Abstract
Background:
Scarring is one of the most dreadful complications of acne for which patients seek surgical treatment.
Objective:
The aim of this research was to study the morphological features of acne scarring and the relationship between severity of acne and its treatment with type and severity of acne scars.
Materials and Methods:
This was a hospital-based, noninterventional, cross-sectional study carried out over a period of 1 month on 100 patients with post-acne scarring. A morphological evaluation of the types, sites, and severity of acne scars was done, and details of the severity and treatment of acne were recorded.
Results:
Of 100 patients included in the study, 61 were male and 39 were females. Females had an earlier onset of acne (15.8 years) as compared to males (16.5 years). The mean duration of active acne was longer in males (99.3 months) than that in females (74.4 months). Male patients had more severe acne vulgaris as compared to females (P = 0.0001). Of 100 patients, 52 started treatment 1 year after the onset of acne, and 18 patients had never taken any anti-acne medication. Morphologically, majority of post-acne scars were ice pick scars in 94% patients, followed by rolling scars in 86%, boxcar scars in 54%, and keloidal scars in 10% patients. Male patients had more severe acne scarring than females (P < 0.05). Of 54 patients with severe acne, 22 progressed to moderate grade and 32 patients progressed to severe grade of acne scarring. Significant reduction in the severity of acne scarring was observed in patients who received isotretinoin as compared to that in patients who received oral antibiotics.
Conclusion:
Majority of patients with active acne delay treatment, which leads to increased acne scarring. Ice pick scars are the most common type of acne scars, and keloidal scars are more common in males. Males have a longer duration of acne, they delay treatment, and have more severe acne scarring. Early introduction of oral isotretinoin may help to reduce the severity of acne scarring. Public education is essential to urge patients to seek early and appropriate treatment of acne that can reduce the incidence and severity of acne scarring and its psychosocial consequences.
Keywords: Acne, acne treatment, boxcar, ice pick, keloidal, post-acne scars, rolling
INTRODUCTION
Acne vulgaris is a chronic inflammatory disorder, which occurs due to retention of desquamated keratinocytes within the pilosebaceous unit forming a microcomedo, which eventually ruptures, leading to inflammation and scarring.[1] It is one of the most common conditions for which patients seek dermatological care.[2] The condition usually starts in adolescence, peaks at the age of 14–19 years with a prevalence of almost 95% and frequently resolves by mid-twenties.[3] Scarring is one of the most dreadful sequelae of acne for which patients frequently seek surgical treatment. It is associated with profound psychosocial disabilities, affecting the quality of life.[4] In patients with active acne, up to 95% of individuals show some degree of facial scarring.[5] Also delayed treatment and severity of acne are associated with a greater extent and severity of scarring. Acne scarring has been categorized into increased tissue formation (including hypertrophic and keloid scars) and more commonly loss of tissue (including ice pick, rolling, and boxcar type scars).[6] Surgical treatment of acne scarring is individualized mainly according to the morphology of acne scars. There are very few Indian studies on the morphological profile of acne scarring and the correlation between onset of treatment and severity of acne with the severity of acne scars. The aim of the study was to address these issues.
MATERIALS AND METHODS
This noninterventional, cross-sectional observation study was performed in the outpatient department of dermatology in a tertiary care hospital in north India, for a period of 1 month. Patients of all age-groups presenting for treatment of acne scars were included in the study. A written informed consent was obtained, and a standardized questionnaire to be answered by patients was formed. The study questionnaire included two different sections. The first section included details of the patient along with the age of onset of active acne and their mean duration, site and type of lesions (photographs with examples of comedones, papules, pustules, and cysts were shown to patients to help them to recognize the features), family history of acne vulgaris, gap between the onset of active acne, and treatment taken and details of treatment taken for active acne were noted. A grading system devised by Lehmann et al.[7] for severity of acne was used [Table 1]. Any history of exposure to drug, sun exposure, seasonal variation, stress, and premenstrual flare were also obtained. Factors pointing toward hormonal imbalance, including obesity, hirsutism, and alopecia, were noted. Second section included morphology, number, and sites of post-acne scars. Grading of severity of acne scars was done using the qualitative global scarring grading system by Goodman and Baron[8] [Table 2].
Table 1.
Lehmann et al.[7] grading system for severity of acne
| Severity of acne | Number of comedones | Number of pseudocysts | Number of inflammatory lesions | Total lesion count |
|---|---|---|---|---|
| Mild acne | <20 | - | <15 | <30 |
| Moderate acne | 20–100 | - | 15–50 | 30–125 |
| Severe acne | >100 | >5 | >50 | >125 |
Table 2.
Goodman and Baron qualitative scarring grading system[8]
| Grades of post-acne scarring | Level of disease | Clinical features |
|---|---|---|
| 1 | Macular | These scars can be erythematous, hyper- or hypopigmented flat marks. They represent a change in color and not of contour or depth. |
| 2 | Mild | Mild atrophy or hypertrophy scars that may not be obvious at social distances of 50 cm or greater and may be covered adequately by makeup or the normal shadow of shaved beard hair in men or normal body hair if extrafacial. |
| 3 | Moderate | Moderate atrophic or hypertrophic scarring that is obvious at social distances of 50 cm or greater and is not covered easily by makeup or the normal shadow of shaved beard hair in men or body hair if extrafacial, but is still able to be flattened by manual stretching of the skin (if atrophic). |
| 4 | Severe | Severe atrophic or hypertrophic scarring that is evident at social distances greater than 50 cm and is not covered easily by makeup or the normal shadow of shaved beard hair in men or body hair if extrafacial and is not able to be flattened by manual stretching of the skin. |
Statistical analysis: The collected data were tabulated in Microsoft Excel worksheet. For constant variables, values (mean and median) and scattering (standard deviation [SD] and range) were calculated. Differential analyses were performed using the parametric, independent two-sample t test and nonparametric Mann–Whitney U test. Computer-based statistical assessments were performed using the Statistical Package for the Social Sciences (SPSS) software, version 18.0 (SPSS, Chicago, Illinois).
RESULTS
A total of 100 patients presenting with post-acne scars were studied, including 61 male and 39 female patients. Male-to-female ratio was 1.56:1. Females had an earlier onset of acne vulgaris, with the mean age of first appearance at 15.8 years (SD, ±3.5 years, range 12–31 years), whereas the mean age of first appearance in males was 16.5 years (SD, ±4.4 years, range 14–33 years) (P < 0.05). When compared between genders, the mean duration of active acne in males was 99.3 months (SD, ±48.7 months, range 6–300 months), which was found to be slightly longer than females, that is, 74.4 months (SD, ±47.7 months, range 5–204 months) (P < 0.05). Family history was positive in 48%.
Of 100 patients, nine patients had given a history of application of topical steroid before the onset of acne. Five patients had onset of acne after intake of oral medications, which included two patients due to oral steroids, two patients due to antitubercular therapy, and one due to anticonvulsant therapy. Photoaggravation was noted in 24 patients. There was seasonal aggravation, most commonly in summer (28%), followed by rainy (10%) and winter season (2%). Seborrheic dermatitis was found to be associated with acne vulgaris in 23 patients. Aggravation of acne due to stress was observed in 22 patients. Premenstrual flare was noted in seven females, of which four were obese, five had associated hirsutism, and three had a history of patterned alopecia.
Face was involved in all the patients with acne vulgaris, which included the cheeks (92%), forehead (42%), jaw (52%), chin (26%), and nose (15%). This was followed by the involvement of the back (58%), chest (25%), and arms (12%) [Table 3]. The most common type of lesion predominantly observed was comedones (92%) followed by papules (88%), pustules (74%), nodules (43%), and cysts (6%). No significant difference was observed between male and female patients with regard to type of acne lesions (P > 0.05) [Figure 1].
Table 3.
Gender-wise distribution of acne
| Site | Male (%) | Female (%) | Total | |
|---|---|---|---|---|
| Face | Cheek | 55 | 36 | 92 |
| Forehead | 31 | 11 | 42 | |
| Jaw | 33 | 19 | 52 | |
| Chin | 10 | 16 | 26 | |
| Nose | 8 | 7 | 15 | |
| Back | 40 | 18 | 58 | |
| Chest | 17 | 8 | 25 | |
| Arm | 8 | 4 | 12 | |
Figure 1.
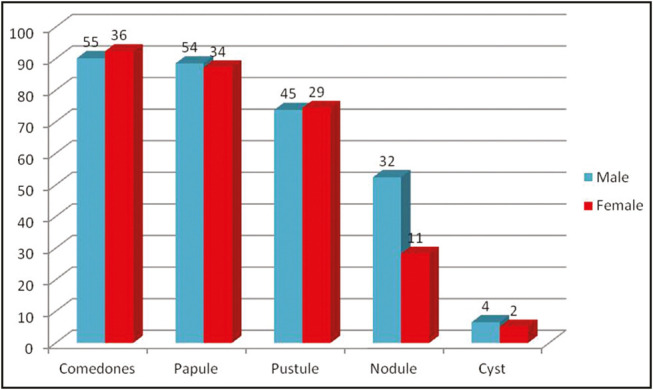
Gender-wise distribution of type of acne
For the severity of acne, Lehmann et al.[7] grading system was used. According to this grading system, a total of 18 patients had mild acne, 28 had moderate acne, and 54 had severe acne. It was observed that male patients had more severe acne vulgaris (P = 0.0001) as compared to females (36 males and 18 females had severe acne vulgaris) [Figure 2].
Figure 2.
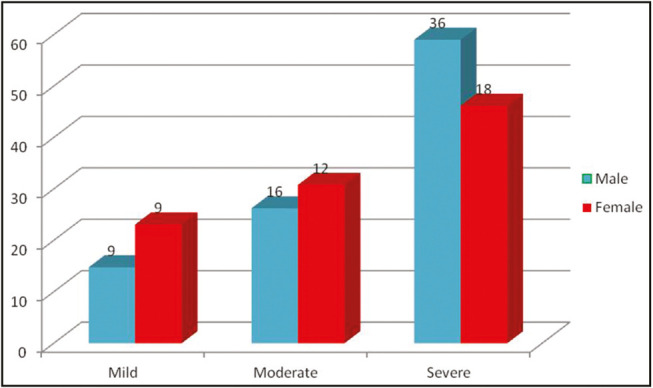
Gender-wise distribution of severity of acne
Treatment was delayed in a majority of patients, where 52 patients had taken treatment 1 year after the onset of acne vulgaris and 18 patients had never taken any treatment. The reasons for delay in treatment included lack of knowledge regarding the natural course of acne (most patients and their caregivers thought acne was a natural phenomenon of growing up and would disappear spontaneously), tried self treatment or that suggested by friends, and not concerned till the development of acne scars. A majority of patients (92%, 53 males and 39 females) said they would have taken treatment earlier by a dermatologist if they knew acne can lead to permanent facial scars. Of 82 patients who had taken treatment, only 11 patients were given oral isotretinoin, 45 had received oral antibiotics, and all of them had received topical treatment.
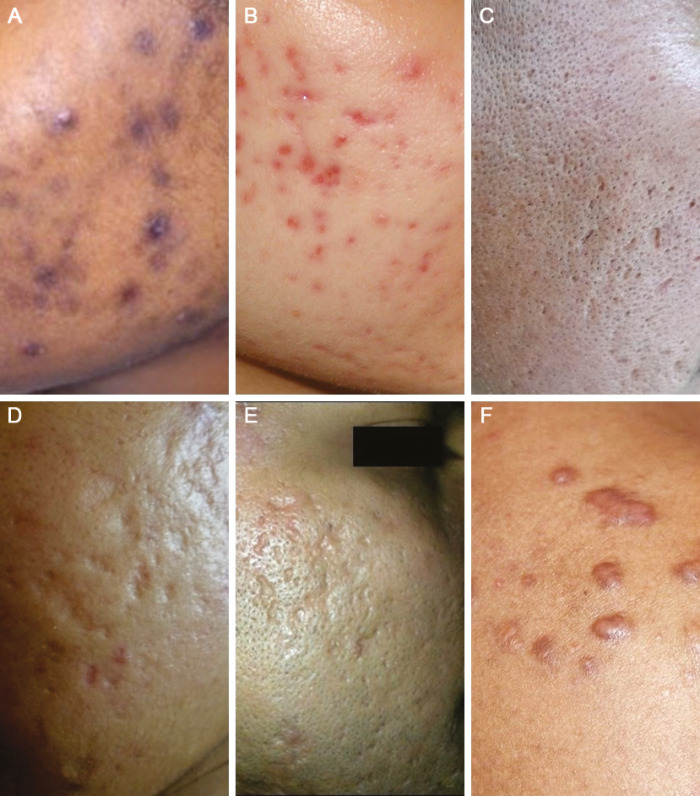
The mean age of presentation of acne scars was 26.2 years (SD, ±4.5 years, range 19–40 years). The mean duration of post-acne scars before seeking treatment in males was slightly longer, 62.5 months (SD, ±11.2 months, range 6–122 months), than that in females, 55.8 months (SD, ±8.4 months, range 2–97 months) (P < 0.05). Majority of post-acne scars were ice pick scars in 94% patients, followed by rolling scars in 86%, boxcar scars in 54%, and keloidal scars in 10% patients [Figure 3A–F]. Post-acne hyperpigmentation was observed in 54% of the patients [Figure 4]. In all the types of scars, the most common area involved was face (92%), followed by back (5%) and chest (3%) [Table 4].
Figure 3.
Morphological types of acne scars. (A) Hyperpigmented macular scars in type 5 skin. (B) Persistent erythematous macules. (C) Ice pick scars. (D) Rolling scars. (E) Boxcar scars. (F) Keloidal scars on the shoulder
Figure 4.
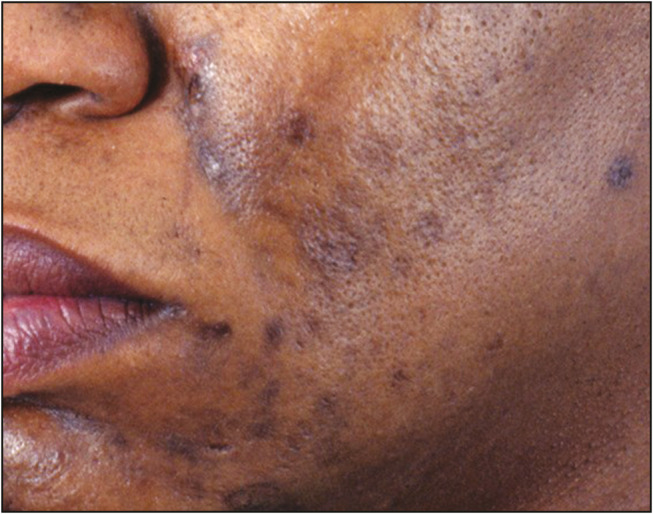
Post-acne pigmentation
Table 4.
Type and site of acne scars
| Type of scar | Site of scar | Male (%) | Female (%) | Total |
|---|---|---|---|---|
| Ice pick scar | Face | 57 | 35 | 94 |
| Chest | 2 | 0 | ||
| Back | 0 | 0 | ||
| Rolling | Face | 49 | 34 | 86 |
| Chest | 1 | 0 | ||
| Back | 2 | 0 | ||
| Boxcar | Face | 37 | 9 | 54 |
| Chest | 2 | 1 | ||
| Back | 2 | 3 | ||
| Hypertrophic/ keloidal scar | Face | 4 | 0 | 10 |
| Chest | 1 | 2 | ||
| Back | 2 | 1 |
According to the Goodman and Baron qualitative grading for acne scar, acne scar was found to be macular in 22 patients, mild in 12 patients, moderate in 29 patients, and severe in 37 patients. It was observed that male patients (28%) were having more severe acne scarring than females (9%) (P < 0.05) [Figure 5]. It was observed that of 11 patients who had taken oral retinoids for acne treatment, six patients developed macular acne scarring, two developed mild acne scar, two developed moderate scar, and one developed severe scarring; whereas of 45 patients who had taken oral antibiotics, 7 patients developed macular acne scarring, 8 developed mild acne scar, 13 developed moderate scar, and 17 developed severe scarring. A significant difference was observed in the severity of scarring in patients who had taken oral retinoids as compared to that in patients who had taken oral antibiotics as a part of treatment (P < 0.05) [Figure 6].
Figure 5.
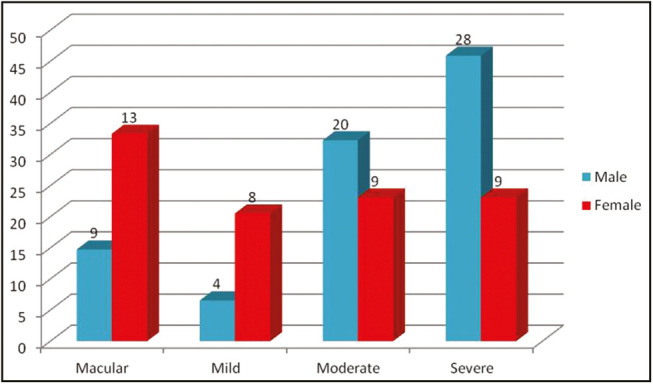
Gender-wise distribution of severity of acne scars
Figure 6.
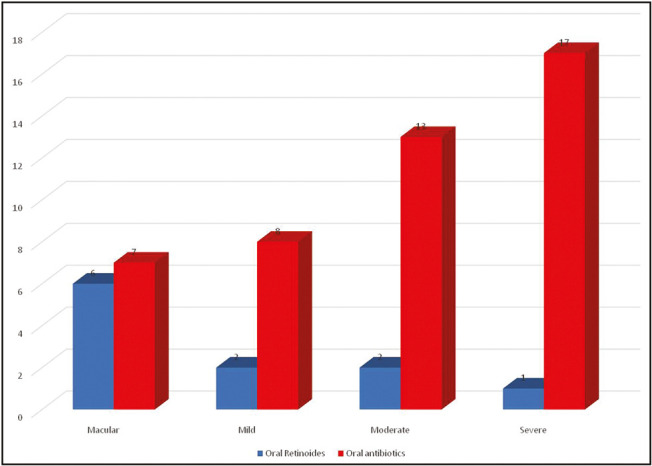
Comparison of severity of acne scars between patients receiving isotretinoin or oral antibiotics
A comparative study was done to assess relationship between severity of acne and severity of acne scarring [Table 5]. Of 18 patients having mild acne, 14 patients progressed to macular acne scarring. Of 54 patients with severe acne, 22 progressed to moderate grade and 32 patients progressed to severe grade of acne scarring.
Table 5.
Comparison between severity of acne vulgaris and severity of acne scarring
| Severity of acne | Severity of acne scarring |
|||
|---|---|---|---|---|
| Macular | Mild | Moderate | Severe | |
| Mild | 14 | 3 | 1 | 0 |
| Moderate | 8 | 9 | 6 | 5 |
| Severe | 0 | 0 | 22 | 32 |
DISCUSSION
Acne vulgaris is the most common chronic skin condition that is virtually universal in the adolescent age-group. Acne scarring is an unfortunate sequelae of acne and is a devastating and permanent complication of acne vulgaris, affecting almost 95% of patients.[9] It may be associated with physical and psychological distress, particularly in young adults, resulting in low self-esteem and diminished quality of life.[10] Majority of the patients (85%) with acne scars have loss of collagen (atrophic scars), whereas a minority of them show hypertrophic scars and keloids.[11] Patients commonly seek surgical treatment for acne scars, which is individualized according to the type and morphology of scars. There are very few Indian studies on the morphological profile of acne scarring and the correlation between severity of acne, delay in treatment with severity of acne scars. We conducted a study on 100 patients who presented to the outpatient department with surgical treatment for post-acne scars. In our study, males were comparatively more than females with male:female ration of 1.56:1. This may be because male patients had more severe acne vulgaris, which progressed to acne scarring as recorded by Kilkenny et al.[12] The mean age of presentation of patient with acne scars was 26.2 years.
In our study, females had an earlier onset of acne vulgaris, with the mean age of first appearance at 15.8 years as compared to males. This may be due to earlier onset of puberty in females. Similar results were also observed by Lucky et al.[13] in their study. In our study, the mean duration of active acne was found to be slightly longer in males (99.3 months) as compared to that in females (74.4 months) (P < 0.05). Conversely, Chuah and Goh[14] observed that mean duration of active acne was slightly longer in females (109.3 months) as compared to that in males (78.4 months).
It has been suggested that genetic factors play a role in acne development and persistence. In our study, family history of acne with first-degree relatives involved was found in 48%. Our results were comparable to a study by Goulden et al.,[15] who observed an incidence of 50% in first-degree relatives. Also in our study, drug-induced acne vulgaris was observed in 14 patients, majority of them were due to use of topical steroids followed by oral steroids, antitubercular drugs, and anticonvulsant therapy. Steroids have been implicated in causation of acne by inducing hypercornification of the upper portion of pilosebaceous unit. In their study, Goulden et al.[15] observed that prednisolone, lithium, anabolic steroids, and phenytoin were causative factors for acne in five patients. Topical steroid abuse also aggravates the severity of acne, leads to delay in treatment, and subsequently delayed healing, and paves the way to increased facial scarring, telangiectasia, and hypertrichosis.
Conventionally, it is observed that acne aggravates in winter, but in most of the Indian studies, it is observed to be aggravated in summer or on photo exposure.[16] Similarly in our study, seasonal aggravation was seen in summer. Motoyoshi[17] suggested that seasonal variation in acne may be due to ultraviolet (UV) radiation–induced generation of squalene peroxides, which are highly comedogenic.
In our study, seborrheic dermatitis was found to be associated with acne vulgaris in 23 patients. Seborrhea is well known to play a central role in both the diseases. Increased level of androgens, increased sensitivity of the sebaceous gland to androgens, or increased local metabolism of androgen hormones in the skin to potent androgen metabolites are postulated mechanisms of pathogenesis of acne vulgaris.[18] Adityan and Thappa[19] also observed similar results in their study. Clinical features suggestive of hyperandrogenism such as premenstrual flare, hirsutism, obesity, and alopecia were observed in total of 12 females (30.77%) in our study. These results were similar to a study conducted by Goulden et al.,[15] who observed that 37% of women in their study had at least one feature of possible hyperandrogenism. It has been suggested that an increased level of androgen and cortisol is associated with chronic stress, leading to aggravation of acne.[20] We observed stress-induced aggravation of acne in 22% of our patients. This is in contrast to a study by Goulden et al.,[15] who reported that in 71% of their patients acne flared with stress.
Acne is a disorder of the pilosebaceous unit. The lesions are found in areas rich in this unit.[21] Similar to literature, we also observed acne on the face in majority of patients, followed by back, chest, and arm. Acne is a polymorphic disease. The most common type of lesion in our study was comedones (92%). These observations are in accordance with data as reported by Kilkenny et al.[12]
According to Lehman grading system for acne, we observed that majority of patients who presented with acne scarring had a history of severe acne (54%). It was also observed that male patients had more severe acne vulgaris as compared to females (P = 0.0001), and thus more severe scarring.
According to various reports, early and effective treatment of acne vulgaris is the most appropriate way to prevent scarring.[22] In our study, 52 patients had a delay of 1 year before receiving treatment for their acne vulgaris, and 18 patients never received any treatment. Of 82 patients who had taken treatment, only 11 patients were given oral isotretinoin, 45 had received oral antibiotics, and all of them had received topical treatment. We also observed that of 18 patients who had never taken any treatment, 12 patients had developed severe grade of acne scarring and 6 patients had developed moderate grade of acne scarring. Similar results were also reported by Chuah and Goh.[14] They observed post-acne scarring in 48% of their patients who had delayed their treatment for 1 year after the onset of their acne vulgaris and in 12 patients (12%) who had never received any treatment. Whereas Layton et al.[5] reported in their study that a time delay up to 3 years between acne onset and adequate treatment is related to the ultimate degree of scarring in both sexes.
As females are cosmetically more concerned, the mean duration of post-acne scars before seeking treatment was slightly shorter in females (55.8 months) than that in males (62.5 months) (P < 0.05). This was in contrast to the study conducted by Chuah and Goh,[14] who found that the mean duration of post-acne scars in females was slightly longer (87.5 months) when compared with that in males (59.7 months) (P < 0.05).
Post-inflammatory hyperpigmentation is a common complication of acne vulgaris in darker skins. We observed it in 54% of our patients. Taylor et al.[23] and Yeung et al.[24] also reported similar incidence of post-acne hyperpigmentation in their study. Most common type of acne scars observed in our study were ice pick scars (94%) followed by rolling scars (86%), boxcar scars (54%), and keloidal scars (10%). When compared between the genders, the difference was not statistically significant (P > 0.05). Adityan and Thappa[19] also reported ice pick scar as the most common type of acne scar in 65.57% patients. Keloidal scars were present on the mandibular region in four patients, and on upper back, shoulder, and chest in six patients, majority of which were males (9 patients). Also patients were more concerned for treatment of facial acne scarring than chest and back scars.
In our study, grading of severity of acne scarring was done according to the Goodman and Baron qualitative grading system. Macular grade was observed in 22 patients, mild in 12 patients, moderate in 29 patients, and severe in 37 patients. Chuah and Goh[14] observed moderate grade (54%) of acne scarring in majority of their patients.
Chronic inflammation in acne causes damage to dermal collagen, which leads to acne scarring. Literature suggests that more severe the inflammation more will be the scarring.[25] In our study, we had observed severe acne scarring in 1 of 11 patients (9.09%) who had taken oral isotretinoin as compared to 17 of 45 patients (37.78%) who had taken oral antibiotic treatment. A significant difference was observed in the severity of acne scarring after oral retinoid treatment as compared to that after oral antibiotics. Also literature suggests that early use of topical retinoids as part of acne treatment helps in significant reduction in chronic inflammation, thereby reducing the severity of acne scarring.[26]
When we compared the severity of acne vulgaris with the severity of acne scarring, we observed that 54 patients with severe acne progressed to acne scarring, of which, 22 had moderate grade of acne scarring and 32 patients had severe grade. Layton et al.[5] also observed that there were significant correlations between the initial acne grade and the overall severity of scarring in all sites and in both sexes (P < 0.01).
There is a lack of knowledge and misconception in the public regarding the cause, natural course of acne, and complications such as permanent scarring. A majority of patients thought that acne was a natural phenomenon of growing up and would disappear on its known. They sought treatment only when it was severe, prolonged, or caused scars. Similar results were reported by Kaushik et al.,[27] in an Indian study, where 88% patients felt that acne was a normal phenomenon, and 64% felt that it would go away. Steroid use was rampant in 50% of their patients. In a review of literature on common misconceptions about acne, Hui[28] reported that there is a paucity of knowledge about acne, particularly with regard to underestimating severity and duration of acne, which can lead to potential severe consequences. Our study brings out the profile of acne scarring and the correlation between severity of acne and delayed treatment with severity of acne scarring, thereby highlighting the need to increase public awareness through education programs on early treatment of acne to reduce the risk and severity of post-acne scarring.
CONCLUSION
Majority of patients with active acne delay treatment, which leads to increased acne scarring. Ice pick scars are the most common type of acne scars, and keloidal scars are more common in males. Males also have a longer duration of acne, delay treatment, and have more severe acne scarring. Early introduction of oral retinoids may help to reduce the severity of acne scarring. Public education is essential to urge patients to seek early and appropriate treatment of acne that can reduce the incidence and intensity of acne scarring and its psychosocial consequences.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Simpson NB, Cunliffe WJ. Disorders of sebaceous glands. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook’s textbook of dermatology. 7th ed. Oxford, UK: Blackwell Publishing; 2004. pp. 43.1–43.75. [Google Scholar]
- 2.Gelmetti CC, Krowchuk DP, Lucky AW. Acne. In: Schachner LA, Katz SI, editors. Pediatric dermatology. 3rd ed. Philadelphia, PA: Mosby; 2003. pp. 589–609. [Google Scholar]
- 3.Burton JL, Cunliffe WJ, Stafford I, Shuster S. The prevalence of acne vulgaris in adolescence. Br J Dermatol. 1971;85:119–26. doi: 10.1111/j.1365-2133.1971.tb07195.x. [DOI] [PubMed] [Google Scholar]
- 4.Rivera AE. Acne scarring: a review and current treatment modalities. J Am Acad Dermatol. 2008;59:659–76. doi: 10.1016/j.jaad.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 5.Layton AM, Henderson CA, Cunliffe WJ. A clinical evaluation of acne scarring and its incidence. Clin Exp Dermatol. 1994;19:303–8. doi: 10.1111/j.1365-2230.1994.tb01200.x. [DOI] [PubMed] [Google Scholar]
- 6.Sánchez Viera M. Management of acne scars: fulfilling our duty of care for patients. Br J Dermatol. 2015;172:47–51. doi: 10.1111/bjd.13650. [DOI] [PubMed] [Google Scholar]
- 7.Lehmann HP, Robinson KA, Andrews JS, Holloway V, Goodman SN. Acne therapy: a methodologic review. J Am Acad Dermatol. 2002;47:231–40. doi: 10.1067/mjd.2002.120912. [DOI] [PubMed] [Google Scholar]
- 8.Goodman GJ, Baron JA. Postacne scarring: a qualitative global scarring grading system. Dermatol Surg. 2006;32:1458–66. doi: 10.1111/j.1524-4725.2006.32354.x. [DOI] [PubMed] [Google Scholar]
- 9.Fife D. Practical evaluation and management of atrophic acne scars: tips for the general dermatologist. J Clin Aesthet Dermatol. 2011;4:50–7. [PMC free article] [PubMed] [Google Scholar]
- 10.Loney T, Standage M, Lewis S. Not just ‘skin deep’: psychosocial effects of dermatological-related social anxiety in a sample of acne patients. J Health Psychol. 2008;13:47–54. doi: 10.1177/1359105307084311. [DOI] [PubMed] [Google Scholar]
- 11.Fabbrocini G, Annunziata MC, D’Arco V, De Vita V, Lodi G, Mauriello MC, et al. Acne scars: pathogenesis, classification and treatment. Dermatol Res Pract. 2010;2010:893080. doi: 10.1155/2010/893080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kilkenny M, Merlin K, Plunkett A, Marks R. The prevalence of common skin conditions in Australian school students: 3. Acne vulgaris. Br J Dermatol. 1998;139:840–5. doi: 10.1046/j.1365-2133.1998.02510.x. [DOI] [PubMed] [Google Scholar]
- 13.Lucky AW, Biro FM, Huster GA, Leach AD, Morrison JA, Ratterman J. Acne vulgaris in premenarchal girls. An early sign of puberty associated with rising levels of dehydroepiandrosterone. Arch Dermatol. 1994;130:308–14. doi: 10.1001/archderm.130.3.308. [DOI] [PubMed] [Google Scholar]
- 14.Chuah SY, Goh CL. The impact of post-acne scars on the quality of life among young adults in Singapore. J Cutan Aesthet Surg. 2015;8:153. doi: 10.4103/0974-2077.167272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goulden V, Clark S, Cunliffe W. Post-adolescent acne: a review of clinical features. Br J Dermatol. 1997;136:66–70. [PubMed] [Google Scholar]
- 16.Sardana K, Sharma RC, Sarkar R. Seasonal variation in acne vulgaris—myth or reality. J Dermatol. 2002;29:484–8. doi: 10.1111/j.1346-8138.2002.tb00313.x. [DOI] [PubMed] [Google Scholar]
- 17.Motoyoshi K. Enhanced comedo formation in rabbit ear skin by squalene and oleic acid peroxides. Br J Dermatol. 1983;109:191–8. doi: 10.1111/j.1365-2133.1983.tb07080.x. [DOI] [PubMed] [Google Scholar]
- 18.Knaggs HE, Wood EJ, Rizer RL, Mills OH. Post-adolescent acne. Int J Cosmet Sci. 2004;26:129–38. doi: 10.1111/j.1467-2494.2004.00210.x. [DOI] [PubMed] [Google Scholar]
- 19.Adityan B, Thappa DM. Profile of acne vulgaris—a hospital-based study from South India. Indian J Dermatol Venereol Leprol. 2009;75:272. doi: 10.4103/0378-6323.51244. [DOI] [PubMed] [Google Scholar]
- 20.Laue L, Peck GL, Loriaux DL, Gallucci W, Chrousos GP. Adrenal androgen secretion in postadolescent acne: increased adrenocortical function without hypersensitivity to adrenocorticotropin. J Clin Endocrinol Metab. 1991;73:380–4. doi: 10.1210/jcem-73-2-380. [DOI] [PubMed] [Google Scholar]
- 21.Zaenglein AL, Graber EM, Thiboutot DM, Strauss JS. Acne vulgaris and acneiform eruptions. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Fitzpatrick’s dermatology in general medicine. 7th ed. New York: McGraw Hill Publishing; 2008. pp. 690–703. [Google Scholar]
- 22.Goodman G. Acne and acne scarring—the case for active and early intervention. Aust Fam Physician. 2006;35:503–4. [PubMed] [Google Scholar]
- 23.Taylor SC, Cook-Bolden F, Rahman Z, Strachan D. Acne vulgaris in skin of color. J Am Acad Dermatol. 2002;46:S98–106. doi: 10.1067/mjd.2002.120791. [DOI] [PubMed] [Google Scholar]
- 24.Yeung CK, Teo LH, Xiang LH, Chan HH. A community based epidemiological study of acne vulgaris in Hong Kong adolescents. Acta Dermatol Venereol. 2002;82:104.7. doi: 10.1080/00015550252948121. [DOI] [PubMed] [Google Scholar]
- 25.Goodman GJ, Baron JA. Postacne scarring—a quantitative global scarring grading system. J Cosmet Dermatol. 2006;5:48–52. doi: 10.1111/j.1473-2165.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- 26.Tan J, Tanghetti E, Baldwin H, Stein Gold L, Lain E. The role of topical retinoids in prevention and treatment of atrophic acne scarring: understanding the importance of early effective treatment. J Drugs Dermatol. 2019;18:255–60. [PubMed] [Google Scholar]
- 27.Kaushik M, Gupta S, Mahendra A. Living with acne: belief and perception in a sample of Indian youths. Indian J Dermatol. 2017;62:491–7. doi: 10.4103/ijd.IJD_100_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hui RW. Common misconceptions about acne vulgaris: a review of the literature. Clin Dermatol Rev. 2017;1:33–6. [Google Scholar]