Abstract
To curb the COVID‐19 pandemic, isolation measures are required. Shared room occupancy is recommended when isolation rooms are insufficient. However, there is little evidence of the applicability of shared and single room occupancy for patients with COVID‐19 to determine whether shared room occupancy is feasible. COVID‐19‐infected patients admitted to the Daegu Dongsan Hospital of Keimyung University from 21 February 2020 to 20 April 2020 were enrolled in the study and randomly assigned to hospital rooms. Clinical symptoms, underlying diseases and epidemiological data of patients were analysed after dividing participants into a shared room occupancy group (group A) and a single room occupancy group (group B). Outcomes analysed included microbiological cure rates, time to clinical symptom improvement, time to defervescence and negative‐to‐positive conversion rates of polymerase chain reaction (PCR) results during hospitalization. A total of 666 patients were included in this study, 535 and 131 patients in groups A and B, respectively. Group B included more underlying conditions, such as pregnancy and solid organ transplantation, and was more closely associated with severe pneumonia during hospitalization. Besides, no statistically significant differences between the two groups in terms of negative PCR rates at HD 7 and 14, conversion rates of PCR results from negative‐to‐positive, as well as time to the improvement of clinical symptoms, and time to defervescence were observed. Our results suggest that the shared room occupancy of patients with mild symptoms could be an alternative to single room occupancy during the COVID‐19 pandemic.
Keywords: airborne infection, COVID‐19, epidemic, quarantine, shared room occupancy, single room occupancy
1. INTRODUCTION
In December 2019, an outbreak of pneumonia with an unknown aetiology was observed in Wuhan, China. The causative agent was soon identified as severe acute respiratory syndrome coronavirus 2, and the disease was named coronavirus disease 2019 (COVID‐19) (Guan et al., 2020). On 19 January 2020, the first case of COVID‐19 in South Korea was reported, and sporadic imported cases have been reported since then. Since the confirmation of a COVID‐19 case on 18 February related to the Shincheonji Church in Daegu, there has been a massive outbreak of the disease, followed by third and fourth generations of community‐acquired transmissions (Korean Society of Infectious Disease et al., 2020).
At that time, individuals underwent screening for COVID‐19 at coronavirus testing centres in Daegu and returned home until they were informed of the results. Those diagnosed with COVID‐19 were recommended home quarantine until hospital beds were available. The number of patients diagnosed increased suddenly; up to 900 patients per day were diagnosed with COVID‐19.
To prevent the spread of COVID‐19, precautions such as maintaining droplet and contact isolation are recommended. Additionally, airborne precautions also need to be taken, such as using a negative pressure isolation room when performing aerosol‐generating procedures (Clinical management of severe acute respiratory infection when novel coronavirus (2019‐nCoV) infection is suspected: interim guidance, 2020). However, due to the lack of negative pressure isolation wards in Daegu, it was impossible for all confirmed patients to be hospitalized in such conditions.
In order to prevent the spread to the community and to provide treatment to confirmed patients, only patients with confirmed mild cases of COVID‐19 were admitted to Daegu Dongsan Hospital (DDH), a general hospital with about 900 beds and only three negative pressure isolation rooms. While patients with severe cases were admitted to tertiary hospitals, patients with mild symptoms, who had been previously admitted to DDH, were transferred to other hospitals. DDH has three buildings: the main hospital, an annex, and a research building. There are six wards on the fifth to eighth floors in the main hospital building and 50–60 beds in each ward. Patients were assigned to hospital rooms randomly. The multiple occupancy rooms accommodate 2–5 patients, whereas the single room occupancy isolates one patient.
On 11 March 2020, the World Health Organization (WHO) declared COVID‐19 a pandemic, with the disease spread globally. If isolation rooms are insufficient, it is recommended that COVID‐19‐infected patients be isolated in cohorts, but there is insufficient evidence to ensure that shared room occupancy is safe. To evaluate the applicability of shared room occupancy compared with single room occupancy, we analysed the clinical characteristics, laboratory findings, and outcomes of patients with COVID‐19 according to medical records of DDH.
2. MATERIALS AND METHODS
2.1. Study design
We conducted a retrospective observational study of the COVID‐19 outbreak at DDH. Patients admitted from 21 February to 20 April were evaluated in this study. Patients under 18 years old were excluded. Patients who were transferred to tertiary hospitals due to deteriorating conditions, or were admitted to residential treatment centres due to improved conditions, were also excluded from the study as the final reverse transcription‐polymerase chain reaction (PCR) results could not be performed. Patients hospitalized for re‐positive PCR results for SARS‐CoV‐2 were excluded. The study was approved by the Institutional Review Board of Dongsan Medical Center (IRB 2020‐03‐027).
2.2. Definitions
The shared room occupancy group, group A, comprised of patients admitted to multiple occupancy rooms. The single room occupancy group, group B, comprised of patients admitted to single occupancy rooms. Fever was defined as a body temperature above 37.8°C. Severe pneumonia was defined by the presence of clinical signs of pneumonia and at least one of the following: respiratory rates >33 breaths/min, severe respiratory distress, or oxygen saturation <93%, according to WHO criteria (“Clinical management of severe acute respiratory infection when novel coronavirus (2019‐nCoV) infection is suspected: interim guidance,” 2020). Clinical improvement was defined as a subjective improvement of a patient's symptoms for 24 hr. PCR testing follow‐ups were performed only in clinically proven cases. Testing was done at least 24 hr after improvement in a patient's symptoms was noticed. Conversion of negative‐to‐positive PCR results was defined as the negative results of the first PCR test changing to positive or indeterminate in subsequent tests. Defervescence was defined as maintenance of body temperature below 37.5°C for more than 48 hr. Time to defervescence was the duration of time from fever to the first instance of body temperature below 37.5°C in patients who satisfied the condition of defervescence.
2.3. Isolation hospital strategy
Only patients diagnosed with COVID‐19 were admitted to this hospital. Patients were randomly assigned to hospital rooms. None of the hospital rooms were equipped with negative pressure. Two to five patients were admitted to each multiple occupancy room, where the spacing between beds was maintained at 1 m or more. In the multiple occupancy rooms, patients were asynchronously admitted. Some patients had only recently had symptoms, while some had had symptoms for a long time, and others were asymptomatic. Patients were instructed to stay inside their rooms, keep curtains closed, stay in their beds, and avoid contact with other patients; only visits to the radiology department on the first floor of the hospital for imaging tests were permitted. Each multiple occupancy room had a shared toilet, and a patient shower facility was available on each floor. Each single occupancy room had a separate toilet and a patient shower facility. Patient movements were tracked using closed‐circuit televisions installed in the corridors.
Medical employees who entered the hospital donned full‐body coverage suits and powered air‐purifying respirators at the entrance. When exiting the hospital, they took off the full‐body coverage suits. Doctors visited patients once a day with full‐body coverage suits, and the patients were called once a day to the annex building for check‐ups. Nurses took turns rounding the wards assigned to them and measured the vital signs of the patients for about 2 hr. When a patient's symptoms, such as fever, chills, cough, sputum, and myalgia, were relieved, the doctors conducted nasopharyngeal swabs after 24 hr. When the PCR test result was negative, the test was performed again after 24 hr. If the PCR result was negative two times consecutively, the doctor released the patient from isolation and discharged the patient.
Each meal was delivered in lunch boxes to the wards from the hospital entrance by a nutrition team. Nurses in each ward then distributed the lunchboxes to patients. After the meal, these boxes were disposed of in waste bins and placed in the corridor outside the rooms; the cleaning team collected the waste bins.
2.4. Data collection
Data on demographics, including gender, age, exposure history, and overseas visits, were collected. Initial vital signs, including body temperature, respiratory rate, pulse rate, and systolic blood pressure, were collected. Clinical findings, including underlying diseases, initial symptoms, oxygen demand, laboratory tests, and PCR results, were collected. The severity of pneumonia was evaluated based on hypoxaemia and oxygen demand. The primary outcome was microbiological, PCR results at hospitalization day (HD) 14, and whether the negative PCR test turned positive during hospitalization. The secondary outcomes were clinical, including death, length of hospitalization, oxygen demand, and fever at HD 7 and HD 14.
2.5. Statistics
Categorical variables were described using frequencies and percentages, while continuous variables were described using mean value, median value, and interquartile range. All statistical analyses were performed using Statistical Package for the Social Sciences 20.0 software (SPSS Inc., IBM, Armonk, NY, USA). The Pearson chi‐square test and Fisher's exact test were used to comparing qualitative variables. For continuous variables, Mann–Whitney U tests were performed if data did not follow a normal distribution, and independent t tests were performed if data did follow a normal distribution. Logistic regression analysis was performed to identify the risk factors of a longer duration of hospital stay (>21 days). For unadjusted comparisons, p < .05 was considered to indicate statistical significance.
3. RESULTS
3.1. Demographics
A total of 786 patients were admitted to our hospital during the study period, 607 patients in shared room occupancy, and 179 in single room occupancy. Of these, 12 patients in shared room occupancy and 11 patients in single room occupancy were under 18 years old and excluded from the study. Forty‐eight patients in shared room occupancy and 10 patients in single room occupancy were transferred to other hospitals or residential treatment centres and, therefore, excluded from the study. Twelve patients in shared room occupancy and 27 patients in single room occupancy who were admitted for re‐positive PCR results for SARS‐CoV‐2 were excluded from the study (Figure 1). Finally, a total of 666 patients were included in this study, 535 patients in group A and 131 patients in group B. The demographic details of both study group patients are given in Table 1. Evaluation of underlying diseases revealed that the incidences of hypertension and neurologic disease were higher in group A, while solid organ transplantation and pregnancy were significantly higher in group B (Table 1).
FIGURE 1.
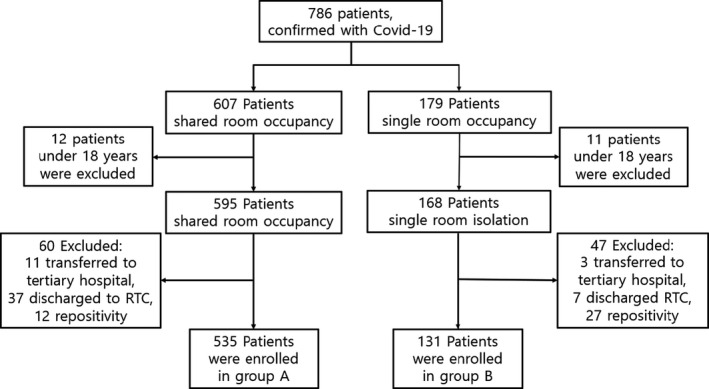
Flow chart showing the number of patients enrolled in this study. A total of 786 patients were admitted to DDH during the study period. Among them, a total of 666 patients were enrolled in this study. RTC: residential treatment centre; group A: patients admitted to multiple occupancy rooms; group B: patients admitted to single occupancy rooms
TABLE 1.
Baseline characteristics
| Group A | Group B | p‐Value | |
|---|---|---|---|
| (n = 535) | (n = 131) | ||
| N (%) | N (%) | ||
| Epidemiology | |||
| Male gender | 156 (29.2%) | 36 (27.5%) | .704 |
| Age | 62 (51–72) | 49 (33–58) | .001 |
| Overseas visit | 10 (1.9%) | 3 (2.3%) | .727* |
| Exposure to confirmed patients | 371 (69.3%) | 81 (61.8%) | .099 |
| Connection with Shincheonji church | 206 (38.5%) | 50 (38.2%) | .943 |
| Underlying diseases | |||
| Hypertension | 174 (32.5%) | 21 (16.0%) | .001 |
| Diabetes mellitus | 96 (17.9%) | 23 (17.6%) | .918 |
| Hyperlipidemia | 58 (10.8%) | 7 (5.3%) | .057 |
| Chronic lung disease | 27 (5.0%) | 7 (5.3%) | .89 |
| Chronic heart disease | 40 (7.5%) | 6 (4.6%) | .241 |
| Cerebrovascular disease | 52 (9.7%) | 3 (2.3%) | .006 |
| Chronic liver disease | 9 (1.7%) | 3 (2.3%) | .712* |
| Chronic renal disease | 2 (0.4%) | 3 (2.3%) | .055* |
| Autoimmune disease | 2 (0.4%) | 2 (1.5%) | .177* |
| Solid tumour | 23 (4.3%) | 5 (3.8%) | .799 |
| Acquired immunodeficiency syndrome | 1 (0.2%) | 0 (0.0%) | .999* |
| Psychiatric disorder | 12 (2.3%) | 1 (0.8%) | .481* |
| Solid organ transplantation | 0 (0.0%) | 3 (2.3%) | .007* |
| Pregnancy | 1 (0.2%) | 9 (6.9%) | .001* |
Values are presented as mean ± standard deviation or number (%) or median (interquartile range); group A: patients admitted to multiple occupancy rooms; group B: patients admitted to single occupancy rooms.
Fisher's exact test.
3.2. Baseline characteristics
Initial symptoms were not different between the two groups. The vital signs measured, such as body temperature, were no different between the two groups. Systolic blood pressure was higher in group A. The median time from symptom onset to admission was six days in group A and seven days in group B. Leukopenia, thrombocytopenia, azotemia, and CRP > 1 mg/dl were not different between the two groups. The rate of pneumonia determined by initial chest X‐rays was not significantly different between the two groups. The rate of pneumonia on chest X‐rays during hospital stay was higher in group A (Table 2).
TABLE 2.
Clinical characteristics of the patients
| Group A | Group B | p‐Value | |
|---|---|---|---|
| (n = 535) | (n = 131) | ||
| N (%) | N (%) | ||
| Initial signs | |||
| Body temperature, °C | 36.8 (36.5–37.2) | 36.7 (36.5–37.1) | .184 |
| Pulse rate | 87.91 ± 15.65 | 89.44 ± 14.68 | .308 |
| Respiratory rate | 20 (18–20) | 20 (18–20) | .543 |
| Systolic blood pressure, mm Hg | 138 (124–153) | 134 (120–144) | .007 |
| Initial symptoms | |||
| Fever | 200 (37.4%) | 43 (32.8%) | .331 |
| Chills | 126 (23.6%) | 33 (25.2%) | .693 |
| Cough | 257 (48.0%) | 55 (42.0%) | .213 |
| Sputum | 208 (38.9%) | 42 (32.1%) | .149 |
| Rhinorrhea | 93 (17.4%) | 26 (19.8%) | .509 |
| Sore throat | 126 (23.6%) | 38 (29.0%) | .194 |
| Myalgia | 189 (35.3%) | 49 (37.4%) | .657 |
| Headache | 137 (31.2%) | 41 (31.3%) | .985 |
| Diarrhoea | 98 (18.3%) | 31 (23.7%) | .165 |
| Dyspnoea | 95 (17.8%) | 25 (19.1%) | .723 |
| Chest pain | 20 (3.7%) | 4 (3.1%) | .999* |
| Symptom onset to admission, days | 6 (4–10) | 7 (4–13) | .195 |
| Laboratory tests | |||
| Leukopenia (WBC < 4,000) | 139 (26.0%) | 35 (26.7%) | .864 |
| Thrombocytopenia (Platelet < 150 K) | 68 (12.7%) | 15 (11.5%) | .696 |
| ANC < 1,000 | 23 (4.3%) | 3 (2.3%) | .287 |
| ALC < 1,000 | 89 (16.6%) | 22 (16.8%) | .965 |
| Azotemia (Serum Creatine > 1.3) | 19 (3.6%) | 7 (5.3%) | .343 |
| CRP > 1 mg/dl | 171 (32.0%) | 40 (30.5%) | .753 |
| Pneumonia on CXR at admission | 269 (50.4%) | 53 (41.1%) | .058 |
| Severe pneumonia | 41 (7.7%) | 12 (9.3%) | .542 |
Values are presented as mean ± standard deviation or number (%) or median (interquartile range); group A: patients admitted to multiple occupancy rooms; group B: patients admitted to single occupancy rooms.
Abbreviations: ALC, absolute lymphocyte count; ALT, alanine transaminase; ANC, absolute neutrophil count; AST, aspartate transaminase; BUN, blood urea nitrogen; CPK, creatinine kinase; CRP, C‐reactive protein; CXR, chest X‐ray; LDH, lactate dehydrogenase; SD, standard deviation.
Fisher's exact test.
3.3. Outcomes
Death was observed in 13 patients (2.4%) in group A and 6 patients (4.6%) in group B. Median duration of hospitalization was 21.5 days in group A and 19.0 days in group B, showing a statistically significant difference (p = .001). No differences were observed in the negative PCR results at HD 14 and PCR conversion from negative‐to‐positive results between the two groups. Oxygen demand at HD 7 and HD 14 was 16.4% and 9.0% in group A and 12.4% and 10.1% in group B, respectively. Fever at HD 7 and HD 14 was 7.3% and 0.4% in group A and 10.1% and 0.9% in group B, respectively (Table 3).
TABLE 3.
Treatment outcomes
| Group A | Group B | p‐Value | |
|---|---|---|---|
| (n = 535) | (n = 131) | ||
| N (%) | N (%) | ||
| Microbiological outcome | |||
| PCR results at HD 14 (total 220) | 44 (20.0%) | 12 (34.3%) | .058 |
| PCR conversion from negative to positive results | 176 (33.1%) | 33 (26.6%) | .16 |
| Clinical outcome | |||
| Oxygen demand at HD 7 | 87 (16.4%) | 16 (12.4%) | .267 |
| Fever at HD 7 | 39 (7.3%) | 13 (10.1%) | .299 |
| Oxygen demand at HD 14 | 47 (9.7%) | 13 (11.5%) | .563 |
| Fever at HD 14 | 2 (0.4%) | 1 (0.9%) | .473* |
| Duration of hospitalization, days | 21.5 (16.0–29.0) | 19.0 (13.0–25.0) | .001 |
| Death | 13 (2.4%) | 6 (4.6%) | .401* |
Values are presented as mean ± standard deviation or number (%) or median (interquartile range); group A: patients admitted to multiple occupancy rooms; group B: patients admitted to single occupancy rooms.
Abbreviations: HD, hospital day; PCR, polymerase chain reaction.
Fisher's exact test.
3.4. Risk factors for longer hospitalization
Multivariate analysis of the subset of variables with a p‐value < .05 in univariate analysis demonstrated that patients with initial body temperature ≥37.5°C (odds ratio [OR], 3.238; 95% confidence interval [CI], 1.679–6.244; p = .001), and pneumonia development during hospital stay (OR, 1.933; 95% CI, 1.333–2.805; p = .001) were the risk factors for longer duration of hospital stay (Table 4).
TABLE 4.
Risk factors for long hospitalization
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Odds ratio [OR] | 95% Confidence Interval [95% CI] | p‐Value | OR | 95% CI | p‐Value | |
| Male | 0.699 | 0.719–1.420 | .015 | 1.031 | 0.715–1.486 | .872 |
| Age over 75 | 0.84 | 0.562–1.253 | .393 | |||
| BT over 37.8°C | 4.05 | 2.139–7.667 | .001 | 3.238 | 1.679–6.244 | .001 |
| Initial abnormal CXR | 1.435 | 1.053–1.956 | .022 | |||
| Pneumonia during hospitalization | 2.049 | 1.465–2.864 | .001 | 1.933 | 1.333–2.805 | .001 |
| HTN | 0.954 | 0.680–1.340 | .786 | |||
| DM | 1.359 | 0.914–2.022 | .13 | |||
| Isolation | 0.616 | 0.411–0.924 | .019 | 0.808 | 0.526–1.242 | .332 |
| CRP > 1 mg/dl | 1.461 | 1.051–2.030 | .024 | 0.898 | 0.610–1.322 | .585 |
Abbreviations: BT, body temperature; CRP, C‐reactive protein; CXR, chest X‐ray; DM, diabetes mellitus; HTN, hypertension.
4. DISCUSSION
In this study, COVID‐19‐infected patients were hospitalized either in single occupancy rooms or in multiple occupancy rooms at our hospital. We found no differences between the two groups in terms of negative PCR results at HD 14, rates of conversion from negative‐to‐positive PCR results, death, oxygen demand, and fever. Although patients with different durations of infection were hospitalized in multiple occupancy rooms, we did not observe any significant influences on the microbiological results between patients with high and low viral shedding. However, the duration of hospitalization was longer in the shared room occupancy group.
To minimize the spread of COVID‐19, self‐isolation and contact precautions are recommended. For patients with respiratory symptoms that may produce airborne droplets, airborne precautions are also considered. Some studies have reported that to control the spread of COVID‐19, early airborne infection isolation is needed (Cheng et al., 2020). Negative pressure room isolation is recommended for patients with COVID‐19 who need aerosol‐generating procedures (Clinical management of severe acute respiratory infection when novel coronavirus (2019‐nCoV) infection is suspected: interim guidance, 2020). In the current pandemic, a shortage of negative pressure isolation rooms is expected around the world, as promulgated by the WHO.
Shared room occupancy has been used for infection control in hospitals; the congregation of confirmed patients in the cohort room prevents the spreading of the disease to other patients in the hospital (Doherty et al., 1998; Hall et al., 1978; Ong et al., 2001). It has been previously used to control droplet infection in cases of seasonal influenza, pertussis, and respiratory syncytial virus (RSV), especially when single rooms have not been available. Influenza and pertussis are mainly spread by aerosols and small droplets of infected fluids; large droplets can transmit RSV. For seasonal influenza outbreaks, guidelines are in place for shared room occupancy in case of unavailability of single rooms (Baek et al., 2014). Shared room occupancy of patients with bronchiolitis in hospitals was also done to prevent cross‐infection during the RSV epidemic (Doherty et al., 1998; Ong et al., 2001). In these previous studies, shared room occupancy was used to protect uninfected patients in hospitals during epidemics of infectious diseases. Shared room occupancy is also used to monitor patients exposed to infectious diseases, to observe the development of symptoms that correlate with the infection, such as fever and respiratory symptoms, as well as to prevent or curtail transmission within the hospital (Chen et al., 2006; Goh et al., 2006; Park et al., 2020; Tan et al., 2004). During the SARS epidemic in 2003, several hospitals in Singapore conducted a cohort quarantine of patients exposed to SARS to prevent intrahospital transmission (Gopalakrishna et al., 2004). During this epidemic, Tan Tock Seng Hospital (TTSH) was designated as a SARS hospital in Singapore. A total of 234 probable SARS patients were admitted to and treated at TTSH and were assigned to single rooms, not a shared room (Deurenberg‐Yap et al., 2005; Leong et al., 2006).
In this study, unlike in previous cohort sequestration, the entire hospital was isolated, and patients who tested positive were isolated and treated. Although older age has been more closely associated with severe COVID‐19 in previous studies (Chen et al., 2020; Guan et al., 2020), we found that the outcomes of the shared room occupancy group were not worse than that of the single room occupancy group, even with the inclusion of older patients. However, the shared room occupancy group showed a longer median duration of hospitalization; this suggests that the virus was transmitted within the shared room occupancy. It could also be attributed to the patients who were transferred from the nursing care facilities. Most of them were bedridden and had several underlying diseases, such as neurologic disease, cardiovascular disease, and solid tumours. In the early days of the COVID‐19 epidemic in Daegu, most patients admitted to our hospitals had mild symptoms, but as the number of confirmed patients increased, patients from nursing facilities were also admitted. They were usually admitted to the multiple occupancy rooms and were categorized in the shared room occupancy group. Among them, patients who requested resuscitation as part of their treatment were transferred to tertiary hospitals, while patients who did not request resuscitation died while undergoing symptomatic treatment.
This study had several limitations. First, patients were restricted to their hospital rooms as much as possible, and one healthcare worker continued to wear one full‐body coverage suit in the hospital. Second, none of the rooms was equipped with negative pressure. Third, mild cases of the disease were included, and severe cases were excluded, which makes it challenging to apply these findings to those with severe symptoms. Despite these limitations, this study is the first to report the feasibility of shared room occupancy in managing the spread of COVID‐19.
The data from this study demonstrate that shared room occupancy may be considered as an alternative to single room occupancy for patients with mild symptoms of COVID‐19 during the epidemic. If patients with mild symptoms are hospitalized in multiple occupancy rooms without negative pressure, patients with severe symptoms who may undergo more aerosol‐producing activities could use negative pressure rooms. This grouping also has the advantage of preventing the community spread of the disease by hospitalizing patients with mild symptoms. However, since the possibility of viral shedding cannot be ruled out in longer durations of hospitalization in the shared room occupancy, single room occupancy is usually considered first. Since this is a novel study on the feasibility of shared room occupancy for COVID‐19, further research is needed to evaluate other factors related to single and multiple occupancy room admissions and to examine the isolation conditions for patients with severe COVID‐19, as well for a larger number of patients with mild symptoms.
CONFLICTS OF INTEREST
All authors report no conflicts of interest relevant to this article.
ETHICAL APPROVAL
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to, and the appropriate ethical review committee approval has been received.
ACKNOWLEDGMENTS
This study was supported by a research grant from the Daegu Medical Association COVID‐19 Scientific Committee. We would like to thank Eun‐Sil Park for data entry. We also acknowledge Editage (www.editage.co.kr) for English language editing.
Hyun M, Lee JY, Kwon YS, et al. COVID‐19: Comparing the applicability of shared room and single room occupancy. Transbound Emerg Dis. 2021;68:2059–2065. 10.1111/tbed.13853
Miri Hyun and Ji Yeon Lee contributed equally to this study.
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
REFERENCES
- Baek, J. H. , Seo, Y. B. , Choi, W. S. , Kee, S. Y. , Jeong, H. W. , Lee, H. Y. , Eun, B. W. , Choo, E. J. , Lee, J. , Kim, S. R. , Kim, Y. K. , Song, J. Y. , Wie, S. H. , Lee, J. S. , Cheong, H. J. , Kim, W. J. ; Transgovernmental Enterprise for Pandemic Influenza in Korea . (2014). Guideline on the prevention and control of seasonal influenza in healthcare setting. Korean Journal of Internal Medicine, 29(2), 265–280. 10.3904/kjim.2014.29.2.265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, M. , Leo, Y. S. , Ang, B. , Heng, B. H. , & Choo, P. (2006). The outbreak of SARS at Tan Tock Seng Hospital—Relating epidemiology to control. Annals of the Academy of Medicine, Singapore, 35(5), 317–325. [PubMed] [Google Scholar]
- Chen, N. , Zhou, M. , Dong, X. , Qu, J. , Gong, F. , Han, Y. , Qiu, Y. , Wang, J. , Liu, Y. , Wei, Y. , Xia, J. , Yu, T. , Zhang, X. , & Zhang, L. (2020). Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet, 395(10223), 507–513. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, V. C. C. , Wong, S. C. , Chen, J. H. K. , Yip, C. C. Y. , Chuang, V. W. M. , Tsang, O. T. Y. , Sridhar, S. , Chan, J. F. W. , Ho, P. L. , & Yuen, K. Y. (2020). Escalating infection control response to the rapidly evolving epidemiology of the coronavirus disease 2019 (COVID‐19) due to SARS‐CoV‐2 in Hong Kong. Infection Control and Hospital Epidemiology, 41(5), 493–498. 10.1017/ice.2020.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical management of severe acute respiratory infection when novel coronavirus (2019‐nCoV) infection is suspected: Interim guidance . (2020). http://www.who.int/doc/default‐source/coronaviruse/clinical‐management‐of‐novel‐cov.pdf
- Deurenberg‐Yap, M. , Foo, L. L. , Low, Y. Y. , Chan, S. P. , Vijaya, K. , & Lee, M. (2005). The Singaporean response to the SARS outbreak: Knowledge sufficiency versus public trust. Health Promotion International, 20(4), 320–326. 10.1093/heapro/dai010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty, J. A. , Brookfield, D. S. , Gray, J. , & McEwan, R. A. (1998). Cohorting of infants with respiratory syncytial virus. Journal of Hospital Infection, 38(3), 203–206. 10.1016/s0195-6701(98)90275-4 [DOI] [PubMed] [Google Scholar]
- Goh, K. T. , Cutter, J. , Heng, B. H. , Ma, S. , Koh, B. K. , Kwok, C. , Toh, C. M. , & Chew, S. K. (2006). Epidemiology and control of SARS in Singapore. Annals of the Academy of Medicine, Singapore, 35(5), 301–316. [PubMed] [Google Scholar]
- Gopalakrishna, G. , Choo, P. , Leo, Y. S. , Tay, B. K. , Lim, Y. T. , Khan, A. S. , & Tan, C. C. (2004). SARS transmission and hospital containment. Emerging Infectious Diseases, 10(3), 395–400. 10.3201/eid1003.030650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan, W. J. , Ni, Z. Y. , Hu, Y. , Liang, W. H. , Ou, C. Q. , He, J. X. , Liu, L. , Shan, H. , Lei, C. L. , Hui, D. S. C. , Du, B. , Li, L. J. , Zeng, G. , Yuen, K. Y. , Chen, R. C. , Tang, C. L. , Wang, T. , Chen, P. Y. , Xiang, J. , …China Medical Treatment Expert Group for Covid‐19 . (2020). Clinical characteristics of Coronavirus Disease 2019 in China. New England Journal of Medicine, 382(18), 1708–1720. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, C. B. , Geiman, J. M. , Douglas, R. G., Jr. , & Meagher, M. P. (1978). Control of nosocomial respiratory syncytial viral infections. Pediatrics, 62(5), 728–732. [PubMed] [Google Scholar]
- Korean Society of Infectious Diseases ; Korean Society of Pediatric Infectious Diseases ; Korean Society of Epidemiology ; Korean Society for Antimicrobial Therapy ; Korean Society for Healthcare‐associated Infection Control and Prevention ; Korea Centers for Disease Control and Prevention . (2020). Report on the Epidemiological Features of Coronavirus Disease 2019 (COVID‐19) Outbreak in the Republic of Korea from January 19 to March 2, 2020. Journal of Korean Medical Science, 35(10), e112. 10.3346/jkms.2020.35.e112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong, H. N. , Earnest, A. , Lim, H. H. , Chin, C. F. , Tan, C. , Puhaindran, M. E. , Tan, A. , Chen, M. I. , & Leo, Y. S. (2006). SARS in Singapore—Predictors of disease severity. Annals of the Academy of Medicine, Singapore, 35(5), 326–331. [PubMed] [Google Scholar]
- Ong, G. M. , Wyatt, D. E. , O'Neill, H. J. , McCaughey, C. , & Coyle, P. V. (2001). A comparison of nested polymerase chain reaction and immunofluorescence for the diagnosis of respiratory infections in children with bronchiolitis, and the implications for a cohorting strategy. Journal of Hospital Infection, 49(2), 122–128. 10.1053/jhin.2001.1044 [DOI] [PubMed] [Google Scholar]
- Park, H. C. , Lee, S.‐H. , Kim, J. , Kim, D. H. , Cho, A. J. , Jeon, H. J. , Oh, J. , Noh, J.‐W. , Jeong, D.‐W. , Kim, Y.‐G. , Lee, C.‐H. , Yoo, K. D. , & Lee, Y.‐K. (2020). Effect of isolation practice on the transmission of middle east respiratory syndrome coronavirus among hemodialysis patients: A 2‐year prospective cohort study. Medicine (Baltimore), 99(3), e18782. 10.1097/MD.0000000000018782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, Y.‐M. , Chow, P. , Tan, B.‐H. , Kurup, A. , Tan, B. , Tan, F. , Seldrup, J. , Heng, D. , Ang, B. , Green, J. , Wong, C.‐Y. , & Soo, K.‐C. (2004). Management of inpatients exposed to an outbreak of severe acute respiratory syndrome (SARS). Journal of Hospital Infection, 58(3), 210–215. 10.1016/j.jhin.2004.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . (2020). Clinical management of severe acute respiratory infection when novel coronavirus (2019‐nCoV) infection is suspected: Interim guidance. https://www.who.int/publications‐detail/clinical‐management‐of‐severe‐acute‐respiratory‐infection‐when‐novel‐coronavirus‐(ncov)‐infection‐is‐suspected
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.


