Abstract
Uveal melanoma is the most common type of intraocular cancer with a low mean annual incidence of 5-10 cases per million. Tumours are located in the choroid (90%), ciliary body (6%) or iris (4%) and of 85% are primary tumours. As in cutaneous melanoma, tumours arise in melanocytes; however, the characteristics of uveal melanoma differ, accounting for 3-5% of melanocytic cancers. Among the numerous risk factors are age, sex, genetic and phenotypic predisposition, the work environment and dermatological conditions. Management is usually multidisciplinary, including several specialists such as ophthalmologists, oncologists and maxillofacial surgeons, who participate in the diagnosis, treatment and complex follow-up of these patients, without excluding the management of the immense emotional burden. Clinically, uveal melanoma generates symptoms that depend as much on the affected ocular globe site as on the tumour size. The anatomopathological study of uveal melanoma has recently benefited from developments in molecular biology. In effect, disease classification or staging according to molecular profile is proving useful for the assessment of this type of tumour. Further, the improved knowledge of tumour biology is giving rise to a more targeted approach to diagnosis, prognosis and treatment development; for example, epigenetics driven by microRNAs as a target for disease control. In the present study, the main epidemiological, clinical, physiopathological and molecular features of this disease are reviewed, and the associations among all these factors are discussed.
Keywords: uveal melanoma, cell transduction pathways, epigenetics, miRNA, immunotherapy
1. Introduction
Although relatively rare, uveal melanoma is the most common type of intraocular tumour with a mean annual incidence of 5-10 cases/1,000,000 individuals. Among all cancers of the eye, 85% are primary tumours of this type and occur in individuals with a mean age of 60 years. The remaining 15% cases are non-Hodgkin lymphomas, retinoblastomas and medulloepitheliomas. Despite these figures, the most frequent tumours affecting the eye are metastases of other types of cancer, mainly lung cancer in males and breast cancer in females (1,2). Uveal melanoma is also a melanocytic cancer, representing approximately 3-5% of all of these cancers, although its characteristic features differ from those of the cutaneous form. Tumours are mainly located in the choroid 85-90%, followed by the ciliary body (6%) and iris (4%). Several studies have demonstrated that both its cell mutation pattern and aetiology have their own characteristics, unrelated in a large measure to those of remaining melanomas. Host susceptibility factors are also fairly specific, and incidence varies according to ethnicity, gender and geographical region (2,3).
Currently, the management approach to uveal melanoma is essentially multidisciplinary, involving ophthalmologists, oncologists and maxillofacial surgeons. Patient management also involves dealing with a heavy emotional burden. Despite intense research into the physiopathology, histology and molecular biology of uveal melanoma, there has been little improvement in its bleak prognosis (4). Patient management thus currently focuses on early detection and aggressive treatment. Notwithstanding, over time, approximately 50% of patients will develop metastatic disease with its ominous prognosis and survival of 6-12 months (5).
When staging a primary uveal melanoma, besides considering its anatomical and pathological features (tumour base diameter, ciliary body involvement, and patterns of extravascular matrix growth, mitosis, and cell morphology), mutations with prognostic value along with their statistics have allowed for an individualized approach able to predict the response to treatment and outcome (6). Clinical manifestations depend on the size and location of the tumour. Often, tumours are incidentally detected in an ophthalmological exam or through symptoms, such as loss of vision, photopsia, myodesopsia or high intraocular pressure (7).
A major characteristic of uveal melanoma is that it differentially affects populations in different geographical regions. Unlike cutaneous melanoma, with an incidence that has risen sharply over the past 30 years, the incidence of uveal melanoma has remained stable over this same period. For example, in Europe, figures range from 2 cases per million per year in Spain, Italy and Portugal, to 9 cases per million in Norway, Denmark or Sweden (8). By contrast, Asia and Africa are less affected. For instance, Korea exhibits an incidence of 0.6 cases/1,000,000 and Africa 0.2 cases/1,000,000 (9). Currently, the world region with the highest number of cases is Australia with 11 cases/million per year (10). To understand the high geographic variation in this disease, it is necessary to examine the associations among possible genetic, phenotypic or occupational risk factors. Accordingly, the present study reviews the main clinical, epidemiological, physiopathological and molecular features that define uveal melanoma.
2. Risk factors
Uveal melanoma has a large number of associated risk factors such as age, sex, genetic or phenotypic predisposition, the work environment and dermatological conditions. While it mainly affects older-aged individuals, an older age is also related to a worse prognosis. The mean age of diagnosis also varies according to the geographical location. In Asia, it tends to affect younger individuals (45-55 years of age), while in Europe or in the USA, it usually presents at around the age of 60 years. It should be mentioned that uveal melanoma in young individuals has also been related to congenital melanocytic syndromes (ocular melanosis and dysplastic nevus syndrome), with a mean onset age of 16 years and a better short-term prognosis owing to its lesser locoregional aggressiveness (11,12). Sex as a risk factor is related to age. For example, in individuals <60 years of age, there is no clear predisposition for any sex and the ratio of affected females to males is 1:1. At more advanced ages, there is a slight predisposition for males, who also exhibit a higher risk of metastasis and therefore, exhibit a higher mortality rate and a worse prognosis (11,13).
As occurs with cutaneous melanoma, uveal melanoma tends to affect Caucasians who represent the great majority of patients. This is due to a series of susceptibility factors for melanocyte lesions, such as fair skin, green or blue eyes and blond or red hair. A higher incidence has also been described in individuals with dysplastic nevus syndrome, multiple nevi, ocular melanosis and freckles; in these subjects it has been related to an early age at onset (14). However, it is not known whether these lesions may be associated with exposure to UV light. According to previous research, not only does the vitreous humour block the actions of light rays in the posterior chamber of the eye, but the crystalline lens/cornea barrier mean there is little support for the theory of mutations triggered by UV radiation (15). Hence, its association with an individual's phenotype may be a susceptibility factor for oncogenic melanocyte mutations and therefore, of the risk of developing uveal melanoma.
A notable risk factor for uveal melanoma is the work environment. Both professional cooks and welders exhibit an up to a 2-fold greater risk of developing uveal melanoma. Researchers have related prolonged exposure to sunflower, olive and other oils while cooking to the production of polycyclic hydrocarbons and complex derived hydrocarbons that function as carcinogens by inducing a state of oxidative stress and damage to DNA repair mechanisms (11). In welders, the association between exposure to UV light while welding and the incidence of uveal melanoma is not clear, as mentioned above. The vitreous humour, lens and cornea play a protective role (15). During heat welding, numerous gases are produced when metals fuse together, giving rise to carcinogenic substances, such as hexavalent chromium, argon, helium, hydrogen fluoride and asbestos (16). Low frequency electric fields are also generated, which also affect cell repair processes and may be related to an increased incidence of a uveal melanoma (12).
Finally, the risk of uveal melanoma in patients with Nevus of Ota is 1/400, which is extremely high compared to subjects without this condition, with an annual incidence of ~1/13,000. In individuals with this nevus, uveal melanoma usually presents at an earlier age and exhibits less aggressive locoregional invasion and a lower incidence of metastasis (17,18). Ocular dysplastic lesions are proliferative non-malignant lesions with atypical characteristics (irregular margins, growth and different tones) that have been linked to a 10-fold greater risk of transformation into uveal melanoma compared with the general population (19). Researchers have demonstrated malignant degeneration in 2 to 5% of patients with an iris nevus. The main risk factors associated with the malignant transformation of an iris nevus are an age <40 years, diffuse lesion appearance, blood detected in the eye fundus and inferior location. By contrast, choroidal nevus, which occurs in ~5% of the population, exhibits a low likelihood of malignant degeneration, approximately 1 case per 9,000 (20). Risk factors for suspecting a malignant choroidal nevus are a thickness >2 mm, the presence of symptoms and orangey colour, among others. It should be underscored that these risk factors generally lead to an earlier appearance of uveal melanoma (11,12).
3. Clinical manifestations
Uveal melanoma generates symptoms depending on the ocular site involved, meaning that most clinical signs are determined by both tumour size and location. Usually patients present with blurred vision, photopsia and/or myodesopsia or are asymptomatic and the uveal melanoma is detected incidentally during a routine ophthalmological examination (7). When the tumour affects the macula, patients exhibit a gradual painless decline in visual acuity. It should also be mentioned that if there is involvement of the iridocorneal angle, signs may be those of acute glaucoma, namely the loss of visual acuity, pain, photopsia and increased intraocular pressure. These symptoms can lead to permanent blindness and are therefore, constitute an ophthalmological emergency. By contrast, the involvement of the iris is usually asymptomatic and presents as a dark growing, invasive hyperpigmented lesion. If the ciliary body is involved, this can compromise the natural lens, causing its subluxation and impaired accommodation, thus interfering with the patient's vision (21). It should be noted that infrequently, intraocular progression can give rise to haemorrhage within the ocular cavity presenting as haemorrhage and exophthalmos. Up to 22% of patients may have systemic manifestations as a consequence of metastatic spread mainly to the liver, and almost 90% succumb to the disease before 5 years following diagnosis (22).
4. Anatomopathological study of uveal melanoma
Callender (23) was the first to establish an anatomopathological classification of these tumours, which was later modified by McLean et al (24), who distinguished between type A fusiform cell, type B fusiform cell, epithelioid cell and mixed tumours. Fusiform type A followed by B tumours were associated with a higher survival rate, and epithelioid cell tumours were associated with the worse prognosis. Mixed tumours were associated with an intermediate outcome (25,26). Another series of histopathological criteria has proven useful to assess disease prognosis in a patient with uveal melanoma. For instance, an elevated microvascular density (MVD) related to tumour irrigation and the presence of a network vascular pattern have been associated with a worse prognosis (27,28). High IGF-1R levels and mean nucleolar diameter have been also related to a lower survival (29,30). The role of some of the more important cell proliferation markers, such as Ki-67 or proliferating cell nuclear antigen (PCNA), have been assessed in uveal melanoma cells, their presence indicating a worse prognosis (31). Finally, localizing some immune system cells, such as lymphocytes or infiltrating macrophages, or the detection of markers like HLA-A have been also associated with a worse prognosis in patients with uveal melanoma (32,33). Notably, the presence of HLA-B has been associated with the epithelioid subtype, which is the histological class exhibiting a lower survival (34).
The anatomopathological study of uveal melanoma has recently benefited from developments in the field of molecular biology. This has meant that currently, classification according to the molecular profile of uveal melanoma has proven more useful than its histological classification, in line with the concept of individualized precision medicine for these patients.
5. Molecular classification of uveal melanoma: Genes involved
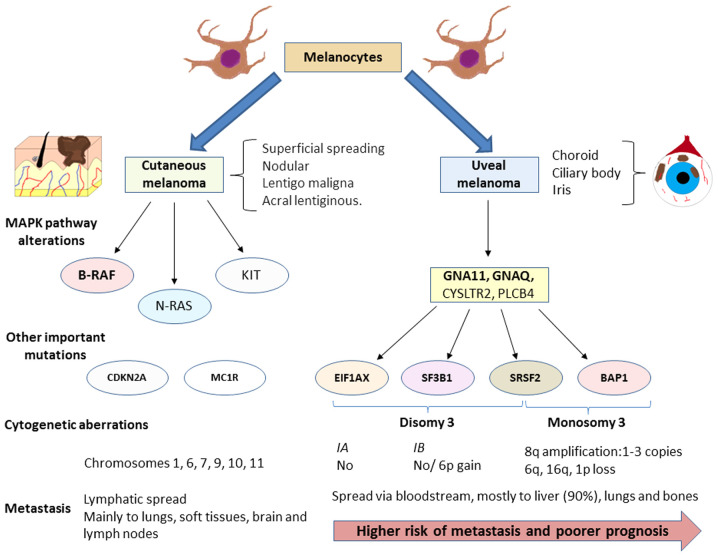
Uveal melanoma is often divided into two categories according to its gene expression profile and to its metastasizing capacity. Hence, class 1 uveal melanomas are associated with a low risk of metastasis and have been linked to a better prognosis, while class 2 tumours feature a high risk of spread and a worse prognosis. In addition, there is significant variation in cytogenetics and expression levels of some genes in the different subtypes; for example, chromosome 3 monosomy is characteristic of class 2 tumours (35). However, this initial classification is insufficient to explain, for example, why some class 1 tumours show a higher risk of metastasis than others.
For this reason, uveal melanoma classification has been extended to include 4 groups: 2 subclasses characterized by chromosome 3 monosomy (M3) with a worse prognosis, and a further 2 subtypes that lack this chromosome abnormality; i.e., with chromosome 3 disomy (D3), with a better prognosis. The first 2 subclasses are associated with a higher metastasis risk and exhibit a loss of or mutation of the gene encoding BRCA-associated protein 1 (BAP1) located on 3p21.1 (NCBI), and conferring a different methylation state to those without this monosomy. Between both M3 subtypes, there is a series of genomic, transcriptional and clinical variations, such as the amplification of 1 to 3 copies of the long arm of chromosome 8 (36).
In turn, the D3 subtypes are divided into IA and IB. The former exhibits no aneuploidy, the least risk of spread and is characterized by a mutation in eukaryotic translation initiation factor 1A X-linked (EIF1AX). Subtype IB, characterized by the possible presence of a total or partial gain of 6p and a higher metastasis risk, features mutations in the splicing factor 3b subunit 1 (SF3B1) gene (37). Furthermore, Field et al (38,39) highlighted the role of gene expression of preferentially expressed antigen in melanoma (PRAME) as an independent biomarker of metastasis frequently found in tumours with a mutation in SF3B1. This marker may also appear in M3 tumours and is also inversely related to mutations in EIF1AX. Mutations in the genes EIF1AX, SF3B1 and BAP1 are mutually exclusive, as well as being key prognostic markers to understand the behaviour of each uveal melanoma subtype (40). Of note, both in D3 uveal melanomas which do not exhibit mutations in SF3B1 or EIF1AX and in M3, which exhibit gain of chromosome 8q, mutations in serine and arginine rich splicing factor 2 (SRSF2) have also been found, indicating a role for this marker in the metastasis of uveal melanoma and its functional analogy with SF3B1 (36).
6. Uveal vs. cutaneous melanoma: Similarities and differences
While cutaneous and uveal melanoma both arise from melanocytes, their molecular profiles, cytogenetic alterations, prognosis and dissemination capacity vary appreciably (Fig. 1). For example, it is known that approximately 50% of cases of uveal melanoma progress to metastasis and the mean survival rate of these patients is 6 to 12 months (13). The most frequent site of spread of these tumours is the liver, though lung and bone metastases are also common (41,42) whereas cutaneous melanoma metastasizes with the same frequency to the lungs, bone, brain and soft tissues and mainly spreads via the lymph system (43).
Figure 1.
Diagram illustrating the diagnostic features of cutaneous melanoma and uveal melanoma. Although both melanomas arise from melanocytes, each one shows its own characteristics while sharing the feature of an altered MAP kinase signalling pathway. Presently, these abnormalities are one of the most promising targets of the treatment of these patients, such as the inhibition of B-RAF for cutaneous melanoma. Nevertheless, as illustrated in the diagram, the mutations that activate this pathway differ and are accompanied by another set of modifications that are also different. In the case of uveal melanoma, these mutations serve to classify tumours into subtypes according to their molecular profile. The four mutations described are mutually exclusive. This molecular classification is also associated with metastasis risk and disease prognosis. The spread of both tumours also differs as cutaneous melanomas usually spread via the lymph while uveal melanomas usually spread via the bloodstream. Uveal melanoma exhibits a high predisposition to spread to the liver, which occurs in 90% of cases. By contrast, cutaneous melanoma may metastasize to the lungs, brain, lymph nodes and soft tissues with almost equal probability. Cytogenetic aberrations are also common in both types of melanoma, although these also differ. GNA, G protein subunit alpha; EIF1AX, eukaryotic translation initiation factor 1A X-linked; SF3B1, splicing factor 3b subunit 1; SRSF2, serine and arginine rich splicing factor 2; BAP1, BRCA-associated protein 1.
As in cutaneous melanoma, in uveal melanoma, the overexpression of the MAPK pathway is observed. However, mutations found in both types of melanoma differ. In the skin form, most frequent abnormalities are found in molecules directly involved in this pathway especially the B-RAF mutation (in 40-60% of cases). In this type of mutation, particularly in residue V600, a worse prognosis has been described (44). In addition, are mutations in other genes, such as NRAS (15-25%) and KIT (39%) are frequent (45). However, it is known that these polymorphisms seldom occur in uveal melanoma (46). The mutations found in this tumour type appear mainly in the genes that code for the α subunit of G, mainly G protein subunit alpha (GNA)11 or GNAQ, detected in up to 90% of cases of uveal melanoma. Furthermore, these mutations seem to play an important role in the onset and progression of uveal melanoma as it has been observed that both abnormalities are not associated with a worse prognosis (47,48). Mutations in other genes have also been observed, such as cysteinyl leukotriene receptor 2 (CYSLTR2; 4%) or phospholipase C beta 4 (PLCB4; 2.5%) (49,50). The mechanisms through which all these alterations affect tumour biology are described below.
In some cases of uveal melanoma, mutations in the telomerase reverse transcriptase (TERT) gene have been described. However, the frequency of this mutation is low, having been found in 1 of 50 uveal melanoma specimens examined by Dono et al (51). Furthermore, this mutation appeared to be associated with a tumour with variations in GNA11 and EIF1AX, that is, it appeared in the least aggressive profile. Nonetheless, this TERT variant has been detected at a higher frequency in both sporadic and familiar cutaneous melanoma (52). The greatest utility of this marker could be in identifying ocular melanoma type as indicated by the study conducted by Griewank et al (53). These authors found that up to 32% of conjunctival melanomas had a mutated TERT promotor, while this polymorphism was absent in 47 uveal melanomas examined. Their findings indicate that the presence or absence of this mutation is able to distinguish between both ocular melanomas and may help explain the different behaviour shown by each one.
7. Biology of uveal melanoma
Roles of inflammation and immune system in uveal melanoma
Hanahan and Weinberg (54) described the main characteristics or hallmarks of tumour cells that form the basis of our under-standing of cancer biology along with the targets of current cancer therapies. The inflammatory response represents one of these hallmarks and its important role in uveal melanoma was reviewed by Bronkhorst and Jager (55). Among other characteristics, the presence of an inflammatory phenotype has been described comprised of different types of lymphocytes and macrophages, along with the increased expression of class 1 and 2 HLA. This phenotype usually appears in M3 tumours as a sign of a worse prognosis (56).
This type of information also provides access to new more effective therapeutic tools for the treatment of uveal melanoma. However, although several studies have shown the efficacy of the key immune response regulators PD-1 and CTLA-4 inhibitors (57,58) in patients with cutaneous melanoma, the response to these molecules in patients with uveal melanoma has not been the same, suggesting the need to gain further insight into the evasive mechanisms of the immune system in uveal melanoma (59). In effect, Mougiakakos et al (60) demonstrated how high levels of cyclooxygenase (COX)-2, a marker of a worse prognosis in these tumours, were associated with elevated Treg levels in uveal melanoma and how this could explain the poor efficacy of antitumour therapies. However, there is a need for further research in this area, as other authors have found no such link between Treg levels and survival in this type of tumour (61,62). Recently, the study conducted by Petralia et al (63) demonstrated how levels of CD47 exhibit a better correlation with elevated levels of Treg and of other inflammatory cells. These results were also reported by Basile et al (64), who also noted that in uveal melanoma, CD200 and HVEM are significantly reduced and that there is an inverse association between the PDL1 levels and mean overall survival (OS), progression-free survival (PFS) and tumour thickness. Notably, the PD-1/PD-L1 levels have been shown to regulate the levels of non-coding RNA in a number of types of cancer, whose importance in uveal melanoma will be subsequently discussed (65). While PD-L1 expression has been reported at the primary tumour site, metastatic uveal melanoma exhibits a low expression of this marker (66). Importantly, the presence of T cells expressing LAG3 rather than CTLA-4 or PD-1 also plays a role in the inflammatory pattern in the microenvironment of primary uveal melanoma (67). Equally, liver metastasized tumours show infiltration of clonally expanded plasma cells, suggesting antibody-mediated immunity. The importance of hepatic stellate cells in liver metastasis has also been reported (68). The paracrine signalling of these cells affects the transcriptional activity of uveal melanoma cells, linked to inflammation and interleukin production. Hence, inflammatory conditions in the primary tumour seem very different to metastasis locations. Collectively, these data provide direction for future treatments pursuing these targets to improve treatment outcomes.
Signalling pathways
As described above, the most frequent mutations that appear in the early development of uveal melanoma are those affecting GPCR receptors, particularly variants of GNA11 or GNAQ. These last 2 genes code for subunit G-α of G proteins and are activated by the serotonin receptor 2A and 2B in the melanocyte (5-HT2A and 5-HT2B (69). Receptor 5-HT2B mutations are often found in a wide variety of tumours and have been linked to a greater metastasis risk (70). Furthermore, GNAQ and GNA11 mutations trigger a wide range of cell signalling cascades, including the PI3K/Akt/mTOR, YAP/TAZ, Wnt/β-catenin, Rac/Rho, Notch and MAPK pathways (71-73). The modification of so many cell signalling pathways notably hinders treatments targeting their inhibition owing to their possible interactions. An example is YAP/TAZ, whose activation occurs independently of HIPPO through its interaction with Rac/Rho, as reported by Feng et al (74). Thus, efforts in therapies targeted at inhibiting these pathways need to assess the cell dynamics of these tumours to increase their efficiency.
Mechanisms involved in metastasis
As previously described, one of the most important mutations found in uveal melanoma and a key point for understanding its biology, particularly its metastasis, is BAP1. BAP1 is a tumour suppressor gene that appears mutated in up to 84% of cases of metastasized uveal melanoma and in 38% of primary uveal melanomas (36,75). BAP1 codes for an enzyme with deubiquitinating capacity that binds to other suppressor proteins, such as BARD1 or BRCA1, generating heterodimers that act as tumour suppressors (76). It has been observed that mutations in the BAP1 germline are associated with a large variety of tumours, including lung adenocarcinoma, menangioma and uveal melanoma (77). Somatic mutations mainly affect premature protein termination or ubiquitin carboxy-terminal hydrolase domains. Among other functions, BAP1 is a key regulator of cell cycle control and transcription, whereby it interacts with histone H2A (78,79). BAP-1 deubiquitinates H2A and its loss has been associated with the death of cells which enter an RNF-2 apoptotic-dependent program (80). However, this mechanism has not been detected in melanocyte lines and it has been described that the loss of BAP-1 leads to defective DNA repair, thus favouring later mutations and cytogenetic aberrations, promoting the metastasis and aggressiveness of tumour cells (81). Matatall et al (82) also examined the role of BAP1 in the differentiation of uveal melanocytes and found that its lack of expression induces a progenitor phenotype in these melanocytes. Furthermore, it has been proposed that the loss of BAP1 can lead to an inflammatory tumour microenvironment (83). Finally, the location of BAP1 also seems to be crucial for metastasis. Szalai et al (84) reported no nuclear immunodetection of BAP1 in approximately 50% of patients with metastatic uveal melanoma, hence supporting the relevance of BAP1 mutations in metastasis.
Another key mutation in uveal melanoma progression is that detected in SF3B1. SF3B1 encodes a component of the spliceosome and its gaining function mutations affect the splicing of several transcripts with effects at different levels (85,86). Yavuzyigitoglu et al (87) confirmed that SF3B1 mutations were important in late metastasis, due to their effects on splicing, which in turn has been associated with a wide range of carcinogenic processes in a number of tumours, including invasion and metastasis (88). In uveal melanoma, SF3B1 splicing defects may play an important role in different processes, probably sharing common oncogenic mechanisms with BAP1 and EIF1AX (89). Mutant SF3B1 is considered to recognise intronic sequences in the bromodomain containing 9 (BRD9), degrading them and affecting the non-canonical barrier-to-autointegration factor complex (ncBAF), thus resulting in the development of myelodysplastic syndrome and uveal melanoma (90). In addition, mutations in SRSF2, U2AF1 and ZRSR2 have also been linked to defective splicing in uveal melanoma. Furthermore, in tumours with mutations in both BAP1 and SF3B1, elevated levels may appear of PRAME, which act as a repressor of retinoic acid signalling and of its receptor, two known tumour suppressors, whose inhibition has been incriminated in a wide variety of cancers (91,92). Mutations affecting EIF1AX, which participate in the onset of translation, has no influence on metastases and more work is needed to establish possible relations between both (86). Of note, EIF1AX mutations seem to exert a synergistic effect on Ras mutations in certain types of tumours, such as ovary and thyroid (93,94). The low proportions of uveal melanoma cell mutations in these genes may explain why EIF1AX is not associated with a greater metastasis risk in the tumours.
Another interesting signalling pathway associated with a number of tumours is that of endothelin 2 and its receptor endothelin receptor type B (EDNRB) associated with a large number of tumours (95,96). EDNRB is a G protein coupled receptor (GPCR) and these proteins play a role in the differentiation of melanocytes (97). Certain studies have found that a lower expression of this receptor in metastasized uveal melanomas indicates a poor prognosis (35,98). However, the mechanism responsible for this remains unclear. As a GPCR, the EDNRB receptor seems capable of activating protein G α subunits, such as GNAQ and GNA11. Urtatiz and Van Raamsdonk (99) proposed that reduced EDNRB receptor expression causes signalling dysregulation mediated by Wt variants and GNAQ/GNA11 mutants. However, in the study by Van Raamsdonk et al (47), it was observed that patients without GNAQ or GNA11 mutations exhibited a worse prognosis. Thus, lower EDNRB expression could be beneficial for patients with mutations in both proteins through their interference with the cell signalling cascade. Further insight into the mechanisms of action of G proteins in cancer and the role of EDNRB in uveal melanoma is required.
The mechanisms whereby uveal melanoma exhibits high tropism for the liver remain elusive. Some authors propose the bloodstream as the dissemination route from the eye to the liver aided by the fenestrated structure of hepatic capillaries (43). In parallel, it has also been hypothesized that it may be the result of increased expression of cMET, a tyrosine kinase inhibitor that is activated by binding to the hepatic growth factor (HGF) receptor produced in the liver that appears elevated in primary uveal melanomas (70,100). Other authors suggest that it is due to the increase in IGF-1/IGF-IR previously described in uveal melanoma (30).
Recent studies have revealed a role of cytokine CXCL12 and its receptor CXCR4, which also interacts with vascular endothelial growth factor (VEGF), potentiating its role in metastasis (101). Furthermore, both this pathway and cMET/HGF have been described to contribute to activation of the pathway PI3K/Akt/mTOR, indicating a worse prognosis for patients with this type of cancer (102). The activation of this pathway by cMET has also been described as a mechanism of resistance to MEK inhibitors (103). A lack of PTEN is also frequent in these tumours, affecting up to 40% of uveal melanomas (104).
Once again, these data suggest the importance of a wide perspective when treating uveal melanoma based on the combination of different therapies to improve their efficacy.
Hypoxia and oxidative stress
Another mechanism which plays a significant role in the development of uveal melanoma is hypoxia. This situation appears in tumours as a consequence of their rapid growth and has been attributed to their metabolic reprogramming (54). Hypoxia is an essential mechanism for a number of carcinogenic processes and is an important factor to consider when designing more effective therapies for various tumours (105). As a response to this setting of hypoxia, factors induced by hypoxia (HIF) will drive a large variety of cell responses among which we find the control of genes and molecules involved in anaerobic metabolism. This is a crucial process in tumour cells (known as the Warburg effect), in metastasis, in cell motility and in angiogenesis (106,107).
Hypoxia-induced factors consist of 2 heterodimer subunits formed by an α subunit (HIF-1 α, HIF-2 α or HIF-3 α) and a β subunit expressed constitutively. In conditions of normoxia, α subunits are degraded by the proteasome following a process of hydroxylation and ubiquitination. In hypoxia, the α subunit joins to the β subunit, recruiting p300/CBP coactivators to bind the hypoxia response element (HRE) present in approximately 100 genes (108). Although the functions of HIF-1 or HIF-2 are still under investigation, they seem more implicated in cancer than the HIF-3 isoform (109).
In uveal melanoma, hypoxia has been associated with numerous alterations. Asnaghi et al (110) detected increased signalling mediated by Notch and the phosphorylation levels of Erk1-2 and Akt. These authors also noted that the inhibition of the Notch pathway partially reduced Erk and Akt phosphorylation, suggesting a need to gain further insight into these targets to delay or avoid tumour dissemination. Furthermore, an increased HIF-1α expression was directly associated with increased levels of markers of cell proliferation (MIB-1), vessel growth (CD31 and VEGF-A) and necrosis; however, it was found to have no effect on patient survival (111).
In a later study, Hu et al (112) assessed the role of hypoxia in the angiogenic phenotype of uveal melanoma by examining another key component, angiopoietin-like 4 (ANGPTL4). In their study, the inhibition of this molecule and of VEGF was found to reduce the angiogenic potential of these tumours. Furthermore, HIF-1α has been demonstrated to contribute to the expression of c-MET and CXCR4. Inhibition with aryl sulphonamide 64B interrupts the interaction between the HIF-1 complex and its coactivators, and therefore reduces its binding to HRE present in the promoters of these genes, diminishing their expression (113).
Recently, Brouwer et al (114,115) observed that in tumours exhibiting M3 and a lack of BAP1 expression, the expression of HIF-1 α was elevated, as was microvascular density and the angiogenic phenotype, while VEGF-B expression was reduced. This suggests a need to address the mechanisms of angiogenesis in these tumours. HIF-1 α expression could not be associated with tumour size, but was related to the presence of T cells and macrophages. Tumour hypoxia also promotes the metabolic programming that tumour cells undergo.
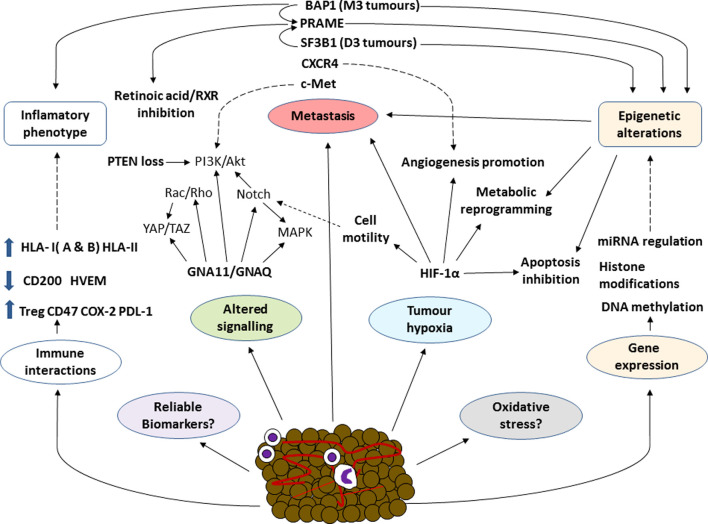
Collectively, these data identify hypoxia as an important factor to consider in the treatment of uveal melanoma, warranting further investigation. Notwithstanding, the mechanisms involved in hypoxia and its possible association with different carcinogenic processes need to be further examined. Some of the more important interactions of the hypoxia-induced factor are summarized in Fig. 2 along with the different biological mechanisms involved in this disease.
Figure 2.
Overall schematic diagram of some of the most significant factors involved in the biology of uveal melanoma. In different types of cancer, associations among the different components of the tumour process are complex explaining the non-success of therapies such as PD-1 inhibition. Some factors involved in uveal melanoma, such as the role of oxidative stress, have been well established. In a cancer as aggressive as uveal melanoma, knowledge of the mechanisms involved and their interactions is essential to develop more effective treatments, predict tumour behaviour and identify new more reliable and accurate biomarkers. BRCA-associated protein 1; PRAME, preferentially expressed antigen in melanoma; SF3B1, splicing factor 3b subunit 1; GNA, G protein subunit alpha; HIF-1α, hypoxia inducible factor α.
Oxidative stress is a cell condition that arises from an imbalance of oxidizing molecules produced mainly via mitochondrial respiration, and of reducing molecules, also known as antioxidants. The main oxidising molecules are reactive oxygen species (ROS) or nitrogen reactive species (NRS), which have been incriminated in a wide variety of diseases, such as Alzheimer's and other neurodegenerative diseases, or cardiovascular diseases, among others (116,117). The role of oxidative stress in the development of cancer is, however, still a somewhat controversial issue. To date, it has been established that oxidative stress can induce a carcinogenic process in early disease stages. For example, it is known that, as with malignant melanoma of the skin, the pheomelanin pigment pathway, which is associated with fairer skin tones and lighter eye colours, may lead to the development of uveal melanoma through a carcinogenesis mechanism independent of UV radiation that eventually gives rise to a process of oxidative damage (43,118). Furthermore, oxidative stress is directly related to an inflammatory response, which can promote the process of carcinogenesis (119).
In more advanced disease stages, this mechanism may block or impair certain key events for tumour development. Accordingly, it is currently proposed that adaptation to oxidative stress is one of the main mechanisms involved in the development of the different cancers (120). Piskounova et al (121) demonstrated that antioxidants, whose function is to minimize oxidative stress in cells, promoted the metastasis of melanoma cells. Recently, Dithmer et al (122) assessed the effects of the VEGF antagonist, bevacizumab, on the survival and proliferation of 5 uveal melanoma tumour lines, simulating a possible complication of the use of ionising radiation to treat primary tumours. The results indicated that this inhibitor exerted a protective effect against the oxidative stress induced by the ionising radiation, highlighting the need for detailed studies designed to unveil the role of oxidative stress in this disease.
Role of epigenetics in the development of uveal melanoma
Epigenetics is another key issue for understanding the factors underlying cancer. Epigenetic mechanisms are varied and include processes, such as DNA methylation, the modification of histones or regulation by non-codifying RNAs, such as microRNAs (miRNAs or miRs), as interesting therapeutic targets for diverse types of cancer (123). In this first section, we focus on the two former mechanisms. The miRNA control of gene transcription is discussed in the subsequent section.
The methylation state is one of the main epigenetic mechanisms. The hypermethylation of the most significant CpG islands through tumour gene suppressor inactivation takes place in numerous cancers including uveal melanoma (124). For example, it is common to observe the hypermethylation of the RASSF1a (Ras association domain family 1 isoform A) gene promotor region in uveal melanoma tumours (125). This gene also appears methylated in a wide variety of tumours, such as cutaneous melanoma, and lung, liver, breast or head and neck cancer, among others, and is a factor for a worse prognosis directly correlated with tumour progression (126). Maat et al (127) examined the role of Ras and EF-hand domain containing (RASEF) as a tumour suppressor gene in 11 uveal melanoma cell lines and 35 samples of primary uveal melanoma, and found that homozygosity in conjunction with hypermethylation was the mechanism whereby RASEF expression was lost, which was associated with a lower survival rate. Similarly, it has been reported that in both cutaneous and uveal melanoma, the hypermethylation of promotor sequences of the genes p16, DcR1 and DcR2 is often observed, directly involved in regulating cell processes, such as senescence and apoptosis (128,129). Of note, it has been observed that this hypermethylation of p16 leads to the phosphorylation of the retinoblastoma protein, which is key for controlling the cell cycle (130). Other important components of the cell cycle that exhibit an upregulated expression in uveal melanoma are Bcl-2, MDM2 and CD1 (102).
Gene hypomethylation is a less frequent epigenetic mechanism than hypermethylation and yet has been related to increased gene expression involved in these PRAME mechanisms or those of the gene deleted in split hand/split foot 1 (DSS1) (39,131). Notably, it is known that the DNA methylation patterns present in M3 tumours with abnormal BAP1 differ from those of D3, which, in turn, also differ between each other according to whether their mutation affects EIF1AX or SF3B1/SRFR2 (132). This could indicate the importance of these genes in epigenetic regulation mechanisms and is also considered an interesting topic of further investigation.
Histone modification is another process with an important role in epigenetic control affecting events, such as methylation, phosphorylation or acetylation. The dysregulation of these mechanisms can lead to the inappropriate activation of oncogenes or in the inactivation of tumour suppressor genes making this an important line of study in the field of cancer (133). In uveal melanoma, the overexpression of transcription factors, such as HES1 has been directly involved in the metastatic capacity of uveal melanoma, suggesting the methylation of the promotor region of histone H3K4 is an inducer of this over-expression (134). In effect, this is another interesting issue to explore in terms of increasing the efficacy of current therapies, especially for metastatic uveal melanomas.
Role of miRNAs in uveal melanoma
Advances in molecular biology have identified an important role of miRNAs in a wide variety of diseases, particularly cancer. Over the past 10 years, the number of studies addressing these molecules has increased exponentially, enhancing the knowledge of their function (135). For example, it is known that miRNAs are a key epigenetic mechanism for the control of gene transcription and may act in some cancer types as tumour suppressors and in others as oncogenes (136,137). In effect, miRNAs are emerging as promising therapeutic targets in various types of cancer and as ever more reliable prognostic factors in individualized precision medicine (138,139). The roles of miRNAs in uveal melanoma as important prognostic and diagnostic markers of tumour onset and progression have been confirmed (140). Some of the miRNAs playing a significant role in uveal melanoma are discussed below.
Yang and Wei (141) compared the expression profiles of miRNAs in 4 uveal melanoma tissues and 4 normal uveal tissues. Their results revealed increased expression levels of miRNAs of the miR-17 family (miRNA-20a, miRNA-106a and miRNA-17) and significant increases in miRNA-21 expression in 4 uveal melanoma cell lines, along with diminished miRNA-145 and miRNA-204 expression. Wang et al (142) elucidated the role of miRNA-21 in uveal melanoma cell metastasis. The results obtained revealed miRNA-21 overexpression following inhibition of p53 expression, which via a series of effector molecules may promote tumour metastasis in vitro. In vivo, its inhibition also leads to a reduced tumour size. Radhakrishnan et al (143) identified 19 miRNAs expressed in metastasized and not in metastatic uveal melanoma, while up to 11 miRNAs were detected only in the metastasized phenotype. Recently, other miRNAs with oncogenic effects have been identified, such as miR-155 (144).
Among the uveal melanoma tumour suppressor miRNAs, miRNA-145 should be mentioned. Li et al (145) found that the insulin 1 receptor substrate (IRS-1) could serve as a therapeutic target to increase the levels of miR-145, which is essential for uveal melanoma cells to enter into apoptosis. Similarly, the members of the miR-34 family of miRNA precursors, miR-34a, miR-34b and miR-34c, have been identified as important tumour suppressors expressed in normal uveal tissue, but not in uveal melanoma following their downregulation of other molecules, such as c-Met, Akt and proteins involved in the cell cycle (146,147). Recently, Serocki et al (148) confirmed the role of miR-17 family miRNAs in controlling HIF expression under conditions of hypoxia. Some miRNAs also reduce the expression of phosphatase and tensin homolog (PTEN), promoting PI3K/AKt/mTOR pathway activation (149,150). In addition, Liu et al (151) described the role of miR-216a-5p as an indicator of a better prognosis due to its inhibitory effect on hexokinase 2, an enzyme overexpressed in a wide array of tumours that is directly related to induction of the Warburg effect. Therapy, pursuing the dysregulation of these miRNAs is a promising approach for the treatment of this type of cancer.
Of note, miRNAs represent one of the various mechanisms involved in the pathogenesis of uveal melanoma, as summarized in Fig. 2. The interactions between all these factors are undoubtedly complex, and future studies will be crucial for a better understanding of such a complex cancer.
8. Blood biomarkers for uveal melanoma
Despite scientific and technological advances that have improved our understanding of various types of cancers, the incidence of uveal melanoma and patient survival has not markedly altered over the past 30 years (152,153). This has determined that the most effective measure against this type of cancer is its early detection. As uveal melanoma tumours spread via the bloodstream, blood biomarkers may be useful to detect metastases early on and to monitor disease progression or the response to treatment (154).
Circulating tumour cells (CTCs) and circulating free DNA (cfDNA) are among the components that may be detected in blood, indicating the presence of a tumour and both are prognostic markers of a variety of cancers (155,156). In uveal melanoma, both the detection of CTCs or cfDNA has proven a reliable indicator of a worse prognosis. Of note, the detection of melanocytic CTCs has exhibited efficacy in arterial blood, but not in veins (157), whereas cfDNA seems more useful in this tumour type, particularly in patients with easily detectable known mutations (158). In effect, today there is an ongoing clinical trial designed to assess the detection and variations produced in blood levels of cfDNA in patients followed before and after undergoing surgery for liver metastasis (NCT02849145).
Characterizing different CTC populations is also crucial for the understand of the biological mechanisms underlying this type of cancer. Schuster et al (159) examined CTCs in 68 patients with uveal melanoma and the gene expression in these cells of tyrosinase and MelanA/MART1. Their results indicated that the presence of CTCs was directly related to the metastatic process and that the detection of these transcripts points to a worse prognosis. Tura et al (160) demonstrated that FISH could be used to examine CTCs in patients with primary uveal melanoma and thus detect the status of chromosome 3. Following a 4-year follow-up period, the results revealed the high reliability of this method to predict the metastases that these patients could develop.
miRNAs can also represent important blood biomarkers detectable in uveal melanoma. Achberger et al (161) identified an association between plasma miRNAs and their variation in a setting of metastasis. Compared to the controls, the levels of miR-20a, -125b, -146a, -155, -181a and -223 were elevated, while those of miRNA-181a were reduced when metastasis appeared. Along these lines, Russo et al (162) found significantly higher blood and tissue levels of miRNA-146a. Furthermore, Eldh et al (163) detected higher levels of exosomes and miRNAs in patients with hepatic metastasis from uveal melanoma compared to patients without metastasis. Based on these data, Stark et al (164) measured the serum levels of up to 17 miRNAs in 65 patients with uveal nevus, localized uveal melanoma and metastasized uveal melanoma. The results served to define a panel of 6 miRNAs (miR-16, miR-145, miR-146a, miR-204, miR-211 and miR-363-3p) that could be used for a precision diagnosis of uveal melanoma with 93% sensitivity and 100% specificity. Collectively, these data indicate a need for advancements in the field of miRNAs, given their great diagnostic and therapeutic value in a disease as complex as uveal cancer.
Apart from these biomarkers, other blood indicators have proven useful in uveal melanoma, such as proteins, glycoproteins and tumour metabolites.
9. Proteomics and metabolomics in uveal melanoma
In the study of cancer, interest in proteomics and metabolomics continues to mount. Tissue and blood samples are the most used for this type of study, as they are minimally invasive and of great clinical value (165). However, these studies still have some limitations, such as a need for greater refinement in the measurement systems used and analytical variations in the data obtained and difficulties in their translation from bench to bedside (166).
The proteins melanoma inhibitory activity (MIA) and OPN (osteopontin) are among the most tested as biomarkers of uveal melanoma and have been directly associated with metas-tasis (167,168). Another biomarker examined is S100-β (169). These latter studies determined that all 3 of these proteins (MIA, OPN and S100-β) combined were able to detect with a high sensitivity the presence of metastases in the liver. However, in the study conducted by Missotten et al (170), no association was observed between this combined biomarker and any clinical or pathological feature of the tumour, questioning its actual prognostic value. Of note, Strobel et al (171) found elevated serum S100-β concentrations in patients with liver metastases from cutaneous melanoma compared to uveal melanoma in which no association was noted. In patients with liver metastasis, increased levels of the oncoprotein, DJ-1/PARK7, the soluble marker, c-Met, and the glycoprotein, ME20-S, have been observed (172-175). Notably, through cell culture techniques, Angi et al (176) compared the proteins secreted by uveal melanoma tumours with a high and low metastasis risk with those secreted by choroidal melanocytes. These authors detected the presence of OPN, MIA, GDF15, PARK7 and ME20, and only recorded significant differences in MIA and GDF15 secretion between cells of uveal melanoma and normal choroidal melanocytes. No differences emerged between the tumours with a high and low risk of metastasis.
Advances in omics-related technologies are proving helpful in the identification of the proteins and metabolites involved in uveal melanoma and in elucidating their roles. In the study by Crabb et al (177), iTRAQ technology was used to examine large numbers of proteins present in 8 samples of metastasized and 7 of non-metastasized uveal melanoma. Their findings identified a need for further investigation into proteins, such as heat shock protein (HSP)β-1 and collagen α3 (VI) as possible biomarkers of these tumours. Shi et al (178), using mass spectrometry and fractioning techniques with magnetic pearls, detected up to 49 differentially expressed peptides in patients with uveal melanoma and healthy controls. Their data indicated that peptides of 1,467 to 9,289 kDa were able to differentiate between patients with uveal melanoma and healthy individuals with a specificity of 100%. These authors also identified precursors of the fibrinogen α chain as possible markers of uveal melanoma. Also, recently Song et al (179) conducted a multiplex immunoassay on serum samples from 48 patients diagnosed with uveal melanoma and 36 healthy controls. Once again, HSPβ-1 and OPN levels proved useful to distinguish between patients and healthy control individuals.
10. Clinical management of uveal melanoma
Risk and prognosis of uveal melanoma
The general prognosis is that 50% of patients will present metastasis within the first 15 years of diagnosis. Once this occurs, the mean life expectancy is between 6 months to 1 year. However, it should be highlighted that the latency period from locoregional disease control until the onset of metastasis can be >25 years, such that patients require exhaustive follow-up over a long period of time. The preferred sites of presenting metastasis are the liver (~60%), lungs (~25%), skin and soft tissues (~10%) and bones (~8%) (13). The genetic analysis of melanocyte lesions has identified that extraocular invasion is related to both the inactivation of the tumour suppressor gene, BAP1 (detected in 85% of cases), and to monosomy 3, as the main risk factors for disease spread (180). Currently, there are no established criteria for the long-term follow-up of patients diagnosed with uveal melanoma. Recommended approaches are imaging techniques conducted every 3 to 12 months. An MRI is the best option both for the detection of liver and extrahepatic metastases, such as those affecting bones or retroperitoneal nodes. A CT scan is also useful for lung node manifestations and larger liver metastases and in patients for whom MRI is not recommended. Ultrasonography exclusively reveals hepatic metastases and PET cannot detect small lesions, the high radiation dose being another major drawback of this technique (181).
Tumour size, extraocular extension, mitotic activity and epithelioid cell type are considered important risk factors for melanoma (182). As previously stated, genetic mutations and chromosome abnormalities are also directly associated with patient outcomes and shed light into the prognosis of uveal melanoma. To examine all these chromosome and molecular features during the management of uveal melanoma, a wide range of methods can be used. The most common approaches are karyotyping, fluorescent in situ hybridisation (FISH) or comparative genome hybridisation (CGH). Further techniques, such as microsatellite analysis, multiple ligation-dependent probe amplification (MLPA) and genome-wide single nucleotide polymorphism can also be used for the genomic study of uveal melanoma. Karyotyping is useful for the detection major chromosome gains or losses. However, minor genetic alterations are not identified. FISH, such as CGH is more accurate in detecting chromosome aberrations in uveal melanoma; however, it is still insufficient for the detection of all chromosome modifications (183). Thus, the study of the molecular biology of uveal melanoma is required for the development of novel techniques. MLPA is currently an interesting option, particularly when combined with clinical and histological data, as it offers information on chromosomes 1, 3, 6 and 8 and it can be used in a wide range of samples, even those subjected to radiotherapy (184,185). Genome expression profiling (GEP) has been however, most successful for the prognosis of uveal melanoma (186). The strengths and limitations of these methods were reviewed by Dogrusöz et al (185). Additionally, whole genome sequencing (WGS) can provide substantial information in uveal melanoma (187) and microarray analysis can also offer whole genome data, partial chromosome defects, loss of heterozygosity or additional challenges not detected by FISH (188).
Pathological and genetic studies require invasive procedures to obtain biopsies; thus, the use of these methods in uveal melanoma is a matter of debate (189). The introduction of non-invasive diagnostic techniques, the validity of these genetic tests and even the emotional and ethical impacts for both patient and physician of the results (190) are some of the limitations of genetic risk determination. Nonetheless, the potential implications of knowledge regarding prognosis could be essential to establish guidelines for the follow-up of patients when the metastatic risk is low and opt for more aggressive treatment options if the risk is high. For instance, the presence of M3 or D3 is critical for the clinical management of uveal melanoma. The detection of the commonly found M3 in small tumours prompts the use of more aggressive treatments in these patients, especially to prevent metastasis (191). If M3 were detected, this could mean the tumour has spread to other organs, and hence, local therapy would not be effective (192). Surveillance in these high-risk patients may be hepatic imaging and liver function tests every 3-6 months (193). The biopsy method must also be considered in the study of M3 in uveal melanoma. Whereas fine needle aspiration (scleral approach) obtains a tumour sample from the base, the transvitreal approach collects the biopsy through the apex returning different results. Because of tumour heterogeneity, the scleral approach is the best method to detect M3 (194). Similarly, BAP1 tumours may have a significant clinical impact in uveal melanoma management, particularly in the development of targeted therapy.
Current and potential therapies
A close association exists between metastatic disease, prognosis and response to therapy. This is due to the fact that considerable advances have been made in the locoregional control of the disease through both conservative techniques (e.g., brachytherapy, external beam radiation therapy or laser photodynamic and photocoagulation therapy) and more aggressive approaches (enucleation) rendering an overall 5-year survival of approximately 80% (4). This survival rate has remained stable over the past 30 years, and developments have therefore consisted mainly of more effective and less aggressive surgical techniques.
Similar to the association existing between the prognosis and metastasis of uveal melanoma, immunotherapy is one of the main pillars of the treatment of disseminated disease. Systemic chemotherapy barely improves the overall prognosis of a patient and the response rate to conventional chemotherapy is <1%. Moreover, there is still no standardized treatment available for the management of metastatic disease that has been able to improve the long-term survival of these patients (195). This has meant that emphasis has been made on the most current treatment option, whereby an immune response is induced based on histological tumour characteristics, T lymphocytes and dendritic cells, and on the different cell signalling pathways. To understand the history of immunotherapy in patients with uveal melanoma, it should be remembered that the first trials involving this approach were conducted in patients with melanoma of the skin. For example, checkpoint inhibitors, mainly anti-CTLA4 (ipilimumab) and anti-PD1 (nivolumab), elicit a response in 40-60% of patients with metastatic cutaneous melanoma. However, in patients with uveal melanoma the response rate is approximately 20-30% (196). This poor response may be attributed to resistance due to the high tumour burden or to a low mutation rate conferring scarce antigenic induction and therefore a poor immune response. It should be emphasized that immunotherapy catalyses an immune reaction against tumour cells, such that a failed response will determine the disease will progress. A new immune approach involves the use of tebentafusp. This agent is based on the immune-mobilizing monoclonal T cell receptor (TCR) formed by a soluble TCR fused with an anti-CD3 presenting to uveal melanoma antigens, leading to T cell activation and triggering the activation of the immune response cascade with the consequence of enhanced tumour lysis (197). This novel treatment gives rise to a response rate of 57 to 71% after 16 weeks (198). It should be noted that this therapy is still under development and is being tested in several types of cancer, and that the long-term response to this new approach remains unknown. Similarly, several coadjuvant therapies have been assessed, such as vaccination with uveal melanoma cell antigens (gp100, t, RNA melanoma or tyrosinases) also targeted at activating the immune response, although this time on the part of dendritic cells. In patients classified as high risk (those with monosomy 3), this type of therapy has given rise to a 3-year survival rate of 79%, although as for tebentafusp, this is still at the clinical trial stage (199). Likewise, molecular targeting mayh be one of the most promising therapies for the management of uveal melanoma. For example, GNAQ and GNA11 pathway inhibitors, such as selumetinib or trametinib, both targeting MEK, have been shown to be successful in some clinical trials (200,201). Despite this, neither selumetinib nor trametinib increase the overall survival rate. This is due to resistance acquired by the tumour to these inhibitors, also observed in cutaneous melanoma (202). Blocking other cell signalling pathways such as cMET/PI3K inhibition in liver metastasis could be a solution to MEK inhibitors resistance (203). Likewise, histone deacetylase (HDAC) inhibitors represent an interesting coadjuvant to MEK inhibitors, also reducing tumour growth in various in vivo models (204). Notably, HDAC inhibitors have exhibited some efficacy in controlling cell differentiation, cell cycle and in the gene expression profile of cultured uveal melanoma cells (205). Importantly, by inhibiting the acetylation of histones, it is possible to reverse the effects of the loss of BAP1 through its effects on the cell cycle, leading to a less aggressive differentiated state (182).
The spliceosome has also been proposed as a potential antitumoral target in a number of types of cancer, as demonstrated by Bonnal et al (206). Examples of SF3B1 inhibitors are sudemycins, spliceostatin A and meayamycin. According to some authors, these compounds act through intron retention (207), whereas others propose massive exon skipping (208), hence interfering with aberrant SF3B1splicing. While further insight is needed into SF3B1 biology to design and predict the desirable effects of its inhibition, its potential as a uveal melanoma target is undeniable (209). Of note, spliceosome inhibitors may also be useful in BAP1 mutant tumours, as they promote c-Myc expression, increasing susceptibility to this therapy (182). Currently, H3B-8800 is being tested in patients with haematological malignancies (NCT02841540). Another undergoing clinical trial involves studying the role of niraparib in patients with uveal melanoma and other tumours featuring BAP1 mutations (NCT03207347), and PRAME is also being targeted in metastatic uveal melanoma through a PRAME-TCR construct (NCT02743611). Viral carriers may be an interesting solution to therapy delivery (210). Ongoing virus-based and other clinical trials for severe uveal melanoma are summarized in Table I. These new treatment lines are indeed a ray of hope that are set to change the fatal prognosis of this disease.
Table I.
Ongoing clinical trials targeting the treatment of metastatic uveal melanoma.
| Name | Identifier | Status | Population | Phase | Purpose |
|---|---|---|---|---|---|
| ENSIGN: Phase II window of opportunity or body radiation therapy and in situ gene therapy followed by nivolumab in metastatic squamous or non-squamous non-small cell lung carcinoma and metastatic uveal melanoma | NCT02831933 | Recruiting | 25 participants with lung squamous cell carcinoma stage IV and non-squamous non-small cell cancer metastatic uveal melanoma trial of stereotactic | Phase 2 | Determine the efficacy and safety of in situ gene therapy and stereotactic body radiation therapy |
| Ipilimumab and nivolumab in combination with immunoembolization for the treatment of metastatic uveal melanoma | NCT03472586 | Recruiting | 35 participants with uveal melanoma and liver metastasis | Phase 2 | Test the use of the monoclonal antibodies ipilimumab and nivolumab and immunoembolization to treat patients with liver metastasis |
| A Phase 1/2 dose-finding study to evaluate the safety, feasibility, and activity of BPX-701, a controllable PRAME T-cell receptor therapy, in HLA-A2+ subjects with AML, previously treated mds, or metastatic uveal melanoma | NCT02743611 | Active, not recruiting | 28 participants with AML, MDS and uveal melanoma | Phase 1 Phase 2 |
Assess the effect of BPX-701 in tumours showing high PRAME expression |
| Phase1b/2 study combining hepatic percutaneous perfusion with ipilimumab plus nivolumab in advanced uveal melanoma | NCT04283890 | Recruiting | 88 participants with metastatic uveal melanoma | Phase 1 Phase 2 |
Assess the use of immunotherapy (ipilimumab with nivolumab) plus chemotherapy (melphalan) |
| Phase Ib Study of cellular adoptive immunotherapy using autologous Cd8+ antigen-specific T cells and anti-Ctla4 for patients with metastatic uveal melanoma | NCT03068624 | Active, not recruiting | 19 participants with metastatic uveal melanoma | Phase 1 | Determine the maximum tolerated dose (MTD) of adoptively transferred SLC45A2-specific cytotoxic T-lymphocytes (CTL) and its combination with cyclophosphamide, aldesleukin and ipilimumab |
| A phase 2 study to evaluate the efficacy and safety of adoptive transfer of autologous tumour infiltrating lymphocytes in patients with metastatic uveal melanoma | NCT03467516 | Recruiting | 59 participants with metastatic uveal melanoma | Phase 2 | Assess the use of TIL in conjunction with TIL high dose aldesleukin |
| Phase I vaccination trial in metastatic uveal melanoma using IKKb-matured dendritic cells loaded with autologous tumour-RNA + RNA coding for defined antigens and driver mutations | NCT04335890 | Recruiting | 12 participants with metastatic uveal melanoma | Phase 1 | Assess the effects of vaccination with IKKb matured dendritic cells loaded with autologous tumour-RNA + RNA coding for defined antigens and driver mutations |
| A phase II study of BVD-523 in metastatic uveal melanoma | NCT03417739 | Active, not recruiting | 13 participants with metastatic uveal melanoma | Phase 2 | Assess the targeting of the MAPK signalling pathway using BVD-523 in advanced uveal melanoma |
| Efficacy study of pembrolizumab with entinostat to treat metastatic melanoma of the eye | NCT02697630 | Active, not recruiting | 29 participants with metastatic uveal melanoma | Phase 2 | Assess the potential combination of entinostat (HDAC inhibitor) and pembrolizumab (immunotherapy) |
| Intravenous and intrathecal nivolumab in treating patients with leptomeningeal disease | NCT03025256 | Recruiting | 30 participants with brain metastasis, among them uveal melanoma | Phase 1 | Compare intrathecal nivolumab and examine how well it acts in combination with intravenous nivolumab when treating patients with leptomeningeal disease |
| Trial of nivolumab in combination with ipilimumab in subjects with previously untreated metastatic uveal melanoma | NCT02626962 | Active, not recruiting | 48 participants with metastatic uveal melanoma | Phase 2 | Assess the impact of nivolumab combined with ipilimumab in subjects with previously untreated, unresectable or metastatic uveal melanoma |
| A study to assess PV-10 chemoablation of cancer of the liver | NCT00986661 | Recruiting | 78 participants with liver metastasis including those with uveal melanoma | Phase 1 | Examine the safety, tolerability, pharmacokinetics and effect of a single intralesional injection of PV-10 on tumour growth in subjects with primary or metastatic liver cancer |
| IN10018 monotherapy and combination therapy for metastatic melanoma | NCT04109456 | Recruiting | 52 participants with metastatic cutaneous or uveal melanoma | Phase 1 | Assess the safety, tolerability and antitumor properties of IN10018 as monotherapy and in combination with cobimetinib in subjects with metastatic uveal melanoma and NRAS-mutant metastatic melanoma |
| Modified virus VSV-IFNbetaTYRP1 in treating patients with stage iii-iv melanoma | NCT03865212 | Recruiting | 72 participants with stage III-IV cutaneous and uveal melanoma | Phase 1 | Confirm the efficacy, side effects and best dose of a modified virus VSV-IFNbetaTYRP1 |
| Yttrium90, ipilimumab, and nivolumab for uveal melanoma with liver metastases | NCT02913417 | Recruiting | 26 participants with liver metastatic uveal melanoma | Phase 1 Phase 2 |
Examine the synergistic effects of SirSpheres Yttrium-90 selective internal hepatic radiation followed by immunotherapy combined with ipilimumab and nivolumab |
| Iodine I 131 monoclonal antibody 3F8 in treating patients with central nervous system cancer or leptomeningeal cancer | NCT00445965 | Active, not recruiting | 78 participants with brain metastasis including those with uveal melanoma | Phase 2 | Assess iodine I 131 monoclonal antibody 3F8 used to treat patients with central nervous system or leptomeningeal cancer |
| Neoadjuvant and adjuvant checkpoint blockade | NCT02519322 | Recruiting | 53 participants with stage III-IV melanomas | Phase 2 | Check the performance of nivolumab with or without ipilimumab or relatlimab before surgery in patients with resectable stage IIIB-IV melanoma |
| Cabozantinib-S-malate compared with temozolomide or dacarbazine in treating patients with metastatic melanoma of the eye that cannot be removed by surgery | NCT01835145 | Active, not recruiting | 47 participants with recurrent/stage III-IV uveal melanoma | Phase 2 | Compare cabozantinib-s-malate with temozolomide or dacarbazine in patients with unresectable metastatic melanoma of the eye |
11. Conclusions and future directions
The present study has provided a comprehensive overview of uveal melanoma, from its biology to the current translational approaches. As discussed herein, uveal melanoma is an own entity in terms of clinical signs, target population, histology and molecular behaviour. The study of novel biological mechanisms possibly involved in uveal melanoma is also a key point for the design of new drugs directed towards targets, such as tumour hypoxia responses mediated by HIF-1α or epigenetic regulation driven by miRNAs. The cellular dynamics of these tumours and the different processes involved in their metastasis are also key topics of further investigation. The search for novel and more reliable blood or tissue biomarkers could be expedited by developments in techniques of proteomics and metabolomics, that will allow for the analysis of larger and more representative samples from patients with uveal melanoma. Risk determination strategies play a crucial role in the management of physicians or researchers and in improving early diagnosis, thus facilitating the follow-up of these patients. Genetics is a determining factor for the uveal melanoma stratification, its behaviour, therapeutic approach, and the emergent development of immunotherapy. New research efforts and current clinical trials must pursue novel therapies targeting the individual set of characteristics of this type of cancer. Uveal melanoma is a fatal cancer and its overall survival rate has not markedly improved over the past 3 decades. As molecular awareness has improved the understanding and management of such a complex disease, extensive research is required to continue to further elucidate the underlying mechanisms and complete them, not only from a biological view, but also with clinical outcomes, supporting the basis of further translational approaches.
Acknowledgments
Not applicable.
Funding
The present study was supported by grants from the B2017/BMD-3804 MITIC-CM (Community of Madrid, Spain), co-financed by the European Development Regional Fund 'A Way to Achieve Europe' (ERDF).
Availability of data and materials
Not applicable.
Authors' contributions
MAO, OFM, SC and MAT were involved in the conceptualization of the study. JB, SC, MAM and MAT were involved in funding acquisition. MAO, SC and MAT were involved in project administration., MAO, OFM and MAT were involved in the investigative aspects of the study. MAO, OFM, NGH, JB, MAM and MAT were involved in data validation. All authors have read and agreed to the published version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Andreoli MT, Mieler WF, Leiderman YI. Epidemiological trends in uveal melanoma. Br J Ophthalmol. 2015;99:1550–1553. doi: 10.1136/bjophthalmol-2015-306810. [DOI] [PubMed] [Google Scholar]
- 2.Maheshwari A, Finger PT. Cancers of the eye. Cancer Metastasis Rev. 2018;37:677–690. doi: 10.1007/s10555-018-9762-9. [DOI] [PubMed] [Google Scholar]
- 3.Carvajal RD, Schwartz GK, Tezel T, Marr B, Francis JH, Nathan PD. Metastatic disease from uveal melanoma: Treatment options and future prospects. Br J Ophthalmol. 2017;101:38–44. doi: 10.1136/bjophthalmol-2016-309034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krantz BA, Dave N, Komatsubara KM, Marr BP, Carvajal RD. Uveal melanoma: Epidemiology, etiology, and treatment of primary disease. Clin Ophthalmol. 2017;11:279–289. doi: 10.2147/OPTH.S89591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seedor RS, Eschelman DJ, Gonsalves CF, Adamo RD, Orloff M, Amjad A, Sharpe-Mills E, Chervoneva I, Shields CL, Shields JA, et al. An Outcome assessment of a single Institution's longitudinal experience with Uveal melanoma patients with liver metastasis. Cancers (Basel) 2020;12:117. doi: 10.3390/cancers12010117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shields CL, Manalac J, Das C, Ferguson K, Shields JA. Choroidal melanoma: Clinical features, classification, and top 10 pseudomelanomas. Curr Opin Ophthalmol. 2014;25:177–185. doi: 10.1097/ICU.0000000000000041. [DOI] [PubMed] [Google Scholar]
- 7.Shields CL, Kaliki S, Furuta M, Mashayekhi A, Shields JA. Clinical spectrum and prognosis of uveal melanoma based on age at presentation in 8,033 cases. Retina. 2012;32:1363–1372. doi: 10.1097/IAE.0b013e31824d09a8. [DOI] [PubMed] [Google Scholar]
- 8.Virgili G, Gatta G, Ciccolallo L, Capocaccia R, Biggeri A, Crocetti E, Lutz JM, Paci E, EUROCARE Working Group Incidence of uveal melanoma in europe. Ophthalmology. 2007;114:2309–2315. doi: 10.1016/j.ophtha.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 9.Park SJ, Oh CM, Kim BW, Woo SJ, Cho H, Park KH. Nationwide incidence of ocular melanoma in South Korea by using the national cancer registry database (1999-2011) Invest Ophthalmol Vis Sci. 2015;56:4719–4724. doi: 10.1167/iovs.15-16532. [DOI] [PubMed] [Google Scholar]
- 10.Singh N, Seregard S, Singh AD. Uveal melanoma: Epidemiologic aspects. Clin Ophthalmic Oncol. 2019:53–69. doi: 10.1007/978-3-030-17879-6_4. [DOI] [Google Scholar]
- 11.Nichols EE, Richmond A, Daniels AB. Disparities in uveal melanoma: Patient characteristics. Semin Ophthalmol. 2016;31:296–303. doi: 10.3109/08820538.2016.1154176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nayman T, Bostan C, Logan P, Burnier MN., Jr Uveal melanoma risk Factors: A systematic review of Meta-analyses. Curr Eye Res. 2017;42:1085–1093. doi: 10.1080/02713683.2017.1297997. [DOI] [PubMed] [Google Scholar]
- 13.Kaliki S, Shields CL, Shields JA. Uveal melanoma: Estimating prognosis. Indian J Ophthalmol. 2015;63:93–102. doi: 10.4103/0301-4738.154367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Jamal RT, Kivelä T. Uveal melanoma among Finnish children and young adults. J AAPOS. 2014;18:61–66. doi: 10.1016/j.jaapos.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Mallet JD, Gendron SP, Drigeard Desgarnier MC, Rochette PJ. Implication of ultraviolet light in the etiology of uveal melanoma: A review. Photochem Photobiol. 2014;90:15–21. doi: 10.1111/php.12161. [DOI] [PubMed] [Google Scholar]
- 16.Mishra S, Bharagava RN. Toxic and genotoxic effects of hexavalent chromium in environment and its bioremediation strategies. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2016;34:1–32. doi: 10.1080/10590501.2015.1096883. [DOI] [PubMed] [Google Scholar]
- 17.Velazquez N, Jones IS. Ocular and oculodermal melanocytosis associated with Uveal melanoma. Ophthalmology. 1983;90:1472–1476. doi: 10.1016/S0161-6420(83)34358-X. [DOI] [PubMed] [Google Scholar]
- 18.Plateroti AM, Scavella V, Abdolrahimzadeh B, Plateroti R, Rahimi S. An update on oculodermal melanocytosis and rare associated conditions. Semin Ophthalmol. 2017;32:524–528. doi: 10.3109/08820538.2015.1118133. [DOI] [PubMed] [Google Scholar]
- 19.Shields CL, Kaliki S, Hutchinson A, Nickerson S, Patel J, Kancherla S, Peshtani A, Nakhoda S, Kocher K, Kolbus E, et al. Iris nevus growth into melanoma: Analysis of 1611 consecutive eyes: The ABCDEF guide. Ophthalmology. 2013;120:766–772. doi: 10.1016/j.ophtha.2012.09.042. [DOI] [PubMed] [Google Scholar]
- 20.Chien JL, Sioufi K, Surakiatchanukul T, Shields JA, Shields CL. Choroidal nevus: A review of prevalence, features, genetics, risks, and outcomes. Curr Opin Ophthalmol. 2017;28:228–237. doi: 10.1097/ICU.0000000000000361. [DOI] [PubMed] [Google Scholar]
- 21.Rodríguez A, Dueñas-Gonzalez A, Delgado-Pelayo S. Clinical presentation and management of uveal melanoma. Mol Clin Oncol. 2016;5:675–677. doi: 10.3892/mco.2016.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nezu N, Goto H, Umazume K, Ueda S, Shibata M. Clinical analysis of uveal melanoma. Nippon Ganka Gakkai Zasshi. 2017;121:413–418. In Japanese. [PubMed] [Google Scholar]
- 23.Callender GR. Malignant melanotic tumors of the eye. A study of histologic types in 111 cases. Trans Am Acad Ophthalmol Otolaryngol. 1931;36:131–140. [Google Scholar]
- 24.McLean IW, Foster WD, Zimmerman LE, Gamel JW. Modifications of Callender's classification of uveal melanoma at the armed forces institute of pathology. Am J Ophthalmol. 1983;96:502–509. doi: 10.1016/S0002-9394(14)77914-0. [DOI] [PubMed] [Google Scholar]
- 25.Seddon JM, Polivogianis L, Hsieh CC, Albert DM, Gamel JW, Gragoudas ES. Death from uveal melanoma. Number of epithelioid cells and inverse SD of nucleolar area as prognostic factors. Arch Ophthalmol. 1987;105:801–806. doi: 10.1001/archopht.1987.01060060087039. [DOI] [PubMed] [Google Scholar]
- 26.Seregard S, Kock E. Prognostic indicators following enucleation for posterior uveal melanoma. A multivariate analysis of Long-term survival with minimized loss to Follow-up. Acta Ophthalmol Scand. 1995;73:340–344. doi: 10.1111/j.1600-0420.1995.tb00039.x. [DOI] [PubMed] [Google Scholar]
- 27.Folberg R, Pe'er J, Gruman LM, Woolson RF, Jeng G, Montague PR, Moninger TO, Yi H, Moore KC. The morpho-logic characteristics of tumor blood vessels as a marker of tumor progression in primary human uveal melanoma: A matched Case-control study. Hum Pathol. 1992;23:1298–1305. doi: 10.1016/0046-8177(92)90299-I. [DOI] [PubMed] [Google Scholar]
- 28.Al-Jamal RT, Mäkitie T, Kivelä T. Nucleolar diameter and microvascular factors as independent predictors of mortality from malignant melanoma of the choroid and ciliary body. Invest Ophthalmol Vis Sci. 2003;44:2381–2389. doi: 10.1167/iovs.02-1215. [DOI] [PubMed] [Google Scholar]
- 29.Moshari A, McLean IW. Uveal melanoma: Mean of the longest nucleoli measured on Silver-stained sections. Invest Ophthalmol Vis Sci. 2001;42:1160–1163. [PubMed] [Google Scholar]
- 30.All-Ericsson C, Girnita L, Seregard S, Bartolazzi A, Jager MJ, Larsson O. Insulin-like growth Factor-1 receptor in uveal melanoma: A predictor for metastatic disease and a potential therapeutic target. Invest Ophthalmol Vis Sci. 2002;43:1–8. [PubMed] [Google Scholar]
- 31.Karlsson M, Boeryd B, Carstensen J, Frånlund B, Gustafsson B, Kågedal B, Sun XF, Wingren S. Correlations of Ki-67 and PCNA to DNA ploidy, S-phase fraction and survival in uveal melanoma. Eur J Cancer. 1996;32A:357–362. doi: 10.1016/0959-8049(95)00562-5. [DOI] [PubMed] [Google Scholar]
- 32.Bronkhorst IH, Ly LV, Jordanova ES, Vrolijk J, Versluis M, Luyten GP, Jager MJ. Detection of M2-macrophages in uveal melanoma and relation with survival. Invest Ophthalmol Vis Sci. 2011;52:643–650. doi: 10.1167/iovs.10-5979. [DOI] [PubMed] [Google Scholar]
- 33.Bronkhorst IH, Jager MJ. Uveal melanoma: The inflammatory microenvironment. J Innate Immun. 2012;4:454–462. doi: 10.1159/000334576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blom DJ, Luyten GP, Mooy C, Kerkvliet S, Zwinderman AH, Jager MJ. Human leukocyte antigen class I expression. Marker of poor prognosis in uveal melanoma. Invest Ophthalmol Vis Sci. 1997;38:1865–1872. [PubMed] [Google Scholar]
- 35.Onken MD, Worley LA, Ehlers JP, Harbour JW. Gene expression profiling in uveal melanoma reveals two molecular classes and predicts metastatic death. Cancer Res. 2004;64:7205–7209. doi: 10.1158/0008-5472.CAN-04-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robertson AG, Shih J, Yau C, Gibb EA, Oba J, Mungall KL, Hess JM, Uzunangelov V, Walter V, Danilova L, et al. Integrative analysis identifies four molecular and clinical subsets in uveal melanoma. Cancer Cell. 2017;32:204–220.e15. doi: 10.1016/j.ccell.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reichstein D. New concepts in the molecular understanding of uveal melanoma. Curr Opin Ophthalmol. 2017;28:219–227. doi: 10.1097/ICU.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 38.Field MG, Decatur CL, Kurtenbach S, Gezgin G, van der Velden PA, Jager MJ, Kozak KN, Harbour JW. PRAME as an independent biomarker for metastasis in uveal melanoma. Clin Cancer Res. 2016;22:1234–1242. doi: 10.1158/1078-0432.CCR-15-2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Field MG, Durante MA, Decatur CL, Tarlan B, Oelschlager KM, Stone JF, Kuznetsov J, Bowcock AM, Kurtenbach S, Harbour JW. Epigenetic reprogramming and aberrant expression of PRAME are associated with increased metastatic risk in class 1 and class 2 uveal melanomas. Oncotarget. 2016;7:59209–59219. doi: 10.18632/oncotarget.10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Decatur CL, Ong E, Garg N, Anbunathan H, Bowcock AM, Field MG, Harbour JW. Driver mutations in uveal melanoma: Associations with gene expression profile and patient outcomes. JAMA Ophthalmol. 2016;134:728–733. doi: 10.1001/jamaophthalmol.2016.0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diener-West M, Reynolds SM, Agugliaro DJ, Caldwell R, Cumming K, Earle JD, Hawkins BS, Hayman JA, Jaiyesimi I, Jampol LM, et al. Development of metastatic disease after enrolment in the COMS trials for treatment of choroidal melanoma: Collaborative ocular melanoma study group report no 26. Arch Ophthalmol. 2005;123:1639–1643. doi: 10.1001/archopht.123.12.1639. [DOI] [PubMed] [Google Scholar]
- 42.Ramaiya KJ, Harbour JW. Current management of uveal melanoma. Expert Rev Ophthalmol. 2007;2:939–946. doi: 10.1586/17469899.2.6.939. [DOI] [Google Scholar]
- 43.Pandiani C, Béranger GE, Leclerc J, Ballotti R, Bertolotto C. Focus on cutaneous and uveal melanoma specificities. Genes Dev. 2017;31:724–743. doi: 10.1101/gad.296962.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ny L, Nyakas M, Hernberg M, Koivunen J, Oddershede L, Yoon MR, Wang X, Guyot P, Geisler J. BRAF mutation as a prognostic marker for survival in malignant melanoma: A systematic review and meta-analysis. J Clin Oncol. 2018;36:e21566. doi: 10.1200/JCO.2018.36.15_suppl.e21566. [DOI] [PubMed] [Google Scholar]
- 45.Wu J, Brunner G, Celebi JT. A melanoma subtype: Uveal melanoma. J Am Acad Dermatol. 2011;64:1185–1186. doi: 10.1016/j.jaad.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saldanha G, Purnell D, Fletcher A, Potter L, Gillies A, Pringle JH. High BRAF mutation frequency does not characterize all melanocytic tumor types. Int J Cancer. 2004;111:705–710. doi: 10.1002/ijc.20325. [DOI] [PubMed] [Google Scholar]
- 47.Van Raamsdonk CD, Griewank KG, Crosby MB, Garrido MC, Vemula S, Wiesner T, Obenauf AC, Wackernagel W, Green G, Bouvier N, et al. Mutations in GNA11 in uveal melanoma. N Engl J Med. 2010;363:2191–2199. doi: 10.1056/NEJMoa1000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van der Kooij MK, Speetjens FM, van der Burg SH, Kapiteijn E. Uveal versus cutaneous melanoma; Same origin, very distinct tumor types. Cancers (Basel) 2019;11:845. doi: 10.3390/cancers11060845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moore AR, Ceraudo E, Sher JJ, Guan Y, Shoushtari AN, Chang MT, Zhang JQ, Walczak EG, Kazmi MA, Taylor BS, et al. Recurrent activating mutations of G-Protein-coupled receptor CYSLTR2 in uveal melanoma. Nat Genet. 2016;48:675–680. doi: 10.1038/ng.3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johansson P, Aoude LG, Wadt K, Glasson WJ, Warrier SK, Hewitt AW, Kiilgaard JF, Heegaard S, Isaacs T, Franchina M, et al. Deep sequencing of uveal melanoma identifies a recurrent mutation in PLCB4. Oncotarget. 2016;7:4624–4631. doi: 10.18632/oncotarget.6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dono M, Angelini G, Cecconi M, Amaro A, Esposito AI, Mirisola V, Maric I, Lanza F, Nasciuti F, Viaggi S, et al. Mutation frequencies of GNAQ, GNA11, BAP1, SF3B1, EIF1AX and TERT in uveal melanoma: Detection of an activating mutation in the TERT gene promoter in a single case of uveal melanoma. Br J Cancer. 2014;110:1058–1065. doi: 10.1038/bjc.2013.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Horn S, Figl A, Rachakonda PS, Fischer C, Sucker A, Gast A, Kadel S, Moll I, Nagore E, Hemminki K, et al. TERT promoter mutations in familial and sporadic melanoma. Science. 2013;339:959–961. doi: 10.1126/science.1230062. [DOI] [PubMed] [Google Scholar]
- 53.Griewank KG, Murali R, Schilling B, Scholz S, Sucker A, Song M, Süsskind D, Grabellus F, Zimmer L, Hillen U, et al. TERT promoter mutations in ocular melanoma distinguish between conjunctival and uveal tumours. Br J Cancer. 2013;109:497–501. doi: 10.1038/bjc.2013.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 55.Bronkhorst IH, Jager MJ. Inflammation in uveal melanoma. Eye (Lond) 2013;27:217–223. doi: 10.1038/eye.2012.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maat W, Ly LV, Jordanova ES, Wolff-Rouendaal D, Schalij-Delfos NE, Jager MJ. Monosomy of chromosome 3 and an inflammatory phenotype occur together in uveal melanoma. Invest Ophthalmol Vis Sci. 2008;49:505–510. doi: 10.1167/iovs.07-0786. [DOI] [PubMed] [Google Scholar]
- 57.Mahoney KM, Freeman GJ, McDermott DF. The next immune-checkpoint inhibitors: PD-1/PD-L1 blockade in melanoma. Clin Ther. 2015;37:764–782. doi: 10.1016/j.clinthera.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yun S, Vincelette ND, Green MR, Wahner Hendrickson AE, Abraham I. Targeting immune checkpoints in unresectable metastatic cutaneous melanoma: A systematic review and Meta-analysis of anti-CTLA-4 and anti-PD-1 agents trials. Cancer Med. 2016;5:1481–1491. doi: 10.1002/cam4.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Javed A, Arguello D, Johnston C, Gatalica Z, Terai M, Weight RM, Orloff M, Mastrangelo MJ, Sato T. PD-L1 expression in tumor metastasis is different between uveal melanoma and cutaneous melanoma. Immunotherapy. 2017;9:1323–1330. doi: 10.2217/imt-2017-0066. [DOI] [PubMed] [Google Scholar]
- 60.Mougiakakos D, Johansson CC, Trocme E, All-Ericsson C, Economou MA, Larsson O, Seregard S, Kiessling R. Intratumoral forkhead box P3-positive regulatory T cells predict poor survival in cyclooxygenase-2-positive uveal melanoma. Cancer. 2010;116:2224–2233. doi: 10.1002/cncr.24999. [DOI] [PubMed] [Google Scholar]
- 61.Lagouros E, Salomao D, Thorland E, Hodge DO, Vile R, Pulido JS. Infiltrative T regulatory cells in enucleated uveal melanomas. Trans Am Ophthalmol Soc. 2009;107:223–228. [PMC free article] [PubMed] [Google Scholar]
- 62.Bronkhorst IH, Vu TH, Jordanova ES, Luyten GP, Burg SH, Jager MJ. Different subsets of tumor-infiltrating lymphocytes correlate with macrophage influx and monosomy 3 in uveal melanoma. Invest Ophthalmol Vis Sci. 2012;53:5370–5378. doi: 10.1167/iovs.11-9280. [DOI] [PubMed] [Google Scholar]
- 63.Petralia MC, Mazzon E, Fagone P, Russo A, Longo A, Avitabile T, Nicoletti F, Reibaldi M, Basile MS. Characterization of the pathophysiological role of CD47 in Uveal melanoma. Molecules. 2019;24:2450. doi: 10.3390/molecules24132450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Basile MS, Mazzon E, Russo A, Mammana S, Longo A, Bonfiglio V, Fallico M, Caltabiano R, Fagone P, Nicoletti F, et al. Differential modulation and prognostic values of immune-escape genes in uveal melanoma. PLoS One. 2019;14:e0210276. doi: 10.1371/journal.pone.0210276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ding L, Lu S, Li Y. Regulation of PD-1/PD-L1 pathway in cancer by noncoding RNAs. Pathol Oncol Res. 2020;26:651–663. doi: 10.1007/s12253-019-00735-9. [DOI] [PubMed] [Google Scholar]
- 66.Javed A, Arguello D, Johnston C, Gatalica Z, Terai M, Weight RM, Orloff M, Mastrangelo MJ, Sato T. PD-L1 expression in tumor metastasis is different between uveal melanoma and cutaneous melanoma. Immunotherapy. 2017;9:1323–1330. doi: 10.2217/imt-2017-0066. [DOI] [PubMed] [Google Scholar]
- 67.Durante MA, Rodriguez DA, Kurtenbach S, Kuznetsov JN, Sanchez MI, Decatur CL, Snyder H, Feun LG, Livingstone AS, Harbour JW. Single-cell analysis reveals new evolutionary complexity in uveal melanoma. Nat Commun. 2020;11(496) doi: 10.1038/s41467-019-14256-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Babchia N, Landreville S, Clément B, Coulouarn C, Mouriaux F. The bidirectional crosstalk between metastatic uveal melanoma cells and hepatic stellate cells engenders an inflammatory microenvironment. Exp Eye Res. 2019;181:213–222. doi: 10.1016/j.exer.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 69.Giulietti M, Vivenzio V, Piva F, Principato G, Bellantuono C, Nardi B. How much do we know about the coupling of G-proteins to serotonin receptors? Mol Brain. 2014;7:49. doi: 10.1186/s13041-014-0049-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tschentscher F, Hüsing J, Holter T, Kruse E, Dresen IG, Jöckel KH, Anastassiou G, Schilling H, Bornfeld N, Horsthemke B, et al. Tumor classification based on gene expression profiling shows that uveal melanomas with and without monosomy 3 represent two distinct entities. Cancer Res. 2003;63:2578–2584. [PubMed] [Google Scholar]
- 71.Bakalian S, Marshall JC, Logan P, Faingold D, Maloney S, Di Cesare S, Martins C, Fernandes BF, Burnier MN., Jr Molecular pathways mediating liver metastasis in patients with uveal melanoma. Clin Cancer Res. 2008;14:951–956. doi: 10.1158/1078-0432.CCR-06-2630. [DOI] [PubMed] [Google Scholar]
- 72.O'Hayre M, Degese MS, Gutkind JS. Novel insights into G protein and G Protein-coupled receptor signaling in cancer. Curr Opin Cell Biol. 2014;27:126–135. doi: 10.1016/j.ceb.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu H, Lei C, Long K, Yang X, Zhu Z, Zhang L, Liu J. Mutant GNAQ promotes cell viability and migration of uveal melanoma cells through the activation of Notch signaling. Oncol Rep. 2015;34:295–301. doi: 10.3892/or.2015.3949. [DOI] [PubMed] [Google Scholar]
- 74.Feng X, Degese MS, Iglesias-Bartolome R, Vaque JP, Molinolo AA, Rodrigues M, Zaidi MR, Ksander BR, Merlino G, Sodhi A, et al. Hippo-independent activation of YAP by the GNAQ uveal melanoma oncogene through a trio-regulated rho GTPase signaling circuitry. Cancer Cell. 2014;25:831–845. doi: 10.1016/j.ccr.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Harbour JW, Onken MD, Roberson ED, Duan S, Cao L, Worley LA, Council ML, Matatall KA, Helms C, Bowcock AM. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science. 2010;330:1410–1413. doi: 10.1126/science.1194472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Coupland SE, Lake SL, Zeschnigk M, Damato BE. Molecular pathology of uveal melanoma. Eye (Lond) 2013;27:230–242. doi: 10.1038/eye.2012.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Abdel-Rahman MH, Pilarski R, Cebulla CM, Massengill JB, Christopher BN, Boru G, Hovland P, Davidorf FH. Germline BAP1 mutation predisposes to uveal melanoma, lung adenocarcinoma, meningioma, and other cancers. J Med Genet. 2011;48:856–859. doi: 10.1136/jmedgenet-2011-100156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Scheuermann JC, de Ayala Alonso AG, Oktaba K, Ly-Hartig N, McGinty RK, Fraterman S, Wilm M, Muir TW, Müller J. Histone H2A deubiquitinase activity of the polycomb repressive complex PR-DUB. Nature. 2010;465:243–247. doi: 10.1038/nature08966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Eletr ZM, Wilkinson KD. An emerging model for BAP1's role in regulating cell cycle progression. Cell Biochem Biophys. 2001;60:3–11. doi: 10.1007/s12013-011-9184-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.He M, Chaurushiya MS, Webster JD, Kummerfeld S, Reja R, Chaudhuri S, Chen YJ, Modrusan Z, Haley B, Dugger DL, et al. Intrinsic apoptosis shapes the tumor spectrum linked to inactivation of the deubiquitinase BAP1. Science. 2019;364:283–285. doi: 10.1126/science.aav4902. [DOI] [PubMed] [Google Scholar]
- 81.Park JJ, Diefenbach RJ, Joshua AM, Kefford RF, Carlino MS, Rizos H. Oncogenic signaling in uveal melanoma. Pigment Cell Melanoma Res. 2018;31:661–672. doi: 10.1111/pcmr.12708. [DOI] [PubMed] [Google Scholar]
- 82.Matatall KA, Agapova OA, Onken MD, Worley LA, Bowcock AM, Harbour JW. BAP1 deficiency causes loss of melanocytic cell identity in uveal melanoma. BMC Cancer. 2013;13:371. doi: 10.1186/1471-2407-13-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Oliva M, Rullan AJ, Piulats JM. Uveal melanoma as a target for Immune therapy. Ann Transl Med. 2016;4:172. doi: 10.21037/atm.2016.05.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Szalai E, Wells JR, Ward L, Grossniklaus HE. Uveal melanoma nuclear BRCA1-associated Protein-1 immunoreactivity is an indicator of metastasis. Ophthalmology. 2018;125:203–209. doi: 10.1016/j.ophtha.2017.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Furney SJ, Pedersen M, Gentien D, Dumont AG, Rapinat A, Desjardins L, Turajlic S, Piperno-Neumann S, de la Grange P, Roman-Roman S, et al. SF3B1 mutations are associated with alternative splicing in uveal melanoma. Cancer Discov. 2013;3:1122–1129. doi: 10.1158/2159-8290.CD-13-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Amaro A, Gangemi R, Piaggio F, Angelini G, Barisione G, Ferrini S, Pfeffer U. The biology of uveal melanoma. Cancer Metastasis Rev. 2017;36:109–140. doi: 10.1007/s10555-017-9663-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yavuzyigitoglu S, Koopmans AE, Verdijk RM, Vaarwater J, Eussen B, van Bodegom A, Paridaens D, Kiliç E, de Klein A, Rotterdam Ocular Melanoma Study Group Uveal melanomas with SF3B1 mutations: A distinct subclass associated with Late-onset metastases. Ophthalmology. 2016;123:1118–1128. doi: 10.1016/j.ophtha.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 88.Coltri PP, Dos Santos MGP, da Silva GHG. Splicing and cancer: Challenges and opportunities. Wiley Interdiscip Rev RNA. 2019;10:e1527. doi: 10.1002/wrna.1527. [DOI] [PubMed] [Google Scholar]
- 89.Alsafadi S, Mobuchon L, Rodrigues M, Stern MH. Uveal melanoma, a model disease for splicing alterations and oncogenesis. Med Sci (Paris) 2018;34:155–160. doi: 10.1051/medsci/20183402013. In French. [DOI] [PubMed] [Google Scholar]
- 90.Tanaka A, Kobayashi S, Xiao M, Inoue D. Understanding and therapeutic targeting of aberrant mRNA splicing mechanisms in oncogenesis. Rinsho Ketsueki. 2020;61:643–650. doi: 10.11406/rinketsu.61.643. In Japanese. [DOI] [PubMed] [Google Scholar]
- 91.Freemantle SJ, Spinella MJ, Dmitrovsky E. Retinoids in cancer therapy and chemoprevention: Promise meets resistance. Oncogene. 2003;22:7305–7315. doi: 10.1038/sj.onc.1206936. [DOI] [PubMed] [Google Scholar]
- 92.Epping MT, Wang L, Edel MJ, Carlee L, Hernandez M, Bernards R. The human tumor antigen PRAME is a dominant repressor of retinoic acid receptor signaling. Cell. 2005;122:835–847. doi: 10.1016/j.cell.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 93.Etemadmoghadam D, Azar WJ, Lei Y, Moujaber T, Garsed DW, Kennedy CJ, Fereday S, Mitchell C, Chiew YE, Hendley J, et al. EIF1AX and NRAS Mutations Co-occur and Cooperate in Low-grade serous ovarian carcinomas. Cancer Res. 2017;77:4268–4278. doi: 10.1158/0008-5472.CAN-16-2224. [DOI] [PubMed] [Google Scholar]
- 94.Krishnamoorthy GP, Davidson NR, Leach SD, Zhao Z, Lowe SW, Lee G, Landa I, Nagarajah J, Saqcena M, Singh K, et al. EIF1AX and RAS mutations cooperate to drive thyroid tumorigenesis through ATF4 and C-MYC. Cancer Discov. 2019;9:264–281. doi: 10.1158/2159-8290.CD-18-0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bagnato A, Rosanò L. The endothelin axis in cancer. Int J Biochem Cell Biol. 2008;40:1443–1451. doi: 10.1016/j.biocel.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 96.Davenport AP, Hyndman KA, Dhaun N, Southan C, Kohan DE, Pollock JS, Pollock DM, Webb DJ, Maguire JJ. Endothelin. Pharmacol Rev. 2016;68:357–418. doi: 10.1124/pr.115.011833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pla P, Alberti C, Solov'eva O, Pasdar M, Kunisada T, Larue L. Ednrb2 orients cell migration towards the dorsolateral neural crest pathway and promotes melanocyte differentiation. Pigment Cell Res. 2005;18:181–187. doi: 10.1111/j.1600-0749.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- 98.Smith SL, Damato BE, Scholes AG, Nunn J, Field JK, Heighway J. Decreased endothelin receptor B expression in large primary uveal melanomas is associated with early clinical metastasis and short survival. Br J Cancer. 2002;87:1308–1313. doi: 10.1038/sj.bjc.6600620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Urtatiz O, Van Raamsdonk CD. Gnaq and Gna11 in the endothelin signaling pathway and melanoma. Front Genet. 2016;7:59. doi: 10.3389/fgene.2016.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mallikarjuna K, Pushparaj V, Biswas J, Krishnakumar S. Expression of epidermal growth factor receptor, ezrin, hepatocyte growth factor, and c-Met in uveal melanoma: An immunohistochemical study. Curr Eye Res. 2007;32:281–290. doi: 10.1080/02713680601161220. [DOI] [PubMed] [Google Scholar]
- 101.Franco R, Botti G, Mascolo M, Loquercio G, Liguori G, Ilardi G, Losito S, La Mura A, Calemma R, Ierano C, et al. CXCR4-CXCL12 and VEGF correlate to uveal melanoma progression. Front Biosci (Elite Ed) 2010;2:13–21. doi: 10.2741/e60. [DOI] [PubMed] [Google Scholar]
- 102.Vivet-Noguer R, Tarin M, Roman-Roman S, Alsafadi S. Emerging therapeutic opportunities based on current knowledge of uveal melanoma Biology. Cancers (Basel) 2019;11:1019. doi: 10.3390/cancers11071019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cheng H, Chua V, Liao C, Purwin TJ, Terai M, Kageyama K, Davies MA, Sato T, Aplin AE. Co-targeting HGF-CMET Signaling with MEK inhibitors in metastatic uveal melanoma. Mol Cancer Ther. 2017;116:516–528. doi: 10.1158/1535-7163.MCT-16-0552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Abdel-Rahman MH, Yang Y, Zhou XP, Craig EL, Davidorf FH, Eng C. High frequency of submicroscopic hemizygous deletion is a major mechanism of loss of expression of PTEN in uveal melanoma. J Clin Oncol. 2006;24:288–295. doi: 10.1200/JCO.2005.02.2418. [DOI] [PubMed] [Google Scholar]
- 105.Walsh JC, Lebedev A, Aten E, Madsen K, Marciano L, Kolb HC. The clinical importance of assessing tumor hypoxia: Relationship of tumor hypoxia to prognosis and therapeutic opportunities. Antioxid Redox Signal. 2014;21:1516–1554. doi: 10.1089/ars.2013.5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Semenza GL. HIF-1: Upstream and downstream of cancer metabolism. Curr Opin Genet Dev. 2010;20:51–56. doi: 10.1016/j.gde.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Burroughs SK, Kaluz S, Wang D, Wang K, Van Meir EG, Wang B. Hypoxia inducible factor pathway inhibitors as anti-cancer therapeutics. Future Med Chem. 2013;5:553–572. doi: 10.4155/fmc.13.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Luke JJ, Triozzi PL, McKenna KC, Van Meir EG, Gershenwald JE, Bastian BC, Gutkind JS, Bowcock AM, Streicher HZ, Patel PM, et al. Biology of advanced uveal melanoma and next steps for clinical therapeutics. Pigment Cell Melanoma Res. 2015;28:135–147. doi: 10.1111/pcmr.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Keith B, Johnson RS, Simon MC. HIF1α and HIF2α: Sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer. 2011;12:9–22. doi: 10.1038/nrc3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Asnaghi L, Lin MH, Lim KS, Lim KJ, Tripathy A, Wendeborn M, Merbs SL, Handa JT, Sodhi A, Bar EE, Eberhart CG. Hypoxia promotes uveal melanoma invasion through enhanced Notch and MAPK activation. PLoS One. 2014;9:e105372. doi: 10.1371/journal.pone.0105372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mouriaux F, Sanschagrin F, Diorio C, Landreville S, Comoz F, Petit E, Bernaudin M, Rousseau AP, Bergeron D, Morcos M. Increased HIF-1α expression correlates with cell proliferation and vascular markers CD31 and VEGF-A in uveal melanoma. Invest Ophthalmol Vis Sci. 2014;55:1277–1283. doi: 10.1167/iovs.13-13345. [DOI] [PubMed] [Google Scholar]
- 112.Hu K, Babapoor-Farrokhran S, Rodrigues M, Deshpande M, Puchner B, Kashiwabuchi F, Hassan SJ, Asnaghi L, Handa JT, Merbs S, et al. Hypoxia-inducible factor 1 upregulation of both VEGF and ANGPTL4 is required to promote the angiogenic phenotype in uveal melanoma. Oncotarget. 2016;7:7816–7828. doi: 10.18632/oncotarget.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dong L, You S, Zhang Q, Osuka S, Devi NS, Kaluz S, Ferguson JH, Yang H, Chen G, Wang B, et al. Arylsulfonamide 64B inhibits Hypoxia/HIF-induced expression of c-Met and CXCR4 and reduces primary tumor growth and metastasis of uveal melanoma. Clin Cancer Res. 2019;25:2206–2218. doi: 10.1158/1078-0432.CCR-18-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Brouwer NJ, Gezgin G, Wierenga APA, Bronkhorst IHG, Marinkovic M, Luyten GPM, Versluis M, Kroes WGM, van der Velden PA, Verdijk RM, Jager MJ. Tumour angiogenesis in uveal melanoma is related to genetic evolution. Cancers (Basel) 2019;11:979. doi: 10.3390/cancers11070979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Brouwer NJ, Wierenga APA, Gezgin G, Marinkovic M, Luyten GPM, Kroes WGM, Versluis M, van der Velden PA, Verdijk RM, Jager MJ. Ischemia is related to tumour genetics in uveal melanoma. Cancers (Basel) 2019;11:1004. doi: 10.3390/cancers11071004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Spector A. Review: Oxidative stress and disease. J Ocul Pharmacol Ther. 2000;16:193–201. doi: 10.1089/jop.2000.16.193. [DOI] [PubMed] [Google Scholar]
- 117.Di Meo S, Reed TT, Venditti P, Victor VM. Role of ROS and RNS sources in physiological and pathological conditions. Oxid Med Cell Longev. 2016;2016:1245049. doi: 10.1155/2016/1245049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mitra D, Luo X, Morgan A, Wang J, Hoang MP, Lo J, Guerrero CR, Lennerz JK, Mihm MC, Wargo JA, et al. An ultraviolet-radiation-independent pathway to melanoma carcinogenesis in the red hair/fair skin background. Nature. 2012;491:449–453. doi: 10.1038/nature11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic Biol Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Andrisic L, Dudzik D, Barbas C, Milkovic L, Grune T, Zarkovic N. Short overview on metabolomics approach to study pathophysiology of oxidative stress in cancer. Redox Biol. 2018;14:47–58. doi: 10.1016/j.redox.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Piskounova E, Agathocleous M, Murphy MM, Hu Z, Huddlestun SE, Zhao Z, Leitch AM, Johnson TM, DeBerardinis RJ, Morrison SJ. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature. 2015;527:186–1891. doi: 10.1038/nature15726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dithmer M, Kirsch AM, Gräfenstein L, Wang F, Schmidt H, Coupland SE, Fuchs S, Roider J, Klettner AK. Uveal melanoma cell under oxidative Stress-influence of VEGF and VEGF-inhibitors. Klin Monbl Augenheilkd. 2019;236:295–307. doi: 10.1055/s-0043-103002. In German. [DOI] [PubMed] [Google Scholar]
- 123.Costa FF. Epigenomics in cancer management. Cancer Manag Res. 2010;2:255–265. doi: 10.2147/CMAR.S7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yang X, Gao L, Zhang S. Comparative Pan-cancer DNA methylation analysis reveals cancer common and specific patterns. Brief Bioinform. 2017;18:761–773. doi: 10.1093/bib/bbw063. [DOI] [PubMed] [Google Scholar]
- 125.Maat W, van der Velden PA, Out-Luiting C, Plug M, Dirks- Mulder A, Jager MJ, Gruis NA. Epigenetic inactivation of RASSF1a in uveal melanoma. Invest Ophthalmol Vis Sci. 2007;48:486–490. doi: 10.1167/iovs.06-0781. [DOI] [PubMed] [Google Scholar]
- 126.Dammann RH, Richter AM, Jiménez AP, Woods M, Küster M, Witharana C. Impact of natural compounds on DNA methylation levels of the tumor suppressor Gene RASSF1A in cancer. Int J Mol Sci. 2017;18:2160. doi: 10.3390/ijms18102160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Maat W, Beiboer SH, Jager MJ, Luyten GP, Gruis NA, van der Velden PA. Epigenetic regulation identifies RASEF as a Tumor-suppressor gene in uveal melanoma. Invest Ophthalmol Vis Sci. 2008;49:1291–1298. doi: 10.1167/iovs.07-1135. [DOI] [PubMed] [Google Scholar]
- 128.Venza M, Visalli M, Biondo C, Lentini M, Catalano T, Teti D, Venza I. Epigenetic regulation of p14ARF and p16INK4A expression in cutaneous and uveal melanoma. Biochim Biophys Acta. 2015;1849:247–256. doi: 10.1016/j.bbagrm.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 129.Venza M, Visalli M, Catalano T, Fortunato C, Oteri R, Teti D, Venza I. Impact of DNA methyltransferases on the epigenetic regulation of tumor necrosis Factor-related Apoptosis-inducing ligand (TRAIL) receptor expression in malignant melanoma. Biochem Biophys Res Commun. 2013;441:743–750. doi: 10.1016/j.bbrc.2013.10.114. [DOI] [PubMed] [Google Scholar]
- 130.Van der Veiden PA, Metzelaar-Blok JA, Bergman W, Hurks H, Frants RR, Gruis NA, Jager MJ. Promoter Hypermethylation: A common cause of reduced P16(INK4a) expression in uveal melanoma. Cancer Res. 2001;61:5303–5306. [PubMed] [Google Scholar]
- 131.Li Y, Jia R, Ge S. Role of Epigenetics in Uveal Melanoma. Int J Biol Sci. 2017;13:426–4332. doi: 10.7150/ijbs.18331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yang ZK, Yang JY, Xu ZZ, Yu WH. DNA Methylation and uveal melanoma. Chin Med J (Engl) 2018;131:845–851. doi: 10.4103/0366-6999.228229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Audia JE, Campbell RM. Histone modifications and cancer. Cold Spring Harb Perspect Biol. 2016;8:a019521. doi: 10.1101/cshperspect.a019521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ding X, Wang X, Lin M, Xing Y, Ge S, Jia R, Zhang H, Fan X, Li J. PAUPAR lncRNA suppresses tumourigenesis by H3K4 demethylation in uveal melanoma. FEBS Lett. 2016;590:1729–1738. doi: 10.1002/1873-3468.12220. [DOI] [PubMed] [Google Scholar]
- 135.Dong R, Liu J, Sun W, Ping W. Comprehensive analysis of aberrantly expressed profiles of lncRNAs and miRNAs with associated ceRNA Network in lung adenocarcinoma and lung squamous cell carcinoma. Pathol Oncol Res. 2020;26:1935–1945. doi: 10.1007/s12253-019-00780-4. [DOI] [PubMed] [Google Scholar]
- 136.Catalanotto C, Cogoni C, Zardo G. MicroRNA in control of gene expression: An overview of nuclear functions. Int J Mol Sci. 2016;17:1712. doi: 10.3390/ijms17101712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rupaimoole R, Slack FJ. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16:203–222. doi: 10.1038/nrd.2016.246. [DOI] [PubMed] [Google Scholar]
- 138.Mishra S, Yadav T, Rani V. Exploring miRNA based approaches in cancer diagnostics and therapeutics. Crit Rev Oncol Hematol. 2016;98:12–23. doi: 10.1016/j.critrevonc.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 139.Lopez-Rincon A, Martinez-Archundia M, Martinez-Ruiz GU, Schoenhuth A, Tonda A. Automatic discovery of 100-miRNA signature for cancer classification using ensemble feature selection. BMC Bioinformatics. 2019;20:480. doi: 10.1186/s12859-019-3050-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li Z, Yu X, Shen J, Jiang Y. MicroRNA dysregulation in uveal melanoma: A new player enters the game. Oncotarget. 2015;6:4562–458. doi: 10.18632/oncotarget.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yang C, Wei W. The miRNA expression profile of the uveal melanoma. Sci China Life Sci. 2011;54:351–358. doi: 10.1007/s11427-011-4149-y. [DOI] [PubMed] [Google Scholar]
- 142.Wang YC, Yang X, Wei WB, Xu XL. Role of microRNA-21 in uveal melanoma cell invasion and metastasis by regulating p53 and its downstream protein. Int J Ophthalmol. 2018;11:1258–1268. doi: 10.18240/ijo.2018.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Radhakrishnan A, Badhrinarayanan N, Biswas J, Krishnakumar S. Analysis of chromosomal aberration (1, 3, and 8) and association of microRNAs in uveal melanoma. Mol Vis. 2009;15:2146–2154. [PMC free article] [PubMed] [Google Scholar]
- 144.Peng J, Liu H, Liu C. MiR-155 Promotes uveal melanoma cell proliferation and invasion by regulating NDFIP1 expression. Technol Cancer Res Treat. 2017;16:1160–1167. doi: 10.1177/1533034617737923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li Y, Huang Q, Shi X, Jin X, Shen L, Xu X, Wei W. MicroRNA 145 may play an important role in uveal melanoma cell growth by potentially targeting insulin receptor substrate 1. Chin Med J (Engl) 2014;127:1410–1416. [PubMed] [Google Scholar]
- 146.Yan D, Zhou X, Chen X, Hu DN, Dong XD, Wang J, Lu F, Tu L, Qu J. MicroRNA-34a inhibits uveal melanoma cell proliferation and migration through downregulation of c-Met. Invest Ophthalmol Vis Sci. 2009;50:1559–1565. doi: 10.1167/iovs.08-2681. [DOI] [PubMed] [Google Scholar]
- 147.Dong F, Lou D. MicroRNA-34b/c suppresses uveal melanoma cell proliferation and migration through multiple targets. Mol Vis. 2012;18:537–546. [PMC free article] [PubMed] [Google Scholar]
- 148.Serocki M, Bartoszewska S, Janaszak-Jasiecka A, Ochocka RJ, Collawn JF, Bartoszewski R. miRNAs regulate the HIF switch during hypoxia: A novel therapeutic target. Angiogenesis. 2018;21:183–202. doi: 10.1007/s10456-018-9600-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sun L, Wang Q, Gao X, Shi D, Mi S, Han Q. MicroRNA-454 functions as an oncogene by regulating PTEN in uveal melanoma. FEBS Lett. 2015;589:2791–2796. doi: 10.1016/j.febslet.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 150.Ling J, Lu P, Zhang Y, Jiang S, Zhang Z. MiR-367 promotes uveal melanoma cell proliferation and migration by regulating PTEN. Genet Mol Res. 2017;16:gmr16039067. doi: 10.4238/gmr16039067. [DOI] [PubMed] [Google Scholar]
- 151.Liu Y, Huo Y, Wang D, Tai Y, Li J, Pang D, Zhang Y, Zhao W, Du N, Huang Y. MiR-216a-5p/Hexokinase 2 axis regulates uveal melanoma growth through modulation of Warburg effect. Biochem Biophys Res Commun. 2018;50:885–892. doi: 10.1016/j.bbrc.2018.05.069. [DOI] [PubMed] [Google Scholar]
- 152.Singh AD, Turell ME, Topham AK. Uveal melanoma: Trends in incidence, treatment, and survival. Ophthalmology. 2011;118:1881–1885. doi: 10.1016/j.ophtha.2011.01.040. [DOI] [PubMed] [Google Scholar]
- 153.Kaliki S, Shields CL. Uveal melanoma: Relatively rare but deadly cancer. Eye (Lond) 2017;31:241–257. doi: 10.1038/eye.2016.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Triozzi PL, Singh AD. Blood biomarkers for uveal melanoma. Future Oncol. 2012;8:205–215. doi: 10.2217/fon.11.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cabel L, Proudhon C, Gortais H, Loirat D, Coussy F, Pierga JY, Bidard FC. Circulating tumor cells: Clinical validity and utility. Int J Clin Oncol. 2017;22:421–430. doi: 10.1007/s10147-017-1105-2. [DOI] [PubMed] [Google Scholar]
- 156.Valpione S, Gremel G, Mundra P, Middlehurst P, Galvani E, Girotti MR, Lee RJ, Garner G, Dhomen N, Lorigan PC, Marais R. Plasma total Cell-free DNA (cfDNA) is a surrogate biomarker for tumour burden and a prognostic biomarker for survival in metastatic melanoma patients. Eur J Cancer. 2018;88:1–9. doi: 10.1016/j.ejca.2017.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Terai M, Mu Z, Eschelman DJ, Gonsalves CF, Kageyama K, Chervoneva I, Orloff M, Weight R, Mastrangelo MJ, Cristofanilli M, Sato T. Arterial blood, rather than venous blood, is a better source for circulating melanoma cells. EBioMedicine. 2015;2:1821–1826. doi: 10.1016/j.ebiom.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bidard FC, Madic J, Mariani P, Piperno-Neumann S, Rampanou A, Servois V, Cassoux N, Desjardins L, Milder M, Vaucher I, et al. Detection rate and prognostic value of circulating tumor cells and circulating tumor DNA in metastatic uveal melanoma. Int J Cancer. 2014;134:1207–1213. doi: 10.1002/ijc.28436. [DOI] [PubMed] [Google Scholar]
- 159.Schuster R, Bechrakis NE, Stroux A, Busse A, Schmittel A, Thiel E, Foerster MH, Keilholz U. Prognostic relevance of circulating tumor cells in metastatic uveal melanoma. Oncology. 2011;80:57–62. doi: 10.1159/000328283. [DOI] [PubMed] [Google Scholar]
- 160.Tura A, Merz H, Reinsberg M, Lüke M, Jager MJ, Grisanti S, Lüke J. Analysis of monosomy-3 in immunomagnetically-isolated circulating melanoma cells in uveal melanoma patients. Pigment Cell Melanoma Res. 2016;29:583–589. doi: 10.1111/pcmr.12507. [DOI] [PubMed] [Google Scholar]
- 161.Achberger S, Aldrich W, Tubbs R, Crabb JW, Singh AD, Triozzi PL. Circulating immune cell and microRNA in patients with uveal melanoma developing metastatic disease. Mol Immunol. 2014;58:182–186. doi: 10.1016/j.molimm.2013.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Russo A, Caltabiano R, Longo A, Avitabile T, Franco LM, Bonfiglio V, Puzzo L, Reibaldi M. Increased levels of miRNA-146a in serum and histologic samples of patients with uveal melanoma. Front Pharmacol. 2016;7:424. doi: 10.3389/fphar.2016.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Eldh M, Olofsson Bagge R, Lässer C, Svanvik J, Sjöstrand M, Mattsson J, Lindnér P, Choi DS, Gho YS, Lötvall J. MicroRNA in exosomes isolated directly from the liver circulation in patients with metastatic uveal melanoma. BMC Cancer. 2014;14:962. doi: 10.1186/1471-2407-14-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Stark MS, Gray ES, Isaacs T, Chen FK, Millward M, McEvoy A, Zaenker P, Ziman M, Soyer HP, Glasson WJ, et al. Panel of Circulating MicroRNAs detects uveal melanoma with high precision. Transl Vis Sci Technol. 2019;8:12. doi: 10.1167/tvst.8.6.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Peng L, Cantor DI, Huang C, Wang K, Baker MS, Nice EC. Tissue and plasma proteomics for early stage cancer detection. Mol Omics. 2018;14:405–423. doi: 10.1039/C8MO00126J. [DOI] [PubMed] [Google Scholar]
- 166.Karimi P, Shahrokni A, Ranjbar MR. Implementation of proteomics for cancer research: Past, present, and future. Asian Pac J Cancer Prev. 2014;15:2433–2438. doi: 10.7314/APJCP.2014.15.6.2433. [DOI] [PubMed] [Google Scholar]
- 167.Reiniger IW, Schaller UC, Haritoglou C, Hein R, Bosserhoff AK, Kampik A, Mueller AJ. 'Melanoma inhibitory activity' (MIA): A promising serological tumour marker in metastatic uveal melanoma. Graefes Arch Clin Exp Ophthalmol. 2005;243:1161–1166. doi: 10.1007/s00417-005-1171-4. [DOI] [PubMed] [Google Scholar]
- 168.Haritoglou I, Wolf A, Maier T, Haritoglou C, Hein R, Schaller UC. Osteopontin and 'melanoma inhibitory activity': Comparison of two serological tumor markers in metastatic uveal melanoma patients. Ophthalmologica. 2009;223:239–243. doi: 10.1159/000206139. [DOI] [PubMed] [Google Scholar]
- 169.Barak V, Frenkel S, Kalickman I, Maniotis AJ, Folberg R, Pe'er J. Serum markers to detect metastatic uveal melanoma. Anticancer Res. 2007;27:1897–1900. [PMC free article] [PubMed] [Google Scholar]
- 170.Missotten GS, Tang NE, Korse CM, Hurks HM, de Wolff-Rouendaal D, Keunen JE, Jager MJ, Bonfrer JM. Prognostic value of S-100-beta serum concentration in patients with uveal melanoma. Arch Ophthalmol. 2003;121:1117–1119. doi: 10.1001/archopht.121.8.1117. [DOI] [PubMed] [Google Scholar]
- 171.Strobel K, Bode B, Dummer R, Veit-Haibach P, Fischer DR, Imhof L, Goldinger S, Steinert HC, von Schulthess GK. Limited value of 18F-FDG PET/CT and S-100B tumour marker in the detection of liver metastases from uveal melanoma compared to liver metastases from cutaneous melanoma. Eur J Nucl Med Mol Imaging. 2009;36:1774–1782. doi: 10.1007/s00259-009-1175-0. [DOI] [PubMed] [Google Scholar]
- 172.Suesskind D, Schatz A, Schnichels S, Coupland SE, Lake SL, Wissinger B, Bartz-Schmidt KU, Henke-Fahle S. GDF-15: A novel serum marker for metastases in uveal melanoma patients. Graefes Arch Clin Exp Ophthalmol. 2012;250:887–895. doi: 10.1007/s00417-011-1786-6. [DOI] [PubMed] [Google Scholar]
- 173.Bande MF, Santiago M, Blanco MJ, Mera P, Capeans C, Rodríguez-Alvarez MX, Pardo M, Piñeiro A. Serum DJ-1/PARK 7 is a potential biomarker of choroidal nevi transformation. Invest Ophthalmol Vis Sci. 2012;53:62–67. doi: 10.1167/iovs.11-7948. [DOI] [PubMed] [Google Scholar]
- 174.Bande MF, Santiago M, Mera P, Piulats JM, Blanco MJ, Rodríguez-Álvarez MX, Capeans C, Piñeiro A, Pardo M. ME20-S as a potential biomarker for the evaluation of uveal melanoma. Invest Ophthalmol Vis Sci. 2015;56:7007–7011. doi: 10.1167/iovs.15-17183. [DOI] [PubMed] [Google Scholar]
- 175.Barisione G, Fabbi M, Gino A, Queirolo P, Orgiano L, Spano L, Picasso V, Pfeffer U, Mosci C, Jager MJ, et al. Potential Role of Soluble c-Met as a New candidate biomarker of metastatic uveal melanoma. JAMA Ophthalmol. 2015;133:1013–1021. doi: 10.1001/jamaophthalmol.2015.1766. [DOI] [PubMed] [Google Scholar]
- 176.Angi M, Kalirai H, Prendergast S, Simpson D, Hammond DE, Madigan MC, Beynon RJ, Coupland SE. In-depth proteomic profiling of the uveal melanoma secretome. Oncotarget. 2016;7:49623–49635. doi: 10.18632/oncotarget.10418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Crabb JW, Hu B, Crabb JS, Triozzi P, Saunthararajah Y, Tubbs R, Singh AD. iTRAQ quantitative proteomic comparison of metastatic and non-metastatic uveal melanoma tumors. PLoS One. 2015;10:e0135543. doi: 10.1371/journal.pone.0135543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Shi XY, Li Q, Wei WB, Tao LM. Peptidome profiling of human serum of uveal melanoma patients based on magnetic bead fractionation and mass spectrometry. Int J Ophthalmol. 2017;10:939–947. doi: 10.18240/ijo.2017.06.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Song J, Merbs SL, Sokoll LJ, Chan DW, Zhang Z. A multi-plex immunoassay of serum biomarkers for the detection of uveal melanoma. Clin Proteomics. 2019;16:10. doi: 10.1186/s12014-019-9230-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Corrêa ZM. Assessing prognosis in uveal melanoma. Cancer Control. 2016;23:93–98. doi: 10.1177/107327481602300202. [DOI] [PubMed] [Google Scholar]
- 181.Balasubramanya R, Selvarajan SK, Cox M, Joshi G, Deshmukh S, Mitchell DG, O'Kane P. Imaging of ocular melanoma metastasis. Br J Radiol. 2016;89:20160092. doi: 10.1259/bjr.20160092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Smit KN, Jager MJ, de Klein A, Kiliç E. Uveal melanoma: Towards a molecular understanding. Prog Retin Eye Res. 2020;75:100800. doi: 10.1016/j.preteyeres.2019.100800. [DOI] [PubMed] [Google Scholar]
- 183.Schopper VJ, Correa ZM. Clinical application of genetic testing for posterior uveal melanoma. Int J Retina Vitreous. 2016;2:4. doi: 10.1186/s40942-016-0030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Damato B, Dopierala JA, Coupland SE. Genotypic profiling of 452 choroidal melanomas with multiplex Ligation-dependent probe amplification. Clin Cancer Res. 2010;16:6083–6092. doi: 10.1158/1078-0432.CCR-10-2076. [DOI] [PubMed] [Google Scholar]
- 185.Dogrusöz M, Jager MJ, Damato B. Uveal melanoma treatment and prognostication. Asia Pac J Ophthalmol (Phila) 2017;6:186–196. doi: 10.22608/APO.201734. [DOI] [PubMed] [Google Scholar]
- 186.Onken MD, Worley LA, Char DH, Augsburger JJ, Correa ZM, Nudleman E, Aaberg TM, Jr, Altaweel MM, Bardenstein DS, Finger PT, et al. Collaborative Ocular Oncology Group report number 1: Prospective validation of a Multi-gene prognostic assay in uveal melanoma. Ophthalmology. 2012;119:1596–1603. doi: 10.1016/j.ophtha.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Royer-Bertrand B, Torsello M, Rimoldi D, El Zaoui I, Cisarova K, Pescini-Gobert R, Raynaud F, Zografos L, Schalenbourg A, Speiser D, et al. Comprehensive genetic landscape of uveal melanoma by whole-genome sequencing. Am J Hum Genet. 2016;99:1190–1198. doi: 10.1016/j.ajhg.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chattopadhyay C, Kim DW, Gombos DS, Oba J, Qin Y, Williams MD, Esmaeli B, Grimm EA, Wargo JA, Woodman SE, Patel SP. Uveal melanoma: From diagnosis to treatment and the science in between. Cancer. 2016;122:2299–2312. doi: 10.1002/cncr.29727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Frizziero L, Midena E, Trainiti S, Londei D, Bonaldi L, Bini S, Parrozzani R. Uveal melanoma biopsy: A review. Cancers (Basel) 2019;11:1075. doi: 10.3390/cancers11081075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Erim Y, Scheel J, Breidenstein A, Metz CH, Lohmann D, Friederich HC, Tagay SP. Psychosocial impact of prognostic genetic testing in the care of uveal melanoma patients: Protocol of a controlled prospective clinical observational. BMC Cancer. 2016;16:408. doi: 10.1186/s12885-016-2479-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Damato B, Coupland SE. Translating uveal melanoma cytogenetics into clinical care. Arch Ophthalmol. 2009;127:423–429. doi: 10.1001/archophthalmol.2009.40. [DOI] [PubMed] [Google Scholar]
- 192.Harbour JW. Molecular prognostic testing and individualized patient care in uveal melanoma. Am J Ophthalmol. 2009;148:823–829.e1. doi: 10.1016/j.ajo.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Aaberg TM, Jr, Cook RW, Oelschlager K, Maetzold D, Rao PK, Mason JO., III Current clinical practice: Differential management of uveal melanoma in the era of molecular tumor analyses. Clin Ophthalmol. 2014;8:2449–2460. doi: 10.2147/OPTH.S70839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Schoenfield L, Pettay J, Tubbs RR, Singh AD. Variation of monosomy 3 status within uveal melanoma. Arch Pathol Lab Med. 2009;133:1219–1222. doi: 10.5858/133.8.1219. [DOI] [PubMed] [Google Scholar]
- 195.Nathan P, Cohen V, Coupland S, Curtis K, Damato B, Evans J, Fenwick S, Kirkpatrick L, Li O, Marshall E, et al. Uveal melanoma UK national guidelines. Eur J Cancer. 2015;51:2404–2412. doi: 10.1016/j.ejca.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 196.Álvarez-Rodríguez B, Latorre A, Posch C, Somoza Á. Recent advances in uveal melanoma treatment. Med Res Rev. 2017;37:1350–1372. doi: 10.1002/med.21460. [DOI] [PubMed] [Google Scholar]
- 197.Damato BE, Dukes J, Goodall H, Carvajal RD. Tebentafusp: T cell redirection for the treatment of metastatic uveal melanoma. Cancers (Basel) 2019;11:971. doi: 10.3390/cancers11070971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Schank TE, Hassel JC. Immunotherapies for the treatment of uveal melanoma-history and future. Cancers (Basel) 2019;11:1048. doi: 10.3390/cancers11081048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Bol K, van den Bosch T, Schreibelt G, Punt C, Figdor C, Paridaens D, de Vries J. Adjuvant dendritic cell vaccination in high-risk uveal melanoma patients. J Immuno Ther Cancer: 2015;3 [Google Scholar]
- 200.Thota R, Johnson DB, Sosman JA. Trametinib in the treatment of melanoma. Expert Opin Biol Ther. 2015;15:735–747. doi: 10.1517/14712598.2015.1026323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Carvajal RD, Piperno-Neumann S, Kapiteijn E, Chapman PB, Frank S, Joshua AM, Piulats JM, Wolter P, Cocquyt V, Chmielowski B, et al. Selumetinib in combination with dacarbazine in patients with metastatic uveal melanoma: A Phase III, Multicenter, randomized trial (SUMIT) J Clin Oncol. 2018;36:1232–1239. doi: 10.1200/JCO.2017.74.1090. [DOI] [PubMed] [Google Scholar]
- 202.Brighton HE, Angus SP, Bo T, Roques J, Tagliatela AC, Darr DB, Karagoz K, Sciaky N, Gatza ML, Sharpless NE, et al. New mechanisms of resistance to MEK inhibitors in melanoma revealed by intravital imaging. Cancer Res. 2018;78:542–557. doi: 10.1158/0008-5472.CAN-17-1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Cheng H, Chua V, Liao C, Purwin TJ, Terai M, Kageyama K, Davies MA, Sato T, Aplin AE. Co-targeting HGF/cMET Signaling with MEK inhibitors in metastatic uveal melanoma. Mol Cancer Ther. 2017;16:516–528. doi: 10.1158/1535-7163.MCT-16-0552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Faião-Flores F, Emmons MF, Durante MA, Kinose F, Saha B, Fang B, Koomen JM, Chellappan SP, Maria-Engler SS, Rix U, et al. HDAC inhibition enhances the in vivo efficacy of MEK inhibitor therapy in uveal melanoma. Clin Cancer Res. 2019;25:5686–5701. doi: 10.1158/1078-0432.CCR-18-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Landreville S, Agapova OA, Matatall KA, Kneass ZT, Onken MD, Lee RS, Bowcock AM, Harbour JW. Histone deacetylase inhibitors induce growth arrest and differentiation in uveal melanoma. Clin Cancer Res. 2012;18:408–416. doi: 10.1158/1078-0432.CCR-11-0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Bonnal S, Vigevani L, Valcárcel J. The spliceosome as a target of novel antitumour drugs. Nat Rev Drug Dis. 2012;11:847–859. doi: 10.1038/nrd3823. [DOI] [PubMed] [Google Scholar]
- 207.Yoshimoto R, Kaida D, Furuno M, Burroughs AM, Noma S, Suzuki H, Kawamura Y, Hayashizaki Y, Mayeda A, Yoshida M. Global analysis of pre-mRNA subcellular localization following splicing inhibition by spliceostatin A. RNA. 2017;23:47–57. doi: 10.1261/rna.058065.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wu G, Fan L, Edmonson MN, Shaw T, Boggs K, Easton J, Rusch MC, Webb TR, Zhang J, Potter PM. Inhibition of SF3B1 by molecules targeting the spliceosome results in massive aberrant exon skipping. RNA. 2018;24:1056–1066. doi: 10.1261/rna.065383.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Zhou Z, Gong Q, Wang Y, Li M, Wang L, Ding H, Li P. The biological function and clinical significance of SF3B1 mutations in cancer. Biomarker Res. 2020;8(38) doi: 10.1186/s40364-020-00220-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.García M, Moreno R, Gil-Martin M, Cascallò M, de Olza MO, Cuadra C, Piulats JM, Navarro V, Domenech M, Alemany R, Salazar R. A Phase 1 Trial of Oncolytic adenovirus ICOVIR-5 administered intravenously to cutaneous and uveal melanoma patients. Hum Gene Ther. 2019;30:352–364. doi: 10.1089/hum.2018.107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.