Abstract
Background
The efficacy of interleukin-6 receptor blockade in hospitalized patients with coronavirus disease 2019 (Covid-19) who are not receiving mechanical ventilation is unclear.
Methods
We performed a randomized, double-blind, placebo-controlled trial involving patients with confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, hyperinflammatory states, and at least two of the following signs: fever (body temperature >38°C), pulmonary infiltrates, or the need for supplemental oxygen in order to maintain an oxygen saturation greater than 92%. Patients were randomly assigned in a 2:1 ratio to receive standard care plus a single dose of either tocilizumab (8 mg per kilogram of body weight) or placebo. The primary outcome was intubation or death, assessed in a time-to-event analysis. The secondary efficacy outcomes were clinical worsening and discontinuation of supplemental oxygen among patients who had been receiving it at baseline, both assessed in time-to-event analyses.
Results
We enrolled 243 patients; 141 (58%) were men, and 102 (42%) were women. The median age was 59.8 years (range, 21.7 to 85.4), and 45% of the patients were Hispanic or Latino. The hazard ratio for intubation or death in the tocilizumab group as compared with the placebo group was 0.83 (95% confidence interval [CI], 0.38 to 1.81; P=0.64), and the hazard ratio for disease worsening was 1.11 (95% CI, 0.59 to 2.10; P=0.73). At 14 days, 18.0% of the patients in the tocilizumab group and 14.9% of the patients in the placebo group had had worsening of disease. The median time to discontinuation of supplemental oxygen was 5.0 days (95% CI, 3.8 to 7.6) in the tocilizumab group and 4.9 days (95% CI, 3.8 to 7.8) in the placebo group (P=0.69). At 14 days, 24.6% of the patients in the tocilizumab group and 21.2% of the patients in the placebo group were still receiving supplemental oxygen. Patients who received tocilizumab had fewer serious infections than patients who received placebo.
Conclusions
Tocilizumab was not effective for preventing intubation or death in moderately ill hospitalized patients with Covid-19. Some benefit or harm cannot be ruled out, however, because the confidence intervals for efficacy comparisons were wide. (Funded by Genentech; ClinicalTrials.gov number, NCT04356937.)
Infections with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (Covid-19), now number more than 7 million in the United States.1 At the peak of the pandemic to date, more than 1000 Americans died from Covid-19 each day, and more than 214,000 had died as of October 13, 2020. After an incubation period, the acute viral phase in patients with symptomatic Covid-19 usually manifests as influenza-like symptoms. In some persons, the illness progresses to hypoxemic respiratory failure.2,3 Evidence suggests that the pathophysiological basis of this profound decline is a severe inflammatory response resembling cytokine release syndrome.4,5 In this phase, patients have markedly abnormal inflammatory markers, including elevated serum interleukin-6, ferritin, and C-reactive protein levels.6-9 Higher concentrations of interleukin-6 in serum are associated with higher levels of SARS-CoV-2 viremia,10 prolonged viral RNA shedding,11 progression to mechanical ventilation,12 and death.13 These findings led us to hypothesize that interleukin-6 receptor blockade might interrupt this inflammatory cascade at a crucial stage.
Evidence from nonrandomized trials and open-label studies has been contradictory,14-33 and published results from randomized, double-blind, placebo-controlled trials have been lacking. We performed the investigator-initiated Boston Area COVID-19 Consortium (BACC) Bay Tocilizumab Trial, a randomized, double-blind, placebo-controlled trial of tocilizumab administered relatively early in the disease course, with the aim of preventing progression of Covid-19. We hypothesized that early intervention with interleukin-6 receptor blockade might limit progression to hypoxemic respiratory failure or death, reduce the risk of clinical worsening, and decrease the duration of supplemental oxygen use.
Methods
Trial Design
We conducted the trial at seven Boston hospitals. The trial was approved by the Mass General Brigham institutional review board and was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. All patients provided written informed consent in keeping with institutional guidelines. The investigators designed the trial, collected the data, and performed the analysis. Genentech funded the trial and provided tocilizumab but had no role in data analysis, data interpretation, or writing of the manuscript. The authors vouch for the accuracy and completeness of the data and for the fidelity of the trial to the protocol, available with the full text of this article at NEJM.org. The trial was overseen by a data and safety monitoring board. All the authors participated in writing the manuscript that was submitted. No one who is not an author contributed to writing the manuscript.
Patients
Patients were eligible for enrollment if they were 19 to 85 years of age and had SARS-CoV-2 infection confirmed by either nasopharyngeal swab polymerase chain reaction or serum IgM antibody assay. Patients had to have at least two of the following signs: fever (body temperature >38°C) within 72 hours before enrollment, pulmonary infiltrates, or a need for supplemental oxygen in order to maintain an oxygen saturation higher than 92%. At least one of the following laboratory criteria also had to be fulfilled: a C-reactive protein level higher than 50 mg per liter, a ferritin level higher than 500 ng per milliliter, a d-dimer level higher than 1000 ng per milliliter, or a lactate dehydrogenase level higher than 250 U per liter. Patients were excluded if they were receiving supplemental oxygen at a rate that exceeded 10 liters per minute, if they had a recent history of treatment with biologic agents or small-molecule immunosuppressive therapy, if they were receiving other immunosuppressive therapy that the investigator believed placed them at higher risk for an infection, or if they had had diverticulitis. The full list of entry criteria is provided in the protocol.
Randomization and Treatment
Patients were randomly assigned in a 2:1 ratio to receive standard care plus a single dose of either tocilizumab (8 mg per kilogram of body weight administered intravenously, not to exceed 800 mg) or placebo; randomization was performed with randomly permuted blocks of sizes 3 and 6. Randomization was stratified according to site. Administration of tocilizumab or placebo was generally complete within 3 hours after informed consent was obtained.
Concomitant Treatment
The results of the Adaptive Covid-19 Treatment Trial (ACTT-1) of remdesivir34 became known during this trial, but the results of the Randomized Evaluation of Covid-19 Therapy (RECOVERY) trial35 regarding the efficacy of dexamethasone were announced afterward. Therefore, some patients received remdesivir as concomitant treatment, whereas no patients received dexamethasone. Antiviral therapy, hydroxychloroquine, and glucocorticoids were permitted as concomitant treatment.
Outcomes
The primary outcome was intubation (or death, for patients who died before intubation) after administration of tocilizumab or placebo, assessed in a time-to-event analysis. The first secondary outcome was clinical worsening, defined on the basis of an ordinal clinical improvement scale. Scores on the scale were defined as follows: 1, discharged or ready for discharge; 2, in (or ready for) a non-ICU hospital ward and not receiving supplemental oxygen; 3, in (or ready for) a non-ICU hospital ward and receiving supplemental oxygen; 4, in the ICU or a non-ICU hospital ward and receiving noninvasive ventilation or high-flow oxygen; 5, in the ICU, intubated, and receiving mechanical ventilation; 6, in the ICU and receiving extracorporeal membrane oxygenation or mechanical ventilation and additional organ support; and 7, death (Table S2 in the Supplementary Appendix, available at NEJM.org). Worsening was defined as an increase by at least 1 point among patients who had been receiving supplemental oxygen at baseline or at least 2 points among those who had not been receiving supplemental oxygen at baseline. The second secondary outcome was discontinuation of supplemental oxygen among patients who had been receiving it at baseline. Both secondary outcomes were assessed in time-to-event analyses. Data from patients who were event-free at the end of follow-up were censored at 28 days (for the primary and first secondary outcome) or at 29 days (for the second secondary outcome). Data from patients who could not be reached for 28-day follow-up were censored at hospital discharge.
Tertiary outcomes were those related to other time-to-event analyses (e.g., improvement, discharge, or death), analyses of duration (supplemental oxygen use, receipt of mechanical ventilation), and binary outcomes (admission to the intensive care unit [ICU] or death). Exploratory analyses addressed the relationships among inflammatory biomarker levels, demographic and clinical features, and efficacy outcomes.
Statistical Analysis
We assumed that the risk of invasive mechanical ventilation or death within 28 days would be 30% in the placebo group12 and that the risk with tocilizumab would be reduced to 15%. With a total of 243 patients, we had 80% power to detect such a difference with the use of a log-rank test, assuming two-sided tests and a significance level of 0.05. A blinded interim analysis for safety was performed.
The statistical analysis and data-management plans are included with the protocol at NEJM.org. The primary efficacy analysis focused on the modified intention-to-treat population, defined as all patients who underwent randomization and received either tocilizumab or placebo before intubation or death. The safety population included all patients who underwent randomization and received either tocilizumab or placebo. For primary and secondary outcomes, the treatment groups were compared with the use of log-rank tests stratified according to site. The three smallest sites were combined. The differences between the treatment groups were estimated as hazard ratios with 95% confidence intervals from stratified Cox proportional hazards models. Estimates of the percentages of patients who had events at specific time points were based on the corresponding Kaplan–Meier curves. The proportional hazards assumption was confirmed through correlation tests between the weighted Schoenfeld residuals and event times.
A single primary analysis was performed with the criterion for statistical significance defined as a two-sided P value of less than 0.05. Testing of the two secondary efficacy outcomes was performed with a Bonferroni–Holm correction to ensure an overall two-sided significance level of less than 0.05. No corrections for multiplicity were used for tertiary and exploratory analyses. Confidence intervals were not adjusted for multiple comparisons and cannot be used to infer effects. We examined whether the treatment effect on the primary outcome varied within subgroups defined on the basis of demographic or clinical risk factors for poor Covid-19 outcomes. Treatment-effect modification was assessed in separate interaction models for each risk factor. Each model was stratified according to site and included age (>65 or ≤65 years), sex, Hispanic or Latino ethnic group, and race (White or non-White) as covariates. We also evaluated the association between these risk factors and the primary outcome in separate main effects models with stratification and covariate adjustment.
Results
Patients
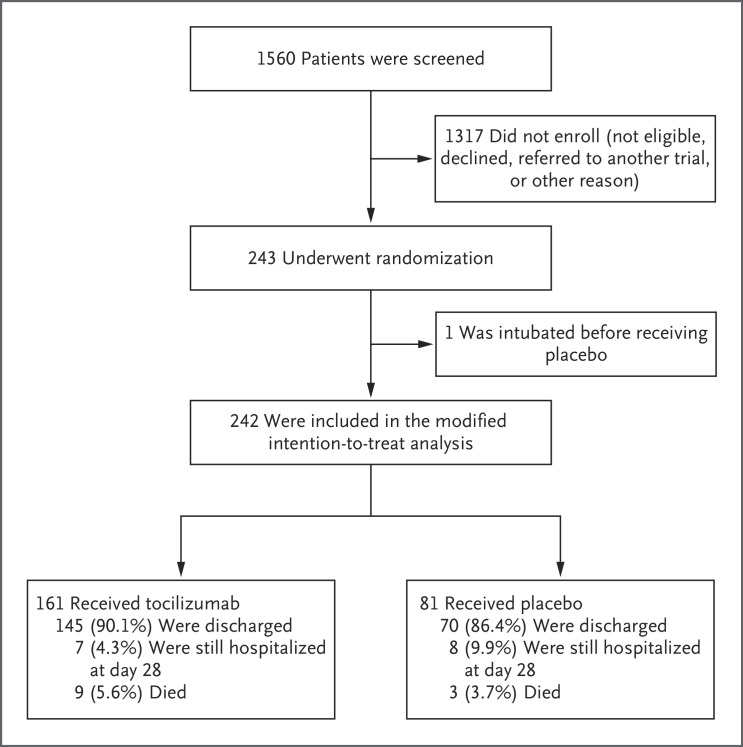
Between April 20 and June 15, 2020, a total of 243 patients were enrolled; 161 received tocilizumab, and 81 received placebo (Figure 1). One patient, who had been intubated before receiving placebo, was excluded from the modified intention-to-treat population but was included in the safety population. The baseline characteristics of the patients are shown in Table 1. In the modified intention-to-treat population, 141 patients (58%) were male and 102 patients (42%) were female. The median age was 59.8 years (interquartile range, 45.3 to 69.4). The youngest patient was 21.7 years of age, and the oldest was 85.4 years of age. In the overall trial population, 45% of patients were Hispanic or Latino, 16% were Black, and 43% were White.
Figure 1. Screening, Randomization, and Outcomes.
The patient who was intubated before receiving placebo was excluded from the modified intention-to-treat population but included in the safety population.
Table 1. Baseline Characteristics of the Patients.*.
| Characteristic | Tocilizumab (N=161) |
Placebo (N=82) |
All Patients (N=243) |
|---|---|---|---|
| Enrolling hospital — no. (%) | |||
| Boston Medical Center | 16 (10) | 7 (9) | 23 (9) |
| Brigham and Women’s Hospital | 17 (11) | 8 (10) | 25 (10) |
| Lahey Hospital | 2 (1) | 2 (2) | 4 (2) |
| Massachusetts General Hospital | 62 (39) | 30 (37) | 92 (38) |
| North Shore Medical Center | 51 (32) | 26 (32) | 77 (32) |
| Newton–Wellesley Hospital | 13 (8) | 7 (9) | 20 (8) |
| St. Elizabeth’s Medical Center | 0 | 2 (2) | 2 (1) |
| Median age (IQR) — yr | 61.6 (46.4–69.7) | 56.5 (44.7–67.8) | 59.8 (45.3–69.4) |
| Age >65 yr — no. (%) | 60 (37) | 22 (27) | 82 (34) |
| Male sex — no. (%) | 96 (60) | 45 (55) | 141 (58) |
| Race or ethnic group — no. (%)† | |||
| American Indian or Alaska Native | 1 (1) | 0 | 1 (<1) |
| Asian | 7 (4) | 2 (2) | 9 (4) |
| Black | 24 (15) | 16 (20) | 40 (16) |
| Native Hawaiian or Pacific Islander | 0 | 1 (1) | 1 (<1) |
| White | 71 (44) | 33 (40) | 104 (43) |
| Other | 35 (22) | 15 (18) | 50 (21) |
| Unknown | 23 (14) | 15 (18) | 38 (16) |
| Hispanic or Latino ethnic group — no. (%)† | |||
| Hispanic or Latino | 70 (43) | 39 (48) | 109 (45) |
| Not Hispanic or Latino | 84 (52) | 35 (43) | 119 (49) |
| Unknown | 7 (4) | 8 (10) | 15 (6) |
| Median BMI (IQR)‡ | 29.9 (26.0–34.2) | 30.2 (25.7–33.8) | 30.1 (25.9–34.2) |
| BMI ≥30 — no. (%)‡ | 80 (50) | 42 (51) | 122 (50) |
| Median time from symptom onset to randomization (IQR) — days | 9.0 (6.0–13.0) | 10.0 (7.0–13.0) | 9.0 (6.0–13.0) |
| Hypertension — no. (%) | 80 (50) | 38 (46) | 118 (49) |
| Heart failure — no. (%) | 17 (11) | 7 (9) | 24 (10) |
| History of myocardial infarction — no. (%) | 15 (9) | 6 (7) | 21 (9) |
| Chronic obstructive pulmonary disorder — no. (%) | 15 (9) | 7 (9) | 22 (9) |
| Asthma — no. (%) | 15 (9) | 7 (9) | 22 (9) |
| Smoking status — no. (%) | |||
| Current smoker | 7 (4) | 0 | 7 (3) |
| Former smoker | 46 (29) | 26 (32) | 72 (30) |
| Lifelong nonsmoker | 99 (61) | 48 (59) | 147 (60) |
| Unknown | 9 (6) | 8 (10) | 17 (7) |
| Diabetes — no. (%) | 45 (28) | 30 (37) | 75 (31) |
| Chronic kidney disease — no. (%) | 29 (18) | 13 (16) | 42 (17) |
| History of cancer — no. (%) | 22 (14) | 8 (10) | 30 (12) |
| Ordinal scale score — no. (%)§ | |||
| 2 | 23 (14) | 15 (18) | 38 (16) |
| 3 | 133 (83) | 61 (74) | 194 (80) |
| 4 | 5 (3) | 5 (6) | 10 (4) |
| 5 | 0 | 1 (1) | 1 (<1) |
| Median laboratory values (IQR)¶ | |||
| Absolute lymphocyte count — cells/mm3 | 1040 (700–1400) | 1030 (680–1360) | 1030 (700–1400) |
| C-reactive protein level — mg/liter | 116.0 (67.1–190.6) | 94.3 (58.4–142.0) | 110.0 (64.9–175.3) |
| Ferritin level — ng/ml | 723 (413–1212) | 686 (382–1228) | 708 (411–1225) |
| d-Dimer level — ng/ml | 857 (536–1695) | 980 (500–1739) | 884 (527–1730) |
| Lactate dehydrogenase level — U/liter | 351 (287–420) | 324 (290–395) | 340 (289–413) |
| Serum interleukin-6 level — pg/ml | 23.6 (14.0–49.9) | 25.4 (14.6–40.3) | 24.4 (14.1–45.5) |
| Erythrocyte sedimentation rate — mm/hr | 61 (42–90) | 63 (42–87) | 61 (42–88) |
| Troponin level — ng/liter | 8 (6–22) | 9 (6–24) | 9 (6–22) |
| NT-proBNP level — pg/ml | 110 (50–438) | 93 (33–431) | 108 (38–437) |
| Procalcitonin level — ng/ml | 0.2 (0.1–0.4) | 0.2 (0.1–0.3) | 0.2 (0.1–0.4) |
Percentages may not total 100 because of rounding. IQR denotes interquartile range, and NT-proBNP N-terminal pro–B-type natriuretic peptide.
Race and ethnic group were reported by the patients.
The body-mass index (BMI) is the weight in kilograms divided by the square of the height in meters.
Scores on the ordinal clinical improvement scale range from 1 to 7, with higher scores indicating worse clinical condition. A score of 2 indicates that the patient was in (or ready for) a non–intensive care unit (ICU) hospital ward and was not receiving supplemental oxygen; a score of 3, that the patient was in (or ready for) a non-ICU hospital ward and was receiving supplemental oxygen; a score of 4, that the patient was in the ICU or in a non-ICU hospital ward and was receiving noninvasive ventilation or high-flow oxygen; and a score of 5, that the patient was in the ICU, intubated, and receiving mechanical ventilation.
Absolute lymphocyte counts were missing for 2 patients (1 in the tocilizumab group and 1 in the placebo group), C-reactive protein levels were missing for 2 patients (1 and 1), ferritin levels were missing for 1 patient (in the placebo group), d-dimer levels were missing for 2 patients (1 and 1), lactate dehydrogenase levels were missing for 3 patients (1 and 2), interleukin-6 levels were missing for 9 patients (6 and 3), erythrocyte sedimentation rates were missing for 24 patients (19 and 5), troponin levels were missing for 9 patients (5 and 4), NT-proBNP levels were missing for 19 patients (12 and 7), and procalcitonin levels were missing for 16 patients (8 and 8).
The body-mass index (the weight in kilograms divided by the square of the height in meters) was at least 30 in 51% of the patients at baseline, 49% of the patients had hypertension, and 31% had known diabetes mellitus. A total of 194 patients (80%) were hospitalized in non-ICU hospital wards and were receiving supplemental oxygen (≤6 liters per minute), delivered by nasal cannula, to maintain an oxygen saturation greater than 92%; 10 (4%) were receiving high-flow oxygen (>6 and ≤10 liters per minute delivered by any device); and 38 (16%) were not receiving supplemental oxygen at baseline. The median concentration of C-reactive protein was 110.0 mg per liter (interquartile range, 64.9 to 175.3); ferritin, 708 ng per milliliter (interquartile range, 411 to 1225); d-dimer, 884 ng per milliliter (interquartile range, 527 to 1730); and lactate dehydrogenase, 340 U per liter (interquartile range, 289 to 413).
Similar percentages of patients in the two groups received remdesivir, hydroxychloroquine, or glucocorticoids. Remdesivir was administered to 77 patients (53 [33%] in the tocilizumab group and 24 [29%] in the placebo group). Hydroxychloroquine was administered to 9 patients (6 [4%] in the tocilizumab group and 3 [4%] in the placebo group). Glucocorticoids were administered to 23 patients (18 [11%] in the tocilizumab group and 5 [6%] in the placebo group).
Primary Outcome
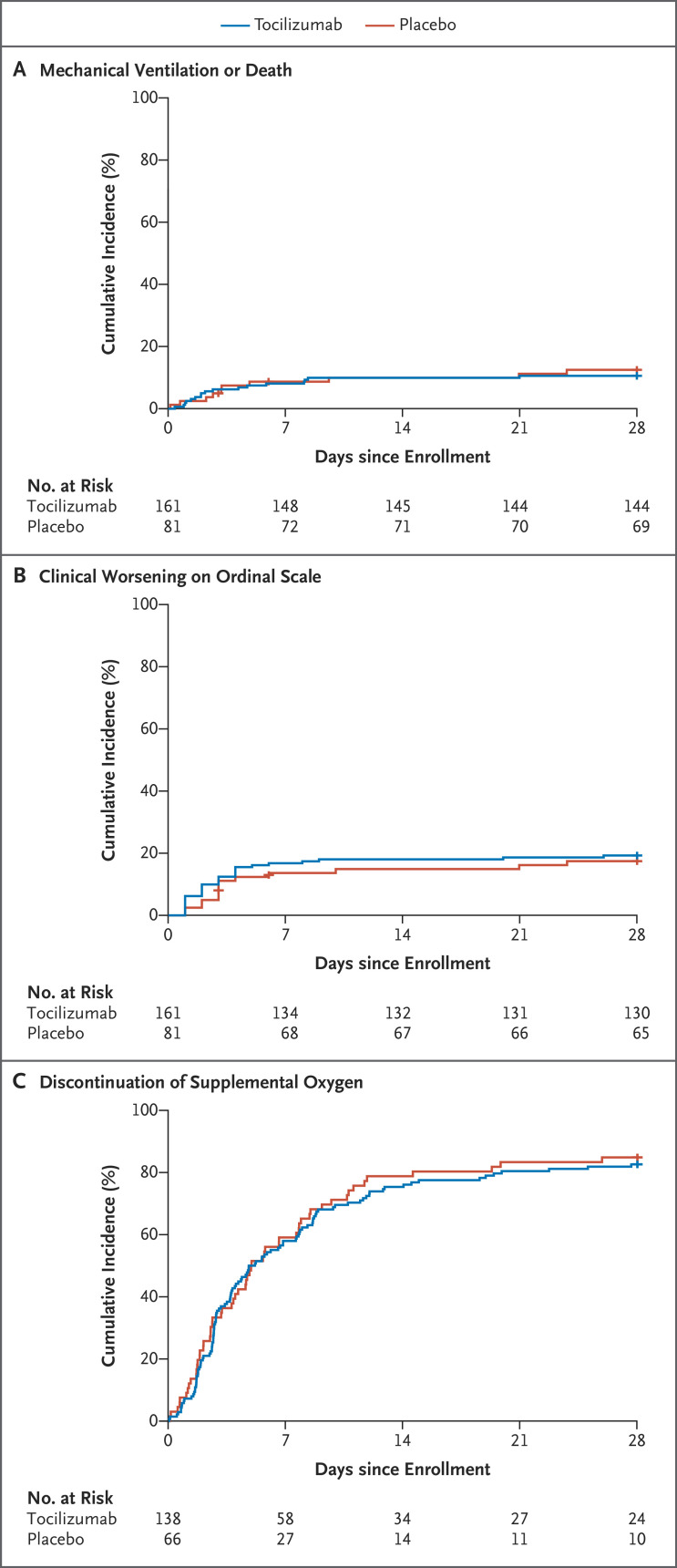
A total of 27 patients (11.2%) were intubated within 28 days or died before intubation (Table 2). At day 28, 17 patients (10.6%) in the tocilizumab group and 10 patients (12.5%) in the placebo group had been intubated or had died (11 were intubated and 6 died without being intubated in the tocilizumab group; 8 were intubated and 2 died without being intubated in the placebo group). The Kaplan–Meier curves for the time to intubation or death are shown in Figure 2A. The hazard ratio for a primary outcome event in the tocilizumab group was 0.83 (95% confidence interval [CI], 0.38 to 1.81; P=0.64 by log-rank test). The hazard ratio was also estimated with adjustment for age, sex, race, Hispanic or Latino ethnic group, diabetes status, and baseline serum interleukin-6 concentration. The adjusted hazard ratio was 0.66 (95% CI, 0.28 to 1.52). The difference between the adjusted and unadjusted hazard ratios was primarily due to the greater percentage of older patients in the tocilizumab group.
Table 2. Time-to-Event Outcomes in the Modified Intention-to-Treat Population.*.
| Outcome | No. of Patients with Event within 28 Days | Percentage of Patients with Event (95% CI)† | Median No. of Days to Event (95% CI) | Hazard Ratio (95% CI) | Log-Rank P Value‡ | |
|---|---|---|---|---|---|---|
| Day 14 | Day 28 | |||||
| Measures of worsening | ||||||
| Primary outcome: mechanical ventilation or death | ||||||
| Tocilizumab | 17 | 9.9 (6.2–15.7) | 10.6 (6.7–16.6) | NR | 0.83 (0.38–1.81) | 0.64 |
| Placebo | 10 | 10.0 (5.1–18.9) | 12.5 (6.9–22.0) | NR | ||
| Secondary outcome: clinical worsening on ordinal scale§ | ||||||
| Tocilizumab | 31 | 18.0 (12.9–24.9) | 19.3 (14.0–26.2) | NR | 1.11 (0.59–2.10) | 0.73 |
| Placebo | 14 | 14.9 (8.7–24.7) | 17.4 (10.7–27.7) | NR | ||
| Tertiary outcome: mechanical ventilation¶ | ||||||
| Tocilizumab | 11 | 6.8 (3.6–11.4) | 6.8 (3.6–11.4) | NR | 0.65 (0.26–1.62) | — |
| Placebo | 8 | 10.0 (4.6–17.7) | 10.0 (4.6–17.7) | NR | ||
| Tertiary outcome: death | ||||||
| Tocilizumab | 9 | 4.4 (2.1–8.9) | 5.6 (3.0–10.5) | NR | 1.52 (0.41–5.61) | — |
| Placebo | 3 | 1.3 (0.2–8.7) | 3.8 (1.2–11.3) | NR | ||
| Measures of improvement | ||||||
| Secondary outcome: discontinuation of supplemental oxygen among patients receiving it at baseline | ||||||
| Tocilizumab | 114 | 75.4 (67.9–82.2) | 82.6 (75.9–88.4) | 5.0 (3.8–7.6) | 0.94 (0.67–1.30) | 0.69 |
| Placebo | 56 | 78.8 (68.3–87.7) | 84.9 (75.2–92.2) | 4.9 (3.8–7.8) | ||
| Tertiary outcome: clinical improvement on ordinal scale§ | ||||||
| Tocilizumab | 147 | 86.3 (80.6–91.1) | 91.3 (86.3–95.1) | 6.0 (5.0–6.0) | 1.06 (0.80–1.41) | — |
| Placebo | 72 | 81.5 (72.4–89.0) | 88.9 (81.0–94.5) | 5.0 (4.0–7.0) | ||
| Tertiary outcome: initial discharge | ||||||
| Tocilizumab | 147 | 86.3 (80.6–91.1) | 91.3 (86.3–95.0) | 6.0 (4.0–7.0) | 1.08 (0.81–1.43) | — |
| Placebo | 72 | 81.5 (72.4–89.0) | 88.9 (81.0–94.5) | 6.0 (5.0–6.0) | ||
The modified intention-to-treat population included the 242 patients (161 in the tocilizumab group and 81 in the placebo group) who underwent randomization and received either tocilizumab or placebo before intubation or death. NR denotes not reached.
Percentages were estimated from the Kaplan–Meier curve.
P values are not reported for tertiary outcomes.
Worsening was defined as an increase in score on the ordinal clinical improvement scale by at least 1 point among patients receiving supplemental oxygen at baseline or at least 2 points among those not receiving supplemental oxygen at baseline. Improvement was defined as an increase in score by at least 2 points.
Results for the time-to-event analysis of mechanical ventilation were obtained with the use of competing-risks analyses with death treated as a competing event. The percentage of patients with an event was estimated from the cumulative incidence function for the event of interest (mechanical ventilation). The cause-specific hazard ratio is reported.
Figure 2. Kaplan–Meier Analyses of Efficacy Outcomes.
Shown are Kaplan–Meier curves for the time-to-event analyses of mechanical ventilation or death (Panel A); clinical worsening, defined as an increase in score on an ordinal clinical improvement scale (scores range from 1 to 7, with higher scores indicating worse clinical condition) by at least 1 point among patients who had been receiving supplemental oxygen at baseline or at least 2 points among those who had not been receiving supplemental oxygen at baseline (Table S2) (Panel B); and discontinuation of supplemental oxygen among patients who had been receiving it at baseline (Panel C).
Secondary Outcomes
The Kaplan–Meier curves for the time to worsening on the ordinal clinical improvement scale are shown in Figure 2B. The hazard ratio for worsening in the tocilizumab group as compared with the placebo group was 1.11 (95% CI, 0.59 to 2.10; P=0.73 by log-rank test) (Table 2). The adjusted hazard ratio was 0.88 (95% CI, 0.45 to 1.72). At 14 days, 18.0% of the patients in the tocilizumab group and 14.9% of the patients in the placebo group had had disease worsening as measured on the ordinal scale. At 28 days, the percentages were 19.3% and 17.4%, respectively.
The Kaplan–Meier curves for the time to discontinuation of supplemental oxygen among patients who had been receiving it at baseline are shown in Figure 2C. The hazard ratio for discontinuation by 28 days in the tocilizumab group as compared with the placebo group was 0.94 (95% CI, 0.67 to 1.30; P=0.69 by log-rank test). The adjusted hazard ratio was 0.95 (95% CI, 0.67 to 1.33). The median time to discontinuation of supplemental oxygen among patients who had been receiving it at baseline was 5.0 days (interquartile range, 3.8 to 7.6) in the tocilizumab group and 4.9 days (interquartile range, 3.8 to 7.8) in the placebo group. At 14 days, 75.4% of patients in the tocilizumab group and 78.8% in the placebo group were no longer receiving supplemental oxygen. At 28 days, the percentages were 82.6% and 84.9%, respectively.
Tertiary and Exploratory Outcomes
Tertiary efficacy outcomes are shown in Table 2 and Table 3. None of the outcomes differed substantially between the treatment groups. The median time to improvement was 6.0 days (95% CI, 5.0 to 6.0) in the tocilizumab group and 5.0 days (95% CI, 4.0 to 7.0) in the placebo group. The median duration of supplemental oxygen use after administration of tocilizumab was 4.0 days; the corresponding measure in the placebo group was 3.9 days. Among the 233 patients who were not in the ICU at enrollment, 25 patients (15.9%) in the tocilizumab group and 12 patients (15.8%) in the placebo group were either admitted to the ICU or died before ICU admission. Among the 19 patients who were intubated, the duration of mechanical ventilation did not differ significantly between the groups (median duration, 15.0 days in the tocilizumab group and 27.9 days in the placebo group). The median time to discharge was 6.0 days in both groups. The results of exploratory analyses of changes in the concentrations of inflammatory markers over time are shown in Table S3.
Table 3. Duration Outcomes and Admission to the ICU or Death in the Modified Intention-to-Treat Population.
| Outcome | Tocilizumab (N=161) |
Placebo (N=81) |
Relative Risk |
|---|---|---|---|
| Median duration of receipt of supplemental oxygen (IQR) — days* | 4.0 (1.8–11.6) | 3.9 (1.1–9.2) | — |
| Median duration of mechanical ventilation (IQR) — days† | 15.0 (12.6–NR) | 27.9 (16.3–NR) | — |
| Admission to ICU or death — % | 15.9 | 15.8 | 0.97 (0.50–1.88) |
Patients who did not receive supplemental oxygen were assigned a value of 0. Patients who died before discontinuation of supplemental oxygen were assigned a value equal to the number of days from when supplemental oxygen began until the end of the 28-day follow-up period.
The median and IQR for duration of mechanical ventilation were estimated from Kaplan–Meier curves generated within patients who received mechanical ventilation (11 in the tocilizumab group and 8 in the placebo group). Data for patients who died without discontinuation of mechanical ventilation were censored at 28 days.
Patient and Treatment Subgroup Analyses
Multivariate, adjusted models showed that patients older than 65 years of age were at greater risk for progression to intubation or death than younger patients (hazard ratio, 3.11; 95% CI, 1.36 to 7.10). In addition, patients with baseline serum interleukin-6 concentrations higher than 40 pg per milliliter were more likely to have progression than those with baseline concentrations at or below 40 pg per milliliter (hazard ratio, 3.03; 95% CI, 1.34 to 6.83). Factors that were not found to affect the risk of intubation or death included male sex (hazard ratio, 1.27; 95% CI, 0.57 to 2.81), Hispanic or Latino ethnic group (hazard ratio, 1.16; 95% CI, 0.47 to 2.85), obesity (hazard ratio, 1.32; 95% CI, 0.60 to 2.90), diabetes (hazard ratio, 1.55; 95% CI, 0.69 to 3.48), and treatment with remdesivir (hazard ratio, 1.95; 95% CI, 0.86 to 4.44). No difference between the subgroups was observed for the treatment effect (Table S4).
Safety
Adverse events are shown in Table 4. No new safety signal for tocilizumab emerged. Neutropenia developed in 22 patients in the tocilizumab group, as compared with only 1 patient in the placebo group (P=0.002), but serious infections occurred in fewer patients in the tocilizumab group (13 [8.1%] vs. 14 [17.3%]; P=0.03). There were 36 serious adverse events in the tocilizumab group, occurring in a total of 28 patients. Of these 36 serious adverse events, 25 were considered by the investigators to be unrelated to tocilizumab, and 11 were considered to be related or possibly related. There were 38 serious adverse events in the placebo group, occurring in 12 patients; 35 events were considered unrelated to placebo, and 3 were considered related or possibly related. All serious adverse events are listed in Table 4. They are also listed separately with additional descriptions in Table S5.
Table 4. Adverse Events in the Safety Population.*.
| Event | Tocilizumab (N=161) |
Placebo (N=82) |
P Value |
|---|---|---|---|
| no. of patients (%) | |||
| Death | 9 (5.6) | 4 (4.9)† | 0.81 |
| Hypersensitivity reaction to infusion | 2 (1.2) | 2 (2.4) | 0.52 |
| Infection of grade ≥3 | 13 (8.1) | 14 (17.1) | 0.03 |
| Grade 3 | 12 (7.5) | 14 (17.1) | |
| Grade 4 | 1 (0.6) | 0 | |
| Myocardial infarction | 0 | 1 (1.2) | 0.15 |
| Deep venous thrombosis | 2 (1.2) | 3 (3.7) | 0.18 |
| Pulmonary embolism | 2 (1.2) | 2 (2.4) | 0.47 |
| Stroke | 2 (1.2) | 0 | 0.31 |
| Seizure | 0 | 1 (1.2) | 0.13 |
| Arterial ischemia | 1 (0.6) | 0 | 0.49 |
| Gastrointestinal perforation | 0 | 0 | — |
| Demyelinating disorder | 0 | 0 | — |
| Elevated liver-function values | |||
| ALT, grade ≥3 | 8 (5.0) | 4 (4.9) | 0.99 |
| Grade 3 | 8 (5.0) | 4 (4.9) | |
| Grade 4 | 0 | 0 | |
| AST, grade ≥3 | 6 (3.7) | 3 (3.7) | 0.99 |
| Grade 3 | 6 (3.7) | 2 (2.4) | |
| Grade 4 | 0 | 1 (1.2) | |
| Neutropenia, grade ≥3 | 22 (13.7) | 1 (1.2) | 0.002 |
| Grade 3 | 21 (13.0) | 1 (1.2) | |
| Grade 4 | 1 (0.6) | 0 | |
| Thrombocytopenia, grade ≥3 | 1 (0.6) | 0 | 0.51 |
| Grade 3 | 1 (0.6) | 0 | |
| Grade 4 | 0 | 0 | |
| Bleeding | 0 | 1 (1.2) | 0.15 |
| Other‡ | 21 (13.0) | 14 (17.1) | 0.15 |
The percentage of patients who had at least one occurrence of each type of adverse event is reported. Grade was calculated as the maximum grade reported across occurrences within a patient. The percentages of patients with adverse events were compared with the use of a Mantel–Haenszel test stratified according to enrolling site without adjustment for multiple comparisons. ALT denotes alanine aminotransferase, and AST aspartate aminotransferase.
One patient who died was intubated before receiving placebo and was excluded from the modified intention-to-treat population but included in the safety population.
Other events are listed in detail in Table S6.
Discussion
Our data do not provide support for the concept that early interleukin-6 receptor blockade is an effective treatment strategy in moderately ill patients hospitalized with Covid-19. The hypothesis underlying our trial was that interleukin-6 receptor blockade in patients with disease that had not yet led to intubation would disrupt the cytokine storm associated with Covid-19, thereby preventing the most severe disease consequences. Findings from this randomized, double-blind, placebo-controlled trial indicated that this intervention had no significant effect on the risk of intubation or death, on disease worsening, on time to discontinuation of supplemental oxygen, or on any of the efficacy outcomes we examined. Because of the width of the confidence intervals for our efficacy comparisons, however, we cannot exclude the possibility that tocilizumab treatment is associated with either some benefit or harm in some patients.
Our results stand in contrast to those of multiple open-label trials and nonrandomized case series, some of which have suggested that interleukin-6 receptor blockade has a substantial positive effect on patients with Covid-19. Reported but still unpublished results of a small number of other randomized trials have been discouraging.36,37 The explanation for the failure of tocilizumab to affect clinical outcomes substantially in our trial is not clear. One possibility is that interleukin-6 and other inflammatory proteins that are observed to be present at elevated levels in patients with Covid-19 represent host responses to the infection, similar to the elevations in cytokine levels seen in patients with endocarditis, sepsis, and other infections, rather than components of a self-amplifying inflammatory loop that would benefit from suppression. Regardless of the explanation, our findings in the context of information about other randomized, blinded trials of interleukin-6 receptor blockade largely undermine the concept that this anticytokine approach is a useful treatment strategy for preventing the evolution of Covid-19 from a moderate to a severe state. It remains possible, however, that patient populations that differ from the one targeted by our trial might benefit from interleukin-6 receptor blockade. It is also possible that other anticytokine approaches or studies in which alternative antiinflammatory or antiviral strategies (or combinations of those strategies) are used will show greater efficacy than we have shown. The overall experience with interleukin-6 receptor blockade, however, underscores the point that any such approach must be subjected to randomized, blinded trials. Moreover, such investigations should be performed early in the course of evaluating each strategy, before the adoption of widespread use.38
Our results confirmed the relationship between older age and poor outcomes in Covid-19 but did not identify separate effects of sex, diabetes, obesity, or Hispanic or Latino ethnic group on prognosis. We also confirmed that patients with higher serum interleukin-6 concentrations at baseline were more likely to have a poor outcome.10,11 Although tocilizumab did not show efficacy in this trial, the drug was not associated with excessive high-grade toxic effects in this population, which was characterized by multiple coexisting conditions. Patients treated with tocilizumab had fewer serious infections than those who received placebo.
Our trial has a number of strengths. It was a randomized, double-blind, placebo-controlled trial focusing on hospitalized patients who had not been intubated. The trial population was also ethnically diverse, including substantial numbers of Hispanic or Latino patients.39,40
The trial also has certain weaknesses. The primary event rate we observed was lower than anticipated, perhaps because of evolving standards of care during the trial. Remdesivir became available early in the trial, and general approaches to management were also approved, including strategies to delay intubation, if possible, rather than intubating early. Nevertheless, 12% of the patients in our trial were intubated or died, and we found little evidence that tocilizumab altered any efficacy outcomes. Despite randomization, imbalance in the percentage of older patients between the treatment groups was observed. This had some effect on the estimated treatment effect on the primary outcome, as evidenced by the difference between the unadjusted and adjusted hazard ratios. However, the confidence intervals in both analyses were wide, and the overall conclusions of the unadjusted and adjusted analyses are consistent.
In this randomized, double-blind, placebo-controlled trial, we did not find any efficacy of interleukin-6 receptor blockade for the treatment of hospitalized patients with Covid-19.
Acknowledgments
We thank the patients who participated in the trial, Payal S. Patel, John B. Montana, and the nurses and staff of the Translational Clinical Research Center at the Massachusetts General Hospital.
Protocol
Supplementary Appendix
Disclosure Forms
Data Sharing Statement
This article was published on October 21, 2020, at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Footnotes
Supported by Genentech.
Dr. Stone reports receiving grant support and consulting fees from Principia Biopharma and Roche, grant support from Viela, advisory board fees and consulting fees from Sanofi, and consulting fees from Chemocentryx, Celgene, AbbVie, Chugai, Gruenthal, GlaxoSmithKline, InflaRx, INSmed, Regeneron, and Roivant; Dr. Frigault, receiving consulting fees and advisory board fees from Novartis and Celgene/BMS and consulting fees from Kite/Gilead and Arcellx; Dr. Healy, receiving grant support from Analysis Group, Celgene (Bristol-Myers Squibb), Verily Life Sciences, Novartis, Merck Serono, and Genzyme; Dr. Dougan, receiving grant support from Novartis and Eli Lilly, grant support and consulting fees from Genentech, consulting fees from ORIC Pharmaceuticals, Partner Therapeutics, and Tillotts Pharma, and advisory board fees from Neoleukin Therapeutics; Dr. Kim, receiving advisory board fees from Biomarin; Dr. Neilan, receiving consulting fees from Parexel and Intrinsic Imaging, advisory board fees from AbbVie, Bristol-Myers Squibb, and H3 Biomedicine, and grant support from AstraZeneca; Dr. Unizony, receiving consulting fees from Janssen and Kiniksa and grant support from Genentech; Dr. Bolster, receiving grant support from Amgen, AbbVie, Pfizer, Cumberland, and Corbus Pharmaceuticals, advisory board fees from Gilead Sciences, consulting fees from Custom Learning Designs, honoraria from Merck Manual, and investments in Johnson & Johnson; Dr. Mansour, receiving consulting fees from Vericel, SmartPharm Therapeutics, Pulsethera, GenMark Diagnostics, Globe Life Sciences, and Day Zero Diagnostics, grant support from Thermo Fisher Scientific and Genentech, advisory board fees from Celularity, holding pending patent 14/110,443 on fungal particles, and pending patent 15/999,463 on cellular therapy for infections. No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Centers for Disease Control. United States COVID-19 cases and deaths by state (https://covid.cdc.gov/covid-data-tracker/).
- 2.Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gandhi RT, Lynch JB, Del Rio C. Mild or moderate Covid-19. N Engl J Med. DOI: 10.1056/NEJMcp2009249. [DOI] [PubMed] [Google Scholar]
- 4.Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:811-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 2020;5:802-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ 2020;369:m1966-m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Lu X, Li Y, et al. Clinical course and outcomes of 344 intensive care patients with COVID-19. Am J Respir Crit Care Med 2020;201:1430-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X, Zhao B, Qu Y, et al. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. Clin Infect Dis 2020. April 17 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin A, He Z-B, Zhang S, Zhang J-G, Zhang X, Yan W-H. Early risk factors for the duration of SARS-CoV-2 viral positivity in COVID-19 patients. Clin Infect Dis 2020. April 27 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herold T, Jurinovic V, Arnreich C, et al. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol 2020;146(1):128-136.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Del Valle DM, Kim-Schulze S, Huang HH, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med 2020. August 24 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guaraldi G, Meschiari M, Cozzi-Lepri A, et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol 2020;2(8):e474-e484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A 2020;117:10970-10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campins L, Boixeda R, Perez-Cordon L, Aranega R, Lopera C, Force L. Early tocilizumab treatment could improve survival among COVID-19 patients. Clin Exp Rheumatol 2020;38:578-578. [PubMed] [Google Scholar]
- 17.Rossotti R, Travi G, Ughi N, et al. Safety and efficacy of anti-IL6-receptor tocilizumab use in severe and critical patients affected by coronavirus disease 2019: a comparative analysis. J Infect 2020;81(4):e11-e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Somers EC, Eschenauer GA, Troost JP, et al. Tocilizumab for treatment of mechanically ventilated patients with COVID-19. Clin Infect Dis 2020. July 11 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Potere N, Di Nisio M, Cibelli D, et al. Interleukin-6 receptor blockade with subcutaneous tocilizumab in severe COVID-19 pneumonia and hyperinflammation: a case-control study. Ann Rheum Dis 2020. July 9 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 20.Antony SJ, Davis MA, Davis MG, et al. Early use of tocilizumab in the prevention of adult respiratory failure in SARS-CoV-2 infections and the utilization of interleukin-6 levels in the management. J Med Virol 2020. July 9 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanz Herrero F, Puchades Gimeno F, Ortega García P, Ferrer Gómez C, Ocete Mochón MD, García Deltoro M. Methylprednisolone added to tocilizumab reduces mortality in SARS-CoV-2 pneumonia: an observational study. J Intern Med 2020. June 30 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jordan SC, Zakowski P, Tran HP, et al. Compassionate use of tocilizumab for treatment of SARS-CoV-2 pneumonia. Clin Infect Dis 2020. June 23 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quartuccio L, Sonaglia A, McGonagle D, et al. Profiling COVID-19 pneumonia progressing into the cytokine storm syndrome: results from a single Italian Centre study on tocilizumab versus standard of care. J Clin Virol 2020;129:104444-104444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rojas-Marte G, Khalid M, Mukhtar O, et al. Outcomes in patients with severe COVID-19 disease treated with tocilizumab: a case-controlled study. QJM 2020;113:546-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Price CC, Altice FL, Shyr Y, et al. Tocilizumab treatment for cytokine release syndrome in hospitalized COVID-19 patients: survival and clinical outcomes. Chest 2020. June 15 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pérez-Sáez MJ, Blasco M, Redondo-Pachón D, et al. Use of tocilizumab in kidney transplant recipients with COVID-19. Am J Transplant 2020. July 12 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knorr JP, Colomy V, Mauriello CM, Ha S. Tocilizumab in patients with severe COVID-19: a single-center observational analysis. J Med Virol 2020. June 17 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campochiaro C, Della-Torre E, Cavalli G, et al. Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur J Intern Med 2020;76:43-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colaneri M, Bogliolo L, Valsecchi P, et al. Tocilizumab for treatment of severe COVID-19 patients: preliminary results from SMAtteo COvid19 REgistry (SMACORE). Microorganisms 2020;8:695-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klopfenstein T, Zayet S, Lohse A, et al. Tocilizumab therapy reduced intensive care unit admissions and/or mortality in COVID-19 patients. Med Mal Infect 2020;50:397-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toniati P, Piva S, Cattalini M, et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun Rev 2020;19:102568-102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alattar R, Ibrahim TBH, Shaar SH, et al. Tocilizumab for the treatment of severe coronavirus disease 2019. J Med Virol 2020. May 5 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Della-Torre E, Campochiaro C, Cavalli G, et al. Interleukin-6 blockade with sarilumab in severe COVID-19 pneumonia with systemic hyperinflammation: an open-label cohort study. Ann Rheum Dis 2020;79:1277-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 — preliminary report. N Engl J Med. DOI: 10.1056/NEJMoa2007764. [DOI] [PubMed] [Google Scholar]
- 35.The RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19 — preliminary report. N Engl J Med. DOI: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park M. US trial investigating sarilumab for COVID-19 stopped. MPR. July 7, 2020 (https://www.empr.com/home/news/drugs-in-the-pipeline/sarilumab-interleukin-6-antagonist-covid19-mechanical-ventilation/).
- 37.Roche. Roche provides an update on the phase III COVACTA trial of Actemra/RoActemra in hospitalised patients with severe COVID-19 associated pneumonia. July 29, 2020 (https://www.roche.com/investors/updates/inv-update-2020-07-29.htm).
- 38.North CM, Dougan ML, Sacks CA. Improving clinical trial enrollment — in the Covid-19 era and beyond. N Engl J Med 2020;383:1406-1408. [DOI] [PubMed] [Google Scholar]
- 39.Dorn AV, Cooney RE, Sabin ML. COVID-19 exacerbating inequalities in the US. Lancet 2020;395:1243-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chastain DB, Osae SP, Henao-Martínez AF, Franco-Paredes C, Chastain JS, Young HN. Racial disproportionality in Covid clinical trials. N Engl J Med 2020;383(9):e59-e59. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.