Abstract
We think that thalassemia is not necessarily a cause of aggravation of the clinical course in COVID‐19; however, certain key factors must be considered, such as the anemic condition, the likely pathogenic role of the virus on hemoglobin, and the hypercoagulable state to prevent any complications.
Keywords: COVID‐19, pneumonia, thalassemia
We think that thalassemia is not necessarily a cause of aggravation of the clinical course in COVID‐19; however, certain key factors must be considered, such as the anemic condition, the likely pathogenic role of the virus on hemoglobin, and the hypercoagulable state to prevent any complications.
1. INTRODUCTION
Few data are available regarding cases of COVID‐19 in patients with thalassemia. We describe a case of a 46‐year‐old man with thalassemia with COVID‐19 pneumonia complicated at illness day 17 by severe plurisegmentary pulmonary microembolism, treated with lopinavir/ritonavir, hydroxychloroquine, ceftriaxone, low‐loss heparin, tocilizumab, and C‐PAP Oxygen therapy.
A novel coronavirus disease (COVID‐19) caused by severe acute respiratory syndrome coronavirus (SARS‐CoV‐2) has now become a pandemic with COVID‐19 affecting a large number of people worldwide.
It has not been showed that patients affected by hemoglobinopathies have a significantly different development of disease and few data are available regarding cases of COVID‐19 in patients with thalassemia. Preliminary data from Italian experience reported 11 cases (as late as 20 April 2020) showing a clinical course not significantly different from the general population. Three of these patients were asymptomatic, one, on treatment with deferiprone, was admitted with fever and agranulocytosis, six were hospitalized but did not require mechanical ventilation. The only patient with more severe symptoms had a history of diffuse large B‐cell lymphoma. 1
B‐thalassemia major is a hereditary disorder caused by mutations in the β globin gene that lead to erythroid expansion in the bone marrow and severe anemia in patients with transfusion‐dependent thalassemia (TDT). Splenectomy, iron overload, and stress erythropoiesis may contribute to increased susceptibility to infections, which are common causes of mortality in patients with thalassemia. Splenectomy promotes a hypercoagulable state because of the decreased ability to scavenge procoagulant red blood cells and activated platelets. 2 In severe patients with COVID‐19, in addition to respiratory symptoms, both thrombosis and pulmonary embolism have been observed. This is in line with the findings that elevated d‐dimer and fibrinogen levels were observed in severe COVID‐19 cases. 3
2. CASE PRESENTATION
We describe a case of a 46‐year‐old man with TDT (IVS‐I 110/IVS‐I 6 mutated; blood group A RH+) from our center where 105 patients with thalassemia, 53 males and 52 females, are followed.
The patient received diagnosis at the age of 3, when he started regular transfusion therapy and chelation, first with deferoxamine, then, at 23, with deferiprone and from the age of 40 with deferasirox. The patient showed regular psychophysical development. At 27 years old, he underwent splenectomy and at 37 cholecystectomy in an emergency. He does not present significant alterations in cardiac and endocrine function. The patient has excellent control of serum and instrumental iron deposits. He received 2 blood units every 2 weeks (pretransfusion hemoglobin mean 10.3 gr/dL) and deferasirox 12 mg/kg/die.
On 9th March, the patient received scheduled transfusion and at examination showed no significant symptoms. The blood count showed hemoglobin value of 10.2 g/dL, white cell count of 8020/mL, with normal leukocyte formula. The following day, he presented fever at home. Given the progressive worsening of symptoms, fever, dry cough, headache, dyspnea, and myalgia, after 3 days he was hospitalized and diagnosed with COVID‐19. The nasopharyngeal swab later performed was positive. Table 1 shows clinical laboratory results during illness (ID) and hospitalization days (HD).
TABLE 1.
Clinical laboratory results during illness days (ID) and hospitalization days (HD)
| Measure | Range | ID −1 |
ID 3 HD 1 |
ID6 HD 3 |
ID 10 HD 7 |
ID 15 HD 12 |
ID17 HD 14 |
ID 25 HD 22 |
ID 29 HD 26 |
ID 30 HD 27 Discharge |
|---|---|---|---|---|---|---|---|---|---|---|
| White cell count (μL) | 3800‐11 000 | 8020 | 9420 | 9590 | 4390 | 8600 | 7490 | 5470 | 5860 | 6570 |
| ‐Red cell count (μL) | 4.2‐5.7 millions | 3.73 | 4.03 | 3.42 | 3.35 | 3.74 | 3.38 | 3.69 | 3.88 | |
| Absolute neutrophil count μL) | 1900‐7400 | 3700 | 4970 | 5890 | 1580 | 3140 | 1870 | 630 | 970 | 722 |
| Absolute lymphocyte count (μL) | 1000‐3900 | 3140 | 3550 | 3080 | 1760 | 3940 | 4200 | 3450 | 3270 | 4065 |
| Platelet count | 150 000‐400 000 | 908 000 | 694 000 | 461 000 | 627 000 | 512 000 | 791 000 | 684 000 | 744 000 | 721 000 |
| Hemoglobin (g/dL) | 13.2‐17 | 10.3 | 11.3 | 9.5 | 9.5 | 10.5 | 9,5 | 10.3 | 10.6 | 12.7 |
| Hematocrit % | 39‐50 | 31.3 | 33.2 | 28.5 | 28.5 | 31.7 | 28.4 | 32.5 | 33.4 | 38 |
| Ferritin (μL) | 300‐400 | 465 | ‐ | 1337 | ‐ | ‐ | ‐ | 893 | ‐ | 893 |
| Blood urea nitrogen (mg/dL) | 10.2‐49.8 | 40 | 21 | 30 | 17 | 26 | ‐ | 24 | 25 | 33 |
| Creatinine (mg/dL) | 0.7‐1.2 | 1.1 | 1.26 | 1.1 | 0.84 | 0.69 | ‐ | 0.76 | 0.79 | 0.8 |
| Total bilirubin (mg/dL) | 0.3‐1.2 | 3.5 | 1.88 | 1.24 | 1.53 | ‐ | ‐ | 2.65 | 1.74 | ‐ |
| Alanine aminotransferase U/L | 10‐40 | 20 | ‐ | 32 | 58 | ‐ | ‐ | 77 | 55 | 72 |
| Aspartate aminotransferase U/L | 9‐45 | 20 | ‐ | 16 | 40 | ‐ | ‐ | 159 | 125 | 150 |
| Fibrinogen | 150‐450 | ‐ | 424 | ‐ | ‐ | ‐ | ‐ | ‐ | 114 | ‐ |
| Dimer | 50‐450 | ‐ | 565 | ‐ | ‐ | 3668 | 4312 | 470 | 321 | 340 |
| Transfusion/units | Yes/2 | Yes/1 | Yes/1 | Yes/1 | Yes/2 |
On HD 3, the patient performed a chest CT scan that showed a picture compatible with bilateral interstitial pneumonia. (Figure 1).
FIGURE 1.
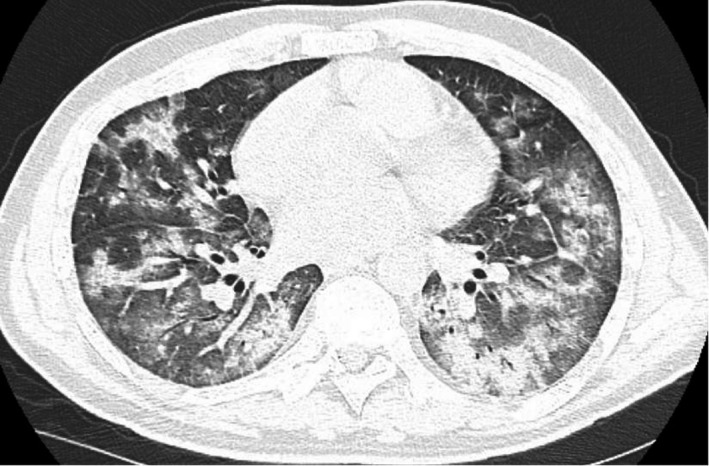
Shows a chest CT scan of the patient indicating severe interstitial pneumonia performed at hospitalization day 3
The patient was treated with lopinavir/ritonavir, hydroxychloroquine, ceftriaxone, low‐loss heparin, and, given the continuation of respiratory symptoms and hemogram analysis alterations, he received 2 administrations of tocilizumab. Oxygen therapy with continuous positive airway pressure (C‐PAP) was performed, with slow but progressive improvement of clinical symptoms.
On HD 3, 7, 13, and 26, the patient underwent transfusion therapy with a pretransfusion hemoglobin value of 9.5 gr/L. On HD 3 and 12, a significative increase of ferritin and of d‐dimer test, respectively, was observed (Table 1). On HD 14, the patient presented leg pain and started therapy with low‐loss heparin. Chest CT scan showed a picture of plurisegmentary pulmonary microembolism. After 27 days of hospitalization, he was discharged with anticoagulant therapy that the patient continues until now (2 months after discharge) for the persistence of alterations shown by Doppler ultrasound lower right limbs.
3. CONCLUSION
Few data are available regarding cases of COVID‐19 in patients with thalassemia. Motta I et al report the experience of 11 cases of patients with thalassemia from the Centers of Italian Hemoglobinopathies Network and conclude that, although preliminary, the data do not indicate increased severity of COVID‐19 in patients with thalassemia. We, on the other hand, describe a case of a 46‐year‐old man with thalassemia with COVID‐19 pneumonia complicated at illness day 17 by severe plurisegmentary pulmonary microembolism, treated with a combination of drugs and oxygen therapy with C‐PAP. During hospitalization, the patient had an increase in transfusion requirement as typically occurs in patients with TDT during infectious process, in fact from HD 1 to HD 3 a decrease of about two grams of hemoglobin is observed. We think that thalassemia is not necessarily a cause of aggravation of the clinical course in COVID‐19; however, certain key factors must be considered, such as the anemic condition, the likely pathogenic role of the virus on hemoglobin, and the hypercoagulable state to prevent any complications in patients with TDT. A recent study showed that the viral proteins orf1ab, ORF3a, and ORF10 could attack the heme group on the beta chain of hemoglobin, forcing the heme iron to dissociate. The authors hypothesize that this effect could play a role in development of severe pneumonia. 4 Also Chen N et al observed that hemoglobin levels of most COVID‐19‐infected patients decreased, and that index values of serum ferritin and heme increased. 5 In addition, specific side effects of chelator drugs like agranulocytosis, increased risk of infection and of acute kidney injury must be considered. Furthermore, thalassemia patients with cardiac iron overload have an additional risk of cardiac complications during severe infections. Adequate transfusion therapy, chelator discontinuation, and starting early anticoagulant therapy are necessary in patient with thalassemia, given the highest risk of severe anemia and thromboembolic complications. A larger number of cases need to be collected to know the impact of COVID‐19 in patients with hemoglobinopathies, yet they need to be considered especially vulnerable.
CONFLICT OF INTEREST
The other authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
MM and MR: wrote the manuscript; AAL, FT, and PP: contributed to clinical follow‐up; AM, GG, and AA: revised the manuscript.
ACKNOWLEDGMENTS
The authors thank all the staff members involved in this case. Published with written consent of the patient.
Marziali M, Ribersani M, Losardo AA, et al. COVID‐19 pneumonia and pulmonary microembolism in a patient with B‐thalassemia major. Clin Case Rep. 2020;8:3139–3142. 10.1002/ccr3.3275
REFERENCES
- 1. Motta I, De Amicis MM, Pinto VM, et al. SARS‐CoV‐2 infection in beta thalassemia: preliminary data from the Italian experience. Am J Hematol. 2020;95(8):E198‐E199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Taher AT, Weatherall DJ, Cappellini MD. Thalassaemia. Lancet. 2018;391(10116):155‐167. [DOI] [PubMed] [Google Scholar]
- 3. Fogarty H, Townsend L, Ni Cheallaigh C, et al. COVID‐19 coagulopathy in caucasian patients. Br J Haematol. 2020;189(6):1044‐1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu W, Li H. COVID‐19: attacks the 1‐beta chain of hemoglobin and captures the porphyrin to inhibit human heme metabolism. ChemRxiv. 10.26434/chemestry.11938173 [DOI] [Google Scholar]
- 5. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]


