Abstract
Objective
The aim of this study was to assess the early operative results of a staged progressive reduction technique using a bilateral external fixator in the treatment of patients with open Lisfranc fracture dislocations.
Methods
In this retrospective study, 21 patients (5 women and 16 men; mean age=44.4 years; age range=24 to 69 years) with open Lisfranc fracture dislocations were included. All the patients were treated in a staged manner from 2012 to 2015. The mean follow-up was 15.4 months (range=12 to 24 months). A two-stage surgical protocol was performed for each patient. At the first stage, a bilateral spanning external fixator was applied across the injured Lisfranc joint, and the length of the disrupted columns was restored by distraction process. Vacuum-assisted closure was used if required. At the second stage, the external fixator was removed, and open reduction and internal fixation were carried out. The time interval between the first and second stages and postoperative complications were documented. To assess the functional status of the patients, the visual analog scale (VAS) and the American Orthopaedic Foot & Ankle Society (AOFAS) midfoot scale were measured at the final follow-up. Radiographic parameters indicating the alignment of the midfoot after the second operation were examined.
Results
Deep infection in one patient and superficial infection in 2 patients were observed. Venous thrombosis was detected in 3 patients. The mean interval between the first and second stages was 18.6 days (range=8 to 48 days). The first metatarso-cuneiform step-off (p=0.002) and the second metatarso-cuneiform step-off (p=0.000) significantly improved at the final follow-up. The mean VAS score was 2.4 (range=0–5), and the mean AOFAS score was 76.3 (range=63 to 97). Primary arthrodesis was performed in seven patients, and six of the remaining 14 patients developed post-traumatic arthritis.
Conclusion
With a low risk of complications, the staged progressive reduction protocol using an adjustable bilateral external fixator can be an effective treatment to achieve and maintain anatomic reduction for patients with open Lisfranc fracture dislocations in a short-time follow-up.
Level of Evidence
Level IV, Therapeutic study
Keywords: Lisfranc injury, Staged management, External fixation, Infection, Anatomical reduction
Introduction
Though rare, Lisfranc fracture dislocations are severe injuries of the midfoot region (1). As proved by earlier studies, the outcome of Lisfranc fracture dislocations is closely related to the extent of restoration and maintenance of anatomic alignment of each column (2–4). Open Lisfranc fracture dislocations are more difficult to treat compared with those injuries with the skin and soft tissue remaining intact, mostly due to the vulnerability of the affected soft tissue to accommodate reduction maneuver and instrument placement to restore and maintain the anatomic alignment, which leaves little safe space for orthopedic surgeons to operate (5). In order to achieve satisfactory outcome and to minimize complication rates, various strategies have been proposed to treat open Lisfranc fracture dislocations, such as primary internal fixation, soft tissue reconstruction, and staged management protocols. Although these strategies are different, they all seek to accomplish immediate anatomic reduction once for all. Yet the necessity of this attempt at the emergency stage is worth debating. It is true that repeated manipulation through the injured soft tissue envelope may cause secondary impairment if edema and inflammation are present. Besides, the internal fixation underneath the injured soft tissue may increase the risk of deep infection. This study on a series of consecutive cases aimed to report the early result of a staged and progressive reduction technique using a bilateral external fixator to treat open Lisfranc fracture dislocations.
Materials and Methods
This study was approved by the Biomedical Research Ethics Committee of West China Hospital of Sichuan University (file number: 1.0; file year: 2019). Clinical data of patients with Lisfranc fracture dislocations, who were over 18 years of age and admitted to our trauma center between January 2012 and December 2015, were retrospectively reviewed by using the hospital information system. Patients who refused to participate, those with incomplete required medical information on record, those who died before definitive internal fixation because of reasons other than the injury itself, and those who received primary amputation for the injury were excluded from the study. All the patients who participated in this study signed the consent form.
First-generation cephalosporin was routinely used for all of the patients, including the ones with grade III open fractures, as soon as the patient arrived (6). In the case that the patient was allergic to cephalosporin, clindamycin was prescribed instead. The antibiotics being used would be changed according to the culture result once there were signs of early infection. The wound of a typical case is demonstrated in Figure 1. Preoperative anteroposterior (AP), lateral, and oblique view radiographs and 3D CT reconstructive images were obtained. The preoperative X-ray and CT reconstructive images of the typical case are shown in Figure 2. Parecoxib was used before surgery for preemptive analgesia if no contraindication was present.
Figure 1. a, b.
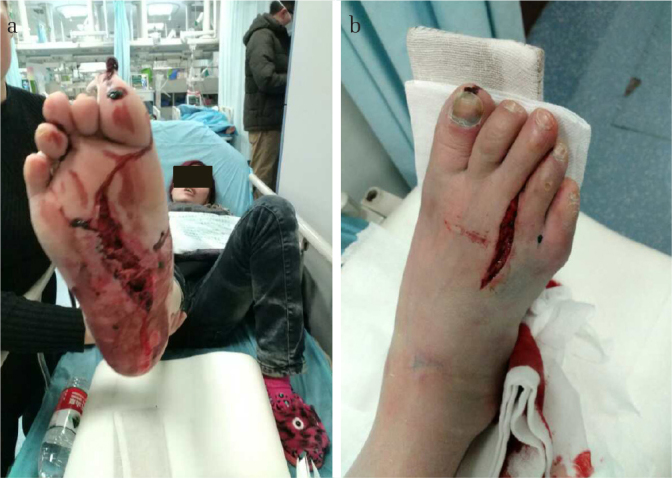
A typical open Lisfranc injury in the current study. A 43-year-old female patient got injured in a car accident. The pictures show the injury with contusion, laceration, and contamination of the right (a) planta pedis and (b) dorsum pedis
Figure 2. a–d.
Preoperative X-ray and CT images of the same foot in Figure 1, showing the fracture and displacement of the first, second, and third tarsometatarsal joints and the talonavicular joint, and fracture of the cuboid
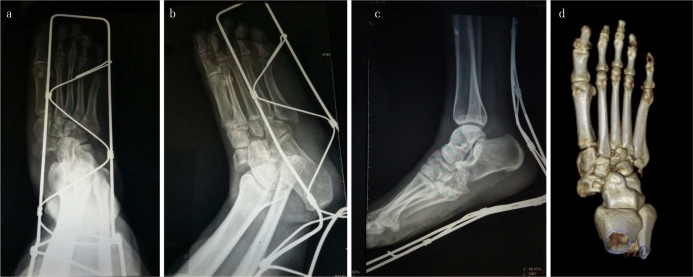
All the operations were performed by the same senior orthopedic surgeon (H.Z.), under general anesthesia, with the patients lying in the supine position. All of the cases were managed in a staged fashion. At the first stage, careful evaluation of the wound was performed according to the presence of tissue defect, the severity of contamination, and the time from injury to the first debridement. Wounds that allowed safe primary closure were closed, and others were sealed with vacuum-assisted closure (VAC) systems (KCI, San Antonio, TX, USA). A spanning bilateral external fixator was applied across the injured Lisfranc joint, with one transfixing pin placed through the calcaneal tuberosity and another one or two through the shaft of the metatarsals. For the cases with disruptions of all three columns, the pins were positioned through all the five metatarsals. For those patients with disruptions of only one or two columns, the pins were inserted through the disrupted columns. Half pins were added to maintain the gross alignment and the relative length of the impaired column if severe comminution and instability of the lateral or medial column were present. The length of the disrupted columns was restored by traction or sometimes over traction, by distraction process of the external fixator. The alignment was reduced by clamping and joystick technique to a relatively normal state to reduce the skin compression or vascular tortuosity. Absolute anatomic reduction was not obligatory at this time. For patients whose reduction was satisfactory during the first-stage operation, the fracture or the dislocated tarsometatarsal (TMT) joint was further stabilized with Kirschner wires (K-wires) in addition to the external fixator. For Gustilo type IIIC cases with vascular impairment and anastomosis, the distraction process was slowly and progressively performed after the operation with intensive monitoring of the acral blood supply to avoid irritation of the anastomotic vessels. The distraction was set back once any sign of circular disturbance of the involved extremity was noticed. The X-ray images of the affected foot in a typical case, where the foot was stabilized with a bilateral external fixator after the first-stage operation, are shown in Figure 3.
Figure 3. a, b.
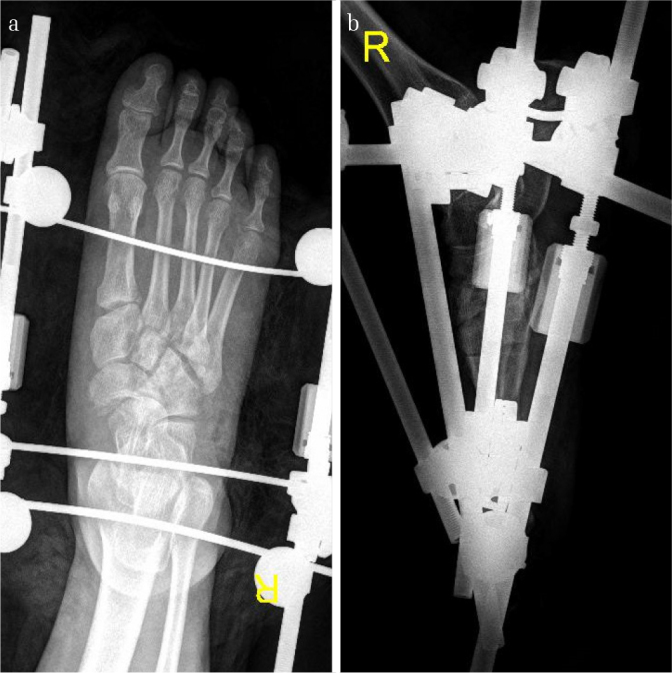
Images of the foot in Figure 1 after immediate wound irrigation and debridement, and the first-stage reduction and fixation with a bilateral external fixator. It could be noted that at this stage, the displaced talonavicular joint was reduced, and the length of the disrupted columns was restored by the external fixator. Note that only the length of the disrupted columns and gross alignment were restored, and anatomical reduction was not achieved at this stage
VAC system was used when there was skin and soft tissue defect or if the wound could not be safely closed at primary intention. Wound debridement was performed approximately every 72 hours until the wound was ready for closure or repair. For the wounds without sign of infection, the external fixator was then removed and the second-stage treatment, which included open reduction and internal fixation using transarticular cannulated screws for the medial and middle columns and K-wires for the lateral column, was carried out as described in our previous study using a dorsal approach overlying the involved column or columns (7). Furthermore, a simultaneous wound closure or repair with skin graft or local tissue flap transfer was performed. For those cases with severely comminuted columns, small unilateral external fixators were used instead. Anatomic reduction was achieved at this stage. For patients with major ligamentous disruptions and multidirectional instability of the Lisfranc joints, a comminuted intraarticular fracture at the base of the first or second metatarsus, or crush injuries of the midfoot with an intraarticular fracture dislocation, a primary arthrodesis of the involved first to third TMT joint or joints was performed (8). For heavily contaminated wounds with a high risk of infection or wounds already showing sign of infection, the external fixator was placed, and gentamicin cement beads were embedded. The wound was sealed with VAC system with continuous instillation using 0.9% saline. Definitive reduction and internal fixation would not be performed until the infection was under control. Figures 4 and 5 demonstrate the post-second-stage operation X-ray images of a typical case with the comminuted lateral column fixed with a small unilateral external fixator and K-wires, before and after the removal of the fixator and the K-wires, respectively.
Figure 4. a, b.
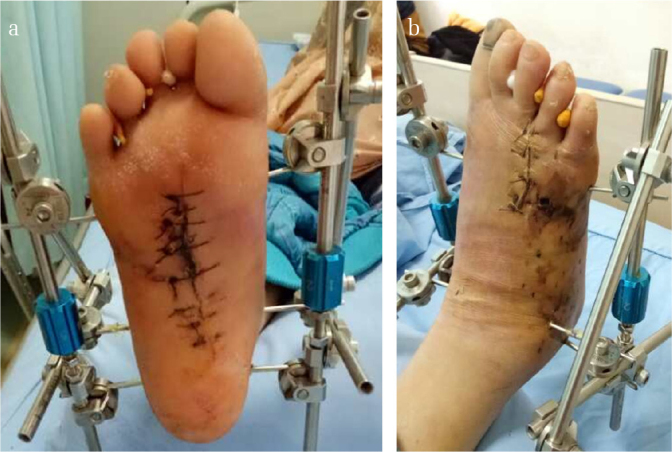
Pictures of the foot in Figure 1 after the first-stage operation, showing the setup of the bilateral fixator. Note that the soft tissue both in the (a) planta pedis and (b) dorsalis pedis healed very well after the setup of the external fixator
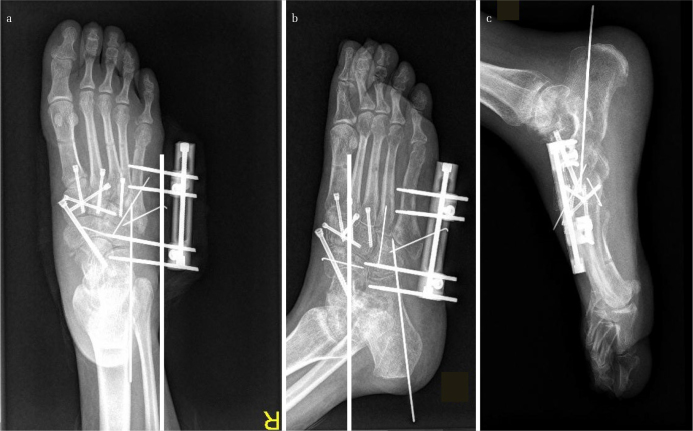
Figure 5. a–c.
Images of the foot in Figure 1 after the bilateral frame was removed, and the tarsometatarsal and the Chopart joints were anatomically reduced and fixed. Excellent reduction and fixation with cannulated screws of the medial and the middle columns could be noted. The fractured talonavicular joint was transfixed with a cannulated screw. For the comminuted lateral column, a small unilateral external fixator together with K-wires was used
After the second-stage operation, cefazolin 2000 mg per 12 hours or cefuroxime 1500 mg per 8 hours was used for 24 hours if no sign of infection was noticed. Anticoagulant therapy with enoxaparin 1 mg/kg for every 24 hours started 12 hours after operation to prevent venous thrombosis. Thromboprophylaxis lasted usually for about two weeks after the surgery. It stopped when the patient regained adequate mobility either by weight bearing or removal of cast immobilization. If venous thrombosis was found, therapeutic dose of low-molecular heparin, that is, enoxaparin 1 mg/kg for every 12 hours, was prescribed. The patient was told to stay in bed with his or her leg elevated. Pressing, moving, or applying hot compresses to the leg was prohibited. The injured limb was routinely elevated and immobilized with a short leg cast for about two weeks. No cast was used for patients in whom small unilateral external fixators were used to fix the comminuted columns. Two weeks later, the cast was removed, and the patients were encouraged to do a range of motion exercises without weight bearing until the 6th to the 8th week, when patients started exercising with partial weight bearing within 10 kg on an arch support. The latter exercise was continued until the third month after the surgery with a gradually increased weight bearing to full dose. The arch support was used for about 3 months. During the follow-up period, radiographs with AP, lateral, and oblique views were obtained at 1, 2, 3, 6, 9, and 12 months after the final operation, followed by yearly radiograph imaging every year after. The patient could return to the hospital anytime if he or she felt any discomfort in the midfoot region. Complications such as infection, osteomyelitis, or posttraumatic arthritis were evaluated. The radiographic criteria for posttraumatic arthritis were the existence of sclerosis, osteophytes, subchondral cysts, and/or joint space narrowing. The functional outcomes were evaluated according to the visual analog scale (VAS) and the American Orthopaedic Foot & Ankle Society (AOFAS) midfoot scale system at every follow-up (9).
Results
A total of 24 patients, including 18 men and six women, were recruited for the study. After screening, three patients were excluded from the study including one patient who was transferred to another trauma center after the initial debridement and external fixation, one patient with Gustilo type IIIC injury who received primary amputation, and another patient who failed to be contacted after discharge due to invalid phone number on record. Finally, there were 21 patients who participated in this study, including 16 men and 5 women, with an average age of 44.4 years (range, 24 to 69 years). After discharge from the hospital, these 21 patients were followed up for an average period of 15.4 months (range, 12 to 24 months). The clinical data of the patients are shown in Table 1. All the Lisfranc fracture dislocations were unilateral. Among them, 6 patients suffered concomitant Chopart injury. The most common cause for the injuries was crushing, followed by pedestrian versus automobile accidents. Motorcycle/electrocycle accidents as well as motor vehicle accidents were also frequently seen. According to the Gustilo-Anderson classification system, there were 3 type II injuries, 6 type IIIA injuries, 11 type IIIB injuries, and 1 type IIIC injury, whereas according to the Hardcastle-Myerson classification system, there were 1 type A1 case, 1 type A2 case, 2 type B1 case, 12 type B2 cases, 2 type C1 cases, and 3 type C2 cases. None of the injuries were solely ligamentous. The average waiting time from injury to the initial debridement was 5.8 hours (range, 3 to 13 hours). Clinical outcomes of these patients are listed in Table 2. As shown in Table 2, 138.9 minutes (range, 98 to 193 minutes) was the average time duration for the first-stage operation, which included maneuvers, such as wound irrigation and debridement, fracture reduction, external fixator installation, and VAC sealing process if necessary. Gentamicin cement beads were embedded in nine patients. Deep infection and superficial infection were found in one and two patients, respectively. No osteomyelitis occurred in this study. Venous thrombosis was found in three patients. However, all the thrombus achieved stable adherence to the vascular wall 2 to 3 weeks later upon treatment. In the patients, neither clinical symptoms nor signs of pulmonary embolism were detected. The average time duration from the first-stage operation to definitive internal fixation was 18.6 days (range, 8 to 48 days). During this period, an average of 2.5 operations were performed (range 1 to 5), most of which were repeated irrigation and debridement. After definitive fixation, as shown in Table 3, no patient was found bearing displacement over 2 mm in the Lisfranc joint region. The first metatarso-cuneiform step-off (p=0.002) and the second metatarso-cuneiform step-off (p=0.000) improved significantly. Seven cases had their TMT joints primarily fused. At the last follow-up, the average VAS score was 2.4 (range, 0 to 5). The average AOFAS score was 76.3 (range, 63 to 97). Signs of posttraumatic arthritis were found in six patients by postoperative imaging at the end of follow-up. The initially achieved reduction was partially lost after removal of the internal fixation instruments in three patients, none of which were more than 2-mm displacement. No significant change was noted in VAS and AOFAS scores in these patients. Therefore, no additional surgical intervention was necessary for these patients. Figure 6 illustrates a general view of the injured foot with weight bearing in a typical case at the end of the follow-up, which was 1 year after the second-stage operation. Figure 7 shows the X-ray images of the foot of the typical case in Figure 1 after the removal of the external fixator. Figure 8 demonstrates the photo images of the foot of the same patient a year after the second-stage internal fixation surgery. Figures 9, 10 and 11 demonstrate the X-ray radiographs of another typical case after the first stage operation, after the second stage operation and at the final follow-up respectively.
Table 1.
Clinical information of the patients included
| Case No. | Age (y) | Gender | Side | Gustilo type | Wound closure | Hardcastle-Myerson type | Part of injury | Cause of injury | TFID (hr) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 31 | M | L | IIIB | skin graft | C2 | Calcaneal fracture, calcaneal-cuboid subluxation, fracture of the medial and middle cuneiforms, fracture of the 1st metatarsal base, complete medial displacement of the 1st metatarsal and lateral displacement of the 2nd to the 5th metatarsals. | Fall | 3 |
| 2 | 36 | M | R | IIIA | Primary closure | B2 | Displacement between the medial and middle cuneiforms, fracture of the 2nd metatarsal base, lateral displacement of the 2nd to the 5th metatarsals. | MCA/EMA | 5 |
| 3 | 47 | F | R | IIIB | Secondary closure | C2 | Fracture of the medial and middle cuneiforms as well as the 1st and 2nd metatarsal base, complete divergence between the 1st and 2nd metatarsals. | PVA | 6 |
| 4 | 69 | F | L | IIIB | Skin graft | B1 | Fracture of the medial cuneiform, medial displacement of the 1st metatarsal. | Crush injury | 5 |
| 5 | 52 | M | R | II | Primary closure | B2 | Fracture of the cuboid and lateral cuneiform, fracture of the 2nd metatarsal base, lateral displacement of the 2nd to the 5th metatarsals. | MCA/EMA | 9 |
| 6 | 51 | M | L | IIIA | Skin graft | A2 | Fracture of the 1st metatarsal base, medial displacement of the 1st to the 5th metatarsals. | PVA | 7 |
| 7 | 37 | M | R | IIIC | Flap | B2 | Fracture of the talus, dislocation of the subtalar and talonavicular joints, fracture of the medial, middle and lateral cuneiforms, fracture of the 5th metatarsal base, lateral displacement of the 2nd to the 5th metatarsals. | Crush injury | 4 |
| 8 | 43 | F | R | IIIA | Primary closure | B2 | Talo-navicular dislocation, calcaneo-cuboid subluxation, naviculo-cuneiform subluxation, fracture of the medial, middle and lateral cuneiforms as well as the cuboid, fracture of the distal 2nd metatarsal and proximal 5th metatarsal, lateral displacement of the 2nd to the 5th metatarsals. | MVA | 7 |
| 9 | 29 | F | L | IIIB | Flap | B1 | Fracture of the 1st metatarsal base, medial displacement of the 1st metatarsal | MVA | 6 |
| 10 | 35 | M | R | IIIB | Flap | C2 | Fracture of the navicular, the medial cuneiform and the 1st metatarsal base, complete divergence between the 1st and 2nd metatarsals. | Crush injury | 4 |
| 11 | 27 | M | L | II | Primary closure | B2 | Fracture of the 2nd metatarsal base, lateral displacement of the 2nd to 5th metatarsals. | Fall | 4 |
| 12 | 61 | M | R | IIIB | Skin graft | B2 | Fracture of the 4th and 5th metatarsals and lateral displacement of the 2nd to the 5th metatarsals. | PVA | 5 |
| 13 | 63 | M | L | IIIA | Primary closure | B2 | Fracture of the 2nd metatarsal base and the middle as well as lateral cuneiforms, lateral displacement of the 2nd to 5th metatarsals. | Crush injury | 13 |
| 14 | 24 | M | L | IIIB | Skin graft | B2 | Displacement between the medial and middle cuneiforms, lateral displacement of the 2nd to the 5th metatarsals. | Crush injury | 8 |
| 15 | 38 | F | R | IIIB | Skin graft | C1 | Fracture of the 1st and 2nd metatarsals, Complete divergence between the 1st and 2nd metatarsals. | MVA | 5 |
| 16 | 56 | M | R | IIIB | Secondary closure | A1 | Displacement of the naviculo-cuneiform joint, medial displacement of the 1st to 5th metatarsals. | PVA | 4 |
| 17 | 59 | M | L | IIIB | Skin graft | B2 | Fracture of the 2nd and 3rd metatarsal base, lateral displacement of the 1st to 5th metatarsals. | MCA/EMA | 6 |
| 18 | 44 | F | R | IIIA | Primary closure | C1 | Fracture of the navicular and medial cuneiform, fracture of the 2nd metataral base, partial divergence between the 1st and 2nd metatarsals. | MVA | 4 |
| 19 | 60 | M | L | II | Primary closure | B2 | Lateral displacement of the 2nd to 5th metatarsals. | MCA/EMA | 5 |
| 20 | 32 | M | R | IIIB | Flap | B2 | Fracture of the medial cuneiform, fracture of the 5th metatarsal base, lateral displacement of the 2nd to 5th metatarsals. | Crush injury | 3 |
| 21 | 39 | M | L | IIIA | Primay closure | B2 | Lateral displacement of the 2nd to 5th metatarsals. | PVA | 8 |
MVA: motor vehicle accident; MCA: motorcycle accident; ECA: electrocycle accident; PVA: pedestrian versus automobile: TFID: time from injury to the first debridement
Table 2.
Clinical outcomes of the patients involved
| Case No. | Duration of the first operation (min) | Time from Ex-Fix to definitive fixation (d) | No. of surgeries between Ex-Fix and definitive fixation | Primary arthrodesis | Complication | Cement embedded | Follow-up period | VAS at the last follow-up | AOFAS at the last follow-up | Time of hospital stay |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 187 | 21 | 3 | √ | √ | 12 | 4 | 73 | 25 | |
| 2 | 122 | 12 | 1 | 15 | 1 | 84 | 16 | |||
| 3 | 134 | 16 | 2 | √ | 24 | 2 | 78 | 21 | ||
| 4 | 139 | 19 | 3 | post-traumatic arthritis, venous thrombosis | 16 | 2 | 74 | 22 | ||
| 5 | 98 | 8 | 1 | 18 | 1 | 92 | 13 | |||
| 6 | 116 | 14 | 2 | 19 | 2 | 97 | 18 | |||
| 7 | 193 | 48 | 5 | √ | superficial infection, venous thrombosis, | √ | 12 | 5 | 63 | 56 |
| 8 | 165 | 23 | 3 | venous thrombosis | √ | 12 | 2 | 75 | 28 | |
| 9 | 145 | 11 | 1 | √ | 12 | 1 | 90 | 14 | ||
| 10 | 147 | 13 | 2 | √ | 15 | 3 | 72 | 17 | ||
| 11 | 109 | 12 | 2 | 19 | 0 | 81 | 16 | |||
| 12 | 126 | 16 | 2 | post-traumatic arthritis | √ | 12 | 3 | 73 | 19 | |
| 13 | 153 | 28 | 4 | Deep infection, post-traumatic arthritis | √ | 18 | 4 | 67 | 32 | |
| 14 | 144 | 21 | 3 | √ | 16 | 2 | 68 | 25 | ||
| 15 | 120 | 25 | 3 | post-traumatic arthritis | 18 | 3 | 75 | 27 | ||
| 16 | 147 | 14 | 2 | post-traumatic arthritis | 15 | 2 | 75 | 18 | ||
| 17 | 163 | 19 | 3 | √ | 12 | 5 | 64 | 23 | ||
| 18 | 134 | 17 | 3 | post-traumatic arthritis | 12 | 3 | 76 | 24 | ||
| 19 | 112 | 10 | 1 | 12 | 1 | 79 | 14 | |||
| 20 | 125 | 26 | 4 | √ | 16 | 2 | 76 | 31 | ||
| 21 | 137 | 17 | 2 | √ | Superficial infection | √ | 19 | 2 | 71 | 21 |
Table 3.
Radiographic outcome of the patients involved
| Variable | Pre-op | Post-op | p |
|---|---|---|---|
| First metatarso-cuneiform step-off (mm) | 1.9±2.3 | 0.4±0.6 | <0.05* |
| Second metatarso-cuneiform step-off (mm) | 3.4±1.5 | 0.7±0.3 | <0.05* |
| First intermetatarsal angle (deg) | 8.9±2.8 | 8.4±1.6 | 0.39 |
| First metatarsal to talus angle (deg) | 10.7±1.9 | 11.8±3.8 | 0.10 |
| Fifth metatarsal to calcaneus angle (deg) | 16.4±4.1 | 15.5±1.2 | 0.28 |
| Second metatarsal length (mm) | 72.5±3.2 | 75.0±3.8 | <0.05* |
| Foot length (mm) | 247.2±10.2 | 247.8±10.3 | <0.05* |
| Second metatarsal length/foot length (%) | 0.29±0.02 | 0.30±0.01 | <0.05* |
“*” stands for difference with statistically significant difference
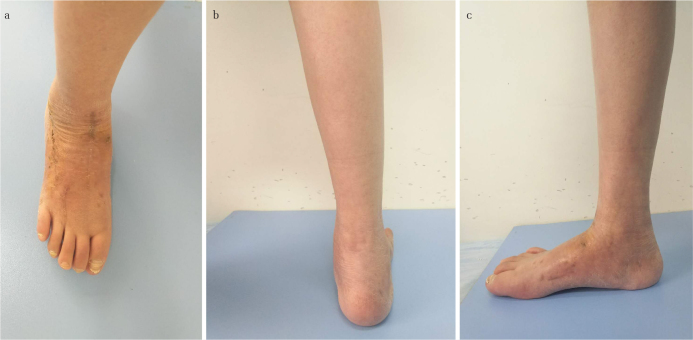
Figure 6. a, b.
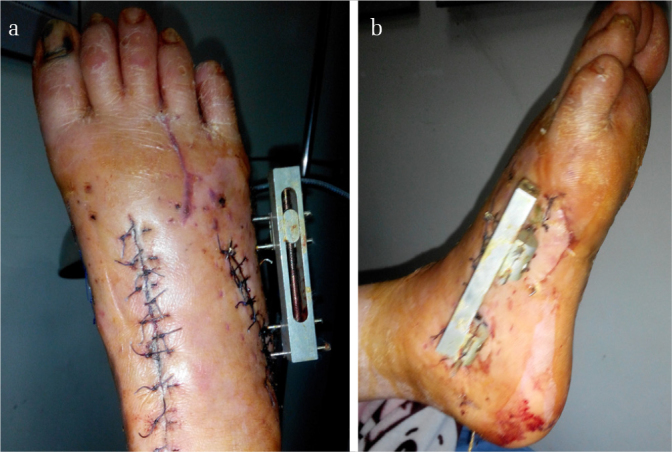
The photograph of the foot in Figure 1 after the second-stage operation. Note that excellent wound healing was achieved in the (a) dorsalis pedis and the (b) small unilateral external fixator still in place across the lateral column in the lateral view
Figure 7. a–c.
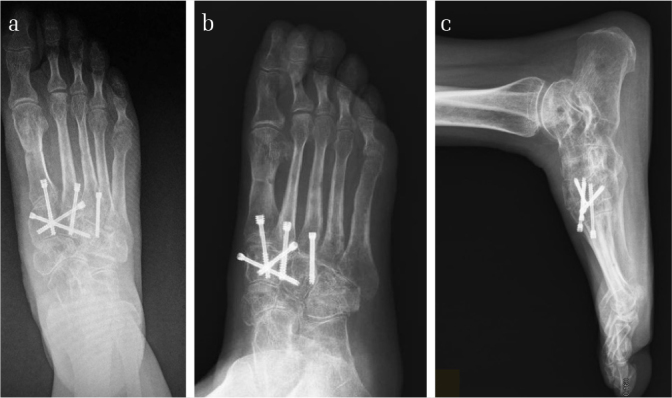
Images of the foot in Figure 1 after the removal of the external fixator, K-wires, and the cannulated screws transfixing the talonavicular joint 7 months later. The postoperative X-ray radiographs showed excellent alignment of the tarsometatarsal joins but narrowed joint space and slightly decalcified bone. The dislocated calcaneocuboid joint was reduced, though there was slight flat foot malformation
Figure 8. a–c.
Images of the foot in Figure 1 a year after the second-stage internal fixation surgery. The general view of the weight-bearing foot shows excellent wound healing in (a) dorsalis pedis, (b) excellent hindfoot alignment, and (c) acceptable height of the medial arch of the foot
Figure 9. a–c.
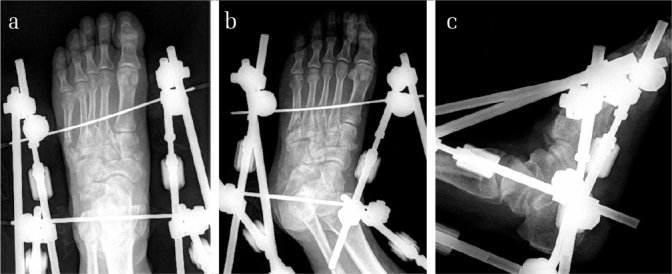
Images of the X-ray radiograph of a 37-year-old male after the first-stage operation. It could also be noted that the displacement of the tarsometatarsal joints and the malalignment of the fracture sites were not anatomically reduced
Figure 10. a–c.
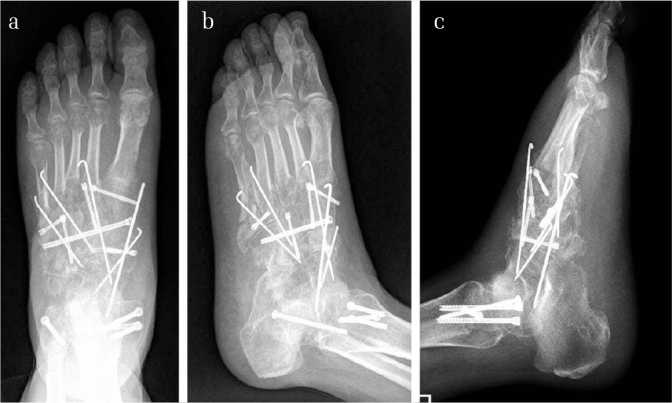
Images of the X-ray radiograph of the same patient after the second-stage operation. The external fixator was changed to internal fixation using cannulated screws and K-wires. It could be noted that the comminuted and displaced middle and lateral columns as well as the cuneonavicular joint were anatomically reduced
Figure 11. a–c.
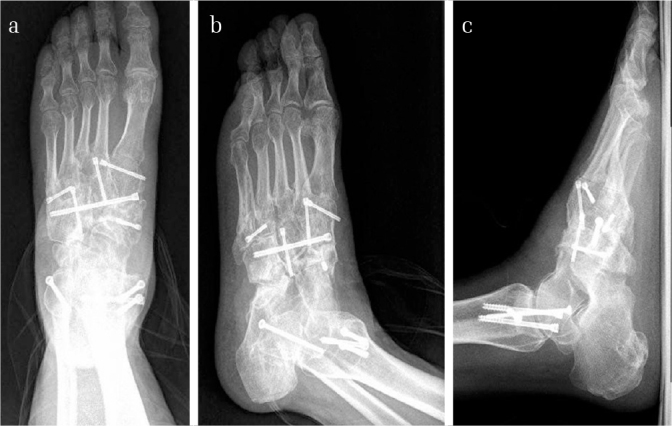
Images of the weight-bearing X-ray radiograph of the same patient at the last follow-up. The K-wires were removed. It could be noted that excellent alignment and bony union were achieved in the disrupted middle and lateral columns in both the AP and the oblique views at this stage (a, b). In the lateral view, it could also be noted that the normal height of the medial arch was maintained even during full weight bearing
Discussion
In this study, we investigated the outcomes of open Lisfranc fracture dislocations repaired with a staged and progressive protocol using an adjustable bilateral external fixator. At the end of follow-up, the patients had a significantly improved first metatarso-cuneiform step-off (p=0.002) and second metatarso-cuneiform step-off (p=0.000). The average VAS score was 2.4, and the average AOFAS score was 76.3. Furthermore, there were three infections in total, including deep infection in one patient and superficial infections in two patients, all of which occurred before the definitive fixation. The results of our study suggest that open Lisfranc fracture dislocations, even those injuries with concomitant vascular injuries, can be treated with the staged and progressive reduction protocol to successfully restore the length of the disrupted columns at the emergency stage and to achieve an excellent reduction of the TMT joint alignment, pain relief, and functional outcome, without increasing the risk of soft tissue compromise and infection.
Treatment of open Lisfranc fracture dislocations is challenging for orthopedic surgeons because of the tenuous soft tissue envelope and the intolerance of nonanatomic alignment due to the highly specific anatomic and biomechanical feature of the midfoot region (10). Efforts have been made to solve this problem with different strategies. Attempts of single-stage primary anatomic reduction and fixation, including external frame fixation as the ultimate procedure, multiple K-wires as the ultimate procedure, and simultaneous screw fixation with flap coverage, were made in early studies. However, the outcomes of these attempts were not satisfactory because various reasons such as unacceptably high risk of persistent mobility (11), incompatibility of the device (5, 12), insufficient stability of the used device, which led to a high risk of flatfoot morbidity (4, 13–15), requirement of complicated technique, risk of flap failure, and inadequate sample size (16).
Furthermore, Gu and Shi proposed a concept of staged management for open Lisfranc fracture dislocations. Their concept was similar to the treatment for high-energy pilon fractures because these two types of fractures shared similar features of intolerance of nonanatomic reduction and the poor soft-tissue coverage (17). The application of staged management for high-energy pilon fractures efficiently reduced the complication rate of the affected soft tissue (18, 19). In the study conducted by Gu and Shi, satisfactory reduction was achieved via the wound or with a joystick technique and provisionally stabilized with K-wires at the first stage, then the K-wires in the medial and middle columns were converted to screws for definitive fixation at the second stage. This staged protocol provided enough time for soft tissue to recover while the bony and articular alignment was stabilized anatomically (17). In the current study, several modifications were made in the surgical protocol proposed by Gu and Shi. Not only the fixation, but also the reduction process was performed in a staged manner. In our protocol, the only goal of the first stage was the restoration of normal length and the gross alignment of the disrupted column or columns instead of direct meticulous anatomic reduction. To achieve this goal, a bilateral adjustable external fixator was used. For those cases with vascular injury and/or anastomosis, the first-stage reduction maneuver was achieved via a slow and gradual distraction process postoperatively using the external fixator to avoid irritation of the vessel. The ultimate anatomic reduction maneuver and definitive internal fixation were not performed until the recovery of the soft tissue envelope to a status ready for closure or repair.
Our strategy has several advantages. First, by using the staged reduction protocol, the iatrogenic damage to the already injured soft tissue envelope during the emergency stage can be minimized, which would reduce the risks of soft tissue compromise and infection. Moreover, the duration of emergency operation can also be shortened, which would result in the shortening of wound exposure time, consequently leading to a lower infection risk. This is especially the case when the open Lisfranc fracture dislocation is just a part of severe, multiple injuries for which damage control is necessary. Though the infection rate in our study was higher than that in Gu and Shi’s study, it is notable that more Gustilo type IIIB and IIIC cases were included in our study, suggesting that patients in our study were more severely injured. Conversion of K-wires to cannulated screws within the relatively short time reported by Gu and Shi in such badly injured and contaminated cases could be risky. It is also worth noting that for the secondary reduction, shortening is the most difficult situation to be corrected because of the contracture of the surrounding tissue, while other malalignments, such as shifting, rotation, and angulation, are relatively easy to handle. Therefore, restoration and maintenance of the length of the disrupted column during the emergency stage will greatly benefit the later open reduction and internal fixation (ORIF). This is the reason why progressive distraction with a spanning external fixator across the Lisfranc joint or/and Chopart joint is needed before ORIF for missed or subtle Lisfranc injuries (7, 20). In the current study, although the included patients were more severely injured compared with Gu and Shi’s report, the Lisfranc fracture dislocations were still anatomically reduced after the second-stage operation without the occurrence of infection later on, and the average AOFAS score was even higher than that reported by Gu and Shi. Despite that the AOFAS score can be influenced by the measurement error and presence of other concomitant injuries in the foot and ankle regions, it still seems that postponing the anatomical reduction manipulation to the time of wound closure or repair does not adversely influence the quality of reduction and functional outcome. Another advantage is that in the cases with vascular injury, progressive reduction with slow and gradual distraction of the external frame could prevent excessive tension on the anastomotic vessels. Intensive monitoring and immediate adjustment of the distraction force when necessary may give the anastomotic vessels a second chance to recover and reduce the risk of vascular irritation and the necrosis of limb or local skin and/or subcutaneous tissue thereafter. The final advantage is that in those cases with significant comminution of the basal metatarsal region, K-wires may not be able to hold the arch height and the relative length of the damaged columns because of the extreme instability of the fracture zone. External fixators can perform better than K-wires in terms of length maintenance. Moreover, in those cases with extensive soft tissue damage and severe contamination, the application of K-wires into the injured area is still risky even when it is not through the impaired soft tissue envelope in terms of infection. Using external fixators instead of K-wires for temporary fixation could avoid the dilemma where infections occur earlier than the time that K-wire could be safely removed without causing loss of reduction.
Previous reports on similar staged reduction strategies have also been found. In 2014, Kadow et al. reported a method of temporary fixation for high-energy Lisfranc injuries using one or two unicolumnar external fixators (21). However, theoretically, their method of fixation could not involve the middle column, which was the most intolerant column to incongruity (22, 23). For cases with transverse ligament laceration, severe comminution or bone defect of the middle column, application of the procedure reported by Kadow et al. would end up in significant shortening and malalignment of this region (3, 12). Furthermore, Ahmed et al. reported a case in which a patient having Lisfranc injury with severe soft tissue injury was treated with temporary application of an Ilizarov external fixator followed by delayed and limited internal fixation (24). In this case, immediate ORIF was not possible because of the full thickness necrosis of the dorsal skin of the affected foot. The method reported in this case highly resembled our strategy, and the patient achieved pain-free weight bearing and good functional outcome. The success reported by these researchers served as further evidence for the effectiveness of staged reduction strategy.
This study has several limitations. First, lack of comparable control group and use of historical control weaken the objectivity of our conclusion. Second, the retrospective design, relatively small sample size, and short follow-up period may cause bias in the study, thus potentially reducing the validity of the study. To verify the superiority of staged reduction strategy over primary anatomical reduction, prospective randomized controlled studies with valid design and adequate sample size will be needed. Third, only first-generation cephalosporin was used for prophylaxis, even for Gustilo type III patients. This may lead to additionally higher risk of infection than there should be.
In conclusion, staged and progressive reduction protocol using an adjustable bilateral external fixator is a promising way to achieve and maintain anatomic reduction, which is essential for good outcome in patients suffering from open Lisfranc fracture dislocations, with low risk of wound complication.
HIGHLIGHTS.
Anatomical reduction at the emergency stage is not always necessary for patients with open Lisfranc fracture-dislocations.
The staged progressive reduction protocol using an adjustable bilateral external fixator is a promising way to achieve and maintain anatomical reduction at a price of relatively low risk of wound complication for open Lisfranc fracture dislocations.
Irritation of the anastomotic vessels can be avoided in patients with Gustilo type IIIC cases with vascular impairment and anastomosis by performing the distraction process slowly and progressively after the operation with intensive monitoring of the acral blood supply.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the Biomedical Research Ethics Committee of West China Hospital of Sichuan University (file number: 1.0; file year: 2019).
Informed Consent: Informed consent was obtained from all the individual participants included in the study.
Author Contributions: Concept - A.T.; Design - A.T., H.A.; Supervision - A.T., Ö.K.; Materials - A.T., H.A., Ü.A., S.H., E.E., Ö.K.; Data Collection and/or Processing - A.T., H.A., Ü.A., S.H., E.E.; Analysis and/or Interpretation - A.T., Ö.K.; Literature Search - A.T., H.A., S.H., E.E.; Writing Manuscript - A.T.; Critical Review - A.T., Ö.K..
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Kalia V, Fishman EK, Carrino JA, Fayad LM. Epidemiology, imaging, and treatment of Lisfranc fracture-dislocations revisited. Skeletal Radiol. 2012;41:129–36. doi: 10.1007/s00256-011-1131-5. [DOI] [PubMed] [Google Scholar]
- 2.Teng AL, Pinzur MS, Lomasney L, Mahoney L, Havey R. Functional outcome following anatomic restoration of tarsal-metatarsal fracture dislocation. Foot Ankle Int. 2002;23:922–6. doi: 10.1177/107110070202301006. [DOI] [PubMed] [Google Scholar]
- 3.Watson TS, Shurnas PS, Denker J. Treatment of Lisfranc joint injury: Current concepts. J Am Acad Orthop Surg. 2010;18:718–28. doi: 10.5435/00124635-201012000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Schepers T, Rammelt S. Classifying the Lisfranc injury: Literature overview and a new classification. FussSprungg. 2018;16:151–9. doi: 10.1016/j.fuspru.2018.07.003. [DOI] [Google Scholar]
- 5.Nithyananth M, Boopalan PR, Titus VT, Sundararaj GD, Lee VN. Long-term outcome of high-energy open Lisfranc injuries: a retrospective study. J Trauma. 2011;70:710–6. doi: 10.1097/TA.0b013e3181f02ab9. [DOI] [PubMed] [Google Scholar]
- 6.Halawi MJ, Morwood MP. Acute management of open fractures: An evidence-based review. Orthopedics. 2015;38:1025–33. doi: 10.3928/01477447-20151020-12. [DOI] [PubMed] [Google Scholar]
- 7.Feng P, Li YX, Li J, et al. Staged management of missed lisfranc injuries: A report of short-term results. Orthop Surg. 2017;9:54–61. doi: 10.1111/os.12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coetzee JC, Ly TV. Treatment of primarily ligamentous Lisfranc joint injuries: primary arthrodesis compared with open reduction and internal fixation. Surgical technique. J Bone Joint Surg Am. 2007;89(Suppl 2 Pt.1):122–7. doi: 10.2106/JBJS.F.01004. [DOI] [PubMed] [Google Scholar]
- 9.Ibrahim T, Beiri A, Azzabi M, Best AJ, Taylor GJ, Menon DK. Reliability and validity of the subjective component of the American Orthopaedic Foot and Ankle Society clinical rating scales. J Foot Ankle Surg. 2007;46:65–74. doi: 10.1053/j.jfas.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Krause F, Schmid T, Weber M. Current Swiss Techniques in Management of Lisfranc Injuries of the Foot. Foot Ankle Clin. 2016;21:335–50. doi: 10.1016/j.fcl.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Chandran P, Puttaswamaiah R, Dhillon MS, Gill SS. Management of complex open fracture injuries of the midfoot with external fixation. J Foot Ankle Surg. 2006;45:308–15. doi: 10.1053/j.jfas.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Qu W, Ni S, Wang Z, et al. Severe open Lisfranc injuries: one-stage operation through internal fixation associated with vacuum sealing drainage. J Orthop Surg Res. 2016;11:134. doi: 10.1186/s13018-016-0471-1. doi: 10.1186/s13018-016-0471-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee CA, Birkedal JP, Dickerson EA, Vieta PA, Jr, Webb LX, Teasdall RD. Stabilization of Lisfranc joint injuries: A biomechanical study. Foot Ankle Int. 2004;25:365–70. doi: 10.1177/107110070402500515. [DOI] [PubMed] [Google Scholar]
- 14.Schepers T, Oprel PP, Van Lieshout EM. Influence of approach and implant on reduction accuracy and stability in lisfranc fracture-dislocation at the tarsometatarsal joint. Foot Ankle Int. 2013;34:705–10. doi: 10.1177/1071100712468581. [DOI] [PubMed] [Google Scholar]
- 15.Efstathopoulos N, Papachristou G, Agoropoulos Z, et al. Open fractures-dislocations of the tarsometatarsal (Lisfranc) joints Report of three cases. Eur J Orthop Surg Traumatol. 1997;7:41–43. doi: 10.1007/BF00578833. [DOI] [Google Scholar]
- 16.Sanli I, Hermus J, Poeze M. Primary internal fixation and soft-tissue reconstruction in the treatment for an open Lisfranc fracture-dislocation. Musculoskelet Surg. 2012;96:59–62. doi: 10.1007/s12306-011-0150-7. [DOI] [PubMed] [Google Scholar]
- 17.Gu W, Shi Z. Staged management of open Lisfranc injuries: Experience from 14 patients. Medicine (Baltimore) 2017;96:e6699. doi: 10.1097/MD.0000000000006699. doi: 10.1097/MD.0000000000006699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sirkin M, Sanders R, DiPasquale T, Herscovici D., Jr A staged protocol for soft tissue management in the treatment of complex pilon fractures. J Orthop Trauma. 2004;18:32–8. doi: 10.1097/00005131-200409001-00005. [DOI] [PubMed] [Google Scholar]
- 19.Tomás-Hernández J. High-energy pilon fractures management: State of the art. EFORT Open Rev. 2017;1:354–61. doi: 10.1302/2058-5241.1.000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tantray MD, Kangoo K, Nazir A, et al. An old mismanaged Lisfranc injury treated by gradual deformity correction followed by the second-stage internal fixation. Strategies Trauma Limb Reconstr. 2017;12:59–62. doi: 10.1007/s11751-016-0273-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kadow TR, Siska PA, Evans AR, Sands SS, Tarkin IS. Staged treatment of high energy midfoot fracture dislocations. Foot Ankle Int. 2014;35:1287–91. doi: 10.1177/1071100714552077. [DOI] [PubMed] [Google Scholar]
- 22.Komenda GA, Myerson MS, Biddinger KR. Results of arthrodesis of the tarsometatarsal joints after traumatic injury. J Bone Joint Surg Am. 1996;78:1665–76. doi: 10.2106/00004623-199611000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Watson TS, Shurnas PS, Denker J. Treatment of Lisfranc joint injury: current concepts. J Am Acad Orthop Surg. 2010;18:718–28. doi: 10.5435/00124635-201012000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed N, Kugan R. Ilizarov frame delayed internal fixation of Lisfranc fracture dislocation with severe soft tissue injury: New technique. Trauma Case Rep. 2015;1:88–94. doi: 10.1016/j.tcr.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]