To the Editor,
We read with great interest the article entitled “Tuberous sclerosis complex (TSC), lymphangioleiomyomatosis, and COVID‐19: The experience of a TSC clinic in Italy” by Peron et al. (2020), which described the features and outcomes of coronavirus disease‐19 (COVID‐19) in patients with tuberous sclerosis complex (TSC) and lymphangioleiomyomatosis (LAM) in Italy, and the potential role of mTOR inhibitors in such infection. Here, we present our experience of patients with LAM and COVID‐19, with an emphasis on the potential influence of the use of mTOR inhibitors.
This was a retrospective study that included patients with LAM who were being followed‐up in our center between March 1, 2020 and August 20, 2020. Patients from all the regions of Brazil were assessed. Data was obtained during regular visits or through phone calls.
A total of 143 women with LAM, including 114 (79.7%) sporadic‐LAM and 29 (20.3%) associated with TSC, were included in the study. Seventy‐three (51%; 95% confidence interval: 42.9–59.1%) patients were using mTOR inhibitors (69 on sirolimus, mean dose of 2 ± 1 mg/day, and four on everolimus, all receiving 10 mg/day). Six (4.2%; 95% confidence interval: 1.9–8.8%) nonsmoking patients had COVID‐19‐compatible symptoms, and a positive polymerase chain reaction (PCR) that confirmed the diagnosis of the infection (Figure 1); three (4.1%) in patients who used mTOR inhibitors and three (4.3%) in those who did not use these drugs (p = .715). Among the six patients who had the diagnosis of COVID‐19 confirmed, three (50%) had renal angiomyolipoma and none of them had any other comorbidity. Only one patient required hospital admission due to hypoxemia. All the patients fully recovered from COVID‐19. Demographic, clinical, and functional characteristics of those with a confirmed diagnosis of COVID‐19 are presented in Table 1. The mean age was 52 ± 9 years and the main clinical manifestations were asthenia (100%), fever (50%), headache (50%), and anosmia (50%).
FIGURE 1.
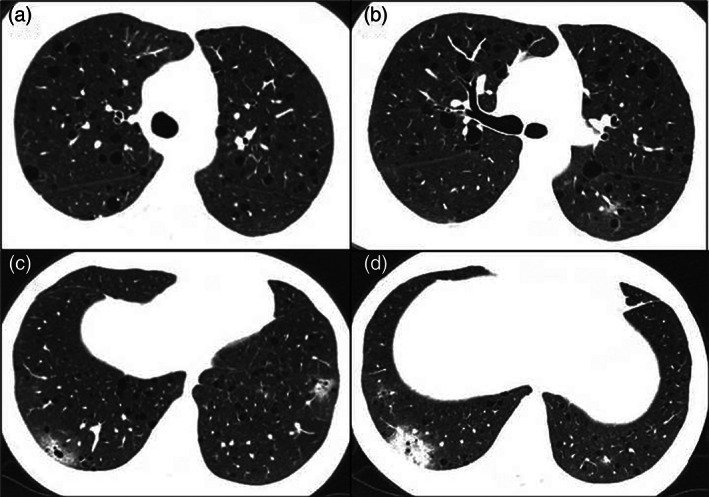
Axial computed tomography images show regular and diffuse pulmonary cysts (a–d), ground‐glass opacities (c), and an area of consolidation (d) in the periphery of the lower lobes in a woman with LAM using everolimus
TABLE 1.
Demographic, clinical, and functional characteristics of patients with LAM with confirmed COVID‐19 (n = 6)
| Patient | LAM | Age (years) | Baseline FEV1 (%predicted) | mTOR inhibitor | Symptoms | Tomographic findings | Hospital admission |
|---|---|---|---|---|---|---|---|
| 1 | TSC‐LAM | 41 | 101 | Everolimus | Fever, asthenia, anosmia and dyspnea mMRC1 for 3 days | Diffuse cysts; discrete areas of ground‐glass opacities and consolidations (extent <25%) | No |
| 2 | TSC‐LAM | 51 | 36 | Sirolimus | Fever, asthenia and dry cough for 3 days | Diffuse cysts; no other finding | Yes; use of supplemental oxygen for 3 days |
| 3 | Sporadic‐LAM | 54 | 72 | No | Fever, asthenia, anosmia and anorexia for 4 days | Diffuse cysts; areas of ground‐glass opacities (extent <25%) | No |
| 4 | Sporadic‐LAM | 61 | 78 | No | Asthenia, headache and diarrhea for 2 days | Diffuse cysts; no other finding | No |
| 5 | Sporadic‐LAM | 44 | 76 | No | Asthenia, dry cough, dyspnea mMRC1 and headache for 7 days | Diffuse cysts; no other finding | No |
| 6 | Sporadic‐LAM | 64 | 48 | Sirolimus | Asthenia, anosmia and headache for 5 days | Diffuse cysts; no other finding | No |
Abbreviations: FEV1, forced expiratory volume in the first second; LAM, lymphangioleiomyomatosis; mMRC, modified Medical Research Council; TSC, tuberous sclerosis complex.
LAM is a low‐grade neoplastic disease that may occur sporadically or be associated with TSC, and is caused by mutations in the TSC genes (TSC1 and TSC2) (McCormack et al., 2016). mTOR inhibitors may be used in LAM for those with progressive lung functional impairment, chylous effusions, or angiomyolipomas (Johnson, Taveira‐DaSilva, & Moss, 2016). To our knowledge, this study was the first to investigate the occurrence of COVID‐19 specifically in a sample of patients with LAM. Peron et al. suggested that TSC and LAM patients do not have an increased risk of developing COVID‐19 and discussed the potential risks and benefits of maintaining the use of mTOR inhibitors regarding such infection (Peron et al., 2020). A relevant limitation raised by the authors was the lack of confirmation of the COVID‐19 diagnosis by PCR in most of the patients (Peron et al., 2020). Recent studies speculate that mTOR inhibitors may have a potential benefit in reducing the viral replication, inflammatory response, and severity associated with COVID‐19 beyond their immunosuppressant action (Terrazzano et al., 2020; Zheng, Li, & Liu, 2020). Recently, LAM Foundation published recommendations based on expert opinion for LAM patients regarding COVID‐19, and stated that the risk of complications was associated with the degree of lung function impairment (The LAM Foundation COVID‐19 Statement, 2020). Additionally, it was recommended to maintain the use of mTOR inhibitors during the outbreak unless there is an active infection, and not to start taking these drugs to prevent or treat COVID‐19 (The LAM Foundation COVID‐19 Statement, 2020). Our findings suggest that the use of mTOR inhibitors do not increase the risk of symptomatic COVID‐19, as the frequency of such infection was similar between patients who were using this class of drugs and those who were not.
Our study has limitations that need to be addressed. This was a single‐center analysis, with a small number of COVID‐19 patients. However, it was performed at a reference center where patients with LAM from all the regions of Brazil were followed up. Additionally, we did not include asymptomatic COVID‐19 patients confirmed by PCR, which may have underestimated the real frequency of the infection in the present study.
In conclusion, our study suggests that LAM is not associated with a higher susceptibility to serious COVID‐19 complications and that mTOR inhibitors do not determine an increase in the risk and severity of the infection. Future multicenter studies are necessary to establish the frequency of COVID‐19 and the role of mTOR inhibitors in reducing the risk and severity of such infection in LAM.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
Bruno Guedes Baldi: study design, data collection, data analysis, writing, and manuscript review. Alexandre Franco Amaral and Phillipe de Figueiredo Braga Colares: data collection, writing, and manuscript review. Ronaldo Adib Kairalla: study design, data analysis, and manuscript review. Martina Rodrigues de Oliveira: data collection, data analysis, writing, and manuscript review. Carlos Roberto Ribeiro Carvalho: study design, data analysis, and manuscript review.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Johnson, S. R. , Taveira‐DaSilva, A. M. , & Moss, J. (2016). Lymphangioleiomyomatosis. Clinics in Chest Medicine, 37(3), 389–403. 10.1016/j.ccm.2016.04.002 [DOI] [PubMed] [Google Scholar]
- McCormack, F. X. , Gupta, N. , Finlay, G. R. , Young, L. R. , Taveira‐DaSilva, A. M. , Glasgow, C. G. , … ATS/JRS Committee on Lymphangioleiomyomatosis . (2016). Official American Thoracic Society/Japanese Respiratory Society Clinical Practice Guidelines: Lymphangioleiomyomatosis diagnosis and management. American Journal of Respiratory and Critical Care Medicine, 194(6), 748–761. 10.1164/rccm.201607-1384ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peron, A. , La Briola, F. , Bruschi, F. , Terraneo, S. , Vannicola, C. , Previtali, R. , … Canevini, M. P. (2020). Tuberous sclerosis complex (TSC), lymphangioleiomyomatosis, and COVID‐19: The experience of a TSC clinic in Italy. American Jounal of Medical Genetics Part A. 10.1002/ajmg.a.61810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrazzano, G. , Rubino, V. , Palatucci, A. T. , Giovazzino, A. , Carriero, F. , & Ruggiero, G. (2020). An open question: Is it rational to inhibit mTor‐dependent pathway as COVID‐19 therapy? Frontiers in Pharmacology, 11, 856. 10.3389/fphar.2020.00856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The LAM Foundation COVID‐19 Statement . (2020, May 15). Retrieved from https://thelamfoundation.org/Portals/0/Files/COVID-19/COVID-19%20Statement%2005-15-2020%20.pdf?ver=2020-05-15-122300-870
- Zheng, Y. , Li, R. , & Liu, S. (2020). Immunoregulation with mTOR inhibitors to prevent COVID‐19 severity: A novel intervention strategy beyond vaccines and specific antiviral medicines. Journal of Medical Virology, 92, 1495–1500. 10.1002/jmv.26009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


