Abstract
Background
Complete recovery of the CD4 T cell count is uncommon among chronically HIV-infected individuals with very low pre-treatment CD4 count. We studied the prevalence of chronically immune recovery and its associated factors including immune characteristics chronic HIV-infected Thais.
Methods
Treatment-naïve participants (n = 375) from the HIV-NAT 006 cohort with a pre-treatment CD4 T cell count after initiating antiretroviral therapy (ART) and having achieved a suppressed viremia (HIV-RNA level < 400 copies/mL) were retrospectively followed at the Thai Red Cross AIDS Research Centre, Bangkok, Thailand. Suboptimal immune recovery (SIR) was defined as having a CD4+ T cell count <200 cells/mm3 for 3 years after ART initiation. A case-control sub-study matched for age, sex and pre-ART CD4 T cell count was conducted to compare immunological characteristics between SIR (n = 17) and non-SIR (n = 24) participants. Immunological biomarkers such as interleukin-7 (IL-7) and soluble CD14 (sCD14) and other covariates including cytomegalovirus (CMV) DNA level, baseline hemoglobin level, hepatitis B and C co-infections, and T cell subsets associated with immune activation and exhaustion were evaluated.
Results
Among 375 participants with pre-ART CD4 T cell counts < 200 cells/mm3, the prevalence of SIR was 39.7%, 19.7% and 7.7% at years 1, 2 and 3 after starting ART, respectively. In a multivariate analysis, a pre-ART CD4 T cell count ≤100 cells/mm3 (adjusted odds ratio [aOR] 9.45, 95% CI 2.92–30.61, p < 0.001), older age (aOR 1.07, 95% CI 1.01–1.13, p = 0.029) and baseline HIV-RNA level (aOR 0.36, 95% CI 0.21–0.59, p < 0.001) were independently associated with SIR at year 3 after ART initiation. In the matched case-control sub-study (cases = 17, controls = 24), there was a higher prevalence of hepatitis C co-infection (18.8% vs. 0%, p = 0.05), lower sCD14 levels (mean, 6.23 vs. 6.27 log10 pg/mL, p = 0.04), lower CD8 T cell counts (mean, 514 vs. 876, p = 0.0003), lower CD4/CD8 T cell ratio (mean, 0.27 vs. 0.41, p = 0.01) and higher expression of PD1 on CD8+ T cells (74.2% vs. 65.1%, p = 0.02) observed in SIR participants compared to their non-SIR counterparts at year 3 after ART initiation.
Conclusions
Nearly 10% of the study participants who had achieved virological suppression failed to recover a CD4 T cell count > 200 cells/mm3 after 3 years of ART which was with a very low pre-ART CD4 T cell count and older age. The long-term clinical outcomes of SIR participants need to be further explored.
Keywords: Suboptimal immune recovery, Immune characteristics, Antiretroviral treatment, Asian
1. Background
The human immunodeficiency virus (HIV) depletes CD4+ T cells which is the major contributing factor leading to the acquired immunodeficiency syndrome (AIDS).1, 2, 3 Early initiation of combination antiretroviral therapy (ART) is associated with lower mortality and reduced incidence of AIDS events among people living with HIV (PLHIV).4,5 Since 2015, the World Health Organization (WHO) guidelines recommend to initiate ART regardless of the CD4 T cell count based on strong evidence of the benefit of early ART initiation.6 Globally, the overall improvement in the quality of HIV diagnosis and treatment has dramatically halted serious AIDS-related deaths and reduced transmission in the past decades. However, in many settings, a subset of PLHIV frequently present with late stage disease and have a low CD4+T cell count when they test. Therefore, ART is initiated in the context of advanced disease and extremely low CD4 T cell counts. These late presenters suffer from opportunistic infections and premature death.7
Early ART initiation improves the CD4 T cell count recovery, immune function, and longevity among PLHIV; however, a certain number of individuals on ART fail to restore their immune function even after achieving virological suppression.8,9 There are several CD4 T cell cut-off levels to indicate suboptimal immune recovery (SIR) post-ART.10, 11, 12, 13 Since a CD4 T cell count has clinical implications and is associated with AIDS and non-AIDS defining illnesses,14,15 we used this CD4 T cell level as the cut-off to indicate SIR. The frequency of SIR ranges from 15-30% and is associated with old age, male sex, low nadir CD4 T cell count and persistent immune activation.8,9,16, 17, 18, 19
The causal link between HIV-associated persistent inflammation/immune activation and disease progression, especially among chronically HIV-infected patients with low CD4 T cell counts, has been previously studied.20 Higher levels of soluble CD14 (sCD14) have been shown to be associated with all-cause mortality in PLHIV.21 Plasma interleukin-7 (IL-7) levels, a gamma chain receptor cytokine for the regulation of CD4+ and CD8+ T-cells, have been suggested to be negatively correlated with the CD4 T cell count and predictive of HIV disease progression.22 The normalization of CD4/CD8 ratio has been suggested to be a biomarker for immune dysfunction. PLHIV with low CD4 T cell counts and a low CD4/CD8 T cell ratio are at higher risk for developing AIDS-related and non-AIDS events.23,24 Moreover, a previous study showed that higher CD38+ expression was also independently associated with reduced survival despite HIV virological suppression, suggesting that immune activation in PLHIV contributes to disease progression.25
Unlike other regions,26 data regarding immune recovery are limited in the Asia region. Although current guidelines recommend ART initiation regardless of the CD4 T cell count, advanced disease and late presentation are still common in this part of the world.27,28 Therefore, it is important to better understand the characteristics and outcomes of PLHIV presenting with a low CD4 T cell count and those with persistent SIR after ART initiation. In this study, we investigated the prevalence of SIR with a CD4 T cell count less than 200 cells/mm3 after 3 years of initiating ART and its associated factors including immune characteristics among HIV-infected Thais.
2. Methods
2.1. Study participants
Data from ART-naïve HIV-infected adults aged ≥18 years old who were enrolled between 2011 to 2013 into the HIV_NAT 006 (the Netherlands-Australia and Thailand collaboration in HIV research) a long-term follow-up HIV cohort in Bangkok, Thailand,23,29,30 were analyzed. ART was initiated at enrollment. Sociodemographic and HIV-related variables such as age, sex, types of ART regimen, HIV-RNA level, CD4 T cell counts and baseline clinical events (including opportunistic infections and tuberculosis) were collected.
2.2. Definitions of suboptimal immune recovery and other variables
In this analysis, we have defined SIR as a CD4 T cell count < 200 cells/mm3 after 3 years of successful ART with suppressed HIV viremia (HIV RNA levels ≤ 400 copies/mL). Hepatitis B (HBV) co-infection was defined as having a positive hepatitis B surface antigen. Hepatitis C (HCV) co-infection was defined by the detection of HBs antigen. HCV co-infection was defined by the presence of HCV antibodies confirmed by HCV viremia. HCV antibodies were tested with a third generation CMIA (ARCHITECT system, Abbott Diagnostics, Wiesbaden, Germany). To confirm HCV infection we quantified HCV RNA with RealTime PCR (m2000 system, Abbott Molecular, Inc., Des Plaines, IL, USA).
2.3. Markers of immune activation, T cell subsets and co-infections
Immunological parameters such as the CD4/CD8 T cell ratio, IL-7, sCD14 and immune-phenotyping were determined in a cross sectional sub-study of a smaller sample size (cases = 17, controls = 24) at year 3 after initiating ART. Cases were defined as participants with < 200 CD4 T cells/mm3 CD4 T cell and controls as those with > 200 CD4 T cells/mm3 CD4 T cell count. Cases and controls were matched by age, sex and pre-ART CD4 T cell. Ages were classified into 5 categories, ≤30, 31–35, 36–40, 41–45, 46–50 years old (five-year age bands). In terms of the CD4 T cell count, it was classified into 4 categories, <50, 50–100, 100–150, 150–200 (50 cells/mm3 CD4 T cell bands). Cases were matched to controls with matching variables. Available stored samples at the time of analysis were included into this sub-study. Site investigators reviewed the selection process.
Frozen peripheral blood mononuclear cells (PBMC) were analyzed using flow cytometry. Cells were stained and analyzed to determine the proportion of CD4+ and CD8+ T cells using 8-colour flow cytometry (FACS Diva, BD Biosciences). T cell maturation subsets such as naïve (TN, CD45RA+CCR7+), central memory (TCM, CD45RA-CCR7+), and transitional memory (TTM, CD45RA-CCR7-) cells. Activated phenotypes of CD4+ and CD8+ T cells were also determined by the proportion of CD38 and HLADR (CD4+CD38+HLA-DR+, and CD8+CD38+HLA-DR+ cells). T cell exhaustion was determined by the percentage of programmed death -1 (PD-1) expression on both CD4+ and CD8+ T cells. Cryopreserved plasma was further tested by immunoassay for IL-7 (R&D Systems, Minneapolis, MN, USA) and sCD14 (R&D Systems, Minneapolis, MN, USA). All participants were tested for co-infections such as cytomegalovirus (CMV) HBV and HCV infections. (CMV), HBV and HCV. CMV DNA polymerase chain reaction (PCR) tests were performed (Roche Molecular Systems, NJ, USA) using plasma samples. The assay used had a CMV DNA limit of detection (LOD) of 44 copies/mL.
2.4. Statistical analysis
Baseline characteristics including age, sex, mode of transmission, body mass index (BMI), pre-ART CD4 T cell count, HIV-RNA level and Centers for Disease Control and Prevention (CDC) HIV staging were compared between participants with or without SIR. Continuous variables were expressed as median (interquartile range, IQR). Differences in continuous and categorical variables between SIR and non-SIR groups were assessed using a Wilcoxon rank sum test and chi-square-test, respectively. The prevalence of SIR among study participants with virological suppression was described at years 1, 2 and 3 after initiating ART. Predictors of SIR were explored by using univariate analysis and then multivariate logistic regression models by adjusting the significant confounders (p < 0.1 from the univariate analysis). Statistical significance was defined as p < 0.05 from the multivariate model. From a total of 375 participants, a cross-sectional case-control sub-study was done in a subset of the participants (cases = 17, controls = 24) that were matched for age, sex and pre-ART CD4 T cell counts to compare immune characteristics of SIR and non-SIR participants. All analyses were carried out using STATA version 13.1 (Stata Corp., College Station, Texas, USA).
2.5. Ethical considerations
All participants gave informed consent. This cohort study was reviewed and approved by the Institutional Review Boards of the Faculty of Medicine, Chulalongkorn University in Bangkok, Thailand.
3. Results
3.1. Participant characteristics
A total of 375 participants who had been treated with ART for ≥3 years were recruited into the study. Baseline characteristics are described in Table 1. The overall median age of participants was 34 (IQR, 30–40) years with median pre-ART CD4 T cell counts of 74 (IQR, 27–131) cells/mm3. 21.3% of participants had CDC stage C. The median baseline HIV-RNA level was 4.8 (IQR, 4.3–5.3) log10 copies/mL. Among study participants, 151 (41.7%) and 159 (43.9%) of them initiated treatment with non-nucleotide reserve transcriptase inhibitors (NNRTI)-based and protease inhibitors (PI)-based regimens, respectively. Proportions of the participants with baseline HBV and HCV co-infections were 20.6% and 6.3%, respectively. 51 (24.5%) participants had detectable CMV DNA levels in the non-SIR group compared to the SIR group (0%).
Table 1.
Participants’ baseline characteristics.
| Suboptimal Immune Response, SIR (n = 29) |
Non-SIR (n = 346) |
Total (n = 375) |
P-value | |
|---|---|---|---|---|
| Sex, n (%) | 0.029 | |||
| Female | 7 (24.1) | 156 (45.1) | 163 (43.5) | |
| Male | 22 (75.9) | 190 (54.9) | 212 (56.5) | |
| Age in years, median (IQR) | 38 [34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44] | 34 [30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40] | 34 [30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40] | 0.015 |
| BMI, median (IQR) | 19.3 (17.9–21.9) N = 11 |
20.5 (18.5–22.6) N = 180 |
20.5 (18.5–22.5) N = 191 |
0.31 |
| Pre-ART CD4 cell count, cells/mm3, median (IQR) | 50 (18–84) | 100 (34–151) | 89 (33–148) | 0.005 |
| Pre-ART HIV-RNA, log10copies/mL, median (IQR) | 4.5 (4.2–4.8) | 4.8 (4.4–5.3) | 4.8 (4.3–5.3) | 0.012 |
| CDC clinical staging, n (%) | 0.44 | |||
| A | 7 (24.1) | 122 (35.3) | 129 (34.4) | |
| B | 14 (48.3) | 152 (43.9) | 166 (44.3) | |
| C | 8 (27.6) | 72 (20.8) | 80 (21.3) | |
| CMV DNA, copies/mL, n (%) | N = 26 | N = 208 | N = 234 | 0.002 |
| <44 (lower limit of detection) | 26 (100.00) | 157 (75.5) | 183 (78.2) | |
| ≥44 | 0 (0.00) | 51 (24.5) | 51 (21.8) | |
| Hepatitis B coinfection, n (%) | 6 (21.4) N = 28 |
61 (20.5) N = 298 |
67 (20.6) N = 326 |
0.90 |
| Hepatitis C coinfection, n (%) | 3 (11.1) N = 27 |
18 (5.8) N = 308 |
21 (6.3) N = 335 |
0.23 |
3.2. Prevalence and factors associated with SIR at 3 year after initiating ART
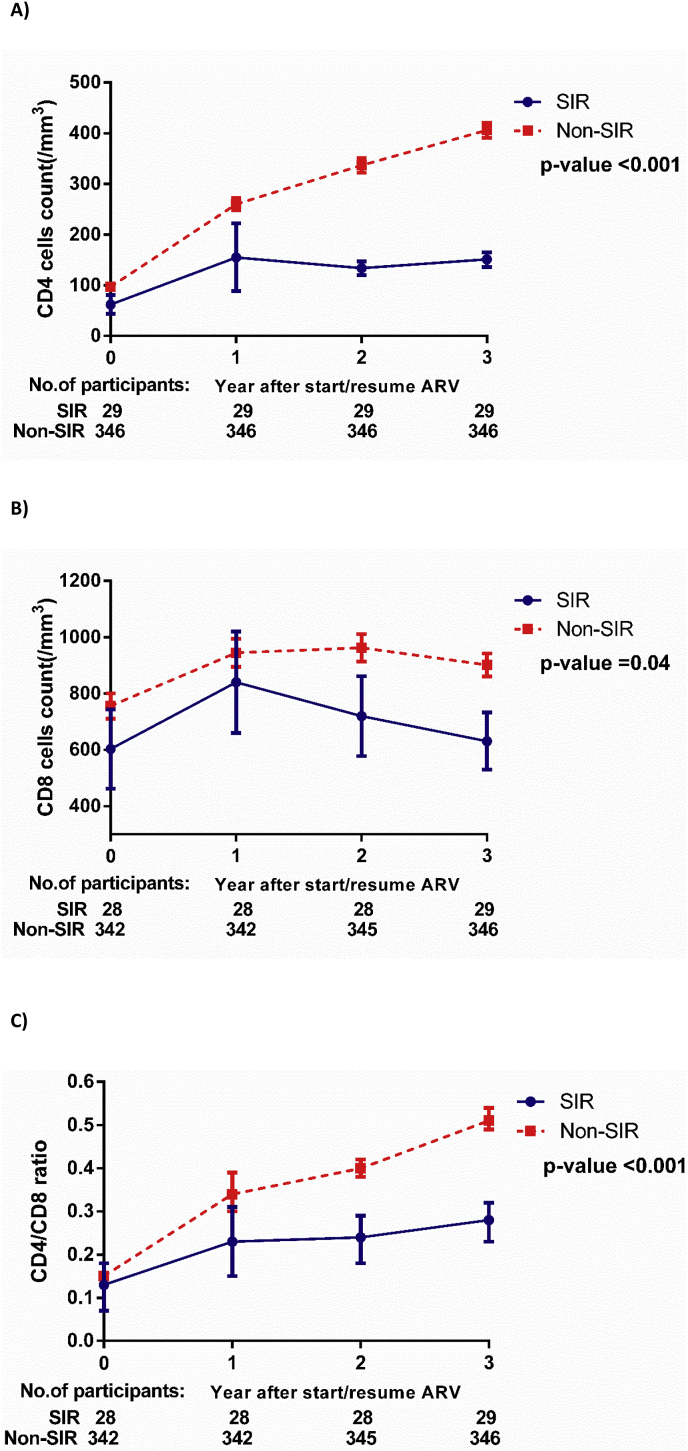
The prevalence of SIR was 39.73%, 19.7% and 7.73% at years 1, 2 and 3 after starting ART, respectively. The CD4 and CD8 T cell counts and CD4/CD8 T cell ratio of participants with SIR and non-SIR at year 1, year 2 and year 3 are described in Fig. 1. Participants in the SIR group had a lower pre-ART CD4 T cell count compared to the non-SIR group (44, IQR, 18–72 vs. 79, IQR, 27–134, p = 0.003) at year 3 after initiating ART. Three years after initiating ART, participants with SIR had significant lower median CD4 T cell counts (161, IQR, 133–181 vs. 375, IQR, 302–490 cells/mm3, p < 0.001) compared to the participants with non-SIR. Moreover, the participants with SIR had lower median CD4/CD8 ratio compared to their counterparts at 3 years after initiating cART (0.24, IQR, 0.2–0.33 vs. 0.45, IQR, 0.35–0.63, p < 0.001) (see Fig. 2).
Fig. 1.
The graphs show CD4 (1 A), CD8 (1 B) T cell counts and CD4/CD8 (1 C) recovery among participants with suboptimal immune response (SIR) and non-SIR group.
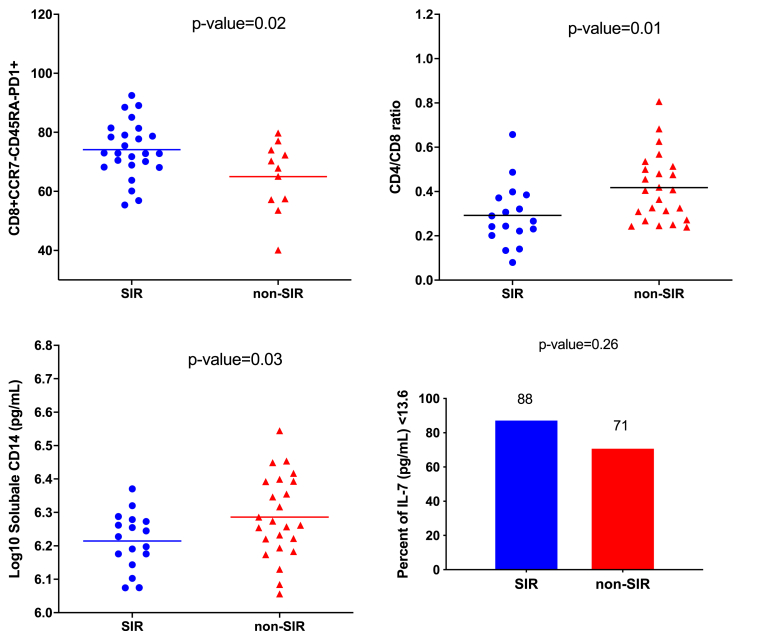
Fig. 2.
Scattered plot shows the immunological characteristics of the matched case-control (case = SIR, control = non-SIR) sub-study at year 3 after initiating ART.
Abbreviations: SIR, suboptimal immune recovery; ART, antiretroviral therapy. The bars in the scattered plots represent the mean values.
At year 3 after initiating ART, male sex, older age, and pre-ART CD4 T cell count <100 cells/mm3 (vs. ≥100 cells/mm3) were associated with SIR in the univariate analysis (Table 2). Baseline HIV-RNA level was negatively associated with SIR. After adjusting for confounders from the univariate analysis, older age (aOR 1.07, 95% CI 1.01–1.13, p = 0.029), and a pre-ART CD4 T cell count <100 cells/mm3 (aOR 9.45, 95% CI 2.92–30.61, p < 0.001, vs. ≥100 cells/mm3) were independently associated with SIR at year 3 after initiating ART. Baseline HIV-RNA level (aOR 0.36, 95% CI 0.21–0.59, p < 0.001) was negatively associated with SIR after adjusting for confounders from the univariate model. We did not find any significant association of viral hepatitis, baseline hemoglobin level and baseline CMV DNA levels with SIR at year 3 after initiating ART.
Table 2.
Univariate and multivariate logistic regression of suboptimal immune recovery (SIR) at year 3 after initiating ART.
| Variables for SIR | Univariate modela |
Multivariate model |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | aOR | 95% CI | p-value | |
| Male | 2.58 | (1.07–6.20) | 0.034 | 1.96 | (0.78–4.94) | 0.155 |
| Age (years) | 1.06 | (1.00–1.11) | 0.027 | 1.07 | (1.01–1.13) | 0.029 |
| Baseline CDC stage C (vs. stage A/B) | 1.45 | (0.62–3.41) | 0.394 | |||
| BMI (kg/m2) | 0.92 | (0.76–1.10) | 0.348 | |||
| Pre-ART CD4 cell count ≤100 101–200 |
3.83 Ref | (1.52–9.65) | 0.004 | 9.45 Ref | (2.92–30.61) | <0.001 |
| Pre-ART HIV-RNA level | 0.54 | (0.36–0.81) | 0.003 | 0.36 | (0.21–0.59) | <0.001 |
| HBV co-infection | 1.06 | (0.41–2.73) | 0.904 | |||
| Baseline Hb | 1.01 | (0.83–1.25) | 0.891 | |||
| HCV co-infection | 2.01 | (0.55–7.32) | 0.288 | |||
OR = odds ratio; CI = confidence interval; Hb = heamoglobin; BMI = body mass index; aOR = adjust odds ratio.
Covariates with p-value less than 0.1 were adjusted in the multivariate models.
3.3. Immune activation and T-cell activation/exhaustion in the matched case-control sub-study
The characteristics of participants in the matched case-control sub-study are described in Table 3. At year 3 after initiating ART, all participants had a suppressed viral load. Immunological parameters were obtained from participants at year 3 after initiating ART. In the matched case-control sub-study (SIR = 17, non-SIR = 24), participants from the SIR group had significantly lower CD8 T cell counts (719 [95% CI, 604–834] vs. 920 [95% CI 614–1226], p = 0.003) and CD4/CD8 T cell ratios. (0.27 [95% CI, 0.22–0.37] vs. 0.41 [95% CI, 0.29–0.51], p = 0.01) compared to the non-SIR group. None of the participants had detectable CMV DNA level at year 3 after initiating ART. However, participants from the SIR group had lower sCD14 levels than those from the non-SIR group (6.23 IQR, 6.18–6.27 vs. 6.27 IQR, 6.21–6.39 log10 pg/mL, p = 0.03). There was no statistical difference in IL-7 levels between the two groups. Additionally, analysis of T cell subsets showed significant higher levels of CD8+ TTM cell exhaustion (CD8+CCR7-CD45RA-PD1+, 74.2% vs. 65.1%, p = 0.02) among participants in the SIR group at year 3 after initiating ART. We did not find any significant differences in T cell activation levels between the two groups. Detailed results of the immune activation and exhaustion markers of T cells are described in the supplemental table (Table S1).
Table 3.
Immunological characteristics among SIR and non-SIR participants at year 3 after initiating ART.
| Age, sex and baseline CD4 count matched case-control |
|||
|---|---|---|---|
| SIR | Non-SIR | P-value1 | |
| Number of participants | N = 17 | N = 24 | |
| Sex, n (%) | 0.80 | ||
| Female | 3 (17.7) | 5 (20.8) | |
| Male | 14 (82.3) | 19 (79.2) | |
| Age in years, median (IQR) | 36 (34–44) | 37(32–42) | 0.72 |
| BMI, median (IQR) | 20.6 (19.3–21.9) | 21.1 (17.9–24.2) | 0.70 |
| Pre-ART CD4 count cells/mm3, median (IQR) | 0.837 | ||
| ≤100 | 4 (23.5) | 5 (20.8) | |
| 101–200 | 13 (76.5) | 19 (79.2) | |
| Pre-ART HIV-RNA level in log10copies/mL, median (IQR) | 4.32 (4.2–4.8) | 5.13 (4.6–5.6) | 0.003 |
| HBV co-infection, n (%) | 3/16 (18.8) | 10/20 (50.00) | 0.083 |
| HCV co-infection, n (%) | 3/16 (18.8) | 0/19 (0.00) | 0.05 |
| IL-7 <13.6 pg/mL, n (%) | 15 (88.2) | 17 (70.8) | 0.262 |
| sCD14 (Log10pg/mL), mean (95% CI) | 6.23 (6.18–6.27) | 6.27 (6.21–6.39) | 0.04 |
| CD4/CD8 ratio, mean (95% CI) | 0.27 (0.22–0.37) | 0.41 (0.29–0.51) | 0.010 |
| CD8 count, mean (95% CI) | 514 (358–695) | 876 (652–1127) | 0.0003 |
| CD8+CCR7-CD45RA-PD1-, mean (95% CI) | 25.1 (20.6–27.9) | 34.9 (28.3–42.5) | 0.02 |
| CD8+CCR7-CD45RA-PD1+, mean (95% CI) | 74.2 (70.5–78.7) | 65.1 (57.2–70.3) | 0.02 |
| CMV DNA, copies/mL, n (%) | |||
| <44 (lower limit of detection) | 17 (100.0) | 24 (100.0) | N/A |
| ≥44 | 0 (0.0) | 0(0.0) | |
4. Discussion
This study shows that nearly 10% of study participants who had achieved virological suppression had SIR with a CD4 T cell count <200 cells/mm3 after continuous ART for 3 years. The prevalence of SIR was 39.73%, 19.7% and 7.73% at years 1, 2 and 3 after starting ART, respectively. The frequency of SIR from this Thai cohort is comparable to that of SIR after 24 months of ART in an comparable to that of an urban cohort from Sub-Sahara Africa after 24 months that used the same CD4 T cell threshold criteria of <200 cells/mm3(12).
SIR participants at year 3 after initiating ART were older, had a lower nadir CD4 T cell count and at year one after ART initiation together with a lower HIV-RNA at baseline. From the multivariate analysis, factors associated with SIR included older age, lower CD4 T cell counts and HIV-RNA level at ART initiation. Previous studies have also shown that age is a predictor of SIR,31,32 even among participants with virological suppression.8 The association of age and SIR with good virological response is possibly due to failing thymic T cell production.8 We did not find an association of viral hepatitis infections (HBV and HCV) with SIR at year 3 after initiating ART. This finding is consistent with a report that showed that neither HBV co-infection or HBeAg reactivity had any impact on CD4 T cell recovery while on ART.33
The prevalence of SIR varied according to different settings.34 For example a study from sub-Sahara Africa reported nearly 15% participants with SIR (using CD4 <200 cells/mm3) at 3 years and 7% at 6 years post-ART initiation.35 This current study showed 7% the predictive value of a low CD4 T cell count at ART initiation. In addition, multiple studies have shown a nearly 8% SIR prevalence after 3 years of ART initiation and how it affects the long-term immune recovery of PLHIV.36, 37, 38 A low baseline CD4 T cell count (<100 cells/mm3) is a strong predictor for SIR in our study. This is in line with the report from the Swiss HIV cohort study which showed that the baseline CD4 T cell count independently predicted the incomplete immune response (defined by CD4 T cell count recovery of <500 cells/mm3) 5 years after initiatingby the ART.32 The study also showed that the annual increase in the CD4 T cell count was relatively smaller in immunological non-responders compared to their counterparts.32
We also found that the baseline HIV-RNA level was negatively correlated with SIR despite the fact that all participants had viral suppression at year 3 after initiating ART. This result is consistent with a recent modelling study that assessed the CD4 recovery over the years after ART initiation; a higher HIV viral load at ART initiation was strongly associated with a higher level of CD4 T cell recovery.39 Previous studies have suggested that a high pre-treatment HIV viral load was a predictor of rapid CD4 T cell recovery because the sequestered T cells may be in higher numbers within the lymphoid tissues in a context of uncontrolled viral replication and could be released quickly into the circulation after ART initiation.40, 41, 42
In our study, baseline CDC grade B or C events were not associated with SIR at year 3 after initiating ART. A previous report showed that 21% of incomplete responders developed clinical CDC grade B or C events but there was no difference in terms of AIDS events between incomplete and complete responders.32 However, in our study, we could not evaluate whether there were any differences in AIDS-related events among participants with and without SIR after initiating ART because of the the small sample size was small.
The CD4/CD8 T cell ratio among SIR participants in the study was significantly lower than non-SIR participants at year 3 after ART initiation. Additionally, we also found that the the CD4/CD8 T cell ratio recovery was much slower in SIR participants (Supplementary Table S2). This finding indicates that there is a high level of immune activation among SIR participants; a previous study reported that the CD4/CD8 T cell ratio recovery was a surrogate marker for chronic inflammation and immune activation.43 We have recently reported that a low CD4/CD8 T cell ratio in the Thai cohort was associated with a lower baseline CD4 T cell count and could predict non-AIDS events after years of follow-up, even in a context of virological suppression.44 The SIR participants whose CD4/CD8 T cell ratio remained low after years of suppressive cART may have poor long-term prognosis such as a higher chance of developing non-AIDS related events and comorbidities. A longer follow-up study is needed.
We did not find a difference in the expression of activation markers (HLA-DR, CD38) on both CD4+ and CD8+ T cells between SIR and non-SIR participants. However, SIR participants had a higher expression of PD-1 on CD8+ T cells which is in line with previous reports; PLHIV with low nadir CD4 T cells are immunologically not responsive because there is intrinsic T cell apoptosis.26,45 Surprisingly, sCD14 levels in our SIR participants were lower than in their non-SIR counterparts at year 3 after initiating ART despite the fact that all participants had undetectable CMV DNA levels. The levels of sCD14 among our study participants (both SIR and non-SIR) were higher than those of healthy HIV-negative Thais (median, 5.9, IQR 5.9–6.0 log10 pg/mL, n = 10) from a previous study.46
Our study was limited by the relatively short duration of follow-up to evaluate CD4 T cell recovery. Second, the sample size of the matched case-control group was small so it was difficult to analyze immune predictors and other parameters for immune recovery. and other parameters. Third, the clinical significance in the difference of the various markers observed between SIR and non-SIR participants in the sub-study was remains uncertain, thus these differences should be interpreted cautiously and further studies should explore the long-term clinical impact between the two groups. Nevertheless, our finding provides with an insight on the immunology of non-responders in an Asian population that was previously unavailable.
In conclusion, SIR after ART initiation is common among participants with a low baseline CD4 T cell count. Nearly 10% of study participants who had achieved virological suppression failed to recover a CD4 T cell count to ≥200 cells/mm3 after 3 years of ART which was associated with very low pre-ART CD4 cell counts (CD4 < 100 cells/mm3) and older age. We also found that SIR participants had low CD4/CD8 T cell ratios and high levels of T cell exhaustion. The long-term clinical outcomes of SIR participants need to be further investigated.
Funding
This work was supported by Ratchadapiseksompotch Fund, Faculty of Medicine, Chulalongkorn University Code: RA 35/53. The funders had no role in the study design, in the collection, analysis and interpretation of data, in the writing of the manuscript, and in the decision to submit the manuscript for publication.
Declaration of competing interest
AA has received honorarium for consultation from ViiV Healthcare. KR received honoraria or consultation fees from Merck, Roche, Jensen-Cilag, Johnson & Johnson, Mylan and GPO (Governmental pharmaceutical organization, Thailand); has participated in a company sponsored speaker’s bureau from Abbott, Gilead, Bristol-Myers Squibb, Merck, Roche, Jensen-Cilag, ViiV Healthcare, and GPO (Governmental pharmaceutical organization); and received Chulalongkorn Academic Advancement into Its 2nd Century Project (CUAASC). JA has received honoraria for participating in advisory meetings from ViIV Healthcare, Merck, Gilead, Roche and AbbVie. The rest of the authors declare no conflict of interest.
Acknowledgements
We would like to thank all of the participants for their contribution to the study. We would also like to thank Pirapon June Ohata for editing the manuscript and the team of HIV-NAT 006 cohort for their hard work.
Footnotes
Some of the data from this manuscript was presented as a poster presentation at the 10th International AIDS Conference on HIV Science, Mexico City, Mexico, July 21–24, 2019.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jve.2020.100005.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Okoye A.A., Picker L.J. CD4(+) T-cell depletion in HIV infection: mechanisms of immunological failure. Immunol. Rev. 2013;254(1):54–64. doi: 10.1111/imr.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klatzmann D., Barre-Sinoussi F., Nugeyre M.T., Danquet C., Vilmer E., Griscelli C. Selective tropism of lymphadenopathy associated virus (LAV) for helper-inducer T lymphocytes. Science. 1984;225(4657):59–63. doi: 10.1126/science.6328660. [DOI] [PubMed] [Google Scholar]
- 3.Masur H., Ognibene F.P., Yarchoan R., Shelhamer J.H., Baird B.F., Travis W. CD4 counts as predictors of opportunistic pneumonias in human immunodeficiency virus (HIV) infection. Ann. Intern. Med. 1989;111(3):223–231. doi: 10.7326/0003-4819-111-3-223. [DOI] [PubMed] [Google Scholar]
- 4.When To Start C. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373(9672):1352–1363. doi: 10.1016/S0140-6736(09)60612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Initiation of antiretroviral therapy in early asymptomatic HIV infection. N. Engl. J. Med. 2015;373(9):795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO . WHO; Geneva: 2015. Guideline on when to Start Antiretroviral Therapy and on Pre-exposure Prophylaxis for HIV. [PubMed] [Google Scholar]
- 7.Phillips A.N., Lundgren J.D. The CD4 lymphocyte count and risk of clinical progression. Curr. Opin. HIV AIDS. 2006;1(1):43–49. doi: 10.1097/01.COH.0000194106.12816.b1. [DOI] [PubMed] [Google Scholar]
- 8.Teixeira L., Valdez H., McCune J.M., Koup R.A., Badley A.D., Hellerstein M.K. Poor CD4 T cell restoration after suppression of HIV-1 replication may reflect lower thymic function. Aids. 2001;15(14):1749–1756. doi: 10.1097/00002030-200109280-00002. [DOI] [PubMed] [Google Scholar]
- 9.Aiuti F., Mezzaroma I. Failure to reconstitute CD4+ T-cells despite suppression of HIV replication under HAART. AIDS Rev. 2006;8(2):88–97. [PubMed] [Google Scholar]
- 10.Tuboi S.H., Brinkhof M.W., Egger M., Stone R.A., Braitstein P., Nash D. Discordant responses to potent antiretroviral treatment in previously naive HIV-1-infected adults initiating treatment in resource-constrained countries: the antiretroviral therapy in low-income countries (ART-LINC) collaboration. J. Acquir. Immune Defic. Syndr. 2007;45(1):52–59. doi: 10.1097/QAI.0b013e318042e1c3. [DOI] [PubMed] [Google Scholar]
- 11.Florence E., Lundgren J., Dreezen C., Fisher M., Kirk O., Blaxhult A. Factors associated with a reduced CD4 lymphocyte count response to HAART despite full viral suppression in the EuroSIDA study. HIV Med. 2003;4(3):255–262. doi: 10.1046/j.1468-1293.2003.00156.x. [DOI] [PubMed] [Google Scholar]
- 12.Nakanjako D., Kiragga A., Ibrahim F., Castelnuovo B., Kamya M.R., Easterbrook P.J. Sub-optimal CD4 reconstitution despite viral suppression in an urban cohort on antiretroviral therapy (ART) in sub-Saharan Africa: frequency and clinical significance. AIDS Res. Ther. 2008;5:23. doi: 10.1186/1742-6405-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaufmann G.R., Furrer H., Ledergerber B., Perrin L., Opravil M., Vernazza P. Characteristics, determinants, and clinical relevance of CD4 T cell recovery to <500 cells/microL in HIV type 1-infected individuals receiving potent antiretroviral therapy. Clin. Infect. Dis. 2005;41(3):361–372. doi: 10.1086/431484. [DOI] [PubMed] [Google Scholar]
- 14.Anglaret X., Minga A., Gabillard D., Ouassa T., Messou E., Morris B. AIDS and non-AIDS morbidity and mortality across the spectrum of CD4 cell counts in HIV-infected adults before starting antiretroviral therapy in Cote d’Ivoire. Clin. Infect. Dis. 2012;54(5):714–723. doi: 10.1093/cid/cir898. an official publication of the Infectious Diseases Society of America. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Opportunistic Infections Project Team of the Collaboration of Observational HIVERiEiE. Young J., Psichogiou M., Meyer L., Ayayi S., Grabar S. CD4 cell count and the risk of AIDS or death in HIV-Infected adults on combination antiretroviral therapy with a suppressed viral load: a longitudinal cohort study from COHERE. PLoS Med. 2012;9(3) doi: 10.1371/journal.pmed.1001194. e1001194-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.d’Arminio Monforte A., Tincati C., Bellistré G.M., Gazzola L., Marchetti G. The absence of CD4+ T cell count recovery despite receipt of virologically suppressive highly active antiretroviral therapy: clinical risk, immunological gaps, and therapeutic options. Clin. Infect. Dis. 2009;48(3):328–337. doi: 10.1086/595851. [DOI] [PubMed] [Google Scholar]
- 17.Baker J.V., Peng G., Rapkin J., Krason D., Reilly C., Cavert W.P. Poor initial CD4+ recovery with antiretroviral therapy prolongs immune depletion and increases risk for AIDS and non-AIDS diseases. J. Acquir. Immune Defic. Syndr. 2008;48(5):541–546. doi: 10.1097/QAI.0b013e31817bebb3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaardbo J.C., Hartling H.J., Gerstoft J., Nielsen S.D. Incomplete immune recovery in HIV infection: mechanisms, relevance for clinical care, and possible solutions. Clin. Dev. Immunol. 2012;2012:670957. doi: 10.1155/2012/670957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thorsteinsson K., Ladelund S., Jensen-Fangel S., Johansen I.S., Katzenstein T.L., Pedersen G. Impact of gender on response to highly active antiretroviral therapy in HIV-1 infected patients: a nationwide population-based cohort study. BMC Infect. Dis. 2012;12:293. doi: 10.1186/1471-2334-12-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Sadr W.M., Lundgren J., Neaton J.D., Gordin F., Abrams D., Arduino R.C. CD4+ count-guided interruption of antiretroviral treatment. N. Engl. J. Med. 2006;355(22):2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 21.Sandler N.G., Wand H., Roque A., Law M., Nason M.C., Nixon D.E. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J. Infect. Dis. 2011;203(6):780–790. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Llano A., Barretina J., Gutierrez A., Blanco J., Cabrera C., Clotet B. Interleukin-7 in plasma correlates with CD4 T-cell depletion and may be associated with emergence of syncytium-inducing variants in human immunodeficiency virus type 1-positive individuals. J. Virol. 2001;75(21):10319–10325. doi: 10.1128/JVI.75.21.10319-10325.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han W.M., Apornpong T., Kerr S.J., Hiransuthikul A., Gatechompol S., Do T. CD4/CD8 ratio normalization rates and low ratio as prognostic marker for non-AIDS defining events among long-term virologically suppressed people living with HIV. AIDS Res. Ther. 2018;15(1):13. doi: 10.1186/s12981-018-0200-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mussini C., Lorenzini P., Cozzi-Lepri A., Lapadula G., Marchetti G., Nicastri E. CD4/CD8 ratio normalisation and non-AIDS-related events in individuals with HIV who achieve viral load suppression with antiretroviral therapy: an observational cohort study. Lancet HIV. 2015;2(3):e98–106. doi: 10.1016/S2352-3018(15)00006-5. [DOI] [PubMed] [Google Scholar]
- 25.Giorgi J.V., Hultin L.E., McKeating J.A., Johnson T.D., Owens B., Jacobson L.P. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J. Infect. Dis. 1999;179(4):859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 26.Nakanjako D., Ssewanyana I., Mayanja-Kizza H., Kiragga A., Colebunders R., Manabe Y.C. High T-cell immune activation and immune exhaustion among individuals with suboptimal CD4 recovery after 4 years of antiretroviral therapy in an African cohort. BMC Infect. Dis. 2011;11:43. doi: 10.1186/1471-2334-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeong S.J., Italiano C., Chaiwarith R., Ng O.T., Vanar S., Jiamsakul A. Late presentation into care of HIV disease and its associated factors in Asia: results of TAHOD. AIDS Res. Hum. Retrovir. 2016;32(3):255–261. doi: 10.1089/aid.2015.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin K.-Y., Cheng C.-Y., Li C.-W., Yang C.-J., Tsai M.-S., Liu C.-E. Trends and outcomes of late initiation of combination antiretroviral therapy driven by late presentation among HIV-positive Taiwanese patients in the era of treatment scale-up. PloS One. 2017;12(6) doi: 10.1371/journal.pone.0179870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Avihingsanon A., Apornpong T., Ramautarsing R.A., Ubolyam S., Tangkijvanich P., Ananworanich J. Decline in serum 25 hydroxyvitamin D levels in HIV-HBV-coinfected patients after long-term antiretroviral therapy. Antivir. Ther. 2014;19(1):41–49. doi: 10.3851/IMP2673. [DOI] [PubMed] [Google Scholar]
- 30.Durier N., Ananworanich J., Apornpong T., Ubolyam S., Kerr S.J., Mahanontharit A. Cytomegalovirus viremia in Thai HIV-infected patients on antiretroviral therapy: prevalence and associated mortality. Clin. Infect. Dis. 2013;57(1):147–155. doi: 10.1093/cid/cit173. [DOI] [PubMed] [Google Scholar]
- 31.Lawn S.D., Myer L., Bekker L.-G., Wood R. CD4 cell count recovery among HIV-infected patients with very advanced immunodeficiency commencing antiretroviral treatment in sub-Saharan Africa. BMC Infect. Dis. 2006;6:59. doi: 10.1186/1471-2334-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaufmann G.R., Furrer H., Ledergerber B., Perrin L., Opravil M., Vernazza P. Characteristics, determinants, and clinical relevance of CD4 T cell recovery to <500 cells/microL in HIV type 1-infected individuals receiving potent antiretroviral therapy. Clin. Infect. Dis. 2005;41(3):361–372. doi: 10.1086/431484. [DOI] [PubMed] [Google Scholar]
- 33.Omland L.H., Weis N., Skinhoj P., Laursen A., Christensen P.B., Nielsen H.I. Impact of hepatitis B virus co-infection on response to highly active antiretroviral treatment and outcome in HIV-infected individuals: a nationwide cohort study. HIV Med. 2008;9(5):300–306. doi: 10.1111/j.1468-1293.2008.00564.x. [DOI] [PubMed] [Google Scholar]
- 34.Maggiolo F., Leone S. CD4+ T lymphocyte recovery in individuals with type 1 human immunodeficiency virus infection. Clin. Infect. Dis. 2010;51(4):465–467. doi: 10.1086/655152. [DOI] [PubMed] [Google Scholar]
- 35.Kroeze S., Ondoa P., Kityo C.M., Siwale M., Akanmu S., Wellington M. Suboptimal immune recovery during antiretroviral therapy with sustained HIV suppression in sub-Saharan Africa. Aids. 2018;32(8):1043–1051. doi: 10.1097/QAD.0000000000001801. [DOI] [PubMed] [Google Scholar]
- 36.Moore R.D., Keruly J.C. CD4+ cell count 6 years after commencement of highly active antiretroviral therapy in persons with sustained virologic suppression. Clin. Infect. Dis. 2007;44(3):441–446. doi: 10.1086/510746. [DOI] [PubMed] [Google Scholar]
- 37.Lok J.J., Bosch R.J., Benson C.A., Collier A.C., Robbins G.K., Shafer R.W. Long-term increase in CD4+ T-cell counts during combination antiretroviral therapy for HIV-1 infection. Aids. 2010;24(12):1867–1876. doi: 10.1097/QAD.0b013e32833adbcf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geng E.H., Neilands T.B., Thiebaut R., Bwana M.B., Nash D., Moore R.D. CD41 T cell recovery during suppression of HIV replication: an international comparison of the immunological efficacy of antiretroviral therapy in North America, Asia and Africa. Int. J. Epidemiol. 2015;44(1):251–263. doi: 10.1093/ije/dyu271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stirrup O.T., Copas A.J., Phillips A.N., Gill M.J., Geskus R.B., Touloumi G. Predictors of CD4 cell recovery following initiation of antiretroviral therapy among HIV-1 positive patients with well-estimated dates of seroconversion. HIV Med. 2018;19(3):184–194. doi: 10.1111/hiv.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bucy R.P., Hockett R.D., Derdeyn C.A., Saag M.S., Squires K., Sillers M. Initial increase in blood CD4(+) lymphocytes after HIV antiretroviral therapy reflects redistribution from lymphoid tissues. J. Clin. Invest. 1999;103(10):1391–1398. doi: 10.1172/JCI5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jarrin I., Pantazis N., Dalmau J., Phillips A.N., Olson A., Mussini C. Does rapid HIV disease progression prior to combination antiretroviral therapy hinder optimal CD4+ T-cell recovery once HIV-1 suppression is achieved? Aids. 2015;29(17):2323–2333. doi: 10.1097/QAD.0000000000000805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diaz M., Douek D.C., Valdez H., Hill B.J., Peterson D., Sanne I. T cells containing T cell receptor excision circles are inversely related to HIV replication and are selectively and rapidly released into circulation with antiretroviral treatment. Aids. 2003;17(8):1145–1149. doi: 10.1097/00002030-200305230-00005. [DOI] [PubMed] [Google Scholar]
- 43.Serrano-Villar S., Moreno S., Fuentes-Ferrer M., Sanchez-Marcos C., Avila M., Sainz T. The CD4:CD8 ratio is associated with markers of age-associated disease in virally suppressed HIV-infected patients with immunological recovery. HIV Med. 2014;15(1):40–49. doi: 10.1111/hiv.12081. [DOI] [PubMed] [Google Scholar]
- 44.Han W.M., Apornpong T., Kerr S.J., Hiransuthikul A., Gatechompol S., Do T. CD4/CD8 ratio normalization rates and low ratio as prognostic marker for non-AIDS defining events among long-term virologically suppressed people living with HIV. AIDS Res. Ther. 2018;15(1):13. doi: 10.1186/s12981-018-0200-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Negredo E., Massanella M., Puig J., Perez-Alvarez N., Gallego-Escuredo J.M., Villarroya J. Nadir CD4 T cell count as predictor and high CD4 T cell intrinsic apoptosis as final mechanism of poor CD4 T cell recovery in virologically suppressed HIV-infected patients: clinical implications. Clin. Infect. Dis. 2010;50(9):1300–1308. doi: 10.1086/651689. [DOI] [PubMed] [Google Scholar]
- 46.Ananworanich J., Sacdalan C.P., Pinyakorn S., Chomont N., de Souza M., Luekasemsuk T. Virological and immunological characteristics of HIV-infected individuals at the earliest stage of infection. J. Virus. Erad. 2016;2(1):43–48. doi: 10.1016/S2055-6640(20)30688-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.