Abstract
Context.
An increasing number of oral cancer treatments require patient adherence and symptom self-management.
Objectives.
The report presents the effects of a medication reminder and symptom management intervention directed at patients initiating new oral oncolytic agents.
Methods.
Patients (N=272) were recruited at six comprehensive cancer centers, interviewed over the telephone following oral agent initiation, and randomized to either standard care or a medication reminder and symptom management intervention. In the intervention arm, the automated system called patients daily to remind them about taking their medications and weekly to assess 18 symptoms and refer patients to a printed Medication Management and Symptom Management Toolkit. Severity of 18 symptoms was also assessed during telephone interviews at week 4 (mid-intervention), week 8 (post-intervention), and at week 12 (follow-up). Adherence was measured using the relative dose intensity (RDI), the ratio of dose taken by patient out of dose prescribed by the oncologist, and assessed using pill counts at weeks 4, 8, and 12 and prescribing information from medical records.
Results.
The RDI was high and did not differ by trial arm. Symptom severity was significantly lower (p<.01) in the experimental arm at week 8, but not at weeks 4 or 12.
Conclusion.
Adherence may be less of a problem than originally anticipated, and intervention was not efficacious possibly due to already high rates of patient adherence to oral oncolytic medication during first 12 weeks. Longer follow-up in future research may identify subgroups of patients who need interventions to sustain adherence.
Keywords: Cancer, Oral Agents, Symptom Management, Adherence
Introduction
An increasing number of cancer treatments are now approved in oral form. Many of these drugs have complicated dosing schedules and require adherence rates of greater than 80% to the Food and Drug Administration (FDA) approved dosing to achieve a therapeutic effect.(1) The transition from infusion chemotherapy to an oral route has significant implications for patients. In exchange for eliminating the need for frequent trips and extended time in infusion units, patients must adhere to their medication regimens and recognize early on the need to manage their symptoms and side effects with less reliance on interactions with oncology professionals. This paper reports the results from a 12-week two-arm randomized trial that tested a telephone adherence and symptom management intervention among patients newly prescribed oral oncolytic agents. If randomized to the intervention arm, patients received telephone daily reminders to take their medication tailored to the specific medication regimen. In addition, each week, telephone symptom assessment was performed for all patients, and those in the intervention arm were asked to use strategies from the printed symptom management Toolkit for symptoms above pre-defined threshold. The following research questions were addressed. First, when compared against standard care and symptom assessments only, did patients who received the reminder and symptom management intervention have better adherence to the medication in the first 12 weeks? Second, did patients who received the reminder and symptom management intervention report lower symptom severity over 12 weeks? Third, are lower symptoms associated with better adherence?
Patient adherence to medications is influenced by multiple factors that lie between the provider prescription and patient consumption.(2–7) This research considers the relative dose intensity (RDI), a ratio of dose consumed by patient to the dose prescribed by the oncologist.(8–10) Unlike medications for other chronic conditions, where dosing is established and remains relatively stable over time, dosing of oral oncolytic agents may undergo adjustments by oncologists due to patients’ conditions and treatment-related symptoms and side effects. As a result, the prescribed doses may change over time creating new challenges for patient adherence and necessitating dynamic calculations of the RDI that account for dose changes. These changes also warrant the considerations of the dose consumed by patients out of the FDA-recommended dose as well as examining symptoms as a potential factor influencing adherence.
Methods
Sample
For this trial patients were recruited between 2013 and 2017 from six NCI-designated Comprehensive Cancer Centers. The Institutional Review Boards at each cancer center approved this trial. The inclusion criteria were: 21 years of age or older, Eastern Cooperative Oncology Group (ECOG) score of 0–2 or Karnofsky score of ≥50, able to read and speak English, had a cellular or land line telephone, and a new prescription of any one of 33 FDA approved oral oncolytic agents selected according to their FDA approval and emerging use in treating prevalent cancers (see Table 1 for 28 agents prescribed to consented patients). Patients were excluded if they were prescribed preventive and adjuvant medications for breast cancer such as tamoxifen, raloxifene, and aromatase inhibitors. The rationale for enrolling patients at the time of initiation of the oral agent was to intervene at the time when patients were forming a new medication-taking behavior in order to prevent sub-optimal adherence. Based on their mechanisms of action, oral oncolytic agents were collapsed into four categories: cytotoxic agents, kinase inhibitors, sex hormone inhibitors, and others. Dosages of these oral oncolytic medications were either: continuous (taken every day); or intermittent with cycles, that included days when a medication was to be taken followed by rest periods. Nurses, clinical pharmacists, or premedical students approached patients who were scheduled to initiate oral agents, verified eligibility criteria, explained the study and obtained informed consent.
Table 1.
Oral agents with counts and percents of patients out of the total sample (N=272) for each drug.
| CYTOTOXICS | |
| Temozolomide | 7 (2.6) |
| Tipiracil & Trifluridine | 1 (0.3) |
| Capecitabine | 92 (33.8) |
| KINASE INHIBITORS | |
| BRC-ABL Tyrosine Kinase Inhibitor | |
| Bosutinib | 1 (0.3) |
| Imatinib | 6 (1.8) |
| Dasatinib | 2 (0.7) |
| Nilotinib | 1 (0.3) |
| VEGF/VEGFR Inhibitor | |
| Axitinib | 3 (1.1) |
| Sorafenib | 11 (4.0) |
| Sunitinib | 8 (2.9) |
| Pazopanib | 22 (8.1) |
| Lenvatinib | 1 (0.3) |
| Regorafenib | 9 (3.3) |
| EGFR HER2/neu | |
| Erlotinib | 5 (1.8) |
| Afatinib | 1 (0.3) |
| Lapatinib | 1 (0.3) |
| ALK Inhibitor | |
| Crizotinib | 4 (1.5) |
| Ceritinib | 1 (0.3) |
| BRAF Inhibitor | |
| Dabrafenib | 7 (2.6) |
| Phospoinositide 3-Kinase Inhibitor | |
| Idelalisib | 1 (0.3) |
| Cyclin Dependent Kinase Inhibitor | |
| Palbociclib | 36 (13.2) |
| Bruton’s Tyrosine Kinase Inhibitor | |
| Ibrutinib | 7 (2.6) |
| SEX HORMONE INHIBITORS | |
| Enzalutamide | 18 (6.6) |
| Abiraterone acetate | 11 (4.0) |
| OTHER | |
| Immunomodulatory | |
| Lenalidomide | 10 (3.7) |
| Pomalidomide | 1 (0.3) |
| mTOR Inhibitors | |
| Everolimus | 11 (4.0) |
| Poly ADP Ribose Polymerase Inhibitor | |
| Olaparib | 1 (0.3) |
Trial design
Recruiters collaborated with cancer center medical oncologists who identified patients initiating one of the selected oral agents. Recruiters approached patients, explained the study and gave consented patients a folder with a copy of their consent form, a passcode patients selected for telephone calls, and numbers to call if they had questions or encountered problems. Patients indicated a preferred time that they wished to be called. Recruiters collected information on dosage, frequency, and cycle of the oral agent, then forwarded this information to the study office. These data were used to program the interactive voice response (IVR) system that made daily medication reminder calls to patients randomized to the intervention arm.
Following enrollment, patients completed a baseline interview within one to three days of starting their oral oncolytic medication. After the initial interview, patients were randomized to either the intervention or standard care control arm using a minimization algorithm that was run from the central study office to ensure concealment. The algorithm balanced trial arms for recruitment location, site of cancer, regimen complexity (continuous vs. intermittent dosing), concurrent intravenous (IV) chemotherapy, and level of depressive symptoms which are known to affect adherence(8) to medications for chronic conditions and enactment of self-management strategies.(11) Subsequent interview data were collected via telephone at week 4 (trial midpoint), week 8 (trial endpoint), and week 12 (follow-up). Interviewers were blinded to trial arm assignment.
Adherence and Symptom Management Intervention
Patients randomized to the intervention arm received daily adherence reminder calls and weekly symptom assessment and management calls delivered by an IVR system.
Adherence reminders.
Each daily reminder call, delivered via a professionally recorded voice, began with a question of whether the oncologist stopped the medication. If yes, all calls were stopped, and the project manager contacted the site to determine if the oral agent stoppage was permanent or temporary. If a patient’s response was “no,” then the patient was reminded to take their oral agent(s) for that day. If a medication had a scheduled rest period, the reminder call was not placed. The goal of the daily reminder intervention was to help patients adopt the behavior of taking the medication as prescribed through establishment of a routine. At the beginning of week 5, patients had the option of continuing daily calls, or reducing calls to every other day.
Symptom assessment and management.
During weeks 2–8, patients in both arms were queried by the IVR as to whether or not they had taken their medications as prescribed and whether they experienced any of 18 cancer- or treatment-related symptoms. If a symptom was present, its severity was assessed on a 1 to 9 scale. For each of the first 8 weekly calls, patients in the intervention arm who reported any symptom at a severity of ≥4 (threshold) were referred to the Medication Management and Symptom Management Toolkit (the Toolkit) (12) which was mailed to them following randomization. The threshold of 4 was selected based on the National Comprehensive Cancer Network for symptom monitoring and management.(13) The Toolkit, written at an 8th grade level, uses a frequently asked question format, defines the symptom, its possible causes, and lists specific evidence-based strategies patients can use to alleviate the symptom.(12–14) Separate sections of the Toolkit were devoted to oral cancer medication management (adherence, storage, disposal, travel, financial assistance). The adherence section emphasized the importance of taking medication as prescribed by the physician, tips for patients on how to maintain adherence, and lists of problems for which patients should contact their oncologist.
Control condition.
Patients randomized to the control arm received weekly standard care and symptom assessment calls delivered by an IVR system.
Measures
Adherence.
Dose taken for each of three 4-week time periods (intake to week 4, week 5 to 8, and week 9 to 12) was determined based on pill counts and prescription label information on the bottle at time of enrollment, weeks 4, 8, and 12, accounting for the number of refills and number of pills per bottle. During 4, 8, and 12 week interviews, patients were asked to pour pills on a clean napkin, use a kitchen utensil to count pills, report the count to the interviewer, and to return pills to their container. Similarly, patients reported information on script labels and counted medications in each blister pack. Dose prescribed for each of the three 4-week time periods was determined based on the health record information, accounting for cycling, dose changes, and temporary and permanent stoppages in each 4-week period. For example, if medication was stopped for one out of four weeks, the prescribed dose was based on three weeks when patient was directed to take the medication by the oncologist. The RDI, ratio of dose taken to dose prescribed (primary outcome), was then calculated for each 4-week time period to reflect patient’s medication taking behavior. To facilitate the interpretation of the RDI, we have also calculated the ratio of dose taken to the FDA-approved dose. The difference between the RDI and the ratio of dose taken to FDA-approved is in the denominator: for the RDI, physician-directed stoppages and dose changes were subtracted to arrive at what the patients were dynamically directed to take by the physicians; whereas the FDA-recommended dose was fixed and based on the medication insert information.
Symptoms.
Eighteen symptoms assessed during interviews and weekly calls were selected based on their reported frequency in the inserts for the selected oral agents and prevalence in our prior research studies(15–18): pain, fatigue, sleep disturbance, anxiety, weakness, headaches, skin rash, numbness or tingling, redness or peeling in hands or feet, swelling, joint pain, mouth sores, lack of appetite, nausea or vomiting, diarrhea, constipation, cough, and shortness of breath. Patients were asked if, in the past seven days, they had experienced each symptom and, if yes, to rate its severity on a scale from 1 to 9 resulting in a summed severity index ranging from 9 to 162. This index was the secondary outcome of the trial and was analyzed at weeks 4, 8, and 12. To facilitate the interpretation, we have also analyzed the number of symptoms at 4 or higher (threshold used in the symptom management intervention) at weeks 4, 8, and 12.
Sociodemographic measures were obtained during baseline interview. Depressive symptoms were assessed during interviews using the Center for Epidemiologic Studies-Depression (CES-D) 20-item scale.(19) Cronbach’s alpha exceeded 0.90 in this sample.
Sample Size
The study was powered using both the continuous RDI as well as indicator of under-adherence defined as RDI<.80.(20) For planning purposes, we used the projected under-adherence rate of 35% and its reduction to 18% for the intervention arm, based on our preliminary work and the literature.(21,22) For power of .80 or greater in two-sided tests comparing two independent proportions (under-adherence rate by trial arm) at 0.05 level of significance, the sample size required to detect this difference was 105 patients per arm. To account for projected 23% attrition, we planned to randomize 274 patients. When adherence was measured using approximately continuous RDI measure, the sample size of 105 per arm was sufficient to detect mean RDI arm differences of 0.39 of the standard deviation at any one time point. For the secondary outcome of symptom severity, the analysis plan included the adjustment for symptom severity at intake, which led to a detectable effect size of 0.36 of the standard deviation at each time point. Detectable effect sizes were even larger in longitudinal analyses that included three repeated measures.
Statistical Analyses
Distributions of the adherence and symptom severity measures were evaluated. Proportions of under-adherent patients for each of three 4-weeks periods were compared using Fisher’s exact tests. Three repeated measures of the RDI and ratio of consumed to FDA-recommended dose (baseline to week 4, week 5 to week 8, and week 9 to week 12) were analyzed using longitudinal linear mixed effects model (LME) with the first-order autoregressive covariance structure. The LME modeling generalizes classical analysis of repeated measures and allows for the modified intent to treat approach under data missing at random (MAR) assumption, so that all patients who completed at least one post-randomization assessment (week 4, 8, or 12) were included, with no need for imputations under MAR. The following covariates were entered in the LME models: trial arm, time, and trial arm by time interaction, and factors used to balance randomization. We have also adjusted for the drug category to control for different types of oral oncolytic medications prescribed to patients. The least square (LS) means by trial arm were output from the LME models at each time, and differences by trial arm were tested at each time point. To gauge the robustness of findings, the comparisons of the adjusted LS means were complemented by the comparisons of the unadjusted means using the two-sample t-tests.
Similar analytic approach was used to evaluate three repeated measures of the summed symptom severity index and the number of symptoms above threshold at weeks 4, 8, and 12, with baseline version of each symptom outcome included as a covariate in the respective model.
Finally, to explore the relationship between symptom severity and adherence, repeated symptom measures at intake and weeks 4 and 8 were entered as a time-varying covariate into the LME models for the repeated measures of RDI described above. The direction and the significance of the association was determined based on the model coefficient for the symptom variable.
Results
The mean sample age was 61 (standard deviation 12), and 50% were males (Table 2). A total of N=233 patients completed week 4; 6 patients who skipped week 4 completed week 8 or 12, thus N=239 were analyzed in the LME models (N=122 intervention, N=117 control). Among those who dropped out, no differences by trial arm were found on sociodemographic, disease, treatment characteristics, or symptom severity at baseline. After the initial 4 weeks of daily reminders, 40% of patients in the intervention arm chose to continue with daily reminders, 38% switched to every other day, and 22% requested that their calls be stopped because they were remembering to take their oral oncolytic medications. According to patient requests, the reminder schedule was adjusted but the symptom management part of the intervention proceeded as planned. There were no differences in RDI at weeks 5–8 or 9–12 among patients according to their choice of frequency of daily reminders after 4 weeks.
Table 2.
Descriptive statistics for the sample at baseline.
| Characteristic | Experimental Arm N (%) | Control Arm N(%) |
| Sex | ||
| Male | 67 (49%) | 69 (51%) |
| Race | ||
| African American | 10 (7.3%) | 12 (9%) |
| Caucasian | 127 (92.7%) | 120 (89%) |
| Other/unknown | 0 (0%) | 3 (2%) |
| Ethnicity | ||
| Hispanic or Latino | 2 (1.5%) | 3 (2%) |
| Level of education | ||
| High school or less | 31 (22.6%) | 40 (29.6%) |
| Some or completed college | 79 (57.7%) | 71 (52.6%) |
| Graduate or professional degree | 25 (18.2%) | 24 (17.8%) |
| Unknown | 2 (1.5%) | 0 (0%) |
| Drug category | ||
| Cytotoxic agents | 50 (36.5%) | 45 (33.3%) |
| Kinase inhibitors | 64 (46.7%) | 63 (46.7%) |
| Sex hormone inhibitors | 14 (10.2%) | 13 (9.6%) |
| Other | 9 (6.6%) | 14 (10.4%) |
| Site of cancer | ||
| Breast | 27 (20%) | 30 (22%) |
| Colorectal | 21 (15%) | 20 (15%) |
| Gastrointestinal | 7 (5%) | 10 (7%) |
| Leukemia | 9 (6.5%) | 7 (5%) |
| Liver | 8 (6%) | 4 (3%) |
| Lung | 5 (4%) | 5 (4%) |
| Lymphoma | 2 (1.5%) | 1 (0.7%) |
| Melanoma | 6 (4%) | 2 (1.5%) |
| Myeloma | 3 (2%) | 4 (3%) |
| Pancreatic | 12 (9%) | 15 (11%) |
| Prostate | 13 (9.5%) | 13 (10%) |
| Renal | 15 (11%) | 9 (7%) |
| Sarcoma | 4 (3%) | 11 (8%) |
| Brain | 1 (0.5%) | 1 (0.7%) |
| Esophageal | 2 (1.5%) | 1 (0.7%) |
| Other | 2 (1.5%) | 2 (1.4%) |
| Mean (StDev) | Mean (StDev) | |
| Age | 60.60 (12.6) | 62.20 (11.9) |
| Number of symptoms above threshold | 3.12 (3.06) | 3.39 (3.27) |
| Summed symptom severity | 22.58 (21.26) | 24.36 (22.47) |
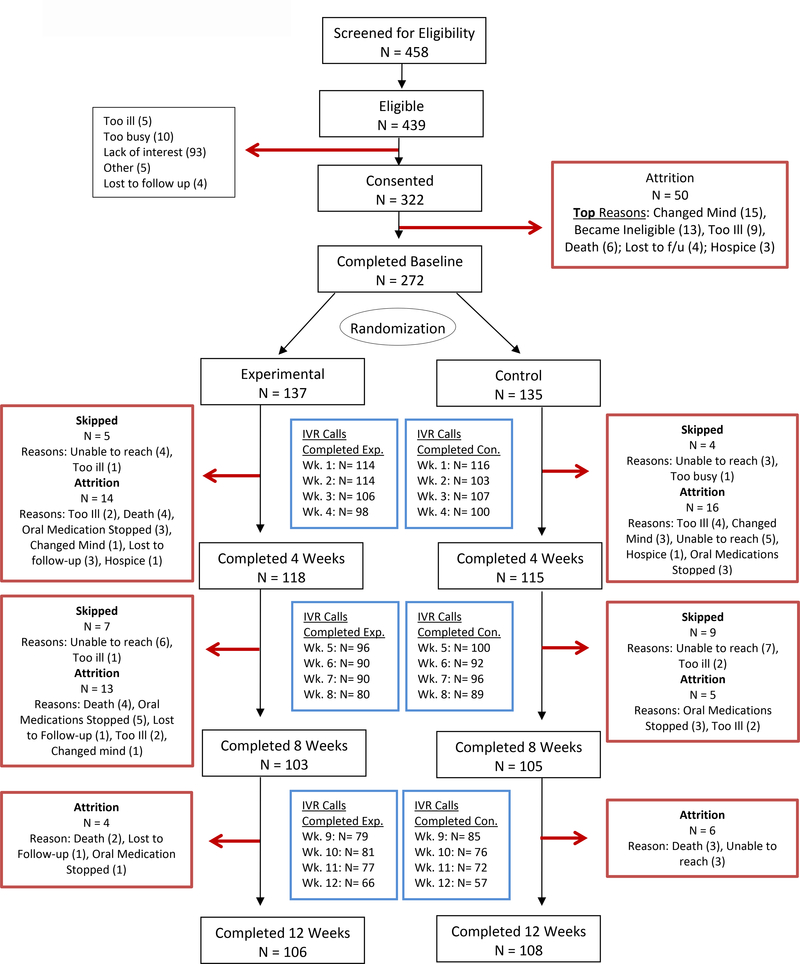
Over 70% of patients completed their weekly calls (Figure 1). Of those who completed the call each week, over 1/3 said that they had used the Toolkit during previous week. Approximately 75% of patients who reported using the Toolkit at each of the weeks 2–8, had at least one symptom above threshold during the previous week, and the remaining 25% used the Toolkit even though their symptoms were below 4 in severity. Among patients who did not use the Toolkit, over 75% stated that the reason was that their symptoms were not bothersome.
Figure 1.
CONSORT diagram.
Only one patient was under-adherent (RDI<0.8) at week 4; 8 patients (4 in each arm) were under-adherent at week 8; and none were under-adherent at week 12, with no differences by trial arm. The analyses of the continuous RDI yielded the same conclusion of high RDI with no differences by trial arm (Table 3). The ratios of dose taken to dose directed by the oncologists (accounting for interruptions) averaged 89–96% in both unadjusted and adjusted longitudinal analyses. The ratio of dose taken by patients to the FDA-recommended averaged 69–75% (Table 3).
Table 3.
Unadjusted and adjusted means of RDI and ratio of dose taken to dose FDA-approved by trial arm and time period.
| Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|
| Experimental arm Mean (SE) | Control arm Mean (SE) | 95% CI for trial arm difference P-value | Experimental arm Mean (SE) | Control arm Mean (SE) | 95% CI for trial arm difference P-value | |
| Baseline to week 4 | ||||||
| RDI | 0.94 (0.01) | 0.95 (0.01) | (−0.03, 0.01) 0.35 | 0.94 (0.01) | 0.95 (0.01) | (−0.04,0.02) 0.44 |
| Ratio of dose taken to FDA-approved | 0.75 (0.03) | 0.82 (0.03) | (−0.14, 0.02) 0.11 | 0.75 (0.03) | 0.80 (0.03) | (−0.13,0.02) 0.16 |
| Weeks 5–8 | ||||||
| RDI | 0.96 (0.01) | 0.97 (0.01) | (−0.10, 0.03) 0.33 | 0.95 (0.01) | 0.97 (0.01) | (−0.04,0.02) 0.50 |
| Ratio of dose taken to FDA-approved | 0.75 (0.033) | 0.77 (0.04) | (−0.11, 0.07) 0.59 | 0.73 (0.03) | 0.76 (0.03) | (−0.11,0.06)) 0.53 |
| Weeks 9–12 | ||||||
| RDI | 0.89 (0.03) | 0.92 (0.03) | (−0.11, 0.07) 0.62 | 0.90 (0.02) | 0.92 (0.02) | (−0.07,0.03) 0.39 |
| Ratio of dose taken to FDA-approved | 0.75 (0.06) | 0.73 (0.06) | (−0.15, 0.19) 0.81 | 0.69 (0.05) | 0.73 (0.05) | (−0.15,0.09) 0.58 |
Symptom severity was significantly lower in the experimental arm at week 8 (immediately post intervention), but not at weeks 4 (mid-intervention) or week 12 (follow-up) (Table 4). The results for the mean number of symptoms above threshold were similar, with the experimental arm having on average one fewer symptom than the control arm at week 8. For both symptom severity index and number of symptoms, differences between trial arm at week 8 were about 1/3 of the standard deviation at baseline, which is often deemed clinically significant in patient-reported outcomes literature.(23) This difference was reduced at week 12 (Table 4).
Table 4.
Unadjusted and adjusted means of symptom outcomes by trial arm and time period.
| Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|
| Experimental Arm Mean (SE) | Control Arm Mean (SE) | 95% CI for trial arm difference P-value | Experimental Arm Mean (SE) | Control Arm Mean (SE) | 95% CI for trial arm difference P-value | |
| Week 4 | ||||||
| Number of symptoms above threshold | 2.58 (0.28) | 3.04 (0.29) | (−1.26, 0.34) 0.26 | 2.46 (0.24) | 2.84 (0.24) | (−0.97,0.21) 0.21 |
| Summed symptom severity | 20.51 (1.98) | 22.86 (1.94) | (−7.82, 3.11) 0.40 | 19.26 (1.57) | 21.74 (1.60) | (−6.02,1.83) 0.21 |
| Week 8 | ||||||
| Number of symptoms above threshold | 1.88 (0.23) | 2.83 (0.26) | (−1.63, −0.26) <.01 | 1.91 (0.24) | 2.72 (0.24) | (−1.41,−0.19) 0.01 |
| Summed symptom severity | 14.56 (1.38) | 20.93 (1.70) | (−10.70, −2.04) <.01 | 14.62 (1.65) | 20.20 (1.65) | (−9.53,−1.38) <.01 |
| Week 12 | ||||||
| Number of symptoms above threshold | 2.18 (0.28) | 2.36 (0.25) | (−0.91, 0.56) 0.64 | 1.94 (0.24) | 2.35 (0.24) | (−1.02,0.21) 0.19 |
| Summed symptom severity | 16.70 (1.79) | 17.35 (1.55) | (−5.32, 4.03) 0.78 | 14.98 (1.63) | 17.47 (1.63) | (−6.75,1.40) 0.22 |
Finally, the association between symptom severity and adherence was in the expected direction, i.e., more severe symptoms at the beginning of the 4-week time period (intake, week 4, or week 8) were associated with lower RDI over the subsequent 4 weeks, but this association was not statistically significant for either the summed severity index or the number of symptoms over threshold.
Discussion
This research considered patient’s adherence to their prescribed dose of new oral oncolytic medication. The RDI was high according to the existing standards.(1) Consistent with other research that reported high rates of adherence to oral oncolytic medications,(24–26) this intervention failed to improve already high adherence observed among patients who received standard instructions and care. Data from this trial indicate that patients had no difficulty remembering to take their medications, and high RDI was sustained over the first three 4-week periods, despite dose adjustments ordered by the oncologists and the complexity of the regimens.
This finding differs from lower adherence rates to medications reported for other chronic conditions.(27,28). Moreover, it was in contrast with the higher non-adherence rates found in our pilot data which were used to inform this trial. One possible explanation is that patients in the pilot study(21,22) were further into their course of treatment and could have been more likely not to adhere to their regimen compared with these patients who were initiating treatment. Second, pilot work adherence was measured using patient self-report, and measurement did not start at the time of medication initiation, which could have led to biased estimates. Third, in this trial patients were queried weekly as to their adherence; this alone may have been adequate to sustain high rates of adherence and render daily reminders unnecessary. Future work might consider a run-in period with monitoring of adherence followed by enrollment of only those patients who demonstrate declining adherence. This would address two questions where, currently there is little evidence: what patient, disease, and treatment characteristics are associated with earlier declines in adherence; and what is the minimal intervention needed to restore and sustain therapeutically effective levels of adherence.
Compared with standard care, the self-management intervention lowered symptom severity at week 8, but this effect was not sustained at week 12. This finding is in contrast with our past work where a similar intervention had sustained effects.(18) The symptom management intervention reduced severity and number of symptoms at week 8, but once the intervention concluded patients were not able to sustain ongoing self-management. Reasons for this could be advanced disease for which oral agents represent the last available line of treatment and prior treatments leading to reduced efficacy of self-care strategies.
A common theme in systematic reviews of strategies to improve adherence among oral oncolytic medications is the low quality of the studies.(24,29,30) This trial overcame some of these weaknesses. Patients were recruited from multiple centers, objective measures of adherence were employed, and use of IVR systems assured consistent assessment and intervention delivery. Multiple drugs were incorporated into the design to extend generalizability of findings over and above a single agent or class of agents. Our analyses involved three time periods since patients’ initiation of new oral oncolytic agents.
Against these strengths, several important limitations are recognized. First, pill counts may not be the best measure of adherence, especially in large studies where counts are conducted monthly and via telephone. In this study, actual pill counts were less of a problem than tracking refills and the combining of existing oral medications with newly received vials of medications.(31) Since we were most interested in establishing adherence and self-management behaviors following the initiation of treatment, this study did not follow patients’ extended course of treatments and thus we do not know their ultimate outcomes or have clinical evidence to define disease progression.
In conclusion, this study confirms other reports of high rates of patient adherence to oral oncolytic medications.(24,26,29,32) Adherence may be less of a problem than originally anticipated.(33) and symptom management requires ongoing support and appears not sustainable by patients alone.
Disclosures and Acknowledgements
Funding: This work was supported by the National Institutes of Health (National Cancer Institute) [grant number 1R01CA162401-01A1].
Footnotes
Conflicts of Interest: The authors do not have any conflicts of interest - financial or other relationships - that could be perceived to influence this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marin D, Bazeos A, Mahon FX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol 2010;28(14):2381–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bestvina CM, Zullig LL, Rushing C, et al. Patient-oncologist cost communication, financial distress, and medication adherence. J Oncol Pract 2014;10(3):162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blaschke TF, Osterberg L, Vrijens B, Urquhart J. Adherence to medications: insights arising from studies on the unreliable link between prescribed and actual drug dosing histories. Annu Rev Pharmacol Toxicol 2012;52:275–301. [DOI] [PubMed] [Google Scholar]
- 4.Hess LM, Louder A, Winfree K, et al. Factors associated with adherence to and treatment duration of erlotinib among patients with non-small cell lung cancer. J Manag Care Spec Pharm 2017;23(6):643–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin C, Clark R, Tu P, Bosworth HB, Zullig LL. Breast cancer oral anti-cancer medication adherence: a systematic review of psychosocial motivators and barriers. Breast Cancer Res Treat 2017;165(2):247–260. [DOI] [PubMed] [Google Scholar]
- 6.Mathes T, Pieper D, Antoine SL, Eikermann M. Adherence influencing factors in patients taking oral anticancer agents: a systematic review. Cancer Epidemiol 2014;38(3):214–226. [DOI] [PubMed] [Google Scholar]
- 7.Seal BS, Anderson S, Shermock KM. Factors associated with adherence rates for oral and intravenous anticancer therapy in commercially insured patients with metastatic colon cancer. J Manag Care Spec Pharm 2016;22(3):227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DiMatteo MR. (2004). Variations in patients’ adherence to medical recommendations: A quantitative review of 50 years of research. Med Care. 2004;42(3):200–209. [DOI] [PubMed] [Google Scholar]
- 9.Ruddy K, Mayer E, Partridge A. Patient adherence and persistance with oral anticancer treatment. CA Cancer J Clin 2009;59(1):56–66. [DOI] [PubMed] [Google Scholar]
- 10.Spoelstra SL, Given CW, Sikorskii A, et al. Proof of concept of a mobile health short message service text message intervention that promotes adherence to oral anticancer agent medications: a randomized controlled trial. Telemed J E Health 2016;22(6):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Given C, Given B, Sikorskii A, et al. Deconstruction of nurse-delivered patient self-management interventions for symptom management: factors related to delivery enactment and response. Ann of Behav Med 2010;40(1):99–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Given BA, Given CW, Majeske C. Medication management & symptom management toolkit. East Lansing, MI: Michigan State University; 2013. [Google Scholar]
- 13.National Comprehensive Cancer Network. Symptoms of cancer and its treatment. Available from: https://www.nccn.org/patients/resources/life_with_cancer/side_effects.aspx Accessed December 27, 2017.
- 14.Oncology Nursing Society. PEP rating system overview. Available from: https://www.ons.org/practice-resources/pep Accessed December 27, 2017.
- 15.Given C, Given B, Rahbar M, et al. Effect of a cognitive behavioral intervention on reducing symptom severity during chemotherapy. J Clin Oncol 2004;22(3):507–516. [DOI] [PubMed] [Google Scholar]
- 16.Given C, Given B, Sikorskii A, et al. Analyzing a symptom management trial: the value of both intention-to-treat and per protocol approaches. Oncol Nurs Forum 2009;36(6):E293–302. [DOI] [PubMed] [Google Scholar]
- 17.Given C, Sikorskii A, Tamkus D, et al. Managing symptoms among breast cancer patients during chemotherapy: results of a two arm behavioral trial. J Clin Oncol 2008;26(36):5855–5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sikorskii A, Given C, Given B, et al. Symptom management for cancer patients: a trial comparing two multimodal interventions. J Pain Symptom Manage 2007;34(3):253–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radloff LS. The CES-D Scale A self-report depression scale for research in the general population. Appl Psychol Meas 1977;1(3):385–401. [Google Scholar]
- 20.Partridge AH, Archer L, Kornblith AB, et al. Adherence and persistence with oral adjuvant chemotherapy in older women with early-stage breast cancer in CALGB 49907: adherence companion study 60104. J Clin Oncol 2010;28(14):2418–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Decker V, Spoelstra S, Miezio E, et al. (2009). A pilot study of an automated voice response system and nursing intervention to monitor adherence to oral chemotherapy agents. Cancer Nurs 2009;32(6):E20–E29. [DOI] [PubMed] [Google Scholar]
- 22.Spoelstra SL, Given BA, Given CW, et al. An intervention to improve adherence and management of symptoms for patients prescribed oral chemotherapy agents: an exploratory study. Cancer Nurs 2013;36(1):18–28. [DOI] [PubMed] [Google Scholar]
- 23.Sloan JA, Cella D, Hays RD. Clinical significance of patient-reported questionnaire data: another step toward consensus. J Clin Epidemiol 2005;58:1217–1219. [DOI] [PubMed] [Google Scholar]
- 24.Zerillo JA, Goldenberg BA, Kotecha RR, et al. Interventions to improve oral chemotherapy safety and quality: a systematic review. JAMA Oncol 2018;4(1):105–117. [DOI] [PubMed] [Google Scholar]
- 25.Gebbia V, Bellavia M, Banna GL, et al. Treatment monitoring program for implementation of adherence to second-line erlotinibe for advanced non-small cell lung cancer. Clin Lung Cancer 2013;14(4):390–398. [DOI] [PubMed] [Google Scholar]
- 26.Timmers L, Boons CC, Kropff F, et al. Adherence and patients’ experiences with the use of oral anticancer agents. Acta Oncol 2014;53(2):259–267. [DOI] [PubMed] [Google Scholar]
- 27.Fuller RH, Perel P, Navarro-Ruan T, et al. Improving medication adherence in patients with cardiovascular disease: a systematic review. Heart 2018;104(15):1238–1243. [DOI] [PubMed] [Google Scholar]
- 28.Spaan P, van Luenen S, Garnefski N, Kraaij V. Psychosocial interventions enhance HIV medication adherence: a systematic review and meta-analysis. J Health Psychol February 2018. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greer JA, Amoyal N, Nisotel L, et al. A systematic review of adherence to oral antineoplastic therapies. Oncologist 2016;21(3):354–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang WC, Chen CY, Lin SJ, Chang CS. Medication adherence to oral anticancer drugs: systematic review. Expert Rev Anticancer Ther 2016;16(4):423–432. [DOI] [PubMed] [Google Scholar]
- 31.Given CW, Given BA, Sikorskii A, Krauss JC, Vachon E. Challenges to the design and testing supportive interventions for cancer patients treated with oral oncolytic agents. Support Care Cancer [In press 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruddy KJ, Pitcher BN, Archer LE, et al. Persistence, adherence, and toxicity with oral CMF in older women with early-stage breast cancer (Adherence Companion Study 60104 for CALGB 49907). Ann Oncol 2012;23(12):3075–3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bassan F, Peter F, Houbre B, et al. Adherence to oral antineoplastic agents by cancer patients: definition and literature review. Eur J Cancer Care (Engl) 2014;23(1):22–35. [DOI] [PubMed] [Google Scholar]