scholar]