Abstract
Oral squamous cell carcinoma (OSCC) is the most prevalent malignancy in head and neck cancer, with high recurrence and mortality. Early diagnosis and efficient therapeutic strategies are vital for the treatment of OSCC patients. Exosomes can be isolated from a broad range of different cell types, implicating them as important factors in the regulation of human physiological and pathological processes. Due to their abundant cargo including proteins, lipids, and nucleic acids, exosomes have played a valuable diagnostic and therapeutic role across multiple diseases, including cancer. In this review, we summarize recent findings concerning the content within and participation of exosomes relating to OSCC and their roles in tumorigenesis, proliferation, migration, invasion, metastasis, and chemoresistance. We conclude this review by looking ahead to their potential utility in providing new methods for treating OSCC to inspire further research in this field.
Keywords: Exosomes, Exosomal cargoes, OSCC, Head and neck squamous carcinoma
Introduction
Extracellular vesicles (EVs) are membrane-bound organelles actively secreted by cells, which can be released by nearly all cell types and detected in many body fluids (Shah, Patel & Freedman, 2018; Shao et al., 2018). The various types of cargo, including proteins, lipids, and nucleic acids within and on EVs surface (Mashouri et al., 2019), vary depending on their parental cells of origin and extracellular environment. This is why they are regarded as a mechanism for intercellular communication (Shah, Patel & Freedman, 2018; Van Niel, D’Angelo & Raposo, 2018). Based on their morphological features and contents, EVs are classified into different groups namely microvesicles (MVs), exosomes, and apoptotic bodies (Gyorgy et al., 2011). Different EV subtypes are thought to be divided based on their physical characteristics (such as size or density) relying on specific secretion mechanism (Mathieu et al., 2019; Théry et al., 2018). Exosomes are one kind of EV with a diameter of <150 nm (Shah, Patel & Freedman, 2018). They origin from intraluminal vesicles (ILVs) secreted by multivesicular bodies (MVBs) through fusing with the plasma membrane (Van Niel, D’Angelo & Raposo, 2018). Exosomes have been shown to play significant roles in regulating physiological and pathological processes, in addition to having great potential in therapeutic development (Ferguson & Nguyen, 2016; Kalluri & LeBleu, 2016; Liao et al., 2019; Mashouri et al., 2019). Exosomes play important function in many diseases (Hadavand & Hasni, 2019; Kadota et al., 2016; Li, Liu & Cheng, 2019; Malm, Loppi & Kanninen, 2016; Zhan et al., 2019). In cancer, their participation in numerous process phases has been observed, including in situ tumorigenesis (Milman, Ginini & Gil, 2019), tumor growth (Matei, Kim & Lyden, 2017; Xue et al., 2017), angiogenesis (Zeng et al., 2018), evasion of immune system (Chen et al., 2018; Kulkarni et al., 2019), resistance to chemotherapeutic agents (Mashouri et al., 2019), and metastasis (Hoshino et al., 2015; Kulkarni et al., 2019; Mashouri et al., 2019). In addition, antitumor effects have also been observed in exosomes (Pakravan et al., 2017; Xie et al., 2019).
OSCC, usually preceded by white or red mucosal changes known as leukoplakia or erythroplakia, respectively, or sometimes a combination of red and white features, is the most prevalent malignancy of the head and neck (Chi, Day & Neville, 2015) characterized by high recurrence and poor prognosis. There are approximately 350,000–400,000 new cases each year with high risk of recurrence (20% to 30%) (Gupta, Johnson & Kumar, 2016). Recently, a close association between OSCC and exosomes biology has been reported. Exosomes can transport between cells and microenvironment and promote the initiation and progression of OSCC directly (Momen-Heravi & Bala, 2018; Razzo et al., 2019); they also modulate OSCC by regulating the immune system, causing metabolic dysfunction and chemoresistance (Cui et al., 2020). They have been developed for applications in the clinic, including as biomarkers for early diagnosis and drug delivery (De Jong et al., 2019; Surman et al., 2019; Zlotogorski-Hurvitz et al., 2016). In this manuscript, we reviewed all these exciting advances. By looking ahead to exosomes’ potential utility in providing new methods for treating OSCC, we intend to inspire further research in this field.
Survey Methodology
We systematically searched with PubMed Advanced Search Builder with the following keywords: (1) OSCC and exosomes, (2) HNSCC and exosomes, (3) proteins in exosomes and OSCC, (4) lipid and exosomes and OSCC, (5) nucleic acids and exosomes in OSCC, (6) non-coding RNA and exosomes in OSCC, (7) mitochondrial DNA and exosomes in OSCC, (8) exosomes and clinic use and OSCC. By reading the titles and abstracts, papers nonrelated with either exosomal cargoes or OSCC or HNSCC were excluded. Besides, papers not published in English were excluded. Our search was not refined by publishing date, journal or impact factor of the journal, authors or authors affiliations.
Exosomes biogenesis
The biogenesis and release of exosomes to the extracellular environment is an ordered process. The first step for exosomes biogenesis is the formation of early endosomes (EEs). By inward budding or endocytosis, primary endocytic vesicles and their contents fuse with each other to form EEs (Huotari & Helenius, 2011; Liao et al., 2019). After the process of primary endocytic vesicles delivering their contents and membranes to EEs in the peripheral cytoplasm over 8–15 min, EEs will then go to their destination (Huotari & Helenius, 2011). One possible destination for EEs is recycling to the plasma membrane, directly or with the help of recycling endosomes (Huotari & Helenius, 2011; Morelli et al., 2004). The other possibility is their conversion to late endosomes (LEs) for additional processing. LEs accumulate ILVs formed by the inward budding of the endosomal membrane (Abels & Breakefield, 2016), which results in the conversion of LEs to MVBs (Abels & Breakefield, 2016; Liao et al., 2019). ILV formation is exquisitely regulated by mechanisms that remain to be fully elucidated, but it is reported to mainly rely on endosomal-sorting complex required for transport (ESCRT)-dependent machinery. Multiprotein ESCRT complexes are composed of ESCRT-0, ESCRT-I, ESCRT-II, and ESCRT-III. These localize on the cytoplasmic side of the endosomal membrane and play unique roles in sorting proteins into ILVs with assistance of accessory proteins (ALIX, VPS4, and VTA1) (Trajkovic et al., 2008). Besides ESCRT-dependent pathway, some evidence revealed that there are alternative, independent pathways regulating ILV biogenesis (Mashouri et al., 2019; McNally & Brett, 2018; Trajkovic et al., 2008). The type of cargo that is sorted into ILVs may relate to the mechanisms involved. With MVBs, there are two notable fates: to either degrade by fusing with lysosomes, or having their ILVs released as exosomes by fusion with the plasma membrane (Grant & Donaldson, 2009; Hessvik & Llorente, 2018).
Exosomes biogenesis starting from the formation of EEs to the final release into extracellular environment is a complex process (Fig. 1). To our knowledge, it can differ between cell types and their cellular physiological or pathological status (Kalluri & LeBleu, 2016; Liao et al., 2019), but much remains to be discovered to fully exploit the potential for exosomes as future clinical therapies (Mashouri et al., 2019).
Figure 1. Exosomes biogenesis and secretion within endosomal system.
This figure is reprinted from the manuscript Role of exosomal proteins in cancer diagnosis by Li et al. (2017b) according to open access licence CC BY 4.0.
Exosomes capsuled protein in OSCC
It is known that exosomes encapsulate genetic material as well as proteins from their cells of origin. Traditionally, the proteins that are encapsulated or transported by exosomes include structural and functional proteins that maintain the basic structure of exosomes and are responsible for regulating fusion, migration, and adhesion to target cells, such as transmembrane protein families (CD9, CD63, CD81 and CD82), molecular chaperones (Hsp70, Hsp90), multi-capsule synthesis proteins (TSG101 and ALIX), membrane carried fusion proteins, and others (Hemler, 2003; Li et al., 2017a; Li et al., 2017b; van Niel et al., 2006). Some exosomes in cancer tissues can carry specific proteins that allow their identification and differentiation from other exosomes, which could help to determine their unique function and potentially act as biomarkers for detecting disease progression (DeRita et al., 2017; Melo et al., 2015; Zhou et al., 2006).
In OSCC, exosomes-loaded protein both promote the tumorigenesis of OSCC and regulate the stromal cells around cancer tissues and support the processes of cancer cell proliferation (Fig. 2).
Figure 2. Functions of exosomes capsuled proteins in OSCC.
Numerous studies have already revealed that epidermal growth factor receptor (EGFR) plays an important role in tumor growth progress including tumorigenesis, tumor invasion and drug resistance (Shostak & Chariot, 2015; Sigismund, Avanzato & Lanzetti, 2018; Yang & Chang, 2018). In OSCC, there is no exception. Overexpression of EGFR activates many signal pathways, such as RAS/MEK/ERK, PI3K/AKT, and JAK/STAT pathways (Byeon, Ku & Yang, 2019; Nakamura et al., 2019; Oliveira-Silva et al., 2016; Zhang et al., 2018). Secretion of EGFR-contained exosomes from OSCC can be increased by EGF’s stimulation. Vesicles internalized by healthy epithelial cells around cancer cells result in epithelial-mesenchymal transition (EMT), turning normal cell into spindle-like cells, which promotes invasion and migration of the cells within the milieu of tumor (Aiello et al., 2018). Fujiwara et al. established a novel mode of tumor therapy with anti-EGFR antibody cetuximab. This drug inhibits EMT in oral epithelial cell by preventing the internalization of OSCC secreted exosomes into epithelial cells (Fujiwara et al., 2018). In the milieu of tumor, fibroblast is another crucial portion for its participation in oral cancer, and evidences show that exosomes can help it function better. Principe et al. compared fibroblasts from resected tumor, adjacent normal tissue in the same patient and conducted a detailed analysis of the cancer associated fibroblasts (CAF) secretome in OSCC. They found that CAF-derived exosomes loaded with cytokines, growth factors or chemokine were richer in tumor tissue than normal tissues, and exosomes play important role in promoting OSCC growth and migration. The major cargo in OSCC exosomes is microfibrillar-associated protein 5 (MFAP5), which is suggested to be a novel prognostic of OSCC and exosomes loaded with those content can be a new treat target (Principe et al., 2018). Jiang et al. found that OSCC cells can secrete exosomes with p-ERK1/2 to adjacent normal fibroblasts, directly active the ERK1/2 signal pathway to down-regulate the expression of Caveolin-1 (CAV1) protein and up regulate the expression of MCT4 and PDK1 in normal fibroblasts. This regulation takes place both in vivo and in vitro and because of it, fibroblasts output much more lactate and cancer cells intake their outputted lactate, which then activates MCT4/MCT1 axis between cancer cell and activated fibroblasts, and, finally, provides sufficient energy for tumor cell growth and reproduction (Jiang et al., 2019).
Interaction between tumor cells and immune system can play a dual role: on one hand, immune cells can recognize and eliminate tumor cells in the early stages of tumor development (Borst et al., 2018; Gardner & Ruffell, 2016; Teng et al., 2015); on the other hand, excessive infiltration of immune cells is related with poor prognosis (Gonzalez, Robles & Werb, 2018; Mantovani et al., 2017). In the microenvironment of tumor, exosomes can mediate the reaction of immune cells (Fig. 2). Kim et al. compared exosomes from normal and OSCC patients. They found that nearly 78% of OSCC patients’ serum-derived exosomes express Fas ligand (FasL) and only 5% exosomes in normal people express FasL. Moreover, co-culturing FasL(+) OSCC serum-derived exosomes with Jurkat cells lead to the apoptosis of immune cells (Kim et al., 2005). Zhang’s group discovered that exosomes derived from supernatant of human oral cancer cells CAL 27 and SCC-25 (ATCC) were rich in interferon regulatory factor 3 (IRF-3) and its phosphorylation which promotes chemokine (C-X-C motif) ligand (CXCL) genes and the expression of the type I interferon (IFN) gene. This strengthenes the biological function of NK cells, including cytotoxicity, proliferation and release of granzyme M and perforin (Wang et al., 2018b). Chen and colleagues found that thrombospondin 1 (THBS1) was transported by OSCC-derived exosomes and could be taken into surrounding macrophages derived from THP-1 and PBMCs resulting in stimulating macrophage transformation into M1-like tumor associated macrophages (TAMs), this process plays a crucial role in controlling OSCC cell migration (Xiao et al., 2018). Besides, recent research indicates that transforming growth factor-beta (TGF-β) is important for tumorigenesis and immunosuppression in the tumor microenvironment (Batlle & Massagué, 2019); however, in OSCC, mutation of loss function in TGFβ type II receptor (T βRII) is quite common. South and colleagues discovered that exosomes extract from stromal fibroblasts isolated from OSCC patients contains T βRII, they can increases TGFβ signaling in OSCC cells devoid of TβRII, which active TGF-β signaling between tumor cells and their surrounding microenvironment (Languino et al., 2016). They also showed that OSCC-derived exosomes were loaded with the C-terminal fragment of desmoglein 2, a highly expressed protein in many kinds of malignant tumors. DSG2 expression in OSCC could promote exosomes secretion through the modification of metalloproteases and repartitioning of Cav1; moreover, these DSG2-CTF-positive exosomes could modulate the microenvironment by converting nearby fibroblasts into TAMs (Overmiller et al., 2017).
In the past few years, several treatments have been conducted to the cancer patients, metastasis is still the major cause of death in most cases. Exosomes and their carriers secreted by cancer cells would participate in pathological generation of blood and lymphatic vessels, which is closely related to the development and progression of tumor tissues (Fu et al., 2019; Mashouri et al., 2019). Vascular endothelial growth factor C (VEGF-C) is universally acknowledged as one of the most effective proteins in promoting lymphatic vessels. It is abundant in lymph node positive metastatic OSCC patients (Lien et al., 2018; Shigetomi et al., 2018) (Fig. 2). Zhong et al. (2019) discovered that increased VEGF-C expression associated with an increased number of salivary exosomes in OSCC tissues. In Kozaki and colleagues’ study, they found that the increase of HSP90-rich exosomes is positively correlated to the increased invasive capacity of OSCC; moreover the knockdown of HSP90α and HSP90β decreased the metastatic capacity and survivability of OSCC cells (Ono et al., 2018). Observing that OSCC LN1-1 cells were more aggressive in lymphatic node metastasis than OEC-M1 cells, Wang et al. (2019a) used stable isotope amino acid labeling to reveal that higher laminin-332 protein levels in tumor cell-derived exosomes was a major cause for the superior lymphangiogenesis ability of OSCC LN1-1 cells, as laminin-332 promotes lymphatic endothelial cell migration and tube formation. Li et al. (2019a) reported that PF4V1, CXCL7, F13A1, and ApoA1 could affect OSCC lymph node metastasis, and the mechanism remains unknown.
Lipid cargo in OSCC exosomes
Exosomes are an alternative means to carrier proteins and lipoproteins for transporting lipids (Record et al., 2014). There are approximately 2000 lipid species identified through comparative lipidomic analyses (Haraszti et al., 2016), which can localize to the membrane and lumen of exosomes. The roles for several kinds of lipids have been reported, including BMP (Bismonoacylglycerophosphate), cholesterols, ceramides, and phosphatidic acid (Record et al., 2014).These lipids play critical roles in exosomes biogenesis and release (Record et al., 2014; Record et al., 2018). And some of them can be unique and help us distinguish different EVs. For example, BMP is recognized as a lipidic molecule required for MVB formation (Subra et al., 2007) and ILV biogenesis (Falguières, Castle & Gruenberg, 2012), however, it is irrelevant to the formations of MVs, which indicates its potential as a biomarker to distinguish exosomes from MVs (Record et al., 2018). Lipid composition is not only different between various EVs, the lipid contents of exosomes are usually different from their parental cells. Llorente et al. (2013) quantified 280 species of lipids from PC-3 prostate cancer cells and their exosomes and found some differences in their lipid composition. This interesting finding indicates that the discrimination of lipids between exosomes and parental cells could play significant roles in many pathophysiologies, which inspires us that by detecting the lipid composition from exosomes and parental cells, it can be a diagnostic as well as therapeutic direction in the future. Over the past few years, lipid metabolic abnormalities have been identified as a feature of tumor cells. Hu et al. (2019) found that some lipid metabolism-related genes in OSCC patients could be used for prognostication. Lipid uptake can also be enhanced in cancer (Tousignant et al., 2019). Pascual et al. (2017) observed a similar phenomenon in OSCC, as they documented a subpopulation of CD44(bright) OSCC cells that express high levels of lipid metabolic genes and can initiate metastasis.
Arachidonic acid (AA) is a free fatty acid that can be transported by exosomes (Subra et al., 2010), and its metabolism is a major dysregulated pathway in cancer cells (Gatto, Schulze & Nielsen, 2016). As the precursor of both leukotrienes and prostaglandins, AA can be transferred between tissues by exosomes contributing to tumor growth and progression (Linton et al., 2018). Exosomes secreted from AsPC-1 cells, a highly metastatic pancreatic ductal adenocarcinoma cell line, were reported to deliver AA to macrophages. The fusogenicity of AsPC-1 exosomes decreased when pretreatment with PLA2 caused the removal of AA, indicating that exosomal AA may enhance crosstalk between cancer cells and TAMs, thus contributing to tumor progression (Linton et al., 2018). Besides AA itself, its products have been detected performing similar functions. Leukotrienes (LTs) are involved in various pathophysiologies such as inflammatory asthma, atherosclerosis, and cancer (Record et al., 2014). Several types of LTs, such as LTB4, LTC4, cysteinyl leukotriene LTC4, and LTC4 synthase, are enriched in exosomes (Esser et al., 2010; Lukic et al., 2019). Exosomes appear to play a role in the biogenesis of LTs. For example, LTA4, the precursor of leukotrienes, only has a five-second half-life in in vitro buffer, but with the protection of exosomes, their half-life can be elongated to several minutes (Esser et al., 2010). Besides assisting the biogenesis of LTs, exosomes seem to play other roles helping LTs performing their pathological functions. Lukic et al. found that exogenous LTC4 generated by monocytic cells can be transformed into pro-tumorigenic LTD4 through gamma-glutamyl transpeptidase-1(GGT-1). GGT-1 is contained within both exosomes and primary cancer cells, which stimulates cancer cell migration and survival (Esser et al., 2010). And since the 5-lipoxygenase (5-Lox) and cyclooxygenase (COX)-2 pathways of arachidonic acid metabolism are involved in oral carcinogenesis (Lai et al., 2018), LTs in exosomes may also play an important role in OSCC. Prostaglandins (PGs) are another product of AA. Exosomes with high concentrations of prostaglandins transport more PGE2 to neighboring cells, enhancing the overall presence of PGE2 in the microenvironment (Subra et al., 2010). Cell motility and metastatic status are impacted by extracellular levels of PGE2 (Nasry, Rodriguez-Lecompte & Martin, 2018). Evidence has shown that exosomes rich in PGE2 participate in tumor immune evasion and promote tumor growth (Record et al., 2014). PGE2-mediated inflammation contributing to OSCC at different stages of carcinogenesis, invasion and metastasis contributes to patient morbidity and mortality (Harada et al., 2017). Abrahao et al. showed that HNSCC cells secrete PGE2. The overexpression of COX-2 in tumor and inflammatory cells, and subsequent increased production of PGE2, may promote HNSCC growth in an autocrine and paracrine way in the microenvironment. Exosomes can therefore be considered a potential medium for autocrine and paracrine regulation (Luga et al., 2012).
Nucleic acids in OSCC exosomes
A diverse collection of nucleic acids, including DNA, coding mRNA, non-coding RNA (ncRNA), micro RNA (miRNA), circular RNA (circRNA), and long non-coding RNA (lncRNA) have been identified in exosomes (Abels & Breakefield, 2016). The nucleic acid content of exosomes is believed to play a significant role in promoting cancer pathogenesis through the oncogenic transformation and transfer of cancer-specific genetic material (Kalluri & LeBleu, 2016). Understanding how nucleic acids transported by exosomes mediate the process of OSCC is critical, and could illuminate strategies for exosomes-based targeted therapy.
DNA
DNA in exosomes has been observed in cell culture supernatant as well as human and mouse biological fluids such as blood, seminal fluid, and urine (Kalluri & LeBleu, 2016). Based on cell origin, it is likely that different types of exosomes contain distinct types of DNA, such as single-stranded DNA (ssDNA), double-stranded DNA (dsDNA), mitochondrial DNA (mtDNA), and of varying states (e.g., fragment length, chromosome-bound) (Kalluri & LeBleu, 2016). Among these various types, dsDNA is the most evaluated (Kahlert et al., 2014).
Exosomal DNA has been found to be involved in immunity regulation (Diamond et al., 2018; Kitai et al., 2017). When tumors were treated by different strategies (e.g., antitumor drugs or radiotherapy), the induced secretion of cancer cell-derived exosomes containing DNA triggered dendritic cell (DC) activation and cytokine production, both of which can have antitumor effects by regulating immune responses (Diamond et al., 2018; Kitai et al., 2017). In addition to its potential therapeutic roles by regulating immune system, DNA in exosomes may become an attractive candidate biomarker for tumor diagnosis because of the inherent stability of DNA within exosomes (Kalluri & LeBleu, 2016). For example, the same mutations in susceptibility genes were found in exosomal DNA and parental cells of pheochromocytomas and paragangliomas (Wang et al., 2018a), and mutant KRAS, TP53, NOTCH1, and BRCA2 DNA in exosomes from pancreatic cancer were also detected (San Lucas et al., 2016; Yang et al., 2017a). Exosomes in OSCC may play similar roles, as mutant genes were discovered in OSCC cells as well (Biswas et al., 2019; Natsuizaka et al., 2017).
Virus infection is closely related to cancers including OSCC. Moreover, viral DNA has also been detected in exosomes from cancer patients (Yang et al., 2017b). For example, Meckes Jr et al. (2010) identified that ERK and PI3K/AKT signaling pathways can be activated if the recipient cells were exposed to exosomes containing major EBV oncogene LMP1. Besides, Human papillomavirus (HPV) is considered a risk factor for OSCC, and its DNA has been found in exosomes from HeLa cells (Mata-Rocha et al., 2020). There are some evidence demonstrating that HPV DNA in exosomes participated in the process of cancer. HPV DNA in plasma-derived exosomes was detected in rectal squamous cell carcinoma patients (Ambrosio et al., 2019). Ambrosio et al. isolated exosomes from the HPV DNA-positive cell line CaSki, which can transfer DNA to normal cell lines. Moreover, circulating exosomes-encapsulated HPV DNA in the blood of neoplastic patients was verified to be transferred to normal and tumor cells at least in vitro. Exosomes containing HPV are involved in HPV-associated carcinogenesis, indicating a potential role in OSCC although there is no direct evidence noted (Guenat et al., 2017).
Mitochondria are major energy generators in cells, and increasing studies have revealed their role in tumor development. Currently, scientists are deepening our understanding of the connections between mitochondria and tumors. For example, cancer cells can obtain mitochondria from healthy cells to achieve chemical resistance (Pasquier et al., 2013), and mitochondrial DAMP provides tumor cells with a possible immune escape mechanism (Zhang, Itagaki & Hauser, 2010a; Zhang et al., 2010b). The transfer of mitochondria or mtDNA is not completed independently, but is carried out by the transporting mediators between cells, such as exosomes and secretory vesicles. In oral cancer, whether exosomal mtDNA participates in tumorigenesis, tumor proliferation, and migration is still vague. However, Uzawa et al. proposed a novel method for detecting mtDNA in OSCC patients and reported significant differences in serum mtDNA levels before and after OSCC patient treatment. They also identified mtDNA as a promising molecular marker for OSCC prognostication (Uzawa et al., 2015). Despite these advances, further exploration into exosomes-derived mtDNA and its application in the diagnosis and treatment of OSCC is necessary.
mRNA
Coding mRNA has been found in exosomes. Transferring mRNA within exosomes can enable mRNA translation into proteins in recipient cells (Huang et al., 2013; Valadi et al., 2007), giving mRNA a potentially powerful role in cell-to-cell communication. Its function has been studied in both healthy and pathological states (Feng et al., 2018; Valadi et al., 2007). As a means for cell-to-cell transportation, the mRNA profiles of tumor-derived exosomes have been evaluated in various cancers such as melanoma (Del Re et al., 2018), glioblastoma (Skog et al., 2008), prostate cancer (Lázaro-Ibáñez et al., 2017), and colorectal cancer (Hao et al., 2017). Moreover, these studies revealed that mRNAs in tumor-derived exosomes can play vital roles in promoting malignant tumor growth, proliferation, and metastasis through suppressing immune responses and resisting antitumor treatment (Del Re et al., 2018; Geis-Asteggiante et al., 2018; Skog et al., 2008).
Salivary liquid biopsy has emerged as an excellent method for disease detection, which is also applied to OSCC (Yakob et al., 2014). Evaluation of the salivary transcriptome from OSCC patients has helped to identify some mRNA biomarkers (Brinkmann et al., 2011). Qadir et al. characterized and compared the transcriptome profiles between exosomes isolated from primary human normal oral keratinocytes (HNOK) and HNSCC cell lines. The results showed that in HNSCC-derived exosomes, the expression of matrix remodeling (EFEMP1, DDK3, SPARC), cell cycle (EEF2K), membrane remodeling (LAMP2, SRPX), differentiation (SPRR2E), apoptosis (CTSC), and transcription/translation (KLF6, PUS7) factors showed significant differences from healthy cell-derived exosomes (Qadir et al., 2018), indicating that cancer cells may confer transcriptome reprogramming through exosomes to enhance cancer-associated pathologies.
ncRNA
Most of the human genome is considered biologically active. However, only a minor fraction of DNA encodes proteins. ncRNAs represent the majority of RNA that is not translated into proteins (Romano et al., 2017). NcRNAs are a category of exosomal cargo under investigation for its complex role in regulating gene expression (Zhang et al., 2015). NcRNA interactions are often interconnected which, when deregulated, could eventually drive tumorigenesis and progression (Chan & Tay, 2018). Identifying ncRNAs and their interactions will help to provide robust biomarkers and new therapeutic targets for more effective cancer therapies, better outcomes, and greater survival (Chan & Tay, 2018; Zhang et al., 2015) (Table 1).
Table 1. NcRNAs regulating the process of OSCC in exosomes.
| NcRNAs | Types of ncRNAs | Pro/ Anti-tumor | Target/ Signal pathway | Functions | Origin of exosomes | Ref. |
|---|---|---|---|---|---|---|
| miR8485 | miRNA | Pro-tumor | — | Promote the carcinogenesis of premalignant lesions, proliferation, migration and invasion of tumor cells | MSCs | Li et al. (2019b) |
| miR-6887-5p | miRNA | Anti-tumor | HBp17/FGFBP-1 | Inhibit tumor cell proliferation, colony formation, then tumor growth | A431 cells | Higaki et al. (2020) |
| miR-142-3p | miRNA | Pro-tumor | TGFBR1 | Cause tumor-promoting changes | Oral dysplasia and OSCC cell lines | Dickman et al. (2017) |
| miR-24-3p | miRNA | Pro-tumor | PER1 | Maintain the proliferation of OSCC cells | Saliva in OSCC patients | He et al. (2020) |
| miR-3188 | miRNA | Anti-tumor | BCL2 | The loss of miR-3188 in exosomes contributes to the malignant phenotypes of HNC cells through the depression of BCL2 | CAFs |
Wang et al. (2019a) Wang et al. (2019a) |
| miR-34a-5p | miRNA | Anti-tumor | AXLAKT/GSK-3beta/ beta-catenin signaling pathway | MiR-34a-5p binds to direct downstream target AXL to suppress OSCC cell proliferation and metastasis | CAFs |
Li et al. (2018a), Li et al. (2018b), Li et al. (2018c) |
| miR3825p | miRNA | Pro-tumor | — | Responsible for OSCC cell migration and invasion | CAFs | Sun et al. (2019) |
| miR-21-5p | miRNA | Pro-tumor | — | Increase metastasis, stemness, chemoresistance and poor survival in patients with OSCC | CAL27 and SCC-15 OSCC cells | Chen et al. (2019) |
| miR-1246 | miRNA | Pro-tumor | DENND2DERK/ AKT pathway | Increase cell motility and invasive ability | HOC313-LM OSCC cells | Sakha et al. (2016) |
| miR-21 | miRNA | Pro-tumor | miR-21/HIF-1alpha/ HIF-2alpha-dependent pathway | MiR-21 can be delivered to normoxic cells to promote prometastatic behaviors | Hypoxic OSCC cells | Li et al. (2016) |
| Pro-tumor | PTEN, PDCD4 | Induce cisplatin resistance of OSCC cells | HSC-3-R and SCC-9-R | Liu et al. (2017) | ||
| miR-200c-3p | miRNA | Pro-tumor | CHD9, WRN | Spread invasive capacity by exosomes in tumor microenvironment | SQUU-B tongue cancer cell clones | Kawakubo-Yasukochi et al. (2018) |
| miR-155 | miRNA | Pro-tumor | — | Lead to mesenchymal transition and increase migratory potential and acquire cells drug-resistant phenotype | Cisplatin resistant OSCC cells | Kirave et al. (2020) |
| miR-200c | miRNA | Anti-tumor | TUBB3, PPP2R1B | Increase the sensitivity of Docetaxel (DTX) resistant HSC-3 cells to DTX | normal tongue epithelial cells (NTECs) | Cui et al. (2020) |
| miR-101-3p | miRNA | Anti-tumor | COL10A1 | Overexpression of miR-101-3p inhibit oral cancer progression and provide a therapeutic target | human bone marrow mesenchymal stem cells (hBMSCs) | Xie et al. (2019) |
| miR-29a-3p | miRNA | Pro-tumor | SOCS1 | Promote M2 subtype macrophage polarization, tumor cell proliferation and invasion | SCC-9 and CAL-27 | Cai et al. (2019) |
| FLJ22447 | lncRNA | Pro-tumor | Lnc-CAF/IL-33 | Reprogram normal fibroblast to CAFs and promote OSCC development | CAFs | Ding et al. (2018) |
miRNA
MiRNA are small ncRNAs around 22 nucleotides long, and they can be divided into oncogenic miRNA and tumor suppressor miRNA (Svoronos, Engelman & Slack, 2016).They perform their post-transcriptional regulatory effects by binding to specific sites known as miRNA response elements (MREs) on their target transcripts, leading to either transcript degradation or translational inhibition (Chan & Tay, 2018). MiRNA regulatory activity in cancer has been widely studied (He et al., 2020; Li et al., 2019b; Sakha et al., 2016).
Exosomal miRNAs in OSCC growth.
MiRNA function in OSCC has been thoroughly discussed (Langevin et al., 2017). Since they are secreted from various types of healthy and tumor cells (Tkach & Théry, 2016), miRNAs in exosomes can play different roles in promoting or inhibiting cancer. Dickman et al. found that miR-142-3p secreted from oral cancer cells promotes cancer cell growth by eliminating the miRNA tumor suppressive effect. Exosomes also promote tumor angiogenesis by releasing miR-142-3p to its microenvironment (Dickman et al., 2017). However, in another study of Higaki et al. (2020), overexpression of miR-6887-5p in SCC/OSCC cells inhibited tumor growth. Similar results document targeting miRNA as a treatment strategy to inhibit tumor growth. Lower levels of miR-3188 were detected in CAFs than normal fibroblasts, and loss of miR-3188 promoted malignant phenotypes in head and neck cancer cells, supporting its consideration as a therapeutic target (Wang et al., 2019b).
Exosomal miRNAs in OSCC cell migration and invasion.
MiRNA in exosomes not only promote or inhibit tumor growth, but have also been shown to participate in OSCC cell migration and invasion. A research compared miRNA profiles in non-invasive SQUU-A and highly invasive SQUU-B tongue cancer cell clones, it was observed that hsa-miR-200c-3p acts within a key pro-invasion role in OSCC. The transfer of miR-200c-3p in exosomes derived from a highly invasive OSCC line can also accelerate the invasion potential of non-invasive counterparts (Kawakubo-Yasukochi et al., 2018). Another research done by Sun et al. also found that miR3825p was overexpressed in CAFs compared with fibroblasts of adjacent normal tissue, and miR3825p overexpression was an important regulatory factor in OSCC cell migration and invasion (Sun et al., 2019). MiRNAs in exosomes can also play like messengers to modulate tumor environment, and then manipulate OSCC cell migration and invasion. Normoxic and hypoxic OSCC-derived exosomes yielded different miRNA profiles, miR-21 showed its most significant role under hypoxic conditions. The loss of miR-21 in hypoxic OSCC cells downregulated miR-21 levels in exosomes and significantly reduced cell migration and invasion. Restoration of miR-21 expression in HIF-1 α- and HIF-2 α-depleted exosomes rescued OSCC cell migration and invasion (Li et al., 2016).
Exosomal miRNAs in OSCC metastasis.
Cancer metastasis is a very complicated process, involving intrinsic and extrinsic mechanisms. Metastasis is also a great challenge in our effort to fight cancer, and it caused poor prognosis of OSCC (Sun et al., 2019). It has been explosively studied and proved miRNAs participated in this process of OSCC. Some studies have noticed that cancer stem cell-derived extracellular vesicles are enriched with miR-21-5p, which is associated with increased potential of OSCC metastasis (Chen et al., 2019). However, some miRNAs have been revealed as performing anti-cancer roles as well. By examining the miRNA profiles of CAF- and normal fibroblast (NF)-derived exosomes, miR-34a-5p expression was found to be significantly decreased, making it an anti-cancer therapeutic target for OSCC (Li et al., 2018c). The complex of metastasis makes it a fascinating field, especially the relations between miRNAs and tumor microenvironment (TME). TME plays a vital role in the progression of OSCC. Recent research has revealed that tumor-derived exosomes (TEX) accumulate in the TME and interact between tumor and healthy stromal cells (Ludwig et al., 2018).Cai et al. cocultured exosomes extracted from OSCC cell lines (SCC-9 and CAL-27) with macrophages (Cai et al., 2019). Their results showed that the upregulation of miR-29a-3p in OSCC-derived exosomes is related to M2 subtype macrophage polarization. After interfering with miR-29a-3p from OSCC, M2 subtype macrophage polarization was inhibited by OSCC-derived exosomes.
Exosomal miRNAs in chemoresistance.
Chemotherapy is a hallmark of fighting cancers. However, Chemoresistance is a significant challenge for OSCC treatment with no clear mechanism. Several studies have shown that miRNAs in exosomes of both healthy and tumor cells can manipulate this phenomenon (Cui et al., 2020; Kirave et al., 2020; Qin et al., 2019). Some miRNAs are upregulated during chemotherapy, which can enhance chemoresistance against antitumor drugs such as cisplatin (CIS) and docetaxel (DTX). Kirave et al. (2020) found that when transferring exosomes from CIS-resistant to CIS-sensitive cells, miR-155 was significantly upregulated in the recipient CIS-sensitive cells. Exosomes isolated from CIS-resistant cell lines contained a higher concentration of miR-21 in accordance with the parental cells’ increased cisplatin resistance, which indicates that miR-21 may be a potential target against chemoresistance (Liu et al., 2017). The underlying mechanism is considered related to EMT and decreased DNA damage in cancer cells (Cui et al., 2020; Kirave et al., 2020). Some miRNAs were downregulated during chemoresistance. For example, downregulation of miR-200c increased resistance to DTX, when miR-200c was transported by exosomes, the results showed the increase of the sensitivity to DTX both in vitro and in vivo, indicating miR-200c could be a therapeutic target of OSCC (Cui et al., 2020).
lncRNA and circRNA
Beyond miRNA, there are other regulatory ncRNAs that perform complex roles in cancer (Morris & Mattick, 2014). One kind, lncRNA, ranging from 200 to >1000 nucleotides, is a novel class in the human genome with seldom to no coding potential (Yang, Wen & Zhu, 2015). They participate in various diseases through interacting with DNA (Lin et al., 2020), RNA, or proteins (Zhang et al., 2014). lncRNA may be involved in cancer cell proliferation (Wang, Li & Shi, 2019; Xu et al., 2019), migration (Xu et al., 2019), invasion (Xiong et al., 2019), metastasis (Jin et al., 2020; Xiong et al., 2019), and antitumor drug resistance (Wang, Li & Shi, 2019). LncRNA has multiple roles in regulating cancers, and the most important one among them is acting as “miRNA sponge” (Tan, Xiang & Xu, 2019; Zhang et al., 2019b). Zhang et al. (2019b) discovered that knockdown of lncRNA UCA1 significantly suppressed TGFβ1-induced tongue cancer cell invasion and eventually induced EMT. TGFβ1-induced EMT and invasion in OSCC are consistent with increased JAG1, whereas miR-124 inhibits its expression. UCA1 binds to miR-124 directly and can downregulate miR-124 expression. This is the basis for lncRNA UCA1’s protumor effect through sponge-like lncRNA-miRNA-mRNA regulation (Zhang et al., 2019b). lncRNA TIRY was also found to act as a miRNA sponge in OSCC by downregulating miR-14 expression in CAF-derived exosomes (Jin et al., 2020). lncRNA FLJ22447 (lnc-CAF) secreted from CAFs regulates NFs to CAFs, and tumor cells increased lnc-CAF levels in stromal fibroblasts via exosomal lnc-CAF as well (Ding et al., 2018). Unlike lncRNA, circRNA consists of a closed continuous loop structure without 5′–3′ polarity or a poly-A tail, which enables its resistance to RNases and higher stability compared with linear RNA (Bai et al., 2019). Similar to lncRNA, circRNA also functions as a miRNA sponge (He et al., 2019). Although the roles of circRNA in exosomes remains unknown, a hypothesis has been introduced by Bai et al. (2019). Some circRNAs may bind to and transport with miRNAs by exosomes. After entering target cells, miRNAs are released to regulate target genes (Jin et al., 2020). CircRNAs in exosomes may therefore enter the recipient cells, bind to miRNAs, and regulate target genes. The roles of circRNAs in OSCC have been investigated by several researchers (Han, Cheng & Li, 2020), and differences in circRNAs profiles between OSCC patients and healthy people have also been distinguished (Qiu et al., 2019; Wei et al., 2020). By performing the function of miRNA sponge, the significance of LncRNA and circRNA has been noted, their discovery in OSCC also indicates that their roles in the pathogenesis cannot be neglected. Although there is still a lack of direct evidence for exo-lncRNA and exo-circRNA regulating OSCC, the evidence presented in other cancers proved it must be a remarkable field in the future research of OSCC as well (Li et al., 2018a; Li et al., 2018b; Ren et al., 2018; Zhang et al., 2019a).
Other ncRNAs
Besides regulatory ncRNA, tRNA and rRNA make up another group of ncRNA referred to as housekeeping ncRNA (Romano et al., 2017). Baglio et al. (2015) defined the exosomes-enclosed RNA species from the full small RNAome of MSC-produced exosomes. Adipose and bone marrow MSC subtypes secrete different tRNA species that may be relevant to clinical applications; however, how tRNAs are transported through exosomes and their influence on the microenvironment in a cell type-dependent manner remains unclear (Baglio et al., 2015). Crescitelli et al. analyzed RNA profiles in different EVs including exosomes. According to their findings, rRNA was primarily detectable in apoptotic bodies, but smaller RNAs without prominent ribosomal RNA peaks in exosomes (Crescitelli et al., 2013). This indicates that exosomes are potentially not carriers of rRNA. Collectively, there is little evidence surrounding the exosomal transportation of tRNA and rRNA, let alone their potential function in modulating cancer and their microenvironment.
Clinical use of exosomes
Exosomes as biomarkers for diseases diagnosis
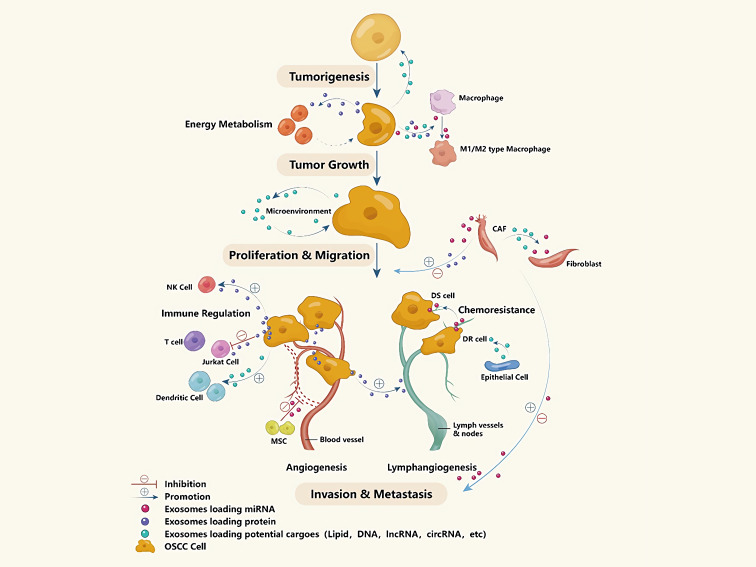
Combining exosomes’ stability and accessibility in various biological fluids like urine, blood (including serum), breast milk, exosomes can be used as biomarkers for various cancers, some can even be used to determine the type and severity of the disease. For example, exosomes carrying Glypican-1 are considered a sensitive indicator of pancreatic cancer in blood samples (Melo et al., 2015). In gastric cancer patients, exosomes expressing CD63 can be isolated from tumor cells cluster but not stromal cells reflects a worse prognosis than the situation that CD63+ exosomes can be find in both cell types (Miki et al., 2018). As for OSCC, exosomes have also been considered as one of the fast-detected methods for they have been discovered in the tumor microenvironment and been discovered play an irreplaceable role in OSCC’s tumorigenesis, tumor proliferation and migration, tumor invasion and metastasis (including angiogenesis and lymphangiogenesis), chemoresistance, and so on (Fig. 3). Many studies have shown that the morphology and content of exosomes isolated from OSCC patients’ saliva and blood are very different from that of healthy people, suggested the possibility of using exosomes for OSCC diagnosed (Zlotogorski-Hurvitz et al., 2016). By using high-resolution AFM, Sharma et al. found the salivary exosomes of oral cancer patients were much larger and amorphous compared with those of healthy people, and also found that CD63 was significantly increased on the surface of cancer exosomes (Sharma et al., 2011). In a study by Rabinowits, tongue squamous cell carcinoma tissue and normal tissue were collected in pairs. They isolated exosomes and found different miRNA loading patterns similar to the loading patterns of blood exosomes, suggesting that circulating exosomes can be a more reliable method in evaluating tumors (Rabinowits et al., 2017). Sanada et al. (2020) examined the expression levels of secreted lysyl-oxidase-like 2 (LOXL2) in pharyngeal and tongue cancer patient serum and found that elevated serum exosomes LOXL2 levels are associated with low-grade oral cancer. All these discoveries of exosomes provide new ideas for the non-invasive disease diagnosis method.
Figure 3. Exosomes’ contents and potential functions in the developing process of OSCC.
Also, the contents of exosomes can indicate tumor invasion capacity and occurrence of distant metastasis, because exosomes always participate in the angiogenesis and lymphangiogenesis of tumor tissues as mentioned before. Li and colleagues (2016) found that miR-21-rich exosomes are associated with increased OSCC invasiveness, and that these exosomes are delivered to normoxic cells to promote prometastatic behaviors. Nakashima et al. utilized integrated microarray profiling technology to analyze the different expression patterns of miRNA between non-invasive and highly invasive tongue cancer cells, observing that hsa-miR-200c-3p was the crucial point in spreading invasive ability (Kawakubo-Yasukochi et al., 2018). Moreover, the quantity and content of exosomes can be used in non-invasive examination for deciding tumor stages and predict the prognosis of treated OSCC patients, and scientists suggested that the combination of different kinds of biomarkers are significantly better than single biomarker in OSCC diagnosing. Ludwig et al. suggest that tumor staging can be understood by exploring the interaction between OSCC cell-derived exosomes and lymphocytes. Exosomes in the plasma of patients with tumors in an uncontrolled phase have greater induction of T cell apoptosis and inhibition of lymphocyte proliferation, which differs from patients without significant disease (Ludwig et al., 2017). The quantity and content of exosomes could predict the prognosis of treated OSCC patients. Liu and Tian compared serum exosomes between laryngeal squamous cell carcinoma patients and vocal cord nodule patients, finding that the expression levels of miR-21 and HOTAIR were higher in exosomes of malignant lesions. Moreover, the serum exosomes of patients with laryngeal cancer in stage III/IV also showed a high level of miR-21 and HOTAIR in exosomes (Wang et al., 2014), suggesting an association between the level of exosomal content and prognosis. Those outcomes are similar to a clinical study performed by Rodríguez Zorrilla et al. (2019), they collected ten OSCC patients’ plasma sample before and after the excision surgery to evaluate the level of plasmatic CD63+ and CAV1+ exosomes levels correlate with patient’s overall survival, they came to an conclusion that the lower expression of CD63+ exosomes after surgery and lower CAV1+ exosomes before surgery would indicate a longer life expectancy.
Exosomes as therapeutic mediums
In terms of exosomal structure, they consist of biogenic lipid bilayers similar to cell membranes, which could protect cargo from degradation and could deliver the drug to target cells. The surface diameter of exosomes is only 40–150 nm, so they are small enough to access most tissue without consumption and degradation by macrophages (De Jong et al., 2019; Kalluri & LeBleu, 2016; Surman et al., 2019; Vader et al., 2016; Yang & Wu, 2018). Moreover, they may exhibit inherent targeting properties, which is determined by lipid composition and protein content (Murphy et al., 2019). With these advantages, they are considered as potential drug delivery systems. At present, clinical trials using exosomes as a drug delivery method against cancer have been gradually increasing. In lung cancer, tumor cell-derived exosomes were extracted from the pleural effusion of lung cancer patients. After modification and loading with the chemotherapy drug methotrexate, they were reinjected into the patient’s chest cavity. It has been observed that exosomes have a safe inhibitory effect on the growth of tumor cells (Guo et al., 2019). In colon cancer, exosomes with carcinoembryonic antigen were isolated from ascites fluid. After combining them with granulocyte macrophage colony stimulating factor, they served as a vaccine to induce a beneficial tumor-specific antitumor cell toxic T lymphocyte response (Dai et al., 2008). In OSCC, the current drug-loading process is mainly based on different carrier systems, such as nanoparticles, nanolipids, and hydrogels, which can alleviate the disadvantage of poor water solubility for oral cancer anti-cancer drugs to a certain extent (Ketabat et al., 2019; Luo et al., 2014; Poonia et al., 2017). Studies have shown that exosomes have been used as carriers for chemotherapeutic agents such as curcumin, DOX, and PTX, thereby reducing their side effects and improving therapeutic efficiency (Batrakova & Kim, 2015; Sun et al., 2010; Tian et al., 2014). Despite this progress, research on exosomes as a drug-loading system is still limited, mainly due to their limited ability to deliver high-dose therapeutic drugs, insufficient basic experiments, and a lack of effective, standardized separation and purification methods (Ludwig, Whiteside & Reichert, 2019; Yang & Wu, 2018).
Conclusions
Researchers are continuing to provide new evidences on the importance of exosomes in cancer tumorigenesis, proliferation, migration, invasion, metastasis, and chemoresistance. Exosomal cargo based drug delivery system is also interesting in the treatment of OSCC because of its numerous type and abundance of cargo found in OSCC-related exosomes, which varies among different states of health and disease. What is more, because of their unique secretion patterns, exosomes can be used as ideal biomarkers for OSCC diagnosis, especially in saliva biopsy, which is a non-invasive method comparing to serum biopsy. With all these possibilities, however, there is still a lot not clearly understand in exosomes generation, secretion, cargo transportation, fusion with target membrane, especially the consistent test standard and separation method of exosomes has not been completely established. A more comprehensive understanding of the complexity of exosomes would help us elucidate disease mechanisms and provide opportunities for the diagnosis and treatment of OSCC. The safety of exosomes is still another question not solved yet. Further studies in this field, such as clinical study or studies using large animal models, are still needed to prove the safety of exosomes in treatment, especially for exosomes secreted from cancer cells. Despite the faced great challenges and difficulty, we still find exosomes’ great potential of usage in OSCC treatment and biopsy. We believe that exosomes can play even more important roles in this field.
Acknowledgments
We acknowledge Dr. Qiang Peng for his valuable suggestion.
Funding Statement
This work was supported by National Natural Science Foundation of China (No. 81700941), the Science and Technology Department of Sichuan Province (No.2020YFS0087) and Sichuan University-Luzhou City cooperation project (No.2018CDLZ-14). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Chengzhi Zhao and Geru Zhang conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, and approved the final draft.
Jialing Liu performed the experiments, prepared figures and/or tables, and approved the final draft.
Chenghao Zhang analyzed the data, prepared figures and/or tables, and approved the final draft.
Yang Yao and Wen Liao conceived and designed the experiments, authored or reviewed drafts of the paper, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
This is a literature review. There is no raw data.
References
- Abels & Breakefield (2016).Abels ER, Breakefield XO. Introduction to extracellular vesicles: biogenesis, RNA cargo selection, content, release, and uptake. Cellular and Molecular Neurobiology. 2016;36:301–312. doi: 10.1007/s10571-016-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello et al. (2018).Aiello NM, Maddipati R, Norgard RJ, Balli D, Li J, Yuan S, Yamazoe T, Black T, Sahmoud A, Furth EE, Bar-Sagi D, Stanger BZ. EMT subtype influences epithelial plasticity and mode of cell migration. Developmental Cell. 2018;45:681–695. doi: 10.1016/j.devcel.2018.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosio et al. (2019).Ambrosio MR, Vernillo R, De Carolis S, Carducci A, Mundo L, Ginori A, Rocca BJ, Nardone V, Lucenti Fei A, Carfagno T, Lazzi S, Cricca M, Tosi P. Putative role of circulating human papillomavirus DNA in the development of primary squamous cell carcinoma of the middle rectum: a case report. Frontiers in Oncology. 2019;9 doi: 10.3389/fonc.2019.00093. Article 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baglio et al. (2015).Baglio SR, Rooijers K, Koppers-Lalic D, Verweij FJ, Pérez Lanzón M, Zini N, Naaijkens B, Perut F, Niessen HW, Baldini N, Pegtel DM. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Research & Therapy. 2015;6 doi: 10.1186/s13287-015-0116-z. Article 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai et al. (2019).Bai H, Lei K, Huang F, Jiang Z, Zhou X. Exo-circRNAs: a new paradigm for anticancer therapy. Molecular Cancer. 2019;18:56. doi: 10.1186/s12943-019-0986-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batlle & Massagué (2019).Batlle E, Massagué J. Transforming growth factor- β signaling in immunity and cancer. Immunity. 2019;50:924–940. doi: 10.1016/j.immuni.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batrakova & Kim (2015).Batrakova EV, Kim MS. Using exosomes, naturally-equipped nanocarriers, for drug delivery. Journal of Controlled Release. 2015;219:396–405. doi: 10.1016/j.jconrel.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas et al. (2019).Biswas NK, Das C, Das S, Maitra A, Nair S, Gupta T, D’Cruz AK, Sarin R, Majumder P. Lymph node metastasis in oral cancer is strongly associated with chromosomal instability and DNA repair defects. International Journal of Cancer. 2019;145:2568–2579. doi: 10.1002/ijc.32305. [DOI] [PubMed] [Google Scholar]
- Borst et al. (2018).Borst J, Ahrends T, Bąbała N, Melief CJM, Kastenmüller W. CD4(+) T cell help in cancer immunology and immunotherapy. Nature Reviews Immunology. 2018;18:635–647. doi: 10.1038/s41577-018-0044-0. [DOI] [PubMed] [Google Scholar]
- Brinkmann et al. (2011).Brinkmann O, Kastratovic DA, Dimitrijevic MV, Konstantinovic VS, Jelovac DB, Antic J, Nesic VS, Markovic SZ, Martinovic ZR, Akin D, Spielmann N, Zhou H, Wong DT. Oral squamous cell carcinoma detection by salivary biomarkers in a Serbian population. Oral Oncology. 2011;47:51–55. doi: 10.1016/j.oraloncology.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byeon, Ku & Yang (2019).Byeon HK, Ku M, Yang J. Beyond EGFR inhibition: multilateral combat strategies to stop the progression of head and neck cancer. Experimental & Molecular Medicine. 2019;51:1–14. doi: 10.1038/s12276-018-0202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai et al. (2019).Cai J, Qiao B, Gao N, Lin N, He W. Oral squamous cell carcinoma-derived exosomes promote M2 subtype macrophage polarization mediated by exosome-enclosed miR-29a-3p. American Journal of Physiology. Cell Physiology. 2019;316:C731–C740. doi: 10.1152/ajpcell.00366.2018. [DOI] [PubMed] [Google Scholar]
- Chan & Tay (2018).Chan JJ, Tay Y. Noncoding RNA:RNA regulatory networks in cancer. International Journal of Molecular Sciences. 2018;19:1310. doi: 10.3390/ijms19051310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2018).Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, Yu Z, Yang J, Wang B, Sun H, Xia H, Man Q, Zhong W, Antelo LF, Wu B, Xiong X, Liu X, Guan L, Li T, Liu S, Yang R, Lu Y, Dong L, McGettigan S, Somasundaram R, Radhakrishnan R, Mills G, Lu Y, Kim J, Chen YH, Dong H, Zhao Y, Karakousis GC, Mitchell TC, Schuchter LM, Herlyn M, Wherry EJ, Xu X, Guo W. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560:382–386. doi: 10.1038/s41586-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2019).Chen JH, Wu ATH, Bamodu OA, Yadav VK, Chao TY, Tzeng YM, Mukhopadhyay D, Hsiao M, Lee JC. Ovatodiolide suppresses oral cancer malignancy by down-regulating exosomal Mir-21/STAT3/β-catenin cargo and preventing oncogenic transformation of normal gingival fibroblasts. Cancer. 2019;12 doi: 10.3390/cancers12010056. Article 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi, Day & Neville (2015).Chi AC, Day TA, Neville BW. Oral cavity and oropharyngeal squamous cell carcinoma–an update. CA: A Cancer Journal for Clinicians. 2015;65:401–421. doi: 10.3322/caac.21293. [DOI] [PubMed] [Google Scholar]
- Crescitelli et al. (2013).Crescitelli R, Lasser C, Szabo TG, Kittel A, Eldh M, Dianzani I, Buzas EI, Lotvall J. Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies microvesicles and exosomes. Journal of Extracellular Vesicles. 2013;2 doi: 10.3402/jev.v2i0.20677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui et al. (2020).Cui J, Wang H, Zhang X, Sun X, Zhang J, Ma J. Exosomal miR-200c suppresses chemoresistance of docetaxel in tongue squamous cell carcinoma by suppressing TUBB3 and PPP2R1B. Aging. 2020;12:6756–6773. doi: 10.18632/aging.103036. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Dai et al. (2008).Dai S, Wei D, Wu Z, Zhou X, Wei X, Huang H, Li G. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Molecular Therapy. 2008;16:782–790. doi: 10.1038/mt.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Re et al. (2018).Del Re M, Marconcini R, Pasquini G, Rofi E, Vivaldi C, Bloise F, Restante G, Arrigoni E, Caparello C, Bianco MG, Crucitta S, Petrini I, Vasile E, Falcone A, Danesi R. PD-L1 mRNA expression in plasma-derived exosomes is associated with response to anti-PD-1 antibodies in melanoma and NSCLC. British Journal of Cancer. 2018;118:820–824. doi: 10.1038/bjc.2018.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRita et al. (2017).DeRita RM, Zerlanko B, Singh A, Lu H, Iozzo RV, Benovic JL, Languino LR. c-Src, insulin-like growth factor I receptor, G-protein-coupled receptor kinases and focal adhesion kinase are enriched into prostate cancer cell exosomes. Journal of Cellular Biochemistry. 2017;118:66–73. doi: 10.1002/jcb.25611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong et al. (2019).De Jong OG, Kooijmans SAA, Murphy DE, Jiang L, Evers MJW, Sluijter JPG, Vader P, Schiffelers RM. Drug delivery with extracellular vesicles: from imagination to innovation. Accounts of Chemical Research. 2019;52:1761–1770. doi: 10.1021/acs.accounts.9b00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond et al. (2018).Diamond JM, Vanpouille-Box C, Spada S, Rudqvist NP, Chapman JR, Ueberheide BM, Pilones KA, Sarfraz Y, Formenti SC, Demaria S. Exosomes shuttle TREX1-sensitive IFN-stimulatory dsDNA from irradiated cancer cells to DCs. Cancer Immunology Research. 2018;6:910–920. doi: 10.1158/2326-6066.Cir-17-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickman et al. (2017).Dickman CT, Lawson J, Jabalee J, MacLellan SA, LePard NE, Bennewith KL, Garnis C. Selective extracellular vesicle exclusion of miR-142-3p by oral cancer cells promotes both internal and extracellular malignant phenotypes. Oncotarget. 2017;8:15252–15266. doi: 10.18632/oncotarget.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding et al. (2018).Ding L, Ren J, Zhang D, Li Y, Huang X, Hu Q, Wang H, Song Y, Ni Y, Hou Y. A novel stromal lncRNA signature reprograms fibroblasts to promote the growth of oral squamous cell carcinoma via LncRNA-CAF/interleukin-33. Carcinogenesis. 2018;39:397–406. doi: 10.1093/carcin/bgy006. [DOI] [PubMed] [Google Scholar]
- Esser et al. (2010).Esser J, Gehrmann U, D’Alexandri FL, Hidalgo-Estévez AM, Wheelock CE, Scheynius A, Gabrielsson S, Rådmark O. Exosomes from human macrophages and dendritic cells contain enzymes for leukotriene biosynthesis and promote granulocyte migration. Journal of Allergy and Clinical Immunology. 2010;126(5):1032–1040. doi: 10.1016/j.jaci.2010.06.039. [DOI] [PubMed] [Google Scholar]
- Falguières, Castle & Gruenberg (2012).Falguières T, Castle D, Gruenberg J. Regulation of the MVB pathway by SCAMP3. Traffic. 2012;13:131–142. doi: 10.1111/j.1600-0854.2011.01291.x. [DOI] [PubMed] [Google Scholar]
- Feng et al. (2018).Feng Y, Lv LL, Wu WJ, Li ZL, Chen J, Ni HF, Zhou LT, Tang TT, Wang FM, Wang B, Chen PS, Crowley SD, Liu BC. Urinary exosomes and exosomal CCL2 mRNA as biomarkers of active histologic injury in IgA nephropathy. American Journal of Pathology. 2018;188:2542–2552. doi: 10.1016/j.ajpath.2018.07.017. [DOI] [PubMed] [Google Scholar]
- Ferguson & Nguyen (2016).Ferguson SW, Nguyen J. Exosomes as therapeutics: the implications of molecular composition and exosomal heterogeneity. Journal of Controlled Release. 2016;228:179–190. doi: 10.1016/j.jconrel.2016.02.037. [DOI] [PubMed] [Google Scholar]
- Fu et al. (2019).Fu M, Gu J, Jiang P, Qian H, Xu W, Zhang X. Exosomes in gastric cancer: roles, mechanisms, and applications. Molecular Cancer. 2019;18 doi: 10.1186/s12943-019-1001-7. Article 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara et al. (2018).Fujiwara T, Eguchi T, Sogawa C, Ono K, Murakami J, Ibaragi S, Asaumi JI, Calderwood SK, Okamoto K, Kozaki KI. Carcinogenic epithelial-mesenchymal transition initiated by oral cancer exosomes is inhibited by anti-EGFR antibody cetuximab. Oral Oncology. 2018;86:251–257. doi: 10.1016/j.oraloncology.2018.09.030. [DOI] [PubMed] [Google Scholar]
- Gardner & Ruffell (2016).Gardner A, Ruffell B. Dendritic cells and cancer immunity. Trends in Immunology. 2016;37:855–865. doi: 10.1016/j.it.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto, Schulze & Nielsen (2016).Gatto F, Schulze A, Nielsen J. Systematic analysis reveals that cancer mutations converge on deregulated metabolism of arachidonate and xenobiotics. Cell Reports. 2016;16:878–895. doi: 10.1016/j.celrep.2016.06.038. [DOI] [PubMed] [Google Scholar]
- Geis-Asteggiante et al. (2018).Geis-Asteggiante L, Belew AT, Clements VK, Edwards NJ, Ostrand-Rosenberg S, El-Sayed NM, Fenselau C. Differential content of proteins, mRNAs, and miRNAs suggests that MDSC and their exosomes may mediate distinct immune suppressive functions. Journal of Proteome Research. 2018;17:486–498. doi: 10.1021/acs.jproteome.7b00646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez, Robles & Werb (2018).Gonzalez H, Robles I, Werb Z. Innate and acquired immune surveillance in the postdissemination phase of metastasis. FEBS Journal. 2018;285:654–664. doi: 10.1111/febs.14325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant & Donaldson (2009).Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nature Reviews Molecular Cell Biology. 2009;10:597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenat et al. (2017).Guenat D, Hermetet F, Prétet J-L, Mougin C. Exosomes and other extracellular vesicles in HPV transmission and carcinogenesis. Viruses. 2017;9:211. doi: 10.3390/v9080211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo et al. (2019).Guo M, Wu F, Hu G, Chen L, Xu J, Xu P, Wang X, Li Y, Liu S, Zhang S, Huang Q, Fan J, Lv Z, Zhou M, Duan L, Liao T, Yang G, Tang K, Liu B, Liao X, Tao X, Jin Y. Autologous tumor cell-derived microparticle-based targeted chemotherapy in lung cancer patients with malignant pleural effusion. Science Translational Medicine. 2019;11:eaat5690. doi: 10.1126/scitranslmed.aat5690. [DOI] [PubMed] [Google Scholar]
- Gupta, Johnson & Kumar (2016).Gupta B, Johnson NW, Kumar N. Global epidemiology of head and neck cancers: a continuing challenge. Oncology. 2016;91:13–23. doi: 10.1159/000446117. [DOI] [PubMed] [Google Scholar]
- Gyorgy et al. (2011).Gyorgy B, Szabo TG, Pasztoi M, Pal Z, Misjak P, Aradi B, Laszlo V, Pallinger E, Pap E, Kittel A, Nagy G, Falus A, Buzas EI. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cellular and Molecular Life Science. 2011;68:2667–2688. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadavand & Hasni (2019).Hadavand M, Hasni S. Exosomal biomarkers in oral diseases. Oral Diseases. 2019;25:10–15. doi: 10.1111/odi.12878. [DOI] [PubMed] [Google Scholar]
- Han, Cheng & Li (2020).Han L, Cheng J, Li A. hsa_circ_0072387 suppresses proliferation, metastasis, and glycolysis of oral squamous cell carcinoma cells by downregulating miR–503-5p. Cancer Biother Radiopharm. 2020;50:3–5. doi: 10.1089/cbr.2019.3371. [DOI] [PubMed] [Google Scholar]
- Hao et al. (2017).Hao YX, Li YM, Ye M, Guo YY, Li QW, Peng XM, Wang Q, Zhang SF, Zhao HX, Zhang H, Li GH, Zhu JH, Xiao WH. KRAS and BRAF mutations in serum exosomes from patients with colorectal cancer in a Chinese population. Oncology Letters. 2017;13:3608–3616. doi: 10.3892/ol.2017.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada et al. (2017).Harada T, Yamamoto H, Kishida S, Kishida M, Awada C, Takao T, Kikuchi A. Wnt5b-associated exosomes promote cancer cell migration and proliferation. Cancer Science. 2017;108:42–52. doi: 10.1111/cas.13109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraszti et al. (2016).Haraszti RA, Didiot MC, Sapp E, Leszyk J, Shaffer SA, Rockwell HE, Gao F, Narain NR, DiFiglia M, Kiebish MA, Aronin N, Khvorova A. High-resolution proteomic and lipidomic analysis of exosomes and microvesicles from different cell sources. Journal of Extracellular Vesicles. 2016;5 doi: 10.3402/jev.v5.32570. Article 32570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He et al. (2019).He T, Li X, Xie D, Tian L. Overexpressed circPVT1 in oral squamous cell carcinoma promotes proliferation by serving as a miRNA sponge. Molecular Medicine Reports. 2019;20:3509–3518. doi: 10.3892/mmr.2019.10615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He et al. (2020).He L, Ping F, Fan Z, Zhang C, Deng M, Cheng B, Xia J. Salivary exosomal miR 24-3p serves as a potential detective biomarker for oral squamous cell carcinoma screening. Biomedicine and Pharmacotherapy. 2020;121 doi: 10.1016/j.biopha.2019.109553. Article 109553. [DOI] [PubMed] [Google Scholar]
- Hemler (2003).Hemler ME. Tetraspanin proteins mediate cellular penetration, invasion, and fusion events and define a novel type of membrane microdomain. Annual Review of Cell and Developmental Biology. 2003;19:397–422. doi: 10.1146/annurev.cellbio.19.111301.153609. [DOI] [PubMed] [Google Scholar]
- Hessvik & Llorente (2018).Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cellular and Molecular Life Science. 2018;75:193–208. doi: 10.1007/s00018-017-2595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higaki et al. (2020).Higaki M, Shintani T, Hamada A, Rosli SNZ, Okamoto T. Eldecalcitol (ED-71)-induced exosomal miR-6887-5p suppresses squamous cell carcinoma cell growth by targeting heparin-binding protein 17/fibroblast growth factor-binding protein-1 (HBp17/FGFBP-1) In Vitro Cellular & Developmental Biology-Animal. 2020;56:222–233. doi: 10.1007/s11626-020-00440-x. [DOI] [PubMed] [Google Scholar]
- Hoshino et al. (2015).Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, Zhang H, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, Labori KJ, Kure EH, Grandgenett PM, Hollingsworth MA, De Sousa M, Kaur S, Jain M, Mallya K, Batra SK, Jarnagin WR, Brady MS, Fodstad O, Muller V, Pantel K, Minn AJ, Bissell MJ, Garcia BA, Kang Y, Rajasekhar VK, Ghajar CM, Matei I, Peinado H, Bromberg J, Lyden D. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu et al. (2019).Hu Q, Peng J, Chen X, Li H, Song M, Cheng B, Wu T. Obesity and genes related to lipid metabolism predict poor survival in oral squamous cell carcinoma. Oral Oncology. 2019;89:14–22. doi: 10.1016/j.oraloncology.2018.12.006. [DOI] [PubMed] [Google Scholar]
- Huang et al. (2013).Huang X, Yuan T, Tschannen M, Sun Z, Jacob H, Du M, Liang M, Dittmar RL, Liu Y, Liang M, Kohli M, Thibodeau SN, Boardman L, Wang L. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genomics. 2013;14:319. doi: 10.1186/1471-2164-14-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huotari & Helenius (2011).Huotari J, Helenius A. Endosome maturation. EMBO Journal. 2011;30:3481–3500. doi: 10.1038/emboj.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang et al. (2019).Jiang E, Xu Z, Wang M, Yan T, Huang C, Zhou X, Liu Q, Wang L, Chen Y, Wang H, Liu K, Shao Z, Shang Z. Tumoral microvesicle-activated glycometabolic reprogramming in fibroblasts promotes the progression of oral squamous cell carcinoma. Faseb Journal. 2019;33:5690–5703. doi: 10.1096/fj.201802226R. [DOI] [PubMed] [Google Scholar]
- Jin et al. (2020).Jin N, Jin N, Bu W, Li X, Liu L, Wang Z, Tong J, Li D. Long non-coding RNA TIRY promotes tumor metastasis by enhancing epithelial-to-mesenchymal transition in oral cancer. Experimental Biology and Medicine. 2020;245:585–596. doi: 10.1177/1535370220903673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadota et al. (2016).Kadota T, Fujita Y, Yoshioka Y, Araya J, Kuwano K, Ochiya T. Extracellular vesicles in chronic obstructive pulmonary disease. International Journal of Molecular Sciences. 2016;17:1801. doi: 10.3390/ijms17111801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlert et al. (2014).Kahlert C, Melo SA, Protopopov A, Tang J, Seth S, Koch M, Zhang J, Weitz J, Chin L, Futreal A, Kalluri R. Identification of double-stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. Journal of Biological Chemistry. 2014;289:3869–3875. doi: 10.1074/jbc.C113.532267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri & LeBleu (2016).Kalluri R, LeBleu VS. Discovery of double-stranded genomic DNA in circulating exosomes. Cold Spring Harbor Symposia on Quantitative Biology. 2016;81:275–280. doi: 10.1101/sqb.2016.81.030932. [DOI] [PubMed] [Google Scholar]
- Kawakubo-Yasukochi et al. (2018).Kawakubo-Yasukochi T, Morioka M, Hazekawa M, Yasukochi A, Nishinakagawa T, Ono K, Kawano S, Nakamura S, Nakashima M. miR-200c-3p spreads invasive capacity in human oral squamous cell carcinoma microenvironment. Molecular Carcinogenesis. 2018;57:295–302. doi: 10.1002/mc.22744. [DOI] [PubMed] [Google Scholar]
- Ketabat et al. (2019).Ketabat F, Pundir M, Mohabatpour F, Lobanova L, Koutsopoulos S, Hadjiiski L, Chen X, Papagerakis P, Papagerakis S. Controlled drug delivery systems for oral cancer treatment-current status and future perspectives. Pharmaceutics. 2019;11:302. doi: 10.3390/pharmaceutics11070302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim et al. (2005).Kim JW, Wieckowski E, Taylor DD, Reichert TE, Watkins S, Whiteside TL. Fas ligand-positive membranous vesicles isolated from sera of patients with oral cancer induce apoptosis of activated T lymphocytes. Clinical Cancer Research. 2005;11:1010–1020. [PubMed] [Google Scholar]
- Kirave et al. (2020).Kirave P, Gondaliya P, Kulkarni B, Rawal R, Garg R, Jain A, Kalia K. Exosome mediated miR-155 delivery confers cisplatin chemoresistance in oral cancer cells via epithelial-mesenchymal transition. Oncotarget. 2020;11:1157–1171. doi: 10.18632/oncotarget.27531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitai et al. (2017).Kitai Y, Kawasaki T, Sueyoshi T, Kobiyama K, Ishii KJ, Zou J, Akira S, Matsuda T, Kawai T. DNA-containing exosomes derived from cancer cells treated with topotecan activate a STING-dependent pathway and reinforce antitumor immunity. Journal of Immunology. 2017;198:1649–1659. doi: 10.4049/jimmunol.1601694. [DOI] [PubMed] [Google Scholar]
- Kulkarni et al. (2019).Kulkarni B, Kirave P, Gondaliya P, Jash K, Jain A, Tekade RK, Kalia K. Exosomal miRNA in chemoresistance, immune evasion, metastasis and progression of cancer. Drug Discovery Today. 2019;24:2058–2067. doi: 10.1016/j.drudis.2019.06.010. [DOI] [PubMed] [Google Scholar]
- Lai et al. (2018).Lai YH, Liu H, Chiang WF, Chen TW, Chu LJ, Yu JS, Chen SJ, Chen HC, Tan BC. MiR-31-5p-ACOX1 axis enhances tumorigenic fitness in oral squamous cell carcinoma via the promigratory prostaglandin E2. Theranostics. 2018;8:486–504. doi: 10.7150/thno.22059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langevin et al. (2017).Langevin S, Kuhnell D, Parry T, Biesiada J, Huang S, Wise-Draper T, Casper K, Zhang X, Medvedovic M, Kasper S. Comprehensive microRNA-sequencing of exosomes derived from head and neck carcinoma cells in vitro reveals common secretion profiles and potential utility as salivary biomarkers. Oncotarget. 2017;8:82459–82474. doi: 10.18632/oncotarget.19614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Languino et al. (2016).Languino LR, Singh A, Prisco M, Inman GJ, Luginbuhl A, Curry JM, South AP. Exosome-mediated transfer from the tumor microenvironment increases TGFβ signaling in squamous cell carcinoma. American Journal of Translational Research. 2016;8:2432–2437. [PMC free article] [PubMed] [Google Scholar]
- Lázaro-Ibáñez et al. (2017).Lázaro-Ibáñez E, Lunavat TR, Jang SC, Escobedo-Lucea C, Oliver-De La Cruz J, Siljander P, Lötvall J, Yliperttula M. Distinct prostate cancer-related mRNA cargo in extracellular vesicle subsets from prostate cell lines. BMC Cancer. 2017;17:92. doi: 10.1186/s12885-017-3087-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2019b).Li W, Han Y, Zhao Z, Ji X, Wang X, Jin J, Wang Q, Guo X, Cheng Z, Lu M, Wang G, Wang Y, Liu H. Oral mucosal mesenchymal stem cellderived exosomes: a potential therapeutic target in oral premalignant lesions. International Journal of Oncology. 2019b;54:1567–1578. doi: 10.3892/ijo.2019.4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2017a).Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in exosome isolation techniques. Theranostics. 2017a;7:789–804. doi: 10.7150/thno.18133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2018b).Li X, Lei Y, Wu M, Li N. Regulation of macrophage activation and polarization by HCC-derived exosomal lncRNA TUC339. International Journal of Molecular Sciences. 2018b;19:2958. doi: 10.3390/ijms19102958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2018a).Li J, Li Z, Jiang P, Peng M, Zhang X, Chen K, Liu H, Bi H, Liu X, Li X. Circular RNA IARS (circ-IARS) secreted by pancreatic cancer cells and located within exosomes regulates endothelial monolayer permeability to promote tumor metastasis. Journal of Experimental & Clinical Cancer Research. 2018a;37 doi: 10.1186/s13046-018-0822-3. Article 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2016).Li L, Li C, Wang S, Wang Z, Jiang J, Wang W, Li X, Chen J, Liu K, Li C, Zhu G. Exosomes derived from hypoxic oral squamous cell carcinoma cells deliver miR-21 to normoxic cells to elicit a prometastatic phenotype. Cancer Research. 2016;76:1770–1780. doi: 10.1158/0008-5472.can-15-1625. [DOI] [PubMed] [Google Scholar]
- Li et al. (2017b).Li W, Li C, Zhou T, Liu X, Liu X, Li X, Chen D. Role of exosomal proteins in cancer diagnosis. Molecular Cancer. 2017b;16:145. doi: 10.1186/s12943-017-0706-8. Article 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Liu & Cheng (2019).Li LM, Liu ZX, Cheng QY. Exosome plays an important role in the development of hepatocellular carcinoma. Pathology, Research and Practice. 2019;215 doi: 10.1016/j.prp.2019.152468. Article 152468. [DOI] [PubMed] [Google Scholar]
- Li et al. (2018c).Li YY, Tao YW, Gao S, Li P, Zheng JM, Zhang SE, Liang J, Zhang Y. Cancer-associated fibroblasts contribute to oral cancer cells proliferation and metastasis via exosome-mediated paracrine miR-34a-5p. EBioMedicine. 2018c;36:209–220. doi: 10.1016/j.ebiom.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2019a).Li C, Zhou Y, Liu J, Su X, Qin H, Huang S, Huang X, Zhou N. Potential markers from serum-purified exosomes for detecting oral squamous cell carcinoma metastasis. Cancer Epidemiology, Biomarkers & Prevention. 2019a;28:1668–1681. doi: 10.1158/1055-9965.EPI-18-1122. [DOI] [PubMed] [Google Scholar]
- Liao et al. (2019).Liao W, Du Y, Zhang C, Pan F, Yao Y, Zhang T, Peng Q. Exosomes: the next generation of endogenous nanomaterials for advanced drug delivery and therapy. Acta Biomater. 2019;86:1–14. doi: 10.1016/j.actbio.2018.12.045. [DOI] [PubMed] [Google Scholar]
- Lien et al. (2018).Lien MY, Tsai HC, Chang AC, Tsai MH, Hua CH, Wang SW, Tang CH. Chemokine CCL4 induces vascular endothelial growth factor C expression and lymphangiogenesis by miR-195-3p in oral squamous cell carcinoma. Front Immunol. 2018;9 doi: 10.3389/fimmu.2018.00412. Article 412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin et al. (2020).Lin K, Song LJ, Ma J, Zhang TS, You DY, He YW. Identification of cancer hallmark-associated gene and lncRNA cooperative regulation pairs and dictate lncRNA roles in oral squamous cell carcinoma. Journal of Cellular and Molecular Medicine. 2020;24:5213–5223. doi: 10.1111/jcmm.15174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton et al. (2018).Linton SS, Abraham T, Liao J, Clawson GA, Butler PJ, Fox T, Kester M, Matters GL. Tumor-promoting effects of pancreatic cancer cell exosomes on THP-1-derived macrophages. PLOS ONE. 2018;13:e0206759. doi: 10.1371/journal.pone.0206759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. (2017).Liu T, Chen G, Sun D, Lei M, Li Y, Zhou C, Li X, Xue W, Wang H, Liu C, Xu J. Exosomes containing miR-21 transfer the characteristic of cisplatin resistance by targeting PTEN and PDCD4 in oral squamous cell carcinoma. Acta Biochimica et Biophysica Sinica. 2017;49:808–816. doi: 10.1093/abbs/gmx078. [DOI] [PubMed] [Google Scholar]
- Llorente et al. (2013).Llorente A, Skotland T, Sylvanne T, Kauhanen D, Rog T, Orlowski A, Vattulainen I, Ekroos K, Sandvig K. Molecular lipidomics of exosomes released by PC-3 prostate cancer cells. Biochimica et Biophysica Acta/General Subjects. 2013;1831:1302–1309. doi: 10.1016/j.bbalip.2013.04.011. [DOI] [PubMed] [Google Scholar]
- Ludwig, Whiteside & Reichert (2019).Ludwig N, Whiteside TL, Reichert TE. Challenges in exosome isolation and analysis in health and disease. International Journal of Molecular Sciences. 2019;20:4684. doi: 10.3390/ijms20194684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig et al. (2018).Ludwig N, Yerneni SS, Razzo BM, Whiteside TL. Exosomes from HNSCC promote angiogenesis through reprogramming of endothelial cells. Molecular Cancer Research. 2018;16:1798–1808. doi: 10.1158/1541-7786.Mcr-18-0358. [DOI] [PubMed] [Google Scholar]
- Luga et al. (2012).Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E, Buchanan M, Hosein AN, Basik M, Wrana JL. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell. 2012;151:1542–1556. doi: 10.1016/j.cell.2012.11.024. [DOI] [PubMed] [Google Scholar]
- Lukic et al. (2019).Lukic A, Wahlund CJE, Gómez C, Brodin D, Samuelsson B, Wheelock CE, Gabrielsson S, Rådmark O. Exosomes and cells from lung cancer pleural exudates transform LTC4 to LTD4, promoting cell migration and survival via CysLT1. Cancer Letters. 2019;444:1–8. doi: 10.1016/j.canlet.2018.11.033. [DOI] [PubMed] [Google Scholar]
- Luo et al. (2014).Luo C, Sun J, Du Y, He Z. Emerging integrated nanohybrid drug delivery systems to facilitate the intravenous-to-oral switch in cancer chemotherapy. Journal of Controlled Release. 2014;176:94–103. doi: 10.1016/j.jconrel.2013.12.030. [DOI] [PubMed] [Google Scholar]
- Malm, Loppi & Kanninen (2016).Malm T, Loppi S, Kanninen KM. Exosomes in Alzheimer’s disease. Neurochemistry International. 2016;97:193–199. doi: 10.1016/j.neuint.2016.04.011. [DOI] [PubMed] [Google Scholar]
- Mantovani et al. (2017).Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nature Reviews Clinical Oncology. 2017;14:399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashouri et al. (2019).Mashouri L, Yousefi H, Aref AR, Ahadi AM, Molaei F, Alahari SK. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Molecular Cancer. 2019;18 doi: 10.1186/s12943-019-0991-5. Article 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata-Rocha et al. (2020).Mata-Rocha M, Rodriguez-Hernandez RM, Chavez-Olmos P, Garrido E, Robles-Vazquez C, Aguilar-Ruiz S, Torres-Aguilar H, Gonzalez-Torres C, Gaytan-Cervantes J, Mejia-Arangure JM, Romero-Tlalolini MLA. Presence of HPV DNA in extracellular vesicles from HeLa cells and cervical samples. Enfermedades Infecciosas Y Microbiologia Clinica. 2020;38:159–165. doi: 10.1016/j.eimc.2019.06.011. [DOI] [PubMed] [Google Scholar]
- Matei, Kim & Lyden (2017).Matei I, Kim HS, Lyden D. Unshielding exosomal RNA unleashes tumor growth and metastasis. Cell. 2017;170:223–225. doi: 10.1016/j.cell.2017.06.047. [DOI] [PubMed] [Google Scholar]
- Mathieu et al. (2019).Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nature Cell Biology. 2019;21:9–17. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- McNally & Brett (2018).McNally EK, Brett CL. The intralumenal fragment pathway mediates ESCRT-independent surface transporter down-regulation. Nature Communications. 2018;9 doi: 10.1038/s41467-018-07734-5. Article 5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meckes Jr et al. (2010).Meckes Jr DG, Shair KH, Marquitz AR, Kung CP, Edwards RH, Raab-Traub N. Human tumor virus utilizes exosomes for intercellular communication. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:20370–20375. doi: 10.1073/pnas.1014194107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo et al. (2015).Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari N, Reissfelder C, Pilarsky C, Fraga MF, Piwnica-Worms D, Kalluri R. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523:177–182. doi: 10.1038/nature14581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki et al. (2018).Miki Y, Yashiro M, Okuno T, Kuroda K, Togano S, Hirakawa K, Ohira M. Clinico-pathological significance of exosome marker CD63 expression on cancer cells and stromal cells in gastric cancer. PLOS ONE. 2018;13:e0202956. doi: 10.1371/journal.pone.0202956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milman, Ginini & Gil (2019).Milman N, Ginini L, Gil Z. Exosomes and their role in tumorigenesis and anticancer drug resistance. Drug Resistance Updates. 2019;45:1–12. doi: 10.1016/j.drup.2019.07.003. [DOI] [PubMed] [Google Scholar]
- Momen-Heravi & Bala (2018).Momen-Heravi F, Bala S. Extracellular vesicles in oral squamous carcinoma carry oncogenic miRNA profile and reprogram monocytes via NF-kappaB pathway. Oncotarget. 2018;9:34838–34854. doi: 10.18632/oncotarget.26208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli et al. (2004).Morelli AE, Larregina AT, Shufesky WJ, Sullivan ML, Stolz DB, Papworth GD, Zahorchak AF, Logar AJ, Wang Z, Watkins SC, Falo Jr LD, Thomson AW. Endocytosis intracellular sorting, and processing of exosomes by dendritic cells. Blood. 2004;104:3257–3266. doi: 10.1182/blood-2004-03-0824. [DOI] [PubMed] [Google Scholar]
- Morris & Mattick (2014).Morris KV, Mattick JS. The rise of regulatory RNA. Nature Reviews Genetics. 2014;15:423–437. doi: 10.1038/nrg3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy et al. (2019).Murphy DE, De Jong OG, Brouwer M, Wood MJ, Lavieu G, Schiffelers RM, Vader P. Extracellular vesicle-based therapeutics: natural versus engineered targeting and trafficking. Experimental & Molecular Medicine. 2019;51:1–12. doi: 10.1038/s12276-019-0223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura et al. (2019).Nakamura H, Tamaki S, Yagyuu T, Yamakawa N, Hatake K, Kirita T. Relationship between egfr expression in oral cancer cell lines and cetuximab antibody-dependent cell-mediated cytotoxicity. Anticancer Research. 2019;39:1275–1282. doi: 10.21873/anticanres.13238. [DOI] [PubMed] [Google Scholar]
- Nasry, Rodriguez-Lecompte & Martin (2018).Nasry WHS, Rodriguez-Lecompte JC, Martin CK. Role of COX-2/PGE2 mediated inflammation in oral squamous cell carcinoma. Cancers. 2018;10 doi: 10.3390/cancers10100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natsuizaka et al. (2017).Natsuizaka M, Whelan KA, Kagawa S, Tanaka K, Giroux V, Chandramouleeswaran PM, Long A, Sahu V, Darling DS, Que J, Yang Y, Katz JP, Wileyto EP, Basu D, Kita Y, Natsugoe S, Naganuma S, Klein-Szanto AJ, Diehl JA, Bass AJ, Wong KK, Rustgi AK, Nakagawa H. Interplay between Notch1 and Notch3 promotes EMT and tumor initiation in squamous cell carcinoma. Nature Communications. 2017;8 doi: 10.1038/s41467-017-01500-9. Article 1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira-Silva et al. (2016).Oliveira-Silva RJ, Carolina de Carvalho A, De Souza Viana L, Carvalho AL, Reis RM. Anti-EGFR Therapy: strategies in head and neck squamous cell carcinoma. Recent Patents on Anti-Cancer Drug Discovery. 2016;11:170–183. doi: 10.2174/1574892811666160309121238. [DOI] [PubMed] [Google Scholar]
- Ono et al. (2018).Ono K, Eguchi T, Sogawa C, Calderwood SK, Futagawa J, Kasai T, Seno M, Okamoto K, Sasaki A, Kozaki K-I. HSP-enriched properties of extracellular vesicles involve survival of metastatic oral cancer cells. Journal of Cellular Biochemistry. 2018;119:7350–7362. doi: 10.1002/jcb.27039. [DOI] [PubMed] [Google Scholar]
- Overmiller et al. (2017).Overmiller AM, Pierluissi JA, Wermuth PJ, Sauma S, Martinez-Outschoorn U, Tuluc M, Luginbuhl A, Curry J, Harshyne LA, Wahl 3rd JK, South AP, Mahoney MG. Desmoglein 2 modulates extracellular vesicle release from squamous cell carcinoma keratinocytes. Faseb Journal. 2017;31:3412–3424. doi: 10.1096/fj.201601138R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakravan et al. (2017).Pakravan K, Babashah S, Sadeghizadeh M, Mowla SJ, Mossahebi-Mohammadi M, Ataei F, Dana N, Javan M. MicroRNA-100 shuttled by mesenchymal stem cell-derived exosomes suppresses in vitro angiogenesis through modulating the mTOR/HIF-1alpha/VEGF signaling axis in breast cancer cells. Cellular Oncology. 2017;40:457–470. doi: 10.1007/s13402-017-0335-7. [DOI] [PubMed] [Google Scholar]
- Pascual et al. (2017).Pascual G, Avgustinova A, Mejetta S, Martin M, Castellanos A, Attolini CS, Berenguer A, Prats N, Toll A, Hueto JA, Bescos C, Di Croce L, Benitah SA. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature. 2017;541:41–45. doi: 10.1038/nature20791. [DOI] [PubMed] [Google Scholar]
- Pasquier et al. (2013).Pasquier J, Guerrouahen BS, Thawadi HAl, Ghiabi P, Maleki M, Abu-Kaoud N, Jacob A, Mirshahi M, Galas L, Rafii S, Le Foll F, Rafii A. Preferential transfer of mitochondria from endothelial to cancer cells through tunneling nanotubes modulates chemoresistance. Journal of Translational Medicine. 2013;11 doi: 10.1186/1479-5876-11-94. Article 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poonia et al. (2017).Poonia M, Ramalingam K, Goyal S, Sidhu SK. Nanotechnology in oral cancer: a comprehensive review. Journal of Oral and Maxillofacial Pathology. 2017;21:407–414. doi: 10.4103/jomfp.JOMFP_29_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Principe et al. (2018).Principe S, Mejia-Guerrero S, Ignatchenko V, Sinha A, Ignatchenko A, Shi W, Pereira K, Su S, Huang SH, O’Sullivan B, Xu W, Goldstein DP, Weinreb I, Ailles L, Liu FF, Kislinger T. Proteomic analysis of cancer-associated fibroblasts reveals a paracrine role for MFAP5 in human oral tongue squamous cell carcinoma. Journal of Proteome Research. 2018;17:2045–2059. doi: 10.1021/acs.jproteome.7b00925. [DOI] [PubMed] [Google Scholar]
- Qadir et al. (2018).Qadir F, Aziz MA, Sari CP, Ma H, Dai H, Wang X, Raithatha D, Da Silva LGL, Hussain M, Poorkasreiy SP, Hutchison IL, Waseem A, Teh MT. Transcriptome reprogramming by cancer exosomes: identification of novel molecular targets in matrix and immune modulation. Molecular Cancer. 2018;17 doi: 10.1186/s12943-018-0846-5. Article 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin et al. (2019).Qin X, Guo H, Wang X, Zhu X, Yan M, Wang X, Xu Q, Shi J, Lu E, Chen W, Zhang J. Exosomal miR-196a derived from cancer-associated fibroblasts confers cisplatin resistance in head and neck cancer through targeting CDKN1B and ING5. Genome Biology. 2019;20 doi: 10.1186/s13059-018-1604-0. Article 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu et al. (2019).Qiu X, Ke X, Ma H, Han L, Chen Q, Zhang S, Da P, Wu H. Profiling and bioinformatics analyses reveal differential expression of circular RNA in tongue cancer revealed by high-throughput sequencing. Journal of Cellular Biochemistry. 2019;120:4102–4112. doi: 10.1002/jcb.27695. [DOI] [PubMed] [Google Scholar]
- Rabinowits et al. (2017).Rabinowits G, Bowden M, Flores LM, Verselis S, Vergara V, Jo VY, Chau N, Lorch J, Hammerman PS, Thomas T, Goguen LA, Annino D, Schoenfeld JD, Margalit DN, Tishler RB, Haddad RI. Comparative analysis of micro RNA expression among benign and malignant tongue tissue and plasma of patients with tongue cancer. Frontiers in Oncology. 2017;7 doi: 10.3389/fonc.2017.00191. Article 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzo et al. (2019).Razzo BM, Ludwig N, Hong CS, Sharma P, Fabian KP, Fecek RJ, Storkus WJ, Whiteside TL. Tumor-derived exosomes promote carcinogenesis of murine oral squamous cell carcinoma. Carcinogenesis. 2019 doi: 10.1093/carcin/bgz124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Record et al. (2014).Record M, Carayon K, Poirot M, Silvente-Poirot S. Exosomes as new vesicular lipid transporters involved in cell–cell communication and various pathophysiologies. Biochimica et Biophysica Acta/General Subjects. 2014;1841:108–120. doi: 10.1016/j.bbalip.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Record et al. (2018).Record M, Silvente-Poirot S, Poirot M, Wakelam MJO. Extracellular vesicles: lipids as key components of their biogenesis and functions. Journal of Lipid Research. 2018;59:1316–1324. doi: 10.1194/jlr.E086173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren et al. (2018).Ren J, Ding L, Zhang D, Shi G, Xu Q, Shen S, Wang Y, Wang T, Hou Y. Carcinoma-associated fibroblasts promote the stemness and chemoresistance of colorectal cancer by transferring exosomal lncRNA H19. Theranostics. 2018;8:3932–3948. doi: 10.7150/thno.25541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez Zorrilla et al. (2019).Rodríguez Zorrilla S, Pérez-Sayans M, Fais S, Logozzi M, Gallas Torreira M, García AGarcía. A pilot clinical study on the prognostic relevance of plasmatic exosomes levels in oral squamous cell carcinoma patients. Cancers. 2019;1:1. doi: 10.3390/cancers11030429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano et al. (2017).Romano G, Veneziano D, Acunzo M, Croce CM. Small non-coding RNA and cancer. Carcinogenesis. 2017;38:485–491. doi: 10.1093/carcin/bgx026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakha et al. (2016).Sakha S, Muramatsu T, Ueda K, Inazawa J. Exosomal microRNA miR-1246 induces cell motility and invasion through the regulation of DENND2D in oral squamous cell carcinoma. Scientific Reports. 2016;6:38750. doi: 10.1038/srep38750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Lucas et al. (2016).San Lucas FA, Allenson K, Bernard V, Castillo J, Kim DU, Ellis K, Ehli EA, Davies GE, Petersen JL, Li D, Wolff R, Katz M, Varadhachary G, Wistuba I, Maitra A, Alvarez H. Minimally invasive genomic and transcriptomic profiling of visceral cancers by next-generation sequencing of circulating exosomes. Annals of Oncology. 2016;27:635–641. doi: 10.1093/annonc/mdv604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig et al. (2017).Ludwig S, Floros T, Theodoraki MN, Hong CS, Jackson EK, Lang S, Whiteside TL. Suppression of lymphocyte functions by plasma exosomes correlates with disease activity in patients with head and neck cancer. Clinical Cancer Research. 2017;23:4843–4854. doi: 10.1158/1078-0432.ccr-16-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanada et al. (2020).Sanada T, Islam A, Kaminota T, Kirino Y, Tanimoto R, Yoshimitsu H, Yano H, Mizuno Y, Okada M, Mitani S, Ugumori T, Tanaka J, Hato N. Elevated exosomal lysyl oxidase like 2 is a potential biomarker for head and neck squamous cell carcinoma. Laryngoscope. 2020;130:e327-e334. doi: 10.1002/lary.28142. [DOI] [PubMed] [Google Scholar]
- Shah, Patel & Freedman (2018).Shah R, Patel T, Freedman JE. Circulating extracellular vesicles in human disease. New England Journal of Medicine. 2018;379:958–966. doi: 10.1056/NEJMra1704286. [DOI] [PubMed] [Google Scholar]
- Shao et al. (2018).Shao H, Im H, Castro CM, Breakefield X, Weissleder R, Lee H. New technologies for analysis of extracellular vesicles. Chemical Reviews. 2018;118:1917–1950. doi: 10.1021/acs.chemrev.7b00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma et al. (2011).Sharma S, Gillespie BM, Palanisamy V, Gimzewski JK. Quantitative nanostructural and single-molecule force spectroscopy biomolecular analysis of human-saliva-derived exosomes. Langmuir: the ACS journal of Surfaces and Colloids. 2011;27:14394–14400. doi: 10.1021/la2038763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi et al. (2018).Shigetomi S, Imanishi Y, Shibata K, Sakai N, Sakamoto K, Fujii R, Habu N, Otsuka K, Sato Y, Watanabe Y, Shimoda M, Kameyama K, Ozawa H, Tomita T, Ogawa K. VEGF-C/Flt-4 axis in tumor cells contributes to the progression of oral squamous cell carcinoma via upregulating VEGF-C itself and contactin-1 in an autocrine manner. American Journal of Cancer Research. 2018;8:2046–2063. [PMC free article] [PubMed] [Google Scholar]
- Shostak & Chariot (2015).Shostak K, Chariot A. EGFR and NF- κB: partners in cancer. Trends Mol Med. 2015;21:385–393. doi: 10.1016/j.molmed.2015.04.001. [DOI] [PubMed] [Google Scholar]
- Sigismund, Avanzato & Lanzetti (2018).Sigismund S, Avanzato D, Lanzetti L. Emerging functions of the EGFR in cancer. Molecular Oncology. 2018;12:3–20. doi: 10.1002/1878-0261.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skog et al. (2008).Skog J, Wurdinger T, Van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry Jr WT, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nature Cell Biology. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subra et al. (2010).Subra C, Grand D, Laulagnier K, Stella A, Lambeau G, Paillasse M, De Medina P, Monsarrat B, Perret B, Silvente-Poirot S, Poirot M, Record M. Exosomes account for vesicle-mediated transcellular transport of activatable phospholipases and prostaglandins. Journal of Lipid Research. 2010;51:2105–2120. doi: 10.1194/jlr.M003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subra et al. (2007).Subra C, Laulagnier K, Perret B, Record M. Exosome lipidomics unravels lipid sorting at the level of multivesicular bodies. Biochimie. 2007;89:205–212. doi: 10.1016/j.biochi.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Sun et al. (2019).Sun LP, Xu K, Cui J, Yuan DY, Zou B, Li J, Liu JL, Li KY, Meng Z, Zhang B. Cancer-associated fibroblast-derived exosomal miR-382-5p promotes the migration and invasion of oral squamous cell carcinoma. Oncology Reports. 2019;42:1319–1328. doi: 10.3892/or.2019.7255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun et al. (2010).Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, Barnes S, Grizzle W, Miller D, Zhang HG. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Molecular Therapy. 2010;18:1606–1614. doi: 10.1038/mt.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surman et al. (2019).Surman M, Drozdz A, Stępień E, Przybyło M. Extracellular vesicles as drug delivery systems - methods of production and potential therapeutic applications. Current Pharmaceutical Design. 2019;25:132–154. doi: 10.2174/1381612825666190306153318. [DOI] [PubMed] [Google Scholar]
- Svoronos, Engelman & Slack (2016).Svoronos AA, Engelman DM, Slack FJ. Oncomir or tumor suppressor? The duplicity of micrornas in cancer. Cancer Research. 2016;76:3666–3670. doi: 10.1158/0008-5472.can-16-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, Xiang & Xu (2019).Tan J, Xiang L, Xu G. LncRNA MEG3 suppresses migration and promotes apoptosis by sponging miR-548d-3p to modulate JAK-STAT pathway in oral squamous cell carcinoma. IUBMB Life. 2019;71:882–890. doi: 10.1002/iub.2012. [DOI] [PubMed] [Google Scholar]
- Teng et al. (2015).Teng MW, Galon J, Fridman WH, Smyth MJ. From mice to humans: developments in cancer immunoediting. Journal of Clinical Investigation. 2015;125:3338–3346. doi: 10.1172/jci80004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théry et al. (2018).Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach JM, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Bedina Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borràs FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, Brennan M, Brigstock DR, Brisson A, Broekman ML, Bromberg JF, Bryl-Górecka P, Buch S, Buck AH, Burger D, Busatto S, Buschmann D, Bussolati B, Buzás EI, Byrd JB, Camussi G, Carter DR, Caruso S, Chamley LW, Chang YT, Chen C, Chen S, Cheng L, Chin AR, Clayton A, Clerici SP, Cocks A, Cocucci E, Coffey RJ, Cordeiro-da Silva A, Couch Y, Coumans FA, Coyle B, Crescitelli R, Criado MF, D’Souza-Schorey C, Das S, Datta Chaudhuri A, De Candia P, De Santana EF, De Wever O, Del Portillo HA, Demaret T, Deville S, Devitt A, Dhondt B, Di Vizio D, Dieterich LC, Dolo V, Dominguez Rubio AP, Dominici M, Dourado MR, Driedonks TA, Duarte FV, Duncan HM, Eichenberger RM, Ekström K, El Andaloussi S, Elie-Caille C, Erdbrügger U, Falcón-Pérez JM, Fatima F, Fish JE, Flores-Bellver M, Försönits A, Frelet-Barrand A, Fricke F, Fuhrmann G, Gabrielsson S, Gámez-Valero A, Gardiner C, Gärtner K, Gaudin R, Gho YS, Giebel B, Gilbert C, Gimona M, Giusti I, Goberdhan DC, Görgens A, Gorski SM, Greening DW, Gross JC, Gualerzi A, Gupta GN, Gustafson D, Handberg A, Haraszti RA, Harrison P, Hegyesi H, Hendrix A, Hill AF, Hochberg FH, Hoffmann KF, Holder B, Holthofer H, Hosseinkhani B, Hu G, Huang Y, Huber V, Hunt S, Ibrahim AG, Ikezu T, Inal JM, Isin M, Ivanova A, Jackson HK, Jacobsen S, Jay SM, Jayachandran M, Jenster G, Jiang L, Johnson SM, Jones JC, Jong A, Jovanovic-Talisman T, Jung S, Kalluri R, Kano SI, Kaur S, Kawamura Y, Keller ET, Khamari D, Khomyakova E, Khvorova A, Kierulf P, Kim KP, Kislinger T, Klingeborn M, Klinke 2nd DJ, Kornek M, Kosanović MM, Kovács Á F, Krämer-Albers EM, Krasemann S, Krause M, Kurochkin IV, Kusuma GD, Kuypers S, Laitinen S, Langevin SM, Languino LR, Lannigan J, Lässer C, Laurent LC, Lavieu G, Lázaro-Ibáñez E, Le Lay S, Lee MS, Lee YXF, Lemos DS, Lenassi M, Leszczynska A, Li IT, Liao K, Libregts SF, Ligeti E, Lim R, Lim SK, Linē A, Linnemannstöns K, Llorente A, Lombard CA, Lorenowicz MJ, Lörincz ÁM, Lötvall J. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. Journal of Extracellular Vesicles. 2018;7 doi: 10.1080/20013078.2018.1535750. Article 153575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian et al. (2014).Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, Wei J, Nie G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35:2383–2390. doi: 10.1016/j.biomaterials.2013.11.083. [DOI] [PubMed] [Google Scholar]
- Tkach & Théry (2016).Tkach M, Théry C. Communication by extracellular vesicles: where we are and where we need to go. Cell. 2016;164:1226–1232. doi: 10.1016/j.cell.2016.01.043. [DOI] [PubMed] [Google Scholar]
- Tousignant et al. (2019).Tousignant KD, Rockstroh A, Taherian Fard A, Lehman ML, Wang C, McPherson SJ, Philp LK, Bartonicek N, Dinger ME, Nelson CC, Sadowski MC. Lipid uptake is an androgen-enhanced lipid supply pathway associated with prostate cancer disease progression and bone metastasis. Molecular Cancer Research. 2019;17:1166–1179. doi: 10.1158/1541-7786.Mcr-18-1147. [DOI] [PubMed] [Google Scholar]
- Trajkovic et al. (2008).Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brugger B, Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- Uzawa et al. (2015).Uzawa K, Kasamatsu A, Baba T, Kimura Y, Nakashima D, Higo M, Sakamoto Y, Ogawara K, Shiiba M, Tanzawa H. Quantitative detection of circulating tumor-derived mitochondrial NADH subunit variants as a potential prognostic biomarker for oral cancer. International Journal of Oncology. 2015;47:1077–1083. doi: 10.3892/ijo.2015.3083. [DOI] [PubMed] [Google Scholar]
- Vader et al. (2016).Vader P, Mol EA, Pasterkamp G, Schiffelers RM. Extracellular vesicles for drug delivery. Advanced Drug Delivery Reviews. 2016;106:148–156. doi: 10.1016/j.addr.2016.02.006. [DOI] [PubMed] [Google Scholar]
- Valadi et al. (2007).Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature Cell Biology. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- Van Niel, D’Angelo & Raposo (2018).Van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nature Reviews Molecular Cell Biology. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- Van Niel et al. (2006).Van Niel G, Porto-Carreiro I, Simoes S, Raposo G. Exosomes: a common pathway for a specialized function. Journal of Biochemistry. 2006;140:13–21. doi: 10.1093/jb/mvj128. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2014).Wang J, Zhou Y, Lu J, Sun Y, Xiao H, Liu M, Tian L. Combined detection of serum exosomal miR-21 and HOTAIR as diagnostic and prognostic biomarkers for laryngeal squamous cell carcinoma. Medical Oncology. 2014;31 doi: 10.1007/s12032-014-0148-8. Article 148. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2018a).Wang L, Li Y, Guan X, Zhao J, Shen L, Liu J. Exosomal double-stranded DNA as a biomarker for the diagnosis and preoperative assessment of pheochromocytoma and paraganglioma. Molecular Cancer. 2018a;17 doi: 10.1186/s12943-018-0876-z. Article 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Li & Shi (2019).Wang X, Li H, Shi J. LncRNA HOXA11-AS promotes proliferation and cisplatin resistance of oral squamous cell carcinoma by suppression of miR-214-3p expression. BioMed Research International. 2019;2019 doi: 10.1155/2019/8645153. Article 8645153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2019a).Wang S-H, Liou G-G, Liu S-H, Chang JS, Hsiao J-R, Yen Y-C, Chen Y-L, Wu W-L, Chang J-Y, Chen Y-W. Laminin γ2-enriched extracellular vesicles of oral squamous cell carcinoma cells enhance in vitro lymphangiogenesis via integrin α3-dependent uptake by lymphatic endothelial cells. International journal of Cancer. 2019a;144:2795–2810. doi: 10.1002/ijc.32027. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2019b).Wang X, Qin X, Yan M, Shi J, Xu Q, Li Z, Yang W, Zhang J, Chen W. Loss of exosomal miR-3188 in cancer-associated fibroblasts contributes to HNC progression. Journal of Experimental & Clinical Cancer Research. 2019b;38 doi: 10.1186/s13046-019-1144-9. Article 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2018b).Wang Y, Qin X, Zhu X, Chen W, Zhang J, Chen W. Oral cancer-derived exosomal NAP1 enhances cytotoxicity of natural killer cells via the IRF-3 pathway. Oral Oncology. 2018b;76:34–41. doi: 10.1016/j.oraloncology.2017.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei et al. (2020).Wei T, Ye P, Yu GY, Zhang ZY. Circular RNA expression profiling identifies specific circular RNAs in tongue squamous cell carcinoma. Molecular Medicine Reports. 2020;21:1727–1738. doi: 10.3892/mmr.2020.10980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao et al. (2018).Xiao M, Zhang J, Chen W, Chen W. M1-like tumor-associated macrophages activated by exosome-transferred THBS1 promote malignant migration in oral squamous cell carcinoma. Journal of Experimental & Clinical Cancer Research. 2018;37:143. doi: 10.1186/s13046-018-0815-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie et al. (2019).Xie C, Du LY, Guo F, Li X, Cheng B. Exosomes derived from microRNA-101-3p-overexpressing human bone marrow mesenchymal stem cells suppress oral cancer cell proliferation, invasion, and migration. Molecular and Cellular Biochemistry. 2019;458:11–26. doi: 10.1007/s11010-019-03526-7. [DOI] [PubMed] [Google Scholar]
- Xiong et al. (2019).Xiong HG, Li H, Xiao Y, Yang QC, Yang LL, Chen L, Bu LL, Zhang WF, Zhang JL, Sun ZJ. Long noncoding RNA MYOSLID promotes invasion and metastasis by modulating the partial epithelial-mesenchymal transition program in head and neck squamous cell carcinoma. Journal of Experimental & Clinical Cancer Research. 2019;38 doi: 10.1186/s13046-019-1254-4. Article 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu et al. (2019).Xu D, Chen Y, Yuan C, Zhang S, Peng W. Long non-coding RNA LINC00662 promotes proliferation and migration in oral squamous cell carcinoma. OncoTargets and Therapy. 2019;12:647–656. doi: 10.2147/ott.S188691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue et al. (2017).Xue M, Chen W, Xiang A, Wang R, Chen H, Pan J, Pang H, An H, Wang X, Hou H, Li X. Hypoxic exosomes facilitate bladder tumor growth and development through transferring long non-coding RNA-UCA1. Molecular Cancer. 2017;16 doi: 10.1186/s12943-017-0714-8. Article 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakob et al. (2014).Yakob M, Fuentes L, Wang MB, Abemayor E, Wong DT. Salivary biomarkers for detection of oral squamous cell carcinoma—current state and recent advances. Current Oral Health Reports. 2014;1:133–141. doi: 10.1007/s40496-014-0014-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang & Chang (2018).Yang CC, Chang KW. Eicosanoids and HB-EGF/EGFR in cancer. Cancer and Metastasis Reviews. 2018;37:385–395. doi: 10.1007/s10555-018-9746-9. [DOI] [PubMed] [Google Scholar]
- Yang & Wu (2018).Yang M, Wu SY. The advances and challenges in utilizing exosomes for delivering cancer therapeutics. Frontiers in Pharmacology. 2018;9 doi: 10.3389/fphar.2018.00735. Article 735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang et al. (2017a).Yang S, Che SP, Kurywchak P, Tavormina JL, Gansmo LB, Correa de Sampaio P, Tachezy M, Bockhorn M, Gebauer F, Haltom AR, Melo SA, LeBleu VS, Kalluri R. Detection of mutant KRAS and TP53 DNA in circulating exosomes from healthy individuals and patients with pancreatic cancer. Cancer Biology & Therapy. 2017a;18:158–165. doi: 10.1080/15384047.2017.1281499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang et al. (2017b).Yang Y, Han Q, Hou Z, Zhang C, Tian Z, Zhang J. Exosomes mediate hepatitis B virus (HBV) transmission and NK-cell dysfunction. Cellular & Molecular Immunology. 2017b;14:465–475. doi: 10.1038/cmi.2016.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Wen & Zhu (2015).Yang Y, Wen L, Zhu H. Unveiling the hidden function of long non-coding RNA by identifying its major partner-protein. Cell & Bioscience. 2015;5 doi: 10.1186/s13578-015-0050-x. Article 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng et al. (2018).Zeng Z, Li Y, Pan Y, Lan X, Song F, Sun J, Zhou K, Liu X, Ren X, Wang F, Hu J, Zhu X, Yang W, Liao W, Li G, Ding Y, Liang L. Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nature Communications. 2018;9:5395. doi: 10.1038/s41467-018-07810-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan et al. (2019).Zhan C, Yang X, Yin X, Hou J. Exosomes and other extracellular vesicles in oral and salivary gland cancers. Oral Diseases. 2019 doi: 10.1111/odi.13172. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2014).Zhang H, Zeitz MJ, Wang H, Niu B, Ge S, Li W, Cui J, Wang G, Qian G, Higgins MJ, Fan X, Hoffman AR, Hu JF. Long noncoding RNA-mediated intrachromosomal interactions promote imprinting at the Kcnq1 locus. Journal of Cell Biology. 2014;204:61–75. doi: 10.1083/jcb.201304152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2019a).Zhang H, Zhu L, Bai M, Liu Y, Zhan Y, Deng T, Yang H, Sun W, Wang X, Zhu K, Fan Q, Li J, Ying G, Ba Y. Exosomal circRNA derived from gastric tumor promotes white adipose browning by targeting the miR-133/PRDM16 pathway. International Journal of Cancer. 2019a;144:2501–2515. doi: 10.1002/ijc.31977. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2015).Zhang J, Li S, Li L, Li M, Guo C, Yao J, Mi S. Exosome and exosomal microRNA: trafficking,s orting, and function. Genomics Proteomics Bioinformatics. 2015;13:17–24. doi: 10.1016/j.gpb.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2018).Zhang M, Han N, Jiang Y, Wang J, Li G, Lv X, Li G, Qiao Q. EGFR confers radioresistance in human oropharyngeal carcinoma by activating endoplasmic reticulum stress signaling PERK-eIF2 α-GRP94 and IRE1 α-XBP1-GRP78. Cancer Med. 2018;7:6234–6246. doi: 10.1002/cam4.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Itagaki & Hauser (2010a).Zhang Q, Itagaki K, Hauser CJ. Mitochondrial DNA is released by shock and activates neutrophils via p38 map kinase. Shock. 2010a;34:55–59. doi: 10.1097/SHK.0b013e3181cd8c08. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2010b).Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010b;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2019b).Zhang TH, Liang LZ, Liu XL, Wu JN, Su K, Chen JY, Zheng QY. LncRNA UCA1/miR-124 axis modulates TGF β1-induced epithelial-mesenchymal transition and invasion of tongue cancer cells through JAG1/Notch signaling. Journal of Cellular Biochemistry. 2019b;120:10495–10504. doi: 10.1002/jcb.28334. [DOI] [PubMed] [Google Scholar]
- Zhong et al. (2019).Zhong W-Q, Ren J-G, Xiong X-P, Man Q-W, Zhang W, Gao L, Li C, Liu B, Sun Z-J, Jia J, Zhang W-F, Zhao Y-F, Chen G. Increased salivary microvesicles are associated with the prognosis of patients with oral squamous cell carcinoma. Journal of Cellular and Molecular Medicine. 2019;23:4054–4062. doi: 10.1111/jcmm.14291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou et al. (2006).Zhou H, Pisitkun T, Aponte A, Yuen PS, Hoffert JD, Yasuda H, Hu X, Chawla L, Shen RF, Knepper MA, Star RA. Exosomal Fetuin-A identified by proteomics: a novel urinary biomarker for detecting acute kidney injury. Kidney International. 2006;70:1847–1857. doi: 10.1038/sj.ki.5001874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotogorski-Hurvitz et al. (2016).Zlotogorski-Hurvitz A, Dayan D, Chaushu G, Salo T, Vered M. Morphological and molecular features of oral fluid-derived exosomes: oral cancer patients versus healthy individuals. Journal of Cancer Research and Clinical Oncology. 2016;142:101–110. doi: 10.1007/s00432-015-2005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The following information was supplied regarding data availability:
This is a literature review. There is no raw data.