Abstract
Background
While pneumonia is the most prevalent healthcare-associated infection following coronary artery bypass surgery grafting (CABG), the relative effectiveness of strategies to reduce its incidence remains unclear. We evaluated the relationship between healthcare-associated infection recommendations and risk of pneumonia following CABG.
Methods
Pneumonia prevention practice recommendations were developed based on literature review and analysis of semi-structured interviews with key healthcare personnel across low (<5.9%), medium (5.9–6.1%) and high (>6.1%) pneumonia rate centers. These practices were implemented among 2,482 patients undergoing CABG from 2016 to 2017 across 18 centers. The independent effect of each practice in reducing pneumonia was assessed using multivariable logistic regression, adjusting for baseline risk and center. A composite (bundle) score was calculated as the number of practices (0 to 4) received by each patient.
Results
Recommended pneumonia prevention practices included: lung protective ventilation management, early extubation, progressive ambulation and avoidance of postoperative bronchodilator therapy. Pneumonia occurred in 2.4% of patients. Lung protective ventilation (ORadj 0.45, 95%CI 0.22–0.92), ambulation (ORadj 0.08, 95%CI 0.04–0.17), and postoperative ventilation less than 6 hours (ORadj 0.47, 95%CI 0.26–0.87) were significantly associated with lower odds of pneumonia; postoperative bronchodilator therapy (ORadj 4.83, 95%CI 2.20–10.7) was significantly associated with higher odds. Risk-adjusted rates of pneumonia, operative mortality, and ICU length of stay were lower in patients with higher bundle scores (all p-trend < 0.01).
Conclusion
These pneumonia prevention recommendations may serve as effective targets for avoiding postoperative healthcare-associated infections.
Keywords: Surgery, Infection control, Quality improvement
Introduction
Postoperative pneumonia is the most prevalent of all healthcare-associated infections following cardiac surgery.[1,2] In patients undergoing coronary artery bypass grafting (CABG), pneumonia is associated with four-fold increased odds of mortality, three-fold increased length of stay, and nearly $10,000 of additional healthcare expenditures.[3,4] Despite its impact on quality, pneumonia rates vary ten-fold across the United States, with approximately 2% of this variation explained by traditional risk factors.[5]
Unfortunately, how to optimally reduce a patient’s risk of pneumonia following cardiac surgery remains incompletely understood. Prior work utilizing clinical databases has identified a myriad of potential strategies, including: prophylactic oral and nasal decontamination, subglottic suctioning, lung protective ventilation, goal-directed extubation protocols, and early postoperative ambulation.[6–11] However, broader adoption of these practices is hampered by an incomplete understanding of how top performing centers achieve superior outcomes. Efforts aimed at reducing unwarranted variability in pneumonia rates would benefit from the identification of significant prevention strategies.
Accordingly, we undertook a multi-phased study across hospitals in the state of Michigan performing CABG surgery. First, we utilized a structured literature review along with semi-structured interviews and site visits to low and high performing centers in Michigan to identify best practices that would be subsequently included within pneumonia prevention recommendations. Second, we evaluated the effectiveness of adherence to these recommendations across centers participating in our statewide quality improvement collaborative.
Patients and Methods
This study was approved by the Institutional Review Board at Michigan Medicine (HUM00084088) and registered at Clinicaltrials.gov (https://clinicaltrials.gov/, NCT02068716).
Patient Population
The Michigan Society of Thoracic and Cardiovascular Surgeons Quality Collaborative (MSTCVS-QC) is a surgeon-led multidisciplinary group encompassing all 33 non-federal hospitals performing adult cardiac surgical procedures in the state of Michigan. All hospitals use the Society of Thoracic Surgeons’ (STS) data collection form and quarterly submit data to both the STS database and the MSTCVS-QC data warehouse. Routine audits demonstrate excellent data integrity (>98% data accuracy).
Quality Improvement Strategy
In the first phase of the study, data from the MSTCVS-QC data warehouse were utilized to identify centers for benchmarking site visits and qualitative interviews (September 2014 to July 2015) to improve our understanding of currently employed pneumonia prevention practices and determinants of their adoption. Centers were chosen based on the STS’ pneumonia rate and included five with low (1.9% – 3.4%), two with medium (5.9% – 6.1%) and three with high (>6.1%) rates (eTable 1). Semi-structured interview guides (eText 1) were developed for clinical and non-clinical team members to focus discussions surrounding areas of clinical practice or organizational structure that may be protective against pneumonia. We employed a Rapid Assessment Process (eFigure 1) to: (i) identify key pneumonia prevention practices, processes and procedures at each hospital and (ii) begin developing an understanding of determinants of center-level differences in pneumonia rates.[12] Recommended pneumonia prevention practices were developed (“pneumonia prevention bundle”, eText 2) based on interview findings and subsequently endorsed by the MSTCVS-QC.
Next, we evaluated whether bundle adoption across MSTCVS-QC centers was associated with lower pneumonia rates. Eighteen of the thirty-three MSTCVS-QC centers agreed to participate (range of center pneumonia rates: 0.0% – 6.8%) in the implementation of the recommended practices over a one-year period (January 1st, 2016 to December 31st, 2016). Nurse coordinators entered data concerning practice adoption of recommended practices into a RedCAP database.[13] Centers received central implementation support from the MSTCVS-QC, including: (i) monthly data feedback regarding their pneumonia rate and adoption of recommended pneumonia prevention practices, (ii) webinars discussing barriers to implementation of the recommended practices and (iii) quarterly in-person meetings. Analysis of this study phase involved 2,482 isolated CABG surgery patients.
Measures
The primary outcome was postoperative pneumonia. Pneumonia diagnosis requires physician or advanced practitioner documentation in the medical record based on laboratory findings (e.g., positive sputum culture results from transtracheal fluid of bronchial washings) and radiologic evidence (e.g., chest radiograph with pulmonary infiltrates consistent with diagnosis of pneumonia).
The primary exposure was the adoption of pneumonia prevention practices, which were combined to create composite interventions:
Nasal antibiotic prophylaxis and oral preparation were combined into a composite ‘preoperative nasal and oral prophylaxis’
Progressive postoperative ambulation was defined as documentation of ambulation and ambulation greater than 150 feet
A composite (bundle) score was calculated as the number of preventive practices (0 to 4) received by each patient. Components of the bundle included: (i) avoidance of postoperative bronchodilator therapy; (ii) preoperative nasal and oral prophylaxis; (iii) progressive postoperative ambulation; (iv) postoperative ventilation < 6 hours.
All other variable definitions are detailed in the STS-ACSD version 1.81 Data Specifications.[14]
Statistical Analysis
Standard descriptive statistics (e.g., chi-square for categorical variables, t-tests/Kruskal-Wallis tests for continuous variables) were used. Univariate logistic regression models were employed.
Patient characteristics and bundle components that were significantly associated with pneumonia were entered as candidate variables in multivariable analysis using forward selection with a threshold p value of 0.05. Our model included center and previously identified preoperative risk factors.[15] Direct standardization was employed to estimate the association of increasing bundle score with adjusted rates of pneumonia, operative mortality and intensive care unit length of stay (hours), respectively.
In a planned sensitivity analysis, we evaluated the relationship between pneumonia prevention practices and postoperative pneumonia among clinically important subgroups (i.e., age, sex, operative status, chronic lung disease).
Statistical analyses were performed using Stata Corporation 14.0 (College Station, TX).[16]
Results
The overall rate of postoperative pneumonia was 2.4% (60 / 2,482).
Site-Visit Findings
We conducted 79 interviews across ten MSTCVS-QC centers selected based on their observed pneumonia rate - five hospitals having low (<5.9%), two having medium (5.9% – 6.1%), and three having high (>6.1%) rates. Interviews revealed considerable variation in pneumonia prevention practices at low versus high pneumonia rate hospitals, as well as in the nature of their implementation (eTable 3).
A list of recommended pneumonia prevention practices emerged from the findings of the interviews, including: preoperative (oral and nasal preparation), intraoperative (blood management; lung protective ventilation) and postoperative (early, goal-directed extubation; subglottic suctioning; daily assessment of oral care; daily spontaneous awakening/breathing trials as well as pairing of the breathing trials with the awakening trials and progressive mobility) phases of care (eText 2).
Cohort characteristics
Average preoperative risk was higher in patients who developed pneumonia (3.9% versus 2.6%, p<0.01, Table 1). Liver disease (p<0.01), requirement of home oxygen therapy (p = 0.03), current cigarette smoking (p = 0.05), and chronic lung disease (p<0.01), history of pneumonia (p<0.01), emergent status (p<0.01), use of an intra-aortic balloon pump (p<0.01) and operative mortality (p<0.01) were more common among patients developing pneumonia. Pneumonia was associated with longer average intensive care unit stays (16.2 days versus 2.6 days), p<0.01.
Table 1.
Univariate Analysis: Cohort characteristics
| Characteristic | All | Pneumonia | P-value | |
|---|---|---|---|---|
| 100.0% (N=2,482) | No, 97.6% (n=2,422) | Yes, 2.4% (n=60) | ||
| Demographics | ||||
| Age | 65.58 (9.93) | 65.54 (9.93) | 67.25 (10.03) | 0.19 |
| Body Mass Index | 31.40 (11.03) |
31.38 (11.11) |
32.35 (7.10) |
0.50 |
| Nonwhite race | 10.11 | 10.12 | 10.00 | 0.99 |
| Laboratory Values | ||||
| Hematocrit | 39.20 (5.40) | 39.19 (5.39) | 39.51 (6.03) | 0.66 |
| White blood cell count | 8.03 (3.30) | 8.02 (3.32) | 8.46 (2.78) | 0.32 |
| Comorbid Disease | ||||
| Dyslipidemia | 91.70 | 91.70 | 91.67 | 0.96 |
| Peripheral vascular disease | 16.44 | 16.43 | 16.67 | 0.96 |
| Cerebrovascular disease | 25.42 | 25.47 | 23.33 | 0.71 |
| Diabetes Mellitus | 48.87 | 48.84 | 50.00 | 0.86 |
| Liver Disease | 2.34 | 2.19 | 8.33 | <0.01 |
| Pulmonary Function | ||||
| Home Oxygen Therapy† | 0.73 | 0.66 | 3.33 | 0.03 |
| History of Pneumonia | ||||
| No | 90.33 | 90.50 | 83.33 | Ref |
| Recent or Remote | 9.31 | 9.17 | 15.00 | 0.12 |
| Current Cigarette Smoking | 22.80 | 22.54 | 33.33 | 0.05 |
| Chronic lung disease | ||||
| None | 71.84 | 72.50 | 45.00 | Ref |
| Mild | 21.27 | 20.77 | 41.67 | <0.01 |
| Mod/Severe | 6.89 | 6.73 | 13.33 | <0.01 |
| Cardiac Function | ||||
| Preoperative intra-aortic balloon pump | 6.04 | 5.82 | 15.00 | <0.01 |
| History of arrhythmia | ||||
| None | 94.20 | 94.38 | 86.67 | Ref |
| Remote | 2.38 | 2.39 | 1.67 | 0.79 |
| Recent | 3.42 | 3.22 | 11.67 | <0.01 |
| Ejection fraction | 52.69 (11.97) | 52.76 (11.95) | 49.56 (12.38) | 0.04 |
| Operative Status | ||||
| Non-emergent | 97.78 | 98.02 | 88.33 | Ref |
| Emergent | 2.14 | 1.90 | 11.67 | <0.01 |
| Preoperative pneumonia risk score | 2.67 (2.45) | 2.64 (2.42) | 3.94 (3.15) | <0.01 |
| Outcomes | ||||
| Operative mortality | 1.69 | 1.49 | 10.00 | <0.01 |
| Intensive care unit length of stay (days) | 2.86 (4.01) | 2.55 (2.91) | 16.17 (12.59) | <0.01 |
Values are percent or mean (SD), unless otherwise noted
Defined as “Dependence on Home Oxygen Therapy”
Association of Pneumonia Prevention Bundle Adoption and Postoperative Pneumonia
We associated bundle adoption with a patient’s risk of pneumonia (Table 2). Lung protective plateau inspiratory pressure (OR 0.39, p=0.003), postoperative ventilation less than 6 hours (OR 0.33, p<0.001), ambulation (OR 0.08, p<0.001), ambulation greater than 150 feet (OR 0.11, p<0.001), and progressive ambulation (OR 0.11, p<0.001) were significantly associated with lower odds of pneumonia. The use of any blood products (OR 2.18, p<0.01), plateau inspiratory pressure (OR 1.06, p<0.01), subglottic suctioning (OR 2.21, p=0.009) and use of postoperative bronchodilators (OR 5.16, p<0.001) were significantly associated with higher odds of pneumonia.
Table 2.
Relationship Between Pneumonia Prevention Practices and Odds of Postoperative Pneumonia
| Characteristic | All | Pneumonia | Odds Ratio (95% CI) | P-Value | |
|---|---|---|---|---|---|
| 100% (N=2,482) | No, 97.6% (n=2,422) | Yes, 2.4% (n=60) | |||
| Preoperative prophylaxis | |||||
| Oral Preparation | 63.82 | 63.79 | 65.00 | 1.00 (0.59–1.72) | 0.98 |
| Nasal Culture | 35.54 | 35.67 | 30.00 | 0.76 (0.44–1.34) | 0.35 |
| Nasal Antibiotic Prophylaxis | 64.34 | 64.45 | 60.00 | 0.86 (0.49–1.51) | 0.60 |
| Composite intervention: Preoperative nasal and oral prophylaxis | 45.33 | 45.50 | 38.33 | 0.73 (0.43–1.26) | 0.26 |
| Intraoperative ventilation | |||||
| Highest Tidal Volume, mL | 608.07 (188.57) | 607.68 (189.13) | 624.14 (164.35) | 1.00 (0.99–1.00) | 0.53 |
| Lung Protective Tidal Volume (< 8 mL/Kg) | 0.78 | 0.75 | 1.69 | 2.27 (0.30–17.31) | 0.43 |
| PEEP, cm H20 | 5.54 (11.30) | 5.55 (11.43) | 5.35 (3.24) | 0.99 (0.95–1.04) | 0.91 |
| Lung Protective PEEP >= 5cm H20) | 82.15 | 82.20 | 80.00 | 0.86 (0.46–1.64) | 0.66 |
| Intraoperative blood products | |||||
| Any blood product | 19.30 | 18.95 | 33.33 | 2.18 (1.26–3.78) | < 0.01 |
| Red blood cells (units) | 0.28 (0.84) | 0.28 (0.84) | 0.46 (0.89) | 1.18 (0.96–1.47) | 0.12 |
| Fresh frozen plasma (units) | 0.07 (0.60) | 0.07 (0.61) | 0.10 (0.40) | 1.06 (0.79–1.42) | 0.69 |
| Platelets (units) | 0.12 (0.55) | 0.12(0.55) | 0.25(0.63) | 1.25 (0.97–1.61) | 0.08 |
| Cryoprecipitate | 0.02 (0.35) | 0.02( 0.35) | 0.06 (0.36) | 1.18 (0.82–1.68) | 0.37 |
| Postoperative ventilation | |||||
| Highest Tidal Volume, mL | 573.38 (162.91) | 572.63 (163.39) | 602.86 (140.76) | 1.00 (0.99–1.00) | 0.20 |
| Lung Protective Tidal Volume (< 8 mL/Kg) | 3.72 | 3.68 | 5.08 | 1.40 (0.43–4.56) | 0.57 |
| PIP, cm H20 | 21.05 (7.54) | 20.95 (7.30) | 25.21 (13.61) | 1.06 (1.03–1.09) | <0.001 |
| Lung Protective PIP <= 30 cm H20) | 87.95 | 88.27 | 75.00 | 0.39 (0.22–0.72) | 0.003 |
| Composite intervention: Appropriate PEEP (> =5 cm) and PIP (<= 30cm) | 71.23 | 71.59 | 56.67 | 0.51 (0.31–0.87) | 0.013 |
| Prolonged ventilation† | |||||
| Postoperative ventilation < 6 hours | 61.20 | 61.85 | 35.00 | 0.33 (0.19–0.57) | <0.001 |
| Daily assessment of oral care with CHG (n = 152) | 76.32 | 74.77 | 80.49 | 0.62 (0.22–1.74) | 0.37 |
| SAT | 48.03 | 47.32 | 50.00 | 0.67 (0.20–2.27) | 0.53 |
| SBT | 57.89 | 57.14 | 60.00 | 0.97 (0.31–3.02) | 0.96 |
| SBT following SAT | 43.71 | 41.07 | 51.28 | 1.30 (0.24–7.02) | 0.76 |
| Subglottic suctioning | 19.94 | 19.65 | 31.67 | 2.21 (1.22–4.01) | 0.009 |
| Use of post-operative bronchodilator | 47.10 | 46.24 | 81.67 | 5.16 (2.67–9.97) | <0.001 |
| Ambulation‡ | |||||
| Ambulation to chair | 95.41 | 95.38 | 96.67 | 0.72 (0.09–5.43) | 0.76 |
| Time to ambulation to chair, (half-days) [median (IQR)] | 1.17 (0.73–1.70) | 1.17 (0.74–1.68) | 1.77 (0.60–6.11) | 1.00 (0.99–1.00) | 0.78 |
| Ambulation | 94.88 | 95.21 | 81.67 | 0.08 (0.04–0.18) | <0.001 |
| Time to ambulation (half-days) [median (IQR)] | 1.08 (0.25–2.61) | 1.01 (0.25–2.56) | 3.33 (0.66–10.14) | 1.00 (0.99–1.00) | 0.63 |
| Ambulation > 150 ft | 75.38 | 76.34 | 36.67 | 0.11 (0.059–0.20) | <0.001 |
| Time to ambulation > 150 ft (half-days) [median (IQR)] | 2.12 (0.54–4.19) | 2.12 (0.51–4.17) | 4.16 (1.42–12.43) | 1.00 (0.99–1.00) | 0.85 |
| Composite intervention: Documentation of progressive postoperative ambulation$ | 74.01 | 74.94 | 36.67 | 0.11 (0.06–0.21) | <0.001 |
Abbreviations: Positive End-Expiratory Pressure (PEEP); Peak Inspiratory Pressure (PIP); Spontaneous awakening trials (SAT); Spontaneous breathing trials (SBT)
Values are percent or mean (SD), unless otherwise noted.
Denominator for “Daily assessment of oral care with CHG”, ”Spontaneous awakening trials”, ”Spontaneous breath trials” was 152 patients. Denominator for “Spontaneous breath trials following Spontaneous awakening trials” was 151 patients.
Ambulation practices were assessed via documentation in the electronic medical record of the following: Ambulation to Chair – Standing, pivoting, and stepping into a bedside chair; Ambulation – Ambulation about the patient’s room, greater than 2 steps; Ambulation > 150ft – Ambulation to a distance greater than 150 feet. Time to ambulation practices were exclusive: i.e. time to ambulation to chair = Date/Time of ambulation to chair – extubation date/time; time to ambulation = Date/time of ambulation – date/time of ambulation to chair; time to ambulation > 150 ft =Date/time of ambulation > 150 ft – date/time of ambulation.
Documentation of Ambulation and ambulation > 150 ft, relative to patients who were documented as having not achieved ambulation or ambulation > 150 ft
Table 3 displays the results from our final regression model. Lung protective ventilation (peak inspiratory pressure < 30 cm H2O) (ORadj 0.45, p = 0.03), postoperative ventilation less than 6 hours (ORadj 0.47, p = 0.02) and progressive postoperative ambulation (ORadj 0.08, p < 0.01) were associated with reduced adjusted odds of pneumonia. Postoperative bronchodilator use was associated with increased adjusted odds of pneumonia (ORadj 4.83, p < 0.01).
Table 3.
Multivariable Model of Odds of Postoperative Pneumonia
| Characteristic | Odds Ratio (95% CI) | P-Value |
|---|---|---|
| PIP ≤ 30 cmH2O | 0.45 (0.22–0.92) | 0.03 |
| Postoperative Ventilation < 6 hours | 0.47 (0.26–0.87) | 0.02 |
| Documentation of Progressive Postoperative Ambulation | 0.08 (0.04–0.17) | <0.001 |
| Use of Postoperative Bronchodilator | 4.83 (2.20–10.7) | <0.001 |
Abbreviation: Inspiratory Pressure (PIP)
For PIP, patients who received PIP ≤ 30 cmH2O were compared to patients who received PIP > 30 cmH2O (reference group)
Adjusted for patient age, race, hematocrit, white blood cell count, dyslipidemia peripheral vascular disease, cerebrovascular disease, diabetes, liver disease, home oxygen therapy, history of pneumonia, current cigarette smoking, chronic lung disease, preoperative intra-aortic balloon pump, history of arrhythmia, ejection fraction, operative status, center
Two percent (2.5%) of patients received a bundle score of zero, 13.3% received one, 34.7% received three and 21.4% received four. Increasing bundle score was inversely associated with a patient’s risk-adjusted rate of postoperative pneumonia (Figure 1a), operative mortality (Figure 1b) and intensive care unit (ICU) length of stay (Figure 1c), all p-trend<0.001.
Figure 1a–c: Relationship between bundles of pneumonia prevention practices and postoperative pneumonia, mortality and intensive care unit length of stay.
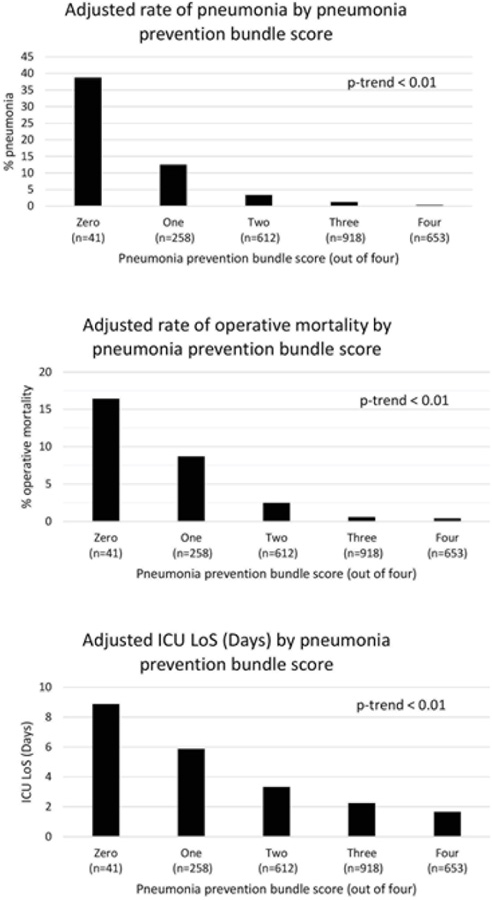
Figure 1a: Postoperative Pneumonia
Figure 1b: Operative Mortality
Figure 1c: Intensive Care Unit Length of Stay (days)
Sensitivity analyses were undertaken by age, sex, chronic lung disease and operative status - the results were qualitatively unchanged (eTable 2).
Comment
In this prospective, multi-institutional study we developed and evaluated the effectiveness of a pneumonia prevention bundle. The choice of specific pneumonia practices was derived out of: (i) the analysis of 79 semi-structured interviews at ten centers having varying infection rates and (ii) a structured literature search. Among 18 centers agreeing to voluntarily implement these practices, bundle adoption was significantly associated with a lower risk adjusted pneumonia rate. Patients receiving a greater bundle score had significantly lower risk-adjusted rates of mortality and intensive care unit length of stay.
We acknowledge several study limitations. First, although our structured literature review and interviews yielded a robust set of candidate pneumonia prevention practices, we recognize that we may not have identified other important practices. Second, we cannot rule out systemic bias in data abstraction attributed to the sole reliance on medical record review for identifying pneumonia prevention practices. Nonetheless, our data coordinating center trained nurse abstractors in using a data dictionary to guide their work (available upon request). Third, while a large, multi-center experience, our findings may not be generalizable outside of Michigan. Fourth, we cannot rule out the effect of unmeasured confounding. For example, our final model revealed a significant adverse association between bronchodilator usage and pneumonia. For example, our final model revealed a significant adverse association between bronchodilator use and pneumonia. We did not collect data concerning the indications for use of this therapy and therefore caution against overinterpretation of this finding. Nonetheless, this result reflects the risk of pneumonia among patients with pre-existing pulmonary disease and the need for heightened vigilance for post-operative pulmonary complications in this patient population.
This study adds to current discourse surrounding potential reasons underlying hospital-level differences in pneumonia rates.[1,2,5] Shih and colleagues reviewed 20,896 patients undergoing isolated CABG across 33 hospitals and found that while predicted rates of healthcare-acquired infections (HAIs) varied 2.8% in absolute terms, observed rates varied by 18.2%.[1] Pneumonia was the most common HAI subtype, a finding further validated through analysis of national STS-ACSD data.[2] High performing hospitals with lower HAI rates were more likely to select appropriate antibiotics and use less blood products. High performing hospitals may thus have structured infection prevention practices. Brescia and colleagues reported that 98% of observed variation in hospital-level rates of postoperative pneumonia is unexplained by traditional STS-ACSD data.[5] Use of the bundle practices may help explain a portion of the unexplained variation.
Our findings highlight several practices which may provide both (i) insight into determinants of variability in pneumonia and (ii) levers for quality improvement. Previous studies have reported significant associations between lung protective ventilation and pneumonia risk. In a randomized trial studying the use of nasal continuous positive airway pressure (nCPAP) among 500 elective cardiac surgery patients, Zarbock and colleagues found that nCPAP was significantly associated with reduced odds of a composite outcome consisting of hypoxemia (PaO2/FiO2 < 100), pneumonia and reintubation (p = 0.03).[17] Sundar and colleagues reported the association of low tidal volume ventilation with extubation time, reintubation, mortality and length of stay in a randomized control trial of 149 patients undergoing cardiac surgery.[11] Patients ventilated with 6 mL/Kg tidal volume, relative to those ventilated with 10 mL/Kg (control group), were significantly less likely to require reintubation (1.3% versus 9.5%, p = 0.03) and were more likely to be extubated at six hours post-procedure (37.3% versus 20.3%, p = 0.02). In their large cohort study, Ladha and colleagues examined the impact of lung protective ventilation (defined as positive end expiratory pressure [PEEP] of 5 cm H2O or more, tidal volume less than 10 mL/Kg, and median plateau pressure < 30 cm H2O) on a composite outcome of pulmonary edema, respiratory failure, pneumonia and reintubation.[18] Among 69,265 patients undergoing non-cardiac surgical procedures, PEEP greater than or equal to 5 cm H2O (OR: 0.91, 95% CI 0.83–0.99) and plateau pressure less than 30 cm H2O (OR: 0.66, 95% CI 0.53–0.81) were independently associated with lower adjusted odds of the composite outcome. Our results, which demonstrate that a plateau pressure < 30 cm H2O is significantly associated with lower adjusted odds of postoperative pneumonia, join the growing body of literature suggesting a potential benefit associated with lung protective ventilation during and following cardiac surgery.
Documentation of progressive, postoperative ambulation was associated with a significant reduction in odds of pneumonia. Recent studies have investigated the effect of perioperative physiotherapy on patient outcomes following cardiac surgery. Herdy and colleagues randomized 56 CABG patients to a rehabilitation or control arm; patients in the rehabilitation arm underwent a progressive, postoperative rehabilitation regime, advancing from passive movements (postoperative day one), to walking, and finally to climbing two flights of stairs (postoperative day five).[7] Rehabilitation patients had lower relative risk of pneumonia (p < 0.01) and were discharged earlier (5.9 ± 1.1 versus 10.3 ± 4.6 days [p < 0.001]) compared to control patients. Unfortunately, few centers currently systematically collect data regarding a patient’s prehabilitation activities or five-meter walk test. Our study was not able to account for differences in either, both of which may serve equally valuable roles in reducing the risk of postoperative pneumonia following CABG.
Our study identified and pursued opportunities for pneumonia prevention across the entire perioperative period. Our Coordinating Center assisted participating centers in the implementation of the pneumonia prevention practices via monthly data feedback, webinars and quarterly in-person meetings. Bundle adoption was associated with lower adjusted odds of pneumonia. Despite central support, appreciable variability in practice persisted. Broader practice adoption may serve as further opportunities for improving the quality of care for our patients.
Supplementary Material
Acknowledgments and Disclosures
This project was funded in part by grant no. R01HS022535 from the Agency for Healthcare Research and Quality (AHRQ) and the US Department of Health and Human Services. The opinions expressed in this document are those of the authors and do not reflect the official position of the AHRQ or the US Department of Health and Human Services. Support for Michigan Society of Thoracic and Cardiovascular Surgeons Quality Collaborative is provided by Blue Cross and Blue Shield of Michigan (BCBSM) and Blue Care Network as part of the BCBSM Value Partnerships program. Although BCBSM and MSTCVS-QC work collaboratively, the opinions, beliefs and viewpoints expressed by the author do not necessarily reflect the opinions, beliefs and viewpoints of BCBSM or any of its employees.
Dr Likosky receives research funding from the Agency for Healthcare Research and Quality (R01HS026003 AHRQ), BCBSM and serves as a consultant for the American Society of Extracorporeal Technology.
Dr Strobel was supported by MICHR TL1 Grant Number: (TL1TR002242).
Footnotes
Presented at the American Heart Association Quality of Care and Outcomes Research Scientific Sessions (April 6–7, 2018) in Arlington, Virginia.
References
- [1].Shih T, Zhang M, Kommareddi M, Boeve TJ, Harrington SD, Holmes RJ, et al. Center-level variation in infection rates after coronary artery bypass grafting. Circ Cardiovasc Qual Outcomes 2014;7:567–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Likosky DS, Wallace AS, Prager RL, Jacobs JP, Zhang M, Harrington SD, et al. Sources of Variation in Hospital-Level Infection Rates After Coronary Artery Bypass Grafting: An Analysis of The Society of Thoracic Surgeons Adult Heart Surgery Database. Ann Thorac Surg 2015;100:1570–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kinlin LM, Kirchner C, Zhang H, Daley J, Fisman DN. Derivation and validation of a clinical prediction rule for nosocomial pneumonia after coronary artery bypass graft surgery. Clin Infect Dis 2010;50:493–501. [DOI] [PubMed] [Google Scholar]
- [4].Thompson MP, Cabrera L, Strobel RJ, Harrington SD, Zhang M, Wu X, et al. Association Between Post-Operative Pneumonia and 90-Day Episode Payments and Outcomes Among Medicare Beneficiaries Undergoing Cardiac Surgery. Circ Cardiovasc Qual Outcomes n.d. [DOI] [PubMed]
- [5].Brescia AA, Rankin JS, Cyr DD, Jacobs JP, Prager RL, Zhang M, et al. Determinants of Variation in Pneumonia Rates After Coronary Artery Bypass Grafting. Ann Thorac Surg 2018;105:513–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bouza E, Pérez MJ, Muñoz P, Rincón C, Barrio JM, Hortal J. Continuous aspiration of subglottic secretions in the prevention of ventilator-associated pneumonia in the postoperative period of major heart surgery. Chest 2008;134:938–46. [DOI] [PubMed] [Google Scholar]
- [7].Herdy AH, Marcchi PLB, Vila A, Tavares C, Collaço J, Niebauer J, et al. Pre- and postoperative cardiopulmonary rehabilitation in hospitalized patients undergoing coronary artery bypass surgery: a randomized controlled trial. Am J Phys Med Rehabil 2008;87:714–9. [DOI] [PubMed] [Google Scholar]
- [8].Lellouche F, Dionne S, Simard S, Bussières J, Dagenais F. High tidal volumes in mechanically ventilated patients increase organ dysfunction after cardiac surgery. Anesthesiology 2012;116:1072–82. [DOI] [PubMed] [Google Scholar]
- [9].Morris AC, Hay AW, Swann DG, Everingham K, McCulloch C, McNulty J, et al. Reducing ventilator-associated pneumonia in intensive care: impact of implementing a care bundle. Crit Care Med 2011;39:2218–24. [DOI] [PubMed] [Google Scholar]
- [10].Segers P, Speekenbrink RGH, Ubbink DT, van Ogtrop ML, de Mol BA. Prevention of nosocomial infection in cardiac surgery by decontamination of the nasopharynx and oropharynx with chlorhexidine gluconate: a randomized controlled trial. JAMA 2006;296:2460–6. [DOI] [PubMed] [Google Scholar]
- [11].Sundar S, Novack V, Jervis K, Bender SP, Lerner A, Panzica P, et al. Influence of low tidal volume ventilation on time to extubation in cardiac surgical patients. Anesthesiology 2011;114:1102–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Beebe J Rapid Assessment Process : An Introduction. Walnut Creek, CA: AltaMira Press; 2001. [Google Scholar]
- [13].Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Society of Thoracic Surgeons Adult Cardiac Surgery Database Version 2.81 Data Specifications n.d. [DOI] [PubMed]
- [15].Strobel RJ, Liang Q, Zhang M, Wu X, Rogers MA, Theurer PF, et al. A Preoperative Risk Model for Postoperative Pneumonia After Coronary Artery Bypass Grafting. Ann Thorac Surg 2016;102:1213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].StataCorp LP. Stata Corporation Stata Statistical Software: Release 14. College Station, TX: n.d. [Google Scholar]
- [17].Zarbock A, Mueller E, Netzer S, Gabriel A, Feindt P, Kindgen-Milles D. Prophylactic nasal continuous positive airway pressure following cardiac surgery protects from postoperative pulmonary complications: a prospective, randomized, controlled trial in 500 patients. Chest 2009;135:1252–9. [DOI] [PubMed] [Google Scholar]
- [18].Ladha K, Vidal Melo MF, McLean DJ, Wanderer JP, Grabitz SD, Kurth T, et al. Intraoperative protective mechanical ventilation and risk of postoperative respiratory complications: hospital based registry study. BMJ 2015;351:h3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


