Abstract
A nonagenarian patient developed a right middle cerebral artery syndrome during recovery after a right internal carotid artery (ICA) balloon angioplasty. Emergent head computed tomography (CT) revealed no acute ischemic changes; CT angiography (CTA) and CT perfusion (CTP) demonstrated a right ICA occlusion with a large right hemispheric predicted core infarct by cerebral blood flow thresholds and minimal mismatch volume. She underwent complete reperfusion in <45 min from symptom onset. Magnetic resonance imaging brain obtained within 48 h showed a decreased infarct volume as that estimated by CTP. This case emphasizes the limitations of estimating the ischemic core with CTP in the golden hour with ultra-early reperfusion and suggests that CTP thresholds should not be used to exclude patients from treatment in the very early time window.
Keywords: Acute ischemic stroke, cerebral blood flow, computed tomography perfusion, endovascular treatment, ghost core infarct
Introduction
Determining the irreversibly injured tissue has major implications for acute stroke management, including the decision to pursue reperfusion therapies, hemorrhagic transformation prognosis, and long-term clinical outcome.[1] Currently, most dedicated stroke centers have incorporated multimodal imaging techniques for acute stroke detection.[2] Although magnetic resonance imaging (MRI) is an imaging modality accepted to evaluate for acute stroke, due to its impracticality, computed tomography (CT) perfusion (CTP)-derived hemodynamic thresholds are being widely incorporated to delineate acute tissue states.[1,2] In addition to being increasingly available, CT angiography (CTA) with CTP is fast, safe, affordable, and obtained simultaneously with a single contrast bolus.[3,4,5] While its effectiveness in selecting patients for mechanical thrombectomy was demonstrated in two of the early window trial (0–6 h),[6,7] its use in routine clinical practice became widely spread after the 2018 American Heart Association Stroke Guidelines recommended the use of perfusion imaging as the preferred selection method for patients with large vessel occlusion presenting between 6 and 24 h after last seen well.[8,9]
CTP provides maps for predicting core and tissue at risk, but the accuracy of prediction is not well established, particularly during the early window.[5] Infarct core areas are predicted using different thresholds of cerebral blood flow (CBF) and cerebral blood volume (CBV). Recent publications suggest that the use of CTP-CBF technique may overestimate infarct core volume in the very early window and with fast complete reperfusion, also known as the ghost infarct core concept.[10,11] While acute stroke management workflow and early recanalization improve, inaccurate calculation of core infarct might deprive eligible patients from reperfusion therapy.[5,12] Herein, we present a case of ultra-early reperfusion in a patient with a right internal carotid artery (ICA) occlusion, in which a preprocedural CTP failed to accurately estimate the core infarct volume.
Case Report
A nonagenarian patient presented to an outside hospital with left facial weakness. She was noted to have hypertension with blood pressure of 240/140 mmHg, for which she received labetalol. This was followed by worsening of her neurological exam with aphasia, left hemiplegia, and right gaze deviation. Head CT was negative for acute findings including no early signs of ischemia. A head-and-neck CTA demonstrated a right ICA occlusion at the cervical bifurcation and no obvious intracranial large vessel occlusion. Intravenous (IV) tissue plasminogen activator (tPA) was administered, and she was then transferred to our comprehensive stroke center for further management. On initial examination, she presented with an NIHSS score of 4 for left facial droop, left arm paresis, and mild dysarthria. Given her fluctuating symptoms, the decision was made to proceed with digital cerebral angiography (DSA) for potential intervention. A 95% stenosis (per NASCET criteria) of the right ICA bifurcation was noted and treated with balloon angioplasty alone, achieving > 50% luminal recanalization and immediate resolution of the symptoms.
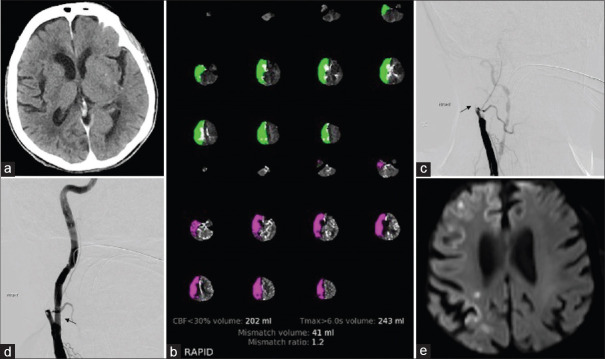
Since the patient received IV tPA, we decided to defer the stent placement for a few days to optimize the antiplatelet regimen. 15 min after the procedure was finalized, she developed acute worsening of her neurological exam with lethargy, dense left hemiplegia, and left hemineglect with a total NIHSS score of 21. She was taken emergently for head CT which did not demonstrate reperfusion hemorrhage or acute ischemia (ASPECTS = 10) [Figure 1a]. Concomitantly, CTA and CTP were performed and automatically reconstructed using an automated perfusion CT software package (RAPID). CTA and CTP demonstrated a complete occlusion of the right ICA with no visualized distal clot, a large core infarct of 202 ml, and hypoperfusion of 243 ml, according to CBF <30% and Tmax>6 s, respectively [Figure 1c]. She was immediately taken back to the operating room, loaded with aspirin and 300 mg of Plavix, and underwent right ICA stent-assisted percutaneous transluminal angioplasty (SAPTA). A complete carotid recanalization without any distal emboli was obtained in <60 min from symptom onset [Figure 1b and d]. An MRI performed 2 days after symptom onset demonstrated scattered small acute infarcts involving the right anterior cerebral artery and middle cerebral artery vascular territories with a total infarct volume of 3.17 cc [Figure 1e].
Figure 1.
(a) Noncontrast computed tomography head, axial image; no evidence of hemorrhage or signs of acute ischemia. (b) RAPID output demonstrates a large area of matched deficit of cerebral blood flow and cerebral blood volume maps, indicative of core infarct in the right anterior cerebral artery and middle cerebral artery territory. (c) Digital cerebral angiography image of the right carotid bifurcation demonstrates occlusion of the internal carotid artery (black arrow). (d) Digital cerebral angiography reveals successful stent placement with little residual stenosis of the right internal carotid artery (black arrow). (e) Magnetic resonance imaging brain, diffusion-weighted imaging acquisition, demonstrated small scattered areas of diffusion restriction indicative of ischemic infarct
The postoperative period was complicated with fluctuation of her neurological status, suspicious of complex partial seizures, and aspiration pneumonia. Her respiratory status gradually deteriorated, requiring noninvasive ventilation with bilevel positive airway pressure. After a 7-day stay in the intensive care unit, the decision was made by the patient's family to pursue comfort care measures. She expired thereafter.
Discussion
Recent literature suggests that CTP thresholds associated with follow-up infarction vary based on the time from symptom onset to imaging.[3,12] The ability of CBV-CTP and CBF-CTP to define irreversible ischemic core decreases with a shorter time from symptom onset to imaging,[10] and its frequency might increase as a result of rapid advances in acute stroke management and an increased frequency of early presentation and reperfusion. Herein, we present a case of ultra-early presentation and reperfusion after carotid stenting, where the core overestimation is evident [Supplementary Table 1]. Although the rate of early or late spontaneous recanalization is in the range of 5%–30%,[13] it is important to define the potentially salvageable tissue to help select patients who are most likely to benefit from intervention.
Supplementary Table 1.
Clinical and imaging findings
| Setting and time from symptom onset | Outside hospital 0 h-2 h | CSC arrival 4 h | 1st endovascular intervention 4 h 40 min-6 h | Recovery period 6 h-6 h 20 min | 2nd endovascular intervention 6 h 20 min | ICU 2 days |
|---|---|---|---|---|---|---|
| Imaging findings | CT Head: no acute ischemic changes (ASPECTS 10) or hemorrhage | DSA: 95% stenosis (per NASCET criteria) of the right ICA bifurcation | CT head: no acute ischemic changes (ASPECTS 10) or hemorrhage | DSA: complete occlusion of the right internal carotid artery at its origin | MRI brain: smgroup scattered infarcts within the right ACA and MCA territories | |
| CTA head and neck: occlusion of the right ICA | →Treated with bgroupoon angioplasty alone, achieving >50% luminal recanalization and immediate resolution of the symptoms | CTA and CTP: occlusion of the right ICA, large core infarct of 202 ml and hypoperfusion of 243 ml, according to CBF<30% and Tmax >6 sec., respectively | →Treated with bgroupoon angioplasty and stenting with improved degree of stenosis and flow with TICI III recanalization | |||
| Clinical findings | Left facial droop and ataxia | NIHSS score of 4: left facial droop, left arm paresis and mild dysarthria | NIHSS score of 21: findings included lethargy, left hemiplegia and left hemineglect | |||
| Symptoms acutely worsened after she received labetalol for elevated blood pressure. She developed aphasia, left hemiplegia and right gaze deviation |
h: hour(s), min: Minutes, CSC: Comprehensive Stroke Center, ICU: Intensive care unit, CT: Computed tomography, ASPECTS: Alberta Stroke Program Early CT Score, CTA: Computed tomography angiography, ICA: Internal carotid artery, DSA: Digital subtraction angiography, NASCET: North American Symptomatic Carotid Endarterectomy Trial, CTP: Computed tomography perfusion, CBF: Cerebral flood flow, Tmax: Time to the maximum of the residue function, TICI: Thrombolysis in Cerebral Infarction, ACA: Anterior cerebral artery, MCA: Middle cerebral artery, NIHSS: National Institutes of Health Stroke Scale
Our institution uses RAPID (iSchemaView, Inc., Menlo Park, California, USA), a fully automated postprocessing software for quantitative CTP analysis, which has been demonstrated to provide rapid and accurate prediction of core stroke volumes.[14,15] RAPID software defines regions of ischemic core as severely reduced CBF (<30% of the total normal tissue) and penumbra as hypoperfused areas were the contrast agent exceeded t seconds (Tmax> 6). However, as evidenced in our case, a CBF <30% threshold might need to be used with caution to predict ischemic core in the ultra-early window. In our case, MRI diffusion-weighted imaging (DWI) revealed a volume of 3.17 cc, while CBF <30% was 202 cc. When adjusting for different CTP thresholds, we notice that more stringent thresholds (CBF <8%; CBV <3%) were better in predicting follow-up infarction in the prefrontal area [Supplementary Figure 1 (207.5KB, tif) ].
A potential explanation is that, while DWI abnormalities capture histological changes related to neuronal death such as cytotoxic edema due to energy failure, CTP-CBF represents a hemodynamic measure that reflects the rate of the contrast (blood) flow through the brain tissue. In the very early stages of the ischemic cascade, neuronal survival is more heterogeneous with prompt recanalization.[12] This case supports such findings, in which previously established CBF <30% thresholds overestimated the final infarct volume in a patient with ultra-early reperfusion. It also illustrates, as previously reported, that CTP perfusion threshold is time dependent in the very early window.[16] Recognition and understanding of this concept are paramount for optimizing the selection and triage of patients who present very early from stroke onset.
Conclusion
Recent changes in acute stroke management have created a pressing need for improved acute stroke detection and patient selection for reperfusion therapies. A misleading overestimation of ischemic core using the current CBF threshold could exclude patients who may potentially benefit from endovascular therapy. With the advent of faster and more efficient endovascular treatment of acute ischemic stroke, lower CTP-CBF thresholds might have to be adapted when CTP is used as a multimodal selection imaging to avoid excluding such patients. Although other advanced imaging techniques could be used to better predict the tissue at risk (e.g., magnetic resonance perfusion-diffusion mismatch), its impracticality and limited availability have limited its widespread adoption. In the early window <6 h after symptom onset, a noncontrast CT head might be preferable to estimate infarcted core and avoid wrong exclusions from mechanical thrombectomy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Examples of distinct computed tomography perfusion parametric maps (a) cerebral blood flow and (b) cerebral blood volume adjusting to different thresholds, depicting a similar infarct volume in the prefrontal region as that present in the (c) magnetic resonance imaging brain, diffusion-weighted imaging acquisition, obtained 2 days after stroke onset. The lesion in the primary motor cortex is not visualized on computed tomography perfusion. Scattered infarcts in the magnetic resonance imaging could not be visualized by either computed tomography perfusion-cerebral blood flow or computed tomography perfusion-cerebral blood volume
References
- 1.d'Esterre CD, Aviv RI, Morrison L, Fainardi E, Lee TY. Acute multi-modal neuroimaging in a porcine model of endothelin-1-induced cerebral ischemia: Defining the acute infarct core. Transl Stroke Res. 2015;6:234–41. doi: 10.1007/s12975-015-0394-x. [DOI] [PubMed] [Google Scholar]
- 2.Abels B, Villablanca JP, Tomandl BF, Uder M, Lell MM. Acute stroke: A comparison of different CT perfusion algorithms and validation of ischaemic lesions by follow-up imaging. Eur Radiol. 2012;22:2559–67. doi: 10.1007/s00330-012-2529-8. [DOI] [PubMed] [Google Scholar]
- 3.d'Esterre CD, Boesen ME, Ahn SH, Pordeli P, Najm M, Minhas P, et al. Time-dependent computed tomographic perfusion thresholds for patients with acute ischemic stroke. Stroke. 2015;46:3390–7. doi: 10.1161/STROKEAHA.115.009250. [DOI] [PubMed] [Google Scholar]
- 4.Limaye K, Bryant A, Bathla G, Dai B, Kasab SA, Shaban A, et al. Computed tomography angiogram derived from computed tomography perfusion done with low iodine volume protocol preserves diagnostic yield for middle cerebral artery-m2 occlusions. J Stroke Cerebrovasc Dis. 2019;28:104458. doi: 10.1016/j.jstrokecerebrovasdis.2019.104458. [DOI] [PubMed] [Google Scholar]
- 5.Konstas AA, Gilberto Gonzalez R, Lev MH. CT Perfusion (CTP) In: Gilberto González R, Hirsch JA, Lev MH, Schaefer PW, Schwamm LH, editors. Acute Ischemic Stroke. 2nd ed. Heidelberg, Germany: Springer-Verlag Berlin Heidelberg; 2011. pp. 83–121. [Google Scholar]
- 6.Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–18. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 7.Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs.t-PA alone in stroke. N Engl J Med. 2015;372:2285–95. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 8.Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378:11–21. doi: 10.1056/NEJMoa1706442. [DOI] [PubMed] [Google Scholar]
- 9.Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378:708–18. doi: 10.1056/NEJMoa1713973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boned S, Padroni M, Rubiera M, Tomasello A, Coscojuela P, Romero N, et al. Admission CT perfusion may overestimate initial infarct core: The ghost infarct core concept. J Neurointerv Surg. 2017;9:66–9. doi: 10.1136/neurintsurg-2016-012494. [DOI] [PubMed] [Google Scholar]
- 11.Martins N, Aires A, Mendez B, Boned S, Rubiera M, Tomasello A, et al. Ghost infarct core and admission computed tomography perfusion: Redefining the role of neuroimaging in acute ischemic stroke. Interv Neurol. 2018;7:513–21. doi: 10.1159/000490117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Najm M, Al-Ajlan FS, Boesen ME, Hur L, Kim CK, Fainardi E, et al. Defining CT perfusion thresholds for infarction in the golden hour and with ultra-early reperfusion. Can J Neurol Sci. 2018;45:339–42. doi: 10.1017/cjn.2017.287. [DOI] [PubMed] [Google Scholar]
- 13.Shah QA. Spontaneous recanalization after complete occlusion of the common carotid artery with subsequent embolic ischemic stroke. J Vasc Interv Neurol. 2009;2:147–51. [PMC free article] [PubMed] [Google Scholar]
- 14.Campbell BC, Yassi N, Ma H, Sharma G, Salinas S, Churilov L, et al. Imaging selection in ischemic stroke: Feasibility of automated CT-perfusion analysis. Int J Stroke. 2015;10:51–4. doi: 10.1111/ijs.12381. [DOI] [PubMed] [Google Scholar]
- 15.Albers GW, Goyal M, Jahan R, Bonafe A, Diener HC, Levy EI, et al. Ischemic core and hypoperfusion volumes predict infarct size in SWIFT PRIME. Ann Neurol. 2016;79:76–89. doi: 10.1002/ana.24543. [DOI] [PubMed] [Google Scholar]
- 16.Qiu W, Kuang H, Lee TY, Boers AM, Brown S, Muir K, et al. Confirmatory study of time-dependent computed tomographic perfusion thresholds for use in acute ischemic stroke. Stroke. 2019;50:3269–73. doi: 10.1161/STROKEAHA.119.026281. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Examples of distinct computed tomography perfusion parametric maps (a) cerebral blood flow and (b) cerebral blood volume adjusting to different thresholds, depicting a similar infarct volume in the prefrontal region as that present in the (c) magnetic resonance imaging brain, diffusion-weighted imaging acquisition, obtained 2 days after stroke onset. The lesion in the primary motor cortex is not visualized on computed tomography perfusion. Scattered infarcts in the magnetic resonance imaging could not be visualized by either computed tomography perfusion-cerebral blood flow or computed tomography perfusion-cerebral blood volume