Abstract
The purpose of this study was to assess Analgesia/Nociception Index (ANI) and bispectral index (BIS) variations in supine and prone position during closed-tracheal suction in intensive care unit (ICU) patients with severe COVID-19 pneumonia requiring myorelaxation and prone positioning. We retrospectively reviewed the data of 15 patients hospitalized in ICU for severe COVID-19 pneumonia requiring sedation, myorelaxation and prone positioning. The BIS, instant ANI (ANIi), mean ANI (ANIm), heart rate (HR), systolic blood pressure (SBP) and SpO2 were retrieved in supine and prone position 1 min before tracheal suction then every minute from the beginning of tracheal suction during 4 min and compared using ANOVA for repeated measures (p < 0.05 considered as statistically significant). Both ANIm and ANIi decreased significantly during tracheal suction with no difference between positions, whereas BIS showed no significant variation within time and between groups. The median [Q1–Q3] ANIm value decreased from 87 [68–98] to 79 [63–09] in supine position and from 79 [63–95] to 78 [66–98] in prone position 2 min after the beginning of tracheal suction. The median [Q1–Q3] ANIi value decreased earlier 1 min after the beginning of tracheal suction from 84 [69–98] to 73 [60–90] in supine position and from 84 [60–99] to 71 [51–88] in prone position. Both HR, SBP and SpO2 varied modestly but significantly during tracheal suction with no difference between positions. Monitoring ANI, but not BIS, may be of interest to detect noxious stimuli such as tracheal suction in ICU myorelaxed patients with severe COVID-19 pneumonia requiring prone positioning.
Electronic supplementary material
The online version of this article (10.1007/s10877-020-00612-w) contains supplementary material, which is available to authorized users.
Keywords: COVID-19, Supine position, Intensive care unit, Tracheal suction, Bispectral index, Analgesia/nociception index
Introduction
The recent COVID-19 pandemic has provided a worldwide dramatic increase in the admission of patients in intensive care unit (ICU) with severe pneumonia [1]. Among them, some required mechanical ventilation because of acute respiratory distress syndrome (ARDS). Although specific data of supportive ICU care for COVID-19 are lacking because of the novelty of the disease, the ventilation strategy for these patients was generally similar to this of ARDS, i.e. focused on the avoidance of lung injury while facilitating gas exchange with lung-protective ventilation, early prone position and use of neuromuscular-blocking agents (NMBAs) [1]. For these patients, deep sedation comprising hypnotic agents and opioids associated to NMBAs is generally required to facilitate mechanical ventilation.
In an effort to optimize the administration of the different sedative drugs, clinical signs but also different monitors may be used, although there are still only few data on this subject. Considering bispectral index (BIS) monitoring for the management of sedation, a recent meta-analysis found insufficient evidence compared to clinical signs because of very low quality of the included studies [2]. Although current guidelines make no recommendation regarding the use of NMBA monitoring in the critical care setting, quantitative monitors providing in particular the train-of-four (TOF) count may be used to determine the level of neuromuscular blockade [3].
Considering analgesia monitoring, a recent monitor called ANI (Analgesia/Nociception Index, MDoloris Medical Systems, France) has been studied in the anesthetic field but only few data is available in ICU [4]. This ANI is a 0–100 index derived from heart rate variability corresponding to the relative parasympathetic tone: high values (above 50) correspond to prominent parasympathetic tone (comfort, analgesia, adequate nociception/antinociception balance), whereas low values (below 50) correspond to prominent sympathetic tone (stress, pain, inadequate nociception/antinociception balance) [4]. The ANI monitor displays constantly the instant ANI (ANIi) calculated every second and the mean ANI (ANIm), corresponding to the mean ANI from the three previous minutes.
We conducted this study to assess ANI and BIS variations in supine and prone position during closed-tracheal suction in sedated and myorelaxed ICU patients with severe COVID-19 pneumonia.
Materials and methods
This study was approved by the Ethics Committee of the French Society of Anesthesia and Intensive Care, CERAR (Comité d’Éthique pour la Recherche en Anesthésie-Réanimation), Paris, France (Approval No. IRB 00010254-2020-076). The Ethics Committee waived the requirement for written informed consent because of the retrospective nature of this study. We retrospectively reviewed the data of patients who were hospitalized in Pierre Oudot hospital center ICU for severe COVID-19 pneumonia requiring sedation, myorelaxation and prone positioning from March to May 2020 during the pandemic in France. The inclusion criteria were severe pneumonia in ICU adult patients with positive SARS-Cov-2 PCR on mechanical ventilation requiring prone positioning (PO2/FiO2 < 150–180) with complete sedation monitoring during closed-tracheal suction, including BIS for narcosis, train-of-four (TOF) for myorelaxation and ANI for analgesia. Closed-tracheal suction is a type of tracheal suction that do not need to be disconnected from the respiratory circuit, which may present several advantages including decreased environmental contamination from respiratory microorganisms [5]. The exclusion criteria comprised patients who did not require myorelaxation or prone positioning and those for whom BIS, ANI or TOF was not used. For each patient meeting the inclusion criteria, one measurement of the following parameters was obtained in supine position and one in the prone position: BIS, TOF, ANI, heart rate (HR), systolic blood pressure (SBP) and SpO2. All measurements were retrieved during 5 min (1 min before tracheal suction then every minute from the beginning of tracheal suction during 4 min) from the electronic file of the patient, except for ANI values retrieved directly from the internal memory of the monitor. These parameters were compared within time between both supine and prone positions using ANOVA for repeated measures, with a value of p < 0.05 considered as statistically significant (MedCalc Statistical Software version 18.2.1, MedCalc Software bvba, Ostend, Belgium).
The patients characteristics (age, weight, BMI….), Simplified Acute Physiology Score (SAPS II) and comorbidities, biological results related to inflammation and sepsis (white blood cell count, C-reactive protein), ventilation parameters (tidal volume, positive end-expiratory pressure [PEEP], respiratory frequency [RF], PO2/FiO2 ratios…), sedation regimens, TOF ratios and use of vasopressors were retrieved at the time of tracheal suction. Data were presented as median [Q1–Q3] for continuous variables or as n (%) for categorical variables.
Results
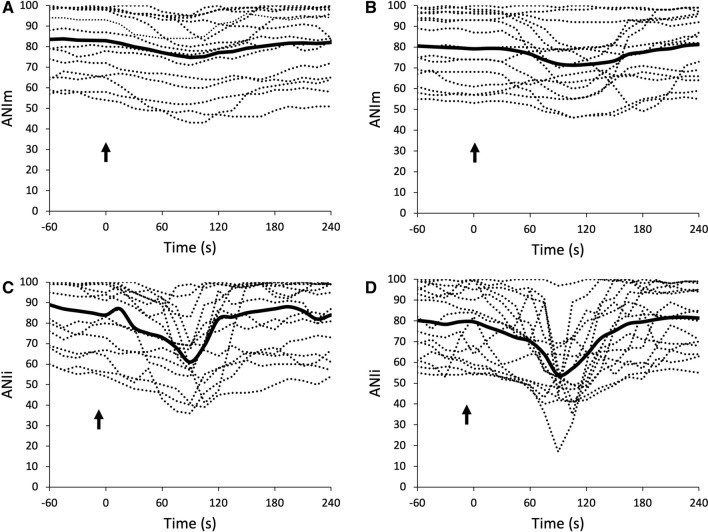
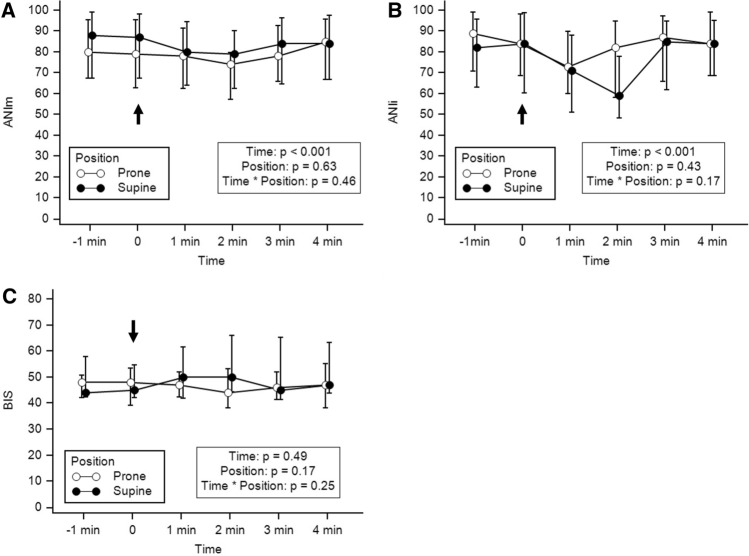
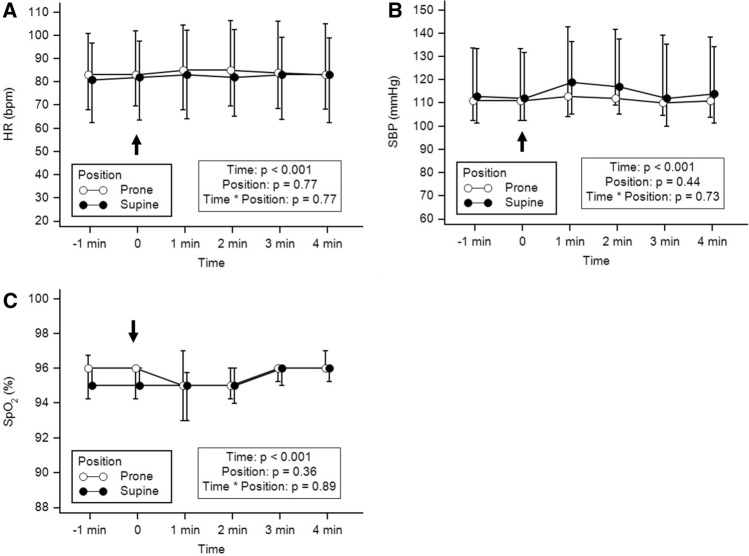
In total, 15 patients could be retrieved and analyzed during the study period. Patient characteristics, SAPS II, biological results, ventilation parameters, sedation regimen, TOF ratios and use of vasopressors are reported in Table 1. The duration of closed-tracheal suction was less than 1 min for each patient. Individual variations of ANIi and ANIm are reported in Fig. 1. Variations of ANI and BIS within time between prone and supine position are reported in Fig. 2. Both ANIm and ANIi decreased significantly during tracheal suction with no difference between prone or supine position, whereas BIS showed no significant variation within time and between positions (Fig. 2). The ANIm value decreased from 87 [68–98] to 79 [63–09] in supine position and from 79 [63–95] to 78 [66–98] in prone position 2 min after the beginning of tracheal suction. The ANIi value decreased earlier from 84 [69–98] to 73 [60–90] in supine position and from 84 [60–99] to 71 [51–88] in prone position 1 min after the beginning of tracheal suction. No significant differences in ANIm and ANIi between patients with or without epinephrine were observed. Variations of HR, SBP and SpO2 are reported in Fig. 3. Both HR, SBP and SpO2 varied modestly but significantly during tracheal suction with no difference between prone and supine position. No complication related to tracheal suction was reported in any patient.
Table 1.
Patient characteristics (n = 15)
| All patients | Position | ||
|---|---|---|---|
| n = 15 | Supine | Prone | |
| Age (years) | 64 [55–67] | ||
| Gender M/F | 11/4 (73/27) | ||
| BMI (kg m−2) | 25 [23–30] | ||
| SAPS II | 59 [47–71] | ||
| Comorbidities | |||
| Hypertension | 4 (27) | ||
| Diabetes | 5 (33) | ||
| Obesity | 3 (20) | ||
| PO2/FiO2 | 150 [147–165] | 238 [203–295] | |
| WBC (G L−1) | 9.8 [6.8–12.8] | ||
| CRP (mg L−1) | 127 [97–198] | ||
| pH | 7.44 [7.37–7.49] | 7.45 [7.42–7.48] | |
| pCO2 (mmHg) | 45 [39–52] | 44 [38–47] | |
| VT (mL kg−1) | 7.1 [6.3–8.1] | 7.1 [6.3–7.7] | |
| PEEP (cmH2O) | 8 [8–10] | 8 [8–10] | |
| Pplat (cmH20) | 25 [21–29] | 26 [23–29] | |
| RF (cycles min−1) | 18 [16–20] | 18 [16–18] | |
| Type of narcotic agent | |||
| Midazolam | 7 (47) | ||
| Propofol | 8 (53) | ||
| Dose of narcotic agent | |||
| Midazolam (µg kg−1 h−1) | 80 [53–98] | 70 [53–98] | |
| Propofol (mg kg−1 h−1) | 2.3 [2.0–2.9] | 2.4 [1.9–2.9] | |
| Type of opioid | |||
| Remifentanil (µg kg−1 min−1) | 7 (47) | ||
| Sufentanil (µg kg−1 min−1) | 8 (53) | ||
| Dose of opioid | |||
| Remifentanil (µg kg−1 min−1) | 0.08 [0.05–0.15] | 0.10 [0.05–0.15] | |
| Sufentanil (µg kg−1 h−1) | 0.30 [0.19–0.50] | 0.24 [0.18–0.49] | |
| Dose of cisatracurium (mg kg−1 h−1) | 0.18 [0.13–0.30] | 0.20 [0.15–0.31] | |
| Use of norepinephrine | 8 (53) | ||
| Norepinephrine dose (µg kg−1 min−1) | 0.14 [0.00–0.31] | 0.15 [0.00–0.29] | |
| TOF ratio | 0 [0–0] | 0 [0–0] | |
Results presented as median [Q1–Q3] or n (%)
BMI body mass index, CRP C-reactive protein, PEEP positive end-expiratory pressure, Pplat plateau pressure, RF respiratory frequency, SAPS II simplified acute physiological score, TOF train-of-four, VT tidal volume, WBC white blood cells
Fig. 1.
Individual variations of ANIm in supine (a) and prone (b) position and of ANIi in supine (c) and prone (d) position. The plain line represents the mean value (n = 15). The arrow represents the beginning of tracheal suction
Fig. 2.
Variations of ANIi (a), ANIm (b) and BIS (c) at each time-point in supine and prone position. The arrow represents the beginning of tracheal suction. Values are presented as median [Q1–Q3]
Fig. 3.
Variations of heart rate (a), systolic blood pressure (b) and SpO2 (c) at each time-point in supine and prone position. The arrow represents the beginning of tracheal suction. Values are presented as median [Q1–Q3]
Discussion
This retrospective study reports for the first time that ANIm and ANIi decrease significantly while BIS shows no variation during closed tracheal suction in both supine and prone position in myorelaxed and sedated ICU patients with SARS-Cov-2 pneumonia requiring mechanical ventilation. This decrease in ANIm and ANIi occurred while HR and SBP exhibited variations related to the noxious stimulus caused by tracheal suction, suggesting that ANI monitoring may help to detect autonomous nervous system response in sedated and non-communicant ICU myorelaxed patients.
ANI has been widely studied during general anesthesia or in awake patients [4, 6–10]. It provides an estimate of the relative parasympathetic tone, varying from 0 (no parasympathetic tone, corresponding to maximal sympathetic activity) to 100 (maximal parasympathetic activity corresponding to no sympathetic tone).
During general anesthesia, an ANI range of [50–80], corresponding to adequate nociception-antinociception balance, is targeted to optimize the administration of analgesic agents and may help to provide improved postoperative analgesia [4]. In awake patients, it seems that ANI is linked to the emotional status, with low values observed during bad emotions like pain, fear or anxiety [4, 11] and high values observed during positive emotions like comfort or analgesia and during the hypnotic state [4, 8, 9, 11].
To date, four studies have reported the use of ANI monitoring in ICU patients [12–15]. In the first study performed in conscious burn patients, the performance of ANI to detect pain during dressing changes procedures was studied, with an area under the receiver-operating characteristics curve (ROC AUC) of 0.76 [95% CI 0.75–0.76] [12].
In the second study, ANIm and ANIi were continuously recorded then compared with the Behavioral Pain Scale (BPS) before, during and after routine care procedures in critically-ill non-comatose non-communicating patients to assess the performance of ANI to predict pain (BPS ≥ 5) [13]. The performance of ANIm to detect BPS ≥ 5 was somewhat poor (ROC AUC = 0.57 [CI 0.53–0.62] and that of ANIi slightly better (ROC AUC = 0.73 [95% CI 0.68–0.77], with 61% sensitivity and 77% specificity at an ANIi threshold of 43. However, the high negative predictive value of 90% observed in this study may show that ANIi may be of highest benefit for excluding significant pain.
Although ANI values were only provided as figures, lower ANIm and ANIi values were observed during tracheal suction than those observed in the current study. This may be explained by differences in sedation protocols (in particular analgesia) between both studies. Indeed, in Chanques’s et al. study [13], sufentanil was administered at a mean rate of 0.1 µg kg−1 min−1, which is third time lower than in the current study (0.33 µg kg−1 min−1 for patients receiving sufentanil in supine position) since our patients suffering from severe SARS-Cov-2 pneumonia required deep sedation and myorelaxation to facilitate mechanical ventilation. As described during general anesthesia, instead of static values of ANI, depending on the balance between analgesia and nociception, it may be of better interest to consider dynamic ANI variations during time to detect the intensity of the nociceptive stimulus, although this should require further study [7].
Another study aimed to determine the effectiveness of ANI in detecting pain assessed by BPS in deeply sedated critically ill patients with or without norepinephrine [14]. The chosen painful stimulus was patient turning for washstand. The authors observed a significant decrease in ANI during the stimulus, with unexpected lower median [Q1–Q3] ANI values in patients without (57 [42–69]) than with 75 (66–80) epinephrine (p < 0.05). Indeed, a decrease in ANI was expected when using norepinephrine, a sympathomimetic agent. In the current study, no difference in ANIm or ANIi was observed whatever the use of norepinephrine. However, the role of norepinephrine on ANI values in ICU patients is still to be determined, and the quite small number of patients included in the current study may prevent any interpretation of this discrepancy.
Indeed, another study has shown decreased median [min–max] ANIi (55 [22–100]) and ANIm (69 [32–100]) during tracheal suctioning with traumatic brain injury, which is similar to the current study [15]. However, no clear difference could be observed without or with epinephrine: median [min–max] ANIi of 56 [30–96] and 50 [22–100], respectively, and median [min–max] ANIm of 66 [32–98] and 69 [46–100], respectively, during tracheal suctioning.
Our study presents certain limitations, in particular because of its retrospective design. Different sedation protocols with different opioid agents (sufentanil and remifentanil) at different rates of administration were used, which renders difficult the interpretation of ANI variations and the generalization of these results. Moreover, the small number of patients in this study, limited by the admission of COVID-19 patients during the pandemic, should make consider our results with caution. Besides, despite requiring no external opening of the ventilatory circuit, the close-tracheal suction procedure in itself leads to an interruption of ventilatory cycles which are tantamount to an apnea which may have affected ANI calculation [16]. A decrease in ANI would also probably be observed during recruitment maneuvers related to the interrupted breathing cycles and not to acute discomfort or nociception, which should require further investigation using a similar methodology. The role of norepinephrine on ANI value is also still unclear and requires further investigation. Further prospective studies with adequate sample sizes should study ANI variations in myorelaxed patients receiving sedation and analgesia with or without norepinephrine and maybe try to evaluate the utility of ANI monitoring to optimize the administration of opioids in ICU patients.
In conclusion and despite these limitations, both ANIm and ANIi decrease during closed-tracheal suction in sedated and myorelaxed ICU patients with severe COVID-19 pneumonia (with earlier decrease within 1 min for ANIi), showing that ANI monitoring may be of interest to detect noxious stimuli in this population. Further study is however required to confirm these results and to evaluate the role of ANI monitoring in the management of analgesia in ICU patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
EB and BA conceived of the presented idea. EB, AF and SL retrieved the retrospective data. EB performed the statistical analysis and wrote the first draft of the manuscript. BA supervised the findings of this work. All authors discussed the results and contributed to the final manuscript.
Funding
This study was performed with institutional sources only.
Compliance with ethical standards
Conflicts of interest
Emmanuel Boselli has received honoraria and travel grants from MDoloris Medical Systems. The other authors have no conflict of interest to declare.
Ethical approval
This study was approved by the Ethics Committee of the French Society of Anesthesia and Intensive Care, CERAR (Comité d’Éthique pour la Recherche en Anesthésie-Réanimation), Paris, France (Approval No. IRB 00010254-2020-076).
Informed consent
The Ethics Committee waived the requirement for written informed consent because of the retrospective nature of this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Phua J, Weng L, Ling L, Egi M, Lim C-M, Divatia JV, et al. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020;8:506–517. doi: 10.1016/S2213-2600(20)30161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shetty RM, Bellini A, Wijayatilake DS, Hamilton MA, Jain R, Karanth S, et al. BIS monitoring versus clinical assessment for sedation in mechanically ventilated adults in the intensive care unit and its impact on clinical outcomes and resource utilization. Cochrane Database Syst Rev. 2018 doi: 10.1002/14651858.CD011240.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Renew JR, Ratzlaff R, Hernandez-Torres V, Brull SJ, Prielipp RC. Neuromuscular blockade management in the critically Ill patient. J Intensive Care. 2020;8:37. doi: 10.1186/s40560-020-00455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boselli E. Intérêt du monitorage du tonus parasympathique relatif par Analgesia/Nociception Index (ANI) chez les patients anesthésiés ou conscients. Douleurs Éval Diagn Trait. 2018;19:205–210. doi: 10.1016/j.douler.2018.07.008. [DOI] [Google Scholar]
- 5.Lorente L, Lecuona M, Jiménez A, Mora ML, Sierra A. Tracheal suction by closed system without daily change versus open system. Intensive Care Med. 2006;32:538–544. doi: 10.1007/s00134-005-0057-6. [DOI] [PubMed] [Google Scholar]
- 6.Ledowski T. Objective monitoring of nociception: a review of current commercial solutions. Br J Anaesth. 2019;123:e312–e321. doi: 10.1016/j.bja.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boselli E, Logier R, Bouvet L, Allaouchiche B. Prediction of hemodynamic reactivity using dynamic variations of Analgesia/Nociception Index (∆ANI) J Clin Monit Comput. 2016;30:977–984. doi: 10.1007/s10877-015-9802-8. [DOI] [PubMed] [Google Scholar]
- 8.Boselli E, Musellec H, Martin L, Bernard F, Fusco N, Guillou N, et al. Effects of hypnosis on the relative parasympathetic tone assessed by ANI (Analgesia/Nociception Index) in healthy volunteers: a prospective observational study. J Clin Monit Comput. 2018;32:487–492. doi: 10.1007/s10877-017-0056-5. [DOI] [PubMed] [Google Scholar]
- 9.Boselli E, Musellec H, Bernard F, Guillou N, Hugot P, Augris-Mathieu C, et al. Effects of hypnosis on the relative parasympathetic tone assessed by ANI (Analgesia/Nociception Index) in healthy volunteers: a prospective observational study. Int J Clin Exp Hypn. 2018;66:134–146. doi: 10.1080/00207144.2018.1421355. [DOI] [PubMed] [Google Scholar]
- 10.De Jonckheere J, Bonhomme V, Jeanne M, Boselli E, Gruenewald M, Logier R, et al. Physiological signal processing for individualized anti-nociception management during general anesthesia: a review. Yearb Med Inform. 2015;24:95–101. doi: 10.15265/IY-2015-004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Jonckheere J, Logier R, Jounwaz R, Vidal R, Jeanne M. From pain to stress evaluation using heart rate variability analysis: development of an evaluation platform. Annu Int Conf IEEE Eng Med Biol. 2010;2010:3852–3855. doi: 10.1109/IEMBS.2010.5627661. [DOI] [PubMed] [Google Scholar]
- 12.Papaioannou V, Chouvarda I, Gaertner E, Benyamina M, Ferry A, Maurel V, et al. Heart rate variability and cardiac baroreflex inhibition-derived index predicts pain perception in burn patients. Burns. 2016;42:1445–1454. doi: 10.1016/j.burns.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 13.Chanques G, Tarri T, Ride A, Prades A, De Jong A, Carr J, et al. Analgesia nociception index for the assessment of pain in critically ill patients: a diagnostic accuracy study. Br J Anaesth. 2017;119:812–820. doi: 10.1093/bja/aex210. [DOI] [PubMed] [Google Scholar]
- 14.Broucqsault-Dédrie C, De Jonckheere J, Jeanne M, Nseir S. Measurement of heart rate variability to assess pain in sedated critically ill patients: a prospective observational study. PLoS ONE. 2016;11:e0147720. doi: 10.1371/journal.pone.0147720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jendoubi A, Abbes A, Ghedira S, Houissa M. Pain measurement in mechanically ventilated patients with traumatic brain injury: behavioral pain tools versus analgesia nociception index. Indian J Crit Care Med. 2017;21:585–588. doi: 10.4103/ijccm.IJCCM_419_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith AL, Owen H, Reynolds KJ. Can short-term heart rate variability be used to monitor fentanyl–midazolam induced changes in ANS preceding respiratory depression? J Clin Monit Comput. 2015;29:393–405. doi: 10.1007/s10877-014-9617-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.