Abstract
Cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors in combination with endocrine therapy are a preferred treatment option for premenopausal and postmenopausal women with hormone receptor–positive (HR+), human epidermal growth factor receptor 2–negative (HER2–) metastatic breast cancer (mBC). Palbociclib is a potent, first-in-class oral inhibitor of CDK4/6. To provide optimal care to patients with HR+/HER2– mBC receiving palbociclib, advanced practitioners require a thorough understanding of the efficacy and adverse event (AE) profile of palbociclib as well as the diverse characteristics and support needs of patients eligible for palbociclib treatment. This Grand Rounds uses two hypothetical patient scenarios to illustrate core issues in the management of premenopausal and postmenopausal patients receiving palbociclib-based therapy for mBC. In addition to providing an overview of key efficacy and safety data, each case offers practical guidance on providing individualized, patient-centered care, the identification and management of treatment-related AEs, management of concomitant medications, and best practices to promote adherence to therapy.
CASE STUDY 1: HAZEL
Hazel (Figure 1) was initially diagnosed with breast cancer at age 65. After completing 5 years of adjuvant therapy with letrozole, Hazel considered herself a cancer survivor. She and her husband had always had an active social life, and Hazel went for daily walks with a group of her friends, two of whom were also breast cancer survivors.
Figure 1.
Hazel: history, assessment, and treatment plan. CAP = chest, abdomen, and pelvis; CT = computed tomography; ER+ = estrogen receptor–positive; HER2– = human epidermal growth factor receptor 2-negative; OTC = over the counter; po = orally; PR+ = progesterone receptor–positive.
Hazel’s recurrence was discovered after she went to her primary care physician for back pain that was interfering with her ability to participate in the walking group and a persistent, nonproductive cough. The diagnosis came just 4 months after the death of Hazel’s husband of 48 years. Still adjusting to that loss and with living alone for the first time in her life, Hazel was shocked that the cancer had returned after she had “done everything I was supposed to do,” and sank into what she called “a funk.”
Hazel’s primary care physician had prescribed venlafaxine shortly after the death of her husband, but Hazel had become increasingly withdrawn, pulling back from most of her social activities and spending much of her time home alone. Her daughter Laura lives approximately 30 minutes away and spends as much time as she can with her mother but is worried that Hazel may not be eating well or taking her medications as prescribed. Concerned that Hazel also seems to be getting more forgetful, Laura recently gave her mother an over-the-counter ginkgo biloba supplement in the hope it might help with her memory, and Hazel has been taking it every night.
CASE STUDY 2: GRACE
Young age is a well-established independent risk factor for more aggressive breast cancer and worse outcomes (Anders, Johnson, Litton, Phillips, & Bleyer, 2009; Fredholm et al., 2009; Fredholm et al., 2016; Kataoka et al., 2016), and premenopausal women who present with early stage disease frequently progress to mBC (Fredholm et al., 2016; Johnson, Anders, Litton, Ruddy, & Bleyer, 2018; Kataoka et al., 2016; Sibylle Loibl et al., 2015). Nonetheless, for Grace, a premenopausal woman with 3 children between the ages of 5 and 14 and a full-time job, discovering that her cancer had progressed was an unexpected blow (Figure 2).
Figure 2.
Grace: history, assessment, and treatment plan. CAP = chest, abdomen, and pelvis; CT = computed tomography; ER+ = estrogen receptor–positive; HER2– = human epidermal growth factor receptor 2–negative; IM = intramuscular; IV = intravenous; PR+ = progesterone receptor–positive; SC = subcutaneous.
The fact that Grace had no family history of breast cancer, no genetic mutations associated with increased breast cancer risk, and had “worked so hard” to adhere to tamoxifen despite struggling with depressive symptoms, hot flashes, and insomnia made the diagnosis feel “random and unfair.” Now that the cancer has become metastatic, Grace is deeply concerned that the side effects of treatment will interfere with her ability to continue in her role as wife and mother. Her insomnia has worsened and she feels “teary a lot of the time.” In an effort to manage these symptoms, Grace has been taking over-the-counter sleep aids and on the recommendation of a friend recently started a St. John’s wort supplement. She had not informed her physician or care team of these changes.
More than 150,000 women in the United States are currently living with metastatic breast cancer (mBC; Mariotto, Etzioni, Hurlbert, Penberthy, & Mayer, 2017). Nearly half of these women are between 50 and 69 years of age, just over 30% are aged 70 years or older, and less than 14% are younger than 50 years of age (Figure 3; Mariotto et al., 2017). Because there are currently no curative treatment options for mBC, the goal of therapy across all age groups is to extend survival while maintaining the quality of patients’ day-to-day lives (National Comprehensive Cancer Network [NCCN], 2020).
Figure 3.
Age distribution of women living with mBC in the United States (2017, estimated; Mariotto et al., 2017). mBC = metastatic breast cancer.
Approximately 60% to 70% of patients with mBC have hormone receptor–positive (HR+), human epidermal growth factor receptor 2–negative (HER2–) tumors (Gong, Liu, Ji, Hu, & Shao, 2017; Howlader et al., 2014; Turner, Neven, Loibl, & Andre, 2016). In this setting, sequential endocrine therapy (ET) is the mainstay of treatment regardless of age or sex, with cytotoxic chemotherapy reserved for patients with symptomatic visceral metastases or disease that is refractory to endocrine agents (Cardoso et al., 2018; NCCN, 2020). Although generally well tolerated, the long-term efficacy of ET is limited by the emergence of endocrine resistance. Estrogen receptor–positive breast tumors have a risk of recurrence of approximately 2% per year (Cadoo, Fornier, & Morris, 2013), and retrospective analyses indicate that about 60% of patients diagnosed with stage I to III HR+/HER2– breast cancer eventually experience distant metastatic recurrence (Malmgren, Hurlbert, Atwood, & Kaplan, 2019).
Amplification of the cyclin D–cyclin-dependent kinase 4 and 6 (CDK4/6)–retinoblastoma pathway is common in HR+ breast cancer and is associated with uncontrolled proliferation of tumor cells (Finn, Aleshin, & Slamon, 2016; Finn et al., 2009; Sammons, Topping, & Blackwell, 2017). Palbociclib (Pfizer Inc, 2019a, 2019b) is a first-in-class oral CDK4/6 inhibitor that acts against this cell cycle dysregulation by inducing temporary cell cycle arrest (Hu et al., 2016; Thangavel et al., 2011). Palbociclib currently is indicated for the treatment of adults with HR+/HER2– advanced or metastatic breast cancer, in combination with an aromatase inhibitor as initial endocrine-based therapy in postmenopausal women and men, or in combination with fulvestrant in patients with disease progression following ET (Pfizer Inc, 2019a; NCCN, 2020).
After the accelerated approval of palbociclib in 2015, CDK4/6 inhibitors in combination with ET rapidly became a preferred treatment option for HR+/HER2– mBC (NCCN, 2020). As of June 2020, palbociclib had been prescribed to over 310,000 patients worldwide, including more than 125,000 in the United States (M. Borland and T. Johnson, personal communication, September, 2020).
The introduction of palbociclib has improved clinical outcomes in patients with HR+/HER2– mBC compared with single-agent ET. However, some of the adverse events (AEs) and drug interactions associated with palbociclib differ from those associated with ET or cytotoxic chemotherapies, and benefit from prompt recognition and management to ensure adherence and to optimize outcomes (Cazzaniga et al., 2019).
Because palbociclib is approved for use in premenopausal and postmenopausal patients, the treatment goals, supportive needs, and risk of toxicities and drug interactions for any given patient may vary depending on where the patient falls on the age spectrum. In addition, like all oral agents, palbociclib is taken outside of the controlled setting of the infusion center. This places the responsibility for proper administration in the hands of the patient, which can be challenging for individuals on multiple medications and may increase the risk of nonadherence (Thomas, Teena, Criner, & Nguyen, 2019).
As clinicians who play key roles not only in patient care but also in training and mentoring members of the oncology care team, advanced practitioners (APs) are in a unique position to improve adherence and to optimize outcomes in patients receiving palbociclib. The hypothetical case studies—Hazel, a 74-year-old postmenopausal woman, and Grace, a 36-year-old premenopausal woman—represent two distinct patient types on the spectrum of HR+/HER2– mBC. The patient cases described in this publication are fictional and do not represent events, or a response, from actual patients. The authors developed these fictional cases for educational purposes only. Although these cases are fictional, they provide useful insights into the clinical management of elderly and premenopausal patients receiving palbociclib for HR+/HER2– mBC and offer practical guidance on providing evidence-based, patient-centered care in real-world treatment settings.
CASE STUDY 1 CONTINUED: HAZEL
Developing the Treatment Plan
When deciding on a treatment plan for older patients such as Hazel, it is important to take into account the wide range of clinical and psychosocial factors that can impact patients’ adherence—and response—to treatment. Care is best provided by a multidisciplinary team that can work in partnership with the patient to develop a plan that addresses her treatment goals and provides the support needed to adhere to the agreed-on treatment plan (Loh et al., 2018).
The oncology care team first conducted a geriatric screening to assess Hazel’s physical limitations, cognitive function, medications, overall mental health, and social support. Although Hazel was generally fit and able to perform all activities of daily living, she was on multiple medications, lived alone, and was still grieving the loss of her husband. The team therefore decided it was important that Hazel’s daughter Laura be involved in the treatment decisions and care. The team also kept Hazel’s primary care provider apprised of every aspect of the treatment plan and connected Hazel with supportive resources in her community.
In the pivotal PALOMA-2 and PALOMA-3 trials (Figure 4, Table 1), 37% of all postmenopausal participants treated with palbociclib were ≥ 65 years, and 10% were ≥ 75 years (Rugo et al., 2018). A pooled analysis of outcomes in older patients enrolled in the full PALOMA trial program found that palbociclib-based combination therapy improved progression-free survival (PFS) across all age groups, with no significant deterioration in well-being or quality of life (Rugo et al., 2018). In addition, real-world data from a cohort of geriatric patients treated with palbociclib at the MD Anderson Cancer Center between January 2015 and October 2017 (N = 605; of whom 160 were aged > 65 years) found no significant differences in palbociclib-related toxicities between patients ≥ 65 years old and those ≥ 70 years old, although patients aged ≥ 70 years were more likely to require early dose reductions and dose delays (Clifton et al., 2019).
Figure 4.
Palbociclib in premenopausal/perimenopausal and postmenopausal mBC: (A) PALOMA-2 (Finn, Martin, et al., 2016; Rugo et al., 2019) and (B) PALOMA-3 study designs (Turner et al., 2015; Turner et al., 2018). CBR = clinical benefit response (confirmed complete response, partial response, or stable disease for ≥ 24 weeks); DoR = duration of response; HER2– = human epidermal growth factor receptor 2-negative; HR+ = hormone receptor-positive; IM = intramuscular; mBC = metastatic breast cancer; NSAI = nonsteroidal aromatase inhibitor; OR = objective response (confirmed complete response or partial response); OS = overall survival; PFS = progression-free survival (time to radiologically confirmed progression per RECIST v1.1 or death during the study); PROs = patient-reported outcomes; qd = once daily; q4w = every 4 weeks; RECIST = Response Evaluation Criteria in Solid Tumors.aAll received goserelin ≥ 4 weeks before study treatment. bAdministered on days 1 and 15 of cycle 1 and on day 1 of each subsequent cycle.
Table 1. Palbociclib in Postmenopausal Patients: PALOMA-2 and PALOMA-3.
| PALOMA-2 (PAL+LET vs. PBO+LET) | PALOMA-3 (PAL+FUL vs. PBO+FUL) | |
|---|---|---|
| Primary analysis | ||
| Postmenopausal women enrolled and treated with PAL, n | 444 | 275 |
| < 65 y, n | 263 | 189 |
| ≥ 65 y, n | 181 | 86 |
| Median PFS in postmenopausal patients, mo, experimental vs. control (HR [95% CI]) | 24.8 vs. 14.5 (0.58 [0.46–0.72]) p < 0.001 | 9.9 vs. 3.9 (0.45 [0.34–0.59]) p < 0.0001 |
| Extended analysis | ||
| Median PFS in postmenopausal patients, mo, experimental vs. control (HR [95% CI]) | 27.6 vs. 14.5 (0.56 [0.46–0.69]) p < .0001 | Not published |
| Median OS in postmenopausal patients, mo, experimental vs. control (HR [95% CI]) | Data not mature | 34.8 vs. 27.1 (0.73 [95% CI, 0.57–0.95]) |
Note. FUL = fulvestrant; HR = hazard ratio; LET = letrozole; OS = overall survival; PAL = palbociclib; PBO = placebo; PFS = progression-free survival (time to radiologically confirmed progression per RECIST v1.1 or death during the study); RECIST = Response Evaluation Criteria in Solid Tumors. Data from Finn et al. (2016); Rugo et al. (2019); Loibl et al. (2017); Turner et al. (2015); Turner et al. (2018).
Based on these data and current evidence-based guidelines (NCCN, 2020), Hazel’s treatment team recommended palbociclib at a dose of 125 mg in combination with the aromatase inhibitor anastrozole (Arimidex), as she had already been treated with adjuvant letrozole. She was also prescribed denosumab (Xgeva) 120 mg subcutaneously every 4 weeks to maintain her bone mineral density and reduce the risk of fractures (Amgen, 2019; Desautels, Harlos, & Jerzak, 2019; NCCN, 2020; Figure 1).
Palbociclib is metabolized primarily by the cytochrome P450 (CYP) 3A pathway and is itself a weak inhibitor of CYP3A4 (Bellet et al., 2019). Although no interactions have been identified between palbociclib and goserelin or ETs, there is a risk of interactions with inhibitors or inducers of CYP3A. Coadministration with inhibitors of CYP3A (e.g., grapefruit juice, some calcium channel blockers, ginkgo biloba) should be avoided because it can increase exposure to palbociclib and increase the risk of toxicity. If unavoidable, palbociclib dosage should be reduced to 75 mg for at least 5 half-lives of elimination of the CYP3A inhibitor after its withdrawal. Conversely, coadministration of palbociclib with a strong inducer of CYP3A (e.g., St. John’s wort) can decrease drug exposure and reduce efficacy (Bellet et al., 2019; Cazzaniga et al., 2019; Pfizer Inc, 2019a).
Because Hazel was receiving medications for hypertension, hypothyroidism, and depression—as well as various OTC preparations—the team conducted a medication reconciliation in consultation with the cancer center’s pharmacist. The pharmacist determined that Hazel’s antidepressant and hypertension medication had the potential to interact with palbociclib and recommended that she be switched from venlafaxine to duloxetine (Cymbalta) and from amlodipine to quinapril (Accupril; Figure 1). She instructed Hazel and her daughter to discontinue the ginkgo biloba and to consult with the treatment team before starting any supplement or herbal medication because even “natural” products can have significant drug interactions. Because it is recommended that palbociclib capsules be taken with food at approximately the same time each day (Bellet et al., 2019; Cazzaniga et al., 2019; Pfizer Inc, 2019a), the pharmacist also sat down with Hazel and her daughter to identify when Hazel typically ate her meals and timed treatment accordingly.
In discussing the treatment plan, Hazel expressed concerns about “losing track” of the 3-week on/1-week off treatment schedule. The pharmacist explained that one advantage of the palbociclib plus letrozole regimen is that each agent is taken only once daily at the same time. She counseled Hazel and her daughter on various techniques for tracking medications, and noted that palbociclib can be supplied in bottles of 21 capsules, thus reinforcing the 21-day dosing. To assist Hazel in keeping track of her medications, the team suggested that Hazel use a pillbox for her palbociclib capsules and letrozole tablets and to set up calendar reminders or alerts on her phone. The team also planned frequent “refreshers” to reinforce appropriate medication practices. Because depression is a risk factor for nonadherence (Bellet et al., 2019; Bender et al., 2014), the team also provided Hazel with information on a local bereavement group and a referral to the cancer center’s psychologist.
Hazel’s Treatment Course
The addition of any targeted agent to ET increases the incidence of AEs, including those that are grade 3 to 4 (Cazzaniga et al., 2019). With palbociclib, the overall incidence of AEs tends to peak within the first 6 months of treatment, with no evidence of cumulative or delayed toxicities (Diéras et al., 2018).
A recent long-term analysis of combined safety data from the PALOMA trial program (N = 872) provides a comprehensive overview of the safety of palbociclib plus ET (Table 2; Diéras et al., 2018). The most common all-grade nonhematologic AEs associated with palbociclib are infections, fatigue, nausea, and stomatitis (Diéras et al., 2018). Most nonhematologic AEs are grade 1 or 2 in severity and can be managed without dose adjustments or interruptions; higher grade AEs can often be resolved with temporary treatment delays or dose reductions (Cazzaniga et al., 2019).
Table 2. Adverse Events Reported by ≥ 15% of Palbociclib-Treated Patients in the PALOMA Trials.
| Adverse event | Percentage reportinga |
|||
|---|---|---|---|---|
| All grades | Grade 1/2 | Grade 3 | Grade 4 | |
| Anyb,c | 99.0 | 21.3 | 62.2 | 13.8 |
| Hematologic | ||||
| Neutropeniad | 80.6 | 15.3 | 55.3 | 10.1 |
| Leukopeniad | 45.2 | 18.5 | 26.1 | 0.6 |
| Anemiad | 27.6 | 23.1 | 4.4 | 0.2 |
| Thrombocytopeniad | 19.0 | 17.1 | 1.6 | 0.3 |
| Nonhematologic | ||||
| Infectionsd | 54.7e | 49.4 | 4.5 | 0.7 |
| Fatigue | 39.2 | 36.7 | 2.3 | 0.2 |
| Nausea | 34.2 | 33.8 | 0.3 | 0 |
| Stomatitisd | 28.9 | 28.2 | 0.7 | 0 |
| Alopecia | 25.9 | 25.9 | f | f |
| Arthralgia | 25.6 | 24.8 | 0.8 | 0 |
| Diarrhea | 24.5 | 23.5 | 1.0 | 0 |
| Headache | 22.5 | 22.1 | 0.3 | 0 |
| Cough | 21.6 | 21.6 | 0 | f |
| Back pain | 19.2 | 17.8 | 1.3 | 0.1 |
| Constipation | 19.2 | 18.9 | 0.2 | 0 |
| Hot flush | 19.0 | 19.0 | 0 | f |
| Vomiting | 17.1 | 16.6 | 0.5 | 0 |
| Rashd | 16.5 | 15.8 | 0.7 | 0 |
| Decreased appetite | 15.8 | 15.0 | 0.8 | 0 |
| Pain in extremity | 14.0 | 13.9 | 0.1 | 0 |
Note. AE = adverse event; CTCAE = Common Terminology Criteria for Adverse Events; MedDRA = Medical Dictionary for Regulatory Activities; PT = preferred term. Information from Diéras et al., 2018.
Grade 5 events were reported for 15 patients (1.7%) as follows: disease progression (6 events), pulmonary embolismd, general physical health deterioration, hepatic failure, acute myocardial infarction, cardiogenic shock, cardiopulmonary failure, cardiovascular insufficiency, death, disseminated intravascular coagulation, and respiratory failure (1 event each).
AEs were graded in accordance with the maximum CTCAE (v4.0) and MedDRA (v19.0) coding dictionary.
Includes data up to 28 days after last dose of study drug.
Cluster terms: Anemia includes the PTs anemia, hematocrit decreased, and hemoglobin decreased; infections includes any reported PT of the system organ class infections and infestations; leukopenia includes the PTs leukopenia and white blood cell count decreased; neutropenia includes the PTs neutropenia and/or neutrophil count decreased; pulmonary embolism includes the PTs pulmonary artery thrombosis, pulmonary embolism, and pulmonary thrombosis; rash includes the PTs dermatitis, dermatitis acneiform, rash, rash erythematous, rash maculopapular, rash papular, rash pruritic, and toxic skin eruption; stomatitis includes the PTs aphthous stomatitis, cheilitis, glossitis, glossodynia, mouth ulceration, mucosal inflammation, oral pain, oropharyngeal discomfort, oropharyngeal pain, and stomatitis; thrombocytopenia includes the PTs platelet count decreased and thrombocytopenia.
A single missing or unknown infection occurred that is not included in the total.
Maximum CTCAE grade does not apply.
Neutropenia was the most commonly reported AE with palbociclib in the PALOMA clinical trial program (reported in 81% of patients). The incidence of any-grade febrile neutropenia, by contrast, was low (1.6%) and declined over the course of the treatment period (Diéras et al., 2018). Although neutropenia is also a hallmark of cytotoxic chemotherapy, the underlying mechanisms of palbociclib-induced neutropenia differ from those seen with chemotherapy. Unlike chemotherapeutic agents—which cause permanent DNA damage and death in bone marrow cells—palbociclib induces a temporary cell cycle arrest that is rapidly reversible when the drug is removed (Cazzaniga et al., 2019; Hu et al., 2016). As a result, palbociclib-induced myelosuppression is manageable with regular monitoring—a complete blood count with differential should be conducted before the start of every cycle, on day 15 of the first 2 cycles, and as clinically indicated (Pfizer Inc, 2019a)—coupled with dose interruptions, dose reductions, or treatment cycle delays as needed (Figure 5; McShane, Wolfe, & Ryan, 2018; Spring, Zangardi, Moy, & Bardia, 2017).
Figure 5.
Dose modifications in the management of palbociclib-related AEs (Pfizer, 2019a; Spring et al., 2017; Thill & Schmidt, 2018). AE = adverse event; ANC = absolute neutrophil count; CBC = complete blood count.aPerform CBC with differential before start of every cycle, on day 15 of first 2 cycles, and as clinically indicated.bApplies to all hematologic AEs except lymphopenia (unless associated with clinical events, e.g., opportunistic infections).
Analyses of data from the PALOMA trial program have shown that AE-related dose modifications (reductions, interruptions, or cycle delays) do not significantly alter the efficacy of palbociclib plus endocrine therapy (Diéras et al., 2017; Sun et al., 2017; Verma et al., 2016; Zheng et al., 2017). The team stressed this point when counseling Hazel and her daughter about the potential side effects of treatment and the possibility of having to adjust her dose. They encouraged Hazel to monitor her temperature daily (to detect possible febrile neutropenia) and to promptly report any symptoms or side effects she might experience.
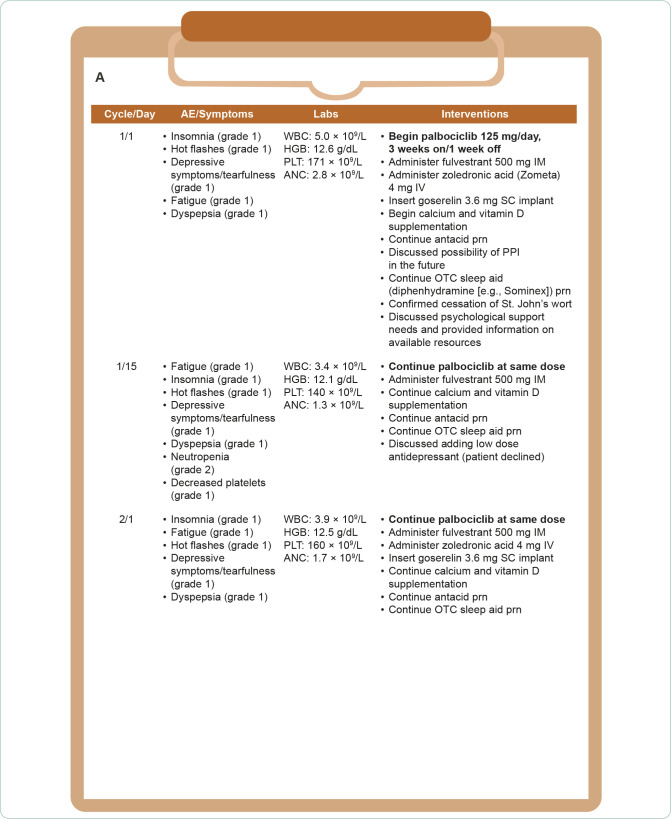
Hazel developed grade 3 neutropenia, grade 3 leukopenia, and grade 1 decreased platelets on day 15 of the first treatment cycle. On recheck at day 22 the neutropenia had worsened to grade 4 and leukopenia persisted at grade 3 (Figure 6A In accordance with evidence-based guidelines, the team delayed the start of cycle 2 for 1 week, at which point Hazel’s absolute neutrophil and white blood cell counts had recovered to grade 1. Cycle 2 was started and palbociclib was reduced to 100 mg/day. Hazel was able to complete 4 treatment cycles with no further grade ≥ 3 hematologic AEs (Figure 6B).
Figure 6A.
Hazel’s treatment course: (A) Cycle 1/Day 1–Cycle 2/Day 1; (B) Cycle 2/Day 15–Cycle 4/Day 1.AE = adverse event; ANC = absolute neutrophil count; CBC = complete blood count; HGB = hemoglobin; PLT = platelets; po = by mouth; prn = as needed; SC = subcutaneous; WBC = white blood cell count.aDelayed 1 week due to prolonged neutropenia and leukopenia.
Figure 6B.
Hazel’s treatment course: (A) Cycle 1/Day 1–Cycle 2/Day 1; (B) Cycle 2/Day 15–Cycle 4/Day 1.AE = adverse event; ANC = absolute neutrophil count; CBC = complete blood count; HGB = hemoglobin; PLT = platelets; po = by mouth; prn = as needed; SC = subcutaneous; WBC = white blood cell count.
Hazel began experiencing gastrointestinal AEs approximately 2 weeks after first starting treatment (Figure 6A). Her nausea (grade 2) was relieved with the addition of an antiemetic and her diarrhea was responsive to loperamide. During cycle 2 Hazel developed grade 1 stomatitis which was helped by dietary modification, improved hydration, and use of a soft toothbrush and soothing oral rinses. By the start of cycle 3 (Figure 6B), Hazel reported that although things “weren’t perfect,” the medication and lifestyle changes had made the symptoms manageable. Even though Hazel still missed her husband very much, the bereavement group had helped her feel more ready to engage with the world. She also recently joined a yoga class for cancer survivors at the community center in an effort to get more exercise without hurting her back.
During the course of Hazel’s treatment, a new tablet formulation of palbociclib became available for use in the United States following approval by the U.S. Food & Drug Administration on November 1, 2019 (FDA, 2019). No change was made to the active ingredient in palbociclib, the dosage strengths available (125 mg, 100 mg, 75 mg), or the dosing schedule (Pfizer Inc, 2019b). The efficacy and safety data in the tablet label are based on the clinical trials conducted with the capsule formulation. The benefit-risk profile of palbociclib tablets is expected to be similar to that of the approved capsules. This new film-coated tablet formulation, when administered with or without food, was shown to be bioequivalent to the palbociclib capsule administered with food. Administration of the tablet formulation with or without food or gastric acid-reducing agents also did not have a clinically meaningful effect on palbociclib drug exposure. Thus, the tablet formulation offers patients the flexibility to take palbociclib with or without food and concomitantly with proton pump inhibitors/antacids regardless of fasting status. Additionally, the tablet formulation does not contain lactose (dairy) or gelatin, which may be important for patients with tolerability issues and/or dietary preferences. The tablets are dispensed in blister packs (three weekly packs per cycle) that are designed to help patients remember their daily dose and stay on track with their treatment. Furthermore, the new blister packaging for the palbociclib tablet formulation was tested with 100 senior citizens, all of whom were able to open the packaging (data on file). The tablets must be stored in the blister pack until it is time for the dose to be taken.
Hazel was switched from palbociclib capsules to palbociclib tablets at the beginning of her fourth treatment cycle. The pharmacist showed her the blister pack and provided her with information on important and relevant changes related to this formulation; the tablets can be taken with or without food, and they must be kept in their original blister pack. She can no longer place her palbociclib into a pillbox but she can utilize the design of the blister pack to help her keep track of her dosing.
CASE STUDY 2 CONTINUED: GRACE
Developing the Treatment Plan
The PALOMA-3 trial (Figure 4) was the basis of the FDA’s approval of palbociclib with fulvestrant for the treatment of advanced HR+/HER2– mBC after progression on prior ET. Premenopausal/perimenopausal women made up 21% (n = 108) of the overall trial population: 8% were ≤ 40 years old, and 31% were ≤ 50 years (Table 3; Loibl et al., 2017). Some patients had been heavily pretreated: one-third had received prior chemotherapy for mBC, and more than half had ≥ 2 prior lines of ET (Cristofanilli et al., 2016; Loibl et al., 2017). In addition, all premenopausal patients were required to start subcutaneous goserelin, a luteinizing hormone-releasing hormone agonist, ≥ 4 weeks before study treatment (Loibl et al., 2017).
Table 3. Palbociclib in Premenopausal Patients: PALOMA-3.
| Primary analysis | |
| Premenopausal/perimenopausal women enrolled and treated with PAL, n | 72 |
| ≤ 40 y, n | 25 |
| > 40–50 y, n | 29 |
| > 50 y, n | 18 |
| Median PFS in premenopausal patients, mo, experimental vs. control (PBO+FUL) (HR [95% CI]) | 9.5 vs. 5.6 (0.50 [0.29–0.87] p = .013 |
| Extended analysis | |
| Median PFS in premenopausal patients, mo, experimental vs. control (HR [95% CI]) | Not published |
| Median OS in premenopausal patients, mo, experimental vs. control (HR [95% CI]) | 38.0 vs. 38.0 (1.07 [0.61–1.86]) |
Note. FUL = fulvestrant; HR = hazard ratio; OS = overall survival; PAL = palbociclib; PBO = placebo; PFS = progression-free survival (time to radiologically confirmed progression per RECIST v1.1 or death during the study); RECIST = Response Evaluation Criteria in Solid Tumors. Data from Loibl et al. (2017); Turner et al. (2015); Turner et al. (2018).
Median PFS among premenopausal/perimenopausal women in PALOMA-3 was 9.5 months in the palbociclib arm and 5.6 months in the placebo arm (HR, 0.50; 95% CI: 0.29–0.87; p = 0.013; Loibl et al., 2017). No statistically significant difference between the palbociclib group and the placebo group in median OS was seen in the small population of premenopausal patients in the PALOMA-3 trial; however, a trend towards improved OS was seen in the overall population (palbociclib group, 34.9 months; placebo group, 28.0 months; p = 0.09; Turner et al., 2018).
No clinically relevant drug interactions have been observed between palbociclib and goserelin, and the frequency of all-grade and serious AEs was similar with palbociclib in both premenopausal and postmenopausal women, as were the rates of dose interruptions (90% and 82%, respectively), dose reductions (42% and 32%, respectively), and AE-related discontinuations (5.6% and 4.7%, respectively; Loibl et al., 2017).
Based on these findings and in accordance with clinical guidelines, the oncology care team recommended that Grace be treated with palbociclib 125 mg orally in combination with fulvestrant (Faslodex) intramuscularly, and with a goserelin (Zoladex) implant subcutaneously every 28 days in order to suppress ovarian function (Figure 2). Because palbociclib is associated with embryo-fetal toxicity, Grace was counseled on the importance of using effective contraception while on treatment and for ≥ 3 weeks after the last dose of palbociclib (Pfizer Inc, 2019a). Because Grace had multiple sites of bone metastases, the treatment plan also included 4 mg zoledronic acid (Zometa), administered intravenously on day 1 of each cycle, with oral calcium (500 mg/day) and vitamin D (400 IU/day) supplementation (Figure 2; Desautels et al., 2019; Novartis Pharmaceuticals Corporation, 2019).
When reviewing Grace’s medication history—including her use of OTC medications and supplements—the team learned of Grace’s recent use of St. John’s wort. Because of the known potential for interaction with palbociclib, Grace was instructed to stop taking the supplement. The team pharmacist also provided education on potential drug/food interactions with palbociclib and the importance of speaking with the team before initiating any supplements or OTC medications. Noting that Grace has been dealing with frequent bouts of gastroesophageal reflux (GERD) and regularly takes antacids (Figure 2), the pharmacist also counseled Grace on lifestyle modifications that could reduce the risk of GERD—including dietary changes and reduced alcohol and coffee intake—and stressed the importance of taking her palbociclib capsules with food.
Grace’s Treatment Course
Grace experienced grade 2 neutropenia during her first 2 treatment cycles, with no dose interruptions or adjustments required (Figure 7A). She did, however, experience continuing difficulties with menopausal symptoms (hot flashes, insomnia, tearfulness) and dyspepsia and became very distressed when she noticed that her hair was thinning (grade 1) midway through cycle 2 (Figure 7B).
Figure 7A.
Grace’s treatment course: (A) Cycle 1/Day 1–Cycle 2/Day 1; (B) Cycle 2/Day 15–Cycle 4/Day 1. AE = adverse event; ANC = absolute neutrophil count; HCT = hematocrit; HGB = hemoglobin; IM = intramuscular; IV = intravenous; OTC = over the counter; PLT = platelets; po = by mouth; PPI = proton pump inhibitor; prn = as needed; SC = subcutaneous; WBC = white blood cell count.
Figure 7B.
Grace’s treatment course: (A) Cycle 1/Day 1–Cycle 2/Day 1; (B) Cycle 2/Day 15–Cycle 4/Day 1. AE = adverse event; ANC = absolute neutrophil count; HCT = hematocrit; HGB = hemoglobin; IM = intramuscular; IV = intravenous; OTC = over the counter; PLT = platelets; po = by mouth; PPI = proton pump inhibitor; prn = as needed; SC = subcutaneous; WBC = white blood cell count.
Despite initial reluctance to seek counseling or consider antidepressant therapy, Grace eventually accepted a referral to the cancer center’s psychologist and filled a prescription for duloxetine. She was also comforted when the medical team explained that the hair thinning seen with palbociclib tends to be mild and rarely noticeable to others; noting that fewer than 3% of patients in the PALOMA trial program experienced grade 2 alopecia (i.e., hair loss of ≥ 50% of what is normal for the individual and is apparent to others; Finn, Martin, et al., 2016; Pfizer Oncology, 2015; Turner et al., 2015; US Department of Health and Human Services, 2009). Like Hazel, by the start of cycle 3, Grace felt that the most troubling symptoms had stabilized and that she was able to anticipate and manage side effects when they did occur. Of note, although Grace had initially insisted she didn’t have time to see a counselor, by the beginning of cycle 4 she admitted that it was helping and that she was feeling less overwhelmed and worried about the future.
Grace switched the formulation of her palbociclib from capsules to tablets at the start of her third cycle. Her oncology AP met with Grace to review the similarities and differences between the palbociclib capsules and tablets and what they meant for her. With the tablet formulation, Grace no longer had to take her dose with meals as clinical studies indicated multiple doses of the proton pump inhibitor rabeprazole coadministered with palbociclib under overnight fasted conditions did not affect the absorption of palbociclib (Pfizer Inc, 2019b). Additionally, histamine 2-receptor antagonists and local antacids are not expected to have an effect on palbociclib exposure (Pfizer Inc, 2019b). Grace completed 4 cycles of therapy without any interruptions or dose reductions (Figure 7B).
DISCUSSION
Although there is still no curative therapeutic option for mBC, targeted therapies and combination regimens, such as palbociclib plus ET, have improved outcomes for women carrying this diagnosis. Of the estimated 150,000 women in the United States who are living with mBC, approximately 1 in 3 is more than 5 years out from diagnosis, and 17% have survived longer than 10 years (Mariotto et al., 2017). To help patients live well with mBC, oncology treatment teams must be prepared to work with patients to manage treatment- and disease-related symptoms. The selection of various therapies to manage symptoms is an ongoing, repeated process in which patients and providers decide together when to stay on treatment and when to modify the treatment plan. The cases presented here describe this process in two hypothetical patients receiving palbociclib-based combination therapy for mBC.
Subgroup analyses from the PALOMA trials and real-world data have demonstrated that the efficacy and safety of palbociclib-based combination therapy is consistent regardless of age or menopausal status (Clifton et al., 2019; Cristofanilli et al., 2016; Pfizer Inc, 2019a; Loibl et al., 2017; Rugo et al., 2018). As a result, 74-year-old Hazel and 36-year-old Grace were both prescribed the recommended palbociclib starting dose of 125 mg (Pfizer Inc, 2019a).
Good communication is particularly important with patients who are receiving oral therapies such as palbociclib because oral agents shift much of the responsibility for administering medication and identifying/monitoring AEs from the treatment team to the patient (Krikorian et al., 2019). Because adherence to oral oncology medications is variable at best—in some studies, nearly half of the patients reported some level of nonadherence (Bender et al., 2014; Thomas et al., 2019)—establishing a rapport and proactively addressing potential barriers to adherence is crucial to an effective treatment plan (Bender et al., 2014; Krikorian et al., 2019). Treatment teams need to be aware of the diverse factors that can increase the risk of nonadherence and be prepared to implement strategies that will help patients feel they are partners in the care process (Krikorian et al., 2019; Miaskowski, Shockney, & Chlebowski, 2008).
Adverse effects are among the most frequently reported reasons for nonadherence to ET (Miaskowski et al., 2008). Clinical trial data indicate that palbociclib-related toxicities can be effectively managed with a combination of support and dose modifications. In the aforementioned long-term analysis of safety data from the PALOMA trial program, 63% of patients remained at the full 125-mg/day dose over a 3-year observation period, and just 8.3% of patients permanently discontinued palbociclib (Diéras et al., 2018). In addition, in the pivotal PALOMA-2 and PALOMA-3 trials, PFS was similar among palbociclib-treated patients who required dose reductions vs. those with no dose reductions, suggesting that dose modifications can reduce the severity and incidence of AEs without reducing treatment efficacy (Dieras et al., 2019; Verma et al., 2016).
Early identification of treatment-related AEs requires the combined efforts of patients and providers. From a provider perspective, it is important to recognize and address patients’ current coping strategies—including self-medication with OTC medications/supplements, herbals, or alcohol—which may necessitate medication changes and/or referral to supportive services. In the hypothetical cases presented here, Grace was using OTC sleep aids, St. John’s wort, and alcohol to address her depression and insomnia, whereas Hazel was taking ginkgo biloba. In both cases, the oncology pharmacist on the team advised discontinuation of the products with potential drug interactions and steered the patients toward more effective treatments and coping mechanisms.
Patient and provider vigilance is particularly important when monitoring for palbociclib-related neutropenia. The teams caring for Grace and Hazel made certain both women understood the risk of neutropenia with palbociclib—including how it differs from chemotherapy-induced neutropenia—and encouraged them to monitor their temperature and report any instances of fever above 100.4°F (Sammons et al., 2017; Thill & Schmidt, 2018; US Department of Health and Human Services, 2017). In keeping with clinical trial and real-world evidence of an increased risk of neutropenia in older patients (Clifton et al., 2019), Hazel experienced persistent neutropenia and leukopenia, which were addressed through treatment delay and dose modification. Nonhematologic AEs in both patients were effectively addressed through supportive measures, including medication and lifestyle changes.
The hypothetical cases of Hazel and Grace describe an effective, multidisciplinary approach to managing combination therapy with palbociclib in patients at opposite ends of the mBC age spectrum. Although dealing with the same diagnosis, Grace and Hazel faced different challenges as they began the treatment process. Hazel was dealing with bereavement, social isolation, managing multiple medications prescribed for mBC, and several comorbid conditions. Grace was struggling with depression, the demands of a young family and stressful job, and her prior negative experiences with chemotherapy and tamoxifen. To ensure that both women obtained the optimal benefits of treatment, Grace and Hazel’s care teams provided counseling and education appropriate to each patient’s needs and offered support, counseling, and referrals to address concomitant issues such as depression (Krikorian et al., 2019; Miaskowski et al., 2008). In Hazel’s case, they also involved her adult daughter in all clinical consultations as an extra layer of support to promote Hazel’s adherence and self-care.
By maintaining clear lines of communication, providing ongoing education and support, and working together to adapt treatment plans to meet patients’ evolving needs, APs and other members of multidisciplinary care teams can empower patients such as Hazel and Grace to be partners in care and complete treatment as planned. l
Acknowledgment
As noted at the beginning of this publication, these fictional case studies do not represent actual patient cases. Editorial and medical writing support was provided by Catherine Grillo and Jill Shults, PhD, of Complete Healthcare Communications, LLC (North Wales, PA), a CHC Group company, and was funded by Pfizer Inc.
Footnotes
Ms. Podsada has served on speakers bureaus for AstraZeneca, Novartis, and Pfizer, Inc. Ms. Ryan is an employee of Pfizer, Inc. Ms. Orbaugh has served on speakers bureaus for Bristol Myers Squibb, Novartis, Pfizer, Inc, and TEVA.
REFERENCES
- Amgen (2019). Xgeva (denosumab) package insert. [Google Scholar]
- Anders, C. K., Johnson, R., Litton, J., Phillips, M., & Bleyer, A. (2009). Breast cancer before age 40 years. Seminars in Oncology, 36(3), 237–249. 10.1053/j.seminoncol.2009.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellet, M., Ahmad, F., Villanueva, R., Valdivia, C., Palomino-Doza, J., Ruiz, A.,...de la Peña, L. (2019). Palbociclib and ribociclib in breast cancer: consensus workshop on the management of concomitant medication. Therapeutic Advances in Medical Oncology, 11 10.1177/1758835919833867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender, C. M., Gentry, A. L., Brufsky, A. M., Casillo, F. E., Cohen, S. M., Dailey, M. M.,...Sereika, S. M. (2014). Influence of patient and treatment factors on adherence to adjuvant endocrine therapy in breast cancer. Oncology Nursing Forum, 41(3), 274–285. 10.1188/14.Onf.274-285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadoo, K. A., Fornier, M. N., & Morris, P. G. (2013). Biological subtypes of breast cancer: current concepts and implications for recurrence patterns. Quarterly Journal of Nuclear Medicine and Molecular Imaging, 57(4), 312–321. [PubMed] [Google Scholar]
- Cardoso, F., Senkus, E., Costa, A., Papadopoulos, E., Aapro, M., André, F.,...Winer, E. P. (2018). 4th ESO–ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4). Annals of Oncology, 29(8), 1634–1657. 10.1093/annonc/mdy192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazzaniga, M. E., Danesi, R., Girmenia, C., Invernizzi, P., Elvevi, A., & Uguccioni, M. (2019). Management of toxicities associated with targeted therapies for HR-positive metastatic breast cancer: a multidisciplinary approach is the key to success. Breast Cancer Research and Treatment, 176(3), 483–494. 10.1007/s10549-019-05261-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton, K., Min, Y., Kimmel, J., Litton, J., Tripathy, D., & Karuturi, M. (2019). Progression-free survival (PFS) and toxicities of palbociclib in a geriatric population. Breast Cancer Research and Treatment, 175(3), 667–674. 10.1007/s10549-019-05181-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristofanilli, M., Turner, N. C., Bondarenko, I., Ro, J., Im, S. A., Masuda, N.,...Slamon, D. (2016). Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncology, 17(4), 425–439. 10.1016/S1470-2045(15)00613-0 [DOI] [PubMed] [Google Scholar]
- Desautels, D. N., Harlos, C. H., & Jerzak, K. J. (2019). Role of bone-modifying agents in advanced cancer. Annals of Palliative Medicine, 9(3). 10.21037/apm.2019.08.07 [DOI] [PubMed] [Google Scholar]
- Diéras, V., Harbeck, N., Joy, A. A., Gelmon, K., Ettl, J., Verma, S.,...Finn, R. (2017). PALOMA-2: Neutropenia patterns in patients with estrogen receptor−positive/human epidermal growth factor receptor 2−negative first-line advanced breast cancer receiving palbociclib plus letrozole. Paper presented at the 42nd Congress of the European Society for Medical Oncology, Madrid, Spain. [Google Scholar]
- Dieras, V., Harbeck, N., Joy, A. A., Gelmon, K., Ettl, J., Verma, S.,...Finn, R. S. (2019). Palbociclib with letrozole in postmenopausal women with ER+/HER2- advanced breast cancer: hematologic safety analysis of the randomized PALOMA-2 trial. Oncologist, 24(12). 10.1634/theoncologist.2019-0019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diéras, V., Rugo, H. S., Schnell, P., Gelmon, K., Cristofanilli, M., Loi, S.,...Finn, R. S. (2018). Long-term pooled safety analysis of palbociclib in combination with endocrine therapy for HR+/HER2- advanced breast cancer. Journal of the National Cancer Institute, 111(4), 419–430. 10.1093/jnci/djy109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn, R. S., Aleshin, A., & Slamon, D. J. (2016). Targeting the cyclin-dependent kinases (CDK) 4/6 in estrogen receptor-positive breast cancers. Breast Cancer Research, 18(1), 17 10.1186/s13058-015-0661-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn, R. S., Dering, J., Conklin, D., Kalous, O., Cohen, D. J., Desai, A. J.,...Slamon, D. J. (2009). PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Research, 11(5), R77 10.1186/bcr2419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn, R. S., Martin, M., Rugo, H. S., Jones, S., Im, S. A., Gelmon, K.,...Slamon, D. J. (2016). Palbociclib and letrozole in advanced breast cancer. New England Journal of Medicine, 375(20), 1925–1936. 10.1056/NEJMoa1607303 [DOI] [PubMed] [Google Scholar]
- Fredholm, H., Eaker, S., Frisell, J., Holmberg, L., Fredriksson, I., & Lindman, H. (2009). Breast cancer in young women: Poor survival despite intensive treatment. PLoS One, 4(11), e7695 10.1371/journal.pone.0007695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm, H., Magnusson, K., Lindstrom, L. S., Garmo, H., Falt, S. E., Lindman, H.,...Fredriksson, I. (2016). Long-term outcome in young women with breast cancer: A population-based study. Breast Cancer Research and Treatment, 160(1), 131–143. 10.1007/s10549-016-3983-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong, Y., Liu, Y. R., Ji, P., Hu, X., & Shao, Z. M. (2017). Impact of molecular subtypes on metastatic breast cancer patients: A SEER population-based study. Scientific Reports, 7, 45411 10.1038/srep45411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlader, N., Altekruse, S. F., Li, C. I., Chen, V. W., Clarke, C. A., Ries, L. A., & Cronin, K. A. (2014). US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. Journal of the National Cancer Institute, 106(5), 5 10.1093/jnci/dju055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, W., Sung, T., Jessen, B., Thibault, S., Finkelstein, M. B., Khan, N. K., & Sacaan, A. I. (2016). Mechanistic investigation of bone marrow suppression associated with palbociclib and its differentiation from cytotoxic chemotherapies. Clinical Cancer Research, 22(8), 2000–2008. https://doi.org/clincanres.1421.2015 [pii] [DOI] [PubMed] [Google Scholar]
- Johnson, R. H., Anders, C. K., Litton, J. K., Ruddy, K. J., & Bleyer, A. (2018). Breast cancer in adolescents and young adults. Pediatric Blood and Cancer, 65(12), e27397 10.1002/pbc.27397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka, A., Iwamoto, T., Tokunaga, E., Tomotaki, A., Kumamaru, H., Miyata, H.,...Tokuda, Y. (2016). Young adult breast cancer patients have a poor prognosis independent of prognostic clinicopathological factors: a study from the Japanese Breast Cancer Registry. Breast Cancer Research and Treatment, 160(1), 163–172. 10.1007/s10549-016-3984-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krikorian, S., Pories, S., Tataronis, G., Caughey, T., Chervinsky, K., Lotz, M.,...Weissmann, L. (2019). Adherence to oral chemotherapy: Challenges and opportunities. Journal of Oncology Pharmacy Practice, 26(7), 1590–1598. 10.1177/1078155218800384 [DOI] [PubMed] [Google Scholar]
- Loh, K. P., Soto-Perez-de-Celis, E., Hsu, T., de Glas, N. A., Battisti, N. M. L., Baldini, C.,...Wildiers, H. (2018). What every oncologist should know about geriatric assessment for older patients with cancer: Young International Society of Geriatric Oncology position paper. Journal of Oncology Practice, 14(2), 85–94. 10.1200/jop.2017.026435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loibl, S., Jackisch, C., Lederer, B., Untch, M., Paepke, S., Kümmel, S.,...von Minckwitz, G. (2015). Outcome after neoadjuvant chemotherapy in young breast cancer patients: A pooled analysis of individual patient data from eight prospectively randomized controlled trials. Breast Cancer Research and Treatment, 152(2), 377–387. 10.1007/s10549-015-3479-z [DOI] [PubMed] [Google Scholar]
- Loibl, S., Turner, N. C., Ro, J., Cristofanilli, M., Iwata, H., Im, S. A.,...Dowsett, M. (2017). Palbociclib combined with fulvestrant in premenopausal women with advanced breast cancer and prior progression on endocrine therapy: PALOMA-3 results. Oncologist, 22(9), 1028–1038. 10.1634/theoncologist.2017-0072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmgren, J., Hurlbert, M., Atwood, M., & Kaplan, H. G. (2019). Examination of a paradox: recurrent metastatic breast cancer incidence decline without improved distant disease survival: 1990–2011. Breast Cancer Research and Treatment, 174(2), 505–514. 10.1007/s10549-018-05090-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariotto, A. B., Etzioni, R., Hurlbert, M., Penberthy, L., & Mayer, M. (2017). Estimation of the number of women living with metastatic breast cancer in the United States. Cancer Epidemiology, Biomarkers, and Prevention, 26(6), 809–815. 10.1158/1055-9965.EPI-16-0889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McShane, T. M., Wolfe, T. A., & Ryan, J. C. (2018). Updates on managing advanced breast cancer with palbociclib combination therapy. Therapeutic Advances in Medical Oncology, 10, 1758835918793849 10.1177/1758835918793849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miaskowski, C., Shockney, L., & Chlebowski, R. T. (2008). Adherence to oral endocrine therapy for breast cancer: a nursing perspective. Clinical Journal of Oncology Nursing, 12(2), 213–221. 10.1188/08.cjon.213-221 [DOI] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network (2020). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Breast Cancer Version 6.2020. [Google Scholar]
- Novartis Pharmaceuticals Corporation (2019). Zometa (zoledronic acid) package insert. [Google Scholar]
- Pfizer Inc (2019a). Ibrance Capsules package insert. [Google Scholar]
- Pfizer Inc (2019b). Ibrance Tablets package insert. [Google Scholar]
- Pfizer Oncology (2015). IBRANCE® Palbociclib 125 mg capsules Dosing and Administration Guide (714136-01). Retrieved from https://www.pfizerpro.com/sites/default/files/ibrance_nurse_dosing_guide.pdf [Google Scholar]
- Rugo, H. S., Finn, R. S., Dieras, V., Ettl, J., Lipatov, O., Joy, A. A.,...Slamon, D. J. (2019). Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Research and Treatment, 174(3), 719–729. 10.1007/s10549-018-05125-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugo, H. S., Turner, N. C., Finn, R. S., Joy, A. A., Verma, S., Harbeck, N.,...Johnston, S. (2018). Palbociclib plus endocrine therapy in older women with HR+/HER2- advanced breast cancer: a pooled analysis of randomised PALOMA clinical studies. European Journal of Cancer, 101, 123–133. 10.1016/j.ejca.2018.05.017 [DOI] [PubMed] [Google Scholar]
- Sammons, S. L., Topping, D. L., & Blackwell, K. L. (2017). HR+, HER2– advanced breast cancer and CDK4/6 inhibitors: Mode of action, clinical activity, and safety profiles. Current Cancer Drug Targets, 17(7), 637–649. 10.2174/1568009617666170330120452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring, L. M., Zangardi, M. L., Moy, B., & Bardia, A. (2017). Clinical management of potential toxicities and drug interactions related to cyclin-dependent kinase 4/6 inhibitors in breast cancer: Practical considerations and recommendations. Oncologist, 22(9), 1039–1048. 10.1634/theoncologist.2017-0142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, W., Yu, Y., Hoffman, J., Turner, N. C., Cristofanilli, M., & Wang, D. (2017). Palbociclib exposure-response analyses in second-line treatment of hormone receptor−positive advanced breast cancer. Paper presented at the American Society of Clinical Oncology Annual Meeting, Chicago, IL, USA. [Google Scholar]
- Thangavel, C., Dean, J. L., Ertel, A., Knudsen, K. E., Aldaz, C. M., Witkiewicz, A. K.,...Knudsen, E. S. (2011). Therapeutically activating RB: Reestablishing cell cycle control in endocrine therapy-resistant breast cancer. Endocrine-Related Cancer, 18(3), 333–345. 10.1530/ERC-10-0262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thill, M., & Schmidt, M. (2018). Management of adverse events during cyclin-dependent kinase 4/6 (CDK4/6) inhibitor-based treatment in breast cancer. Therapeutic Advances in Medical Oncology, 10, 1758835918793326 10.1177/1758835918793326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, S. A., Teena, J., Criner, E., & Nguyen, T. M. (2019). Challenges to oral chemotherapy adherence. US Pharmacist, 44(6), HS-9-HS-12. Retrieved from https://www.uspharmacist.com/article/challenges-to-oral-chemotherapy-adherence [Google Scholar]
- Turner, N. C., Neven, P., Loibl, S., & Andre, F. (2016). Advances in the treatment of advanced oestrogen-receptor-positive breast cancer. Lancet, 389(10087), 2403–2414. 10.1016/S0140-6736(16)32419-9 [DOI] [PubMed] [Google Scholar]
- Turner, N. C., Ro, J., Andre, F., Loi, S., Verma, S., Iwata, H.,...PALOMA3 Study Group (2015). Palbociclib in hormone-receptor-positive advanced breast cancer. New England Journal of Medicine, 373(3), 209–219. 10.1056/NEJMoa1505270 [DOI] [PubMed] [Google Scholar]
- Turner, N. C., Slamon, D. J., Ro, J., Bondarenko, I., Im, S. A., Masuda, N.,...Cristofanilli, M. (2018). Overall survival with palbociclib and fulvestrant in advanced breast cancer. New England Journal of Medicine, 379(20), 1926–1936. 10.1056/NEJMoa1810527 [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services (2009). Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0 (No. 03-5410). Retrieved from Bethesda, MD: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf [Google Scholar]
- US Department of Health and Human Services (2017). Common terminology criteria for adverse events (CTCAE). Version 5.0. Retrieved from https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_8.5x11.pdf [Google Scholar]
- US Food and Drug Administration (2019). IBRANCE® Tablets NDA 212436 Approval. Retrieved from https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2019/212436Orig1s000ltr.pdf [Google Scholar]
- Verma, S., Bartlett, C. H., Schnell, P., DeMichele, A. M., Loi, S., Ro, J.,...Rugo, H. S. (2016). Palbociclib in combination with fulvestrant in women with hormone receptor-positive/HER2-negative advanced metastatic breast cancer: detailed safety analysis from a multicenter, randomized, placebo-controlled, phase III study (PALOMA-3). Oncologist, 21(10), 1165–1175. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27368881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, J., Yu, Y., Durairaj, C., Amantea, M., Dieras, V., Finn, R., & Wang, D. (2017). Palbociclib exposure-response analyses in treatment of hormone receptor–positive, human epidermal growth factor receptor 2–negative advanced breast cancer. Paper presented at the 40th Annual San Antonio Breast Cancer Symposium, San Antonio, TX. [Google Scholar]