Abstract
Objective and Design
A randomized, open-label pilot study in individuals treated with antiretroviral therapy (ART) since acute HIV infection (AHI) with a regimen including a histone deacetylase inhibitor to induce HIV from latency and control HIV replication during subsequent treatment interruption (TI).
Methods
Fifteen participants who initiated ART at AHI were randomized to vorinostat/hydroxychloroquine/maraviroc (VHM) plus ART (n = 10) or ART alone (n = 5). The VHM arm received three 14-day vorinostat cycles within 10 weeks before TI. ART was resumed for plasma viral load (VL) > 1,000 HIV RNA copies/mL. Primary outcome was proportion of participants on VHM + ART versus ART only with VL < 50 copies/mL for 24 weeks after TI.
Results
Fifteen participants on ART (median: 178 weeks: range 79–295) enrolled. Two on VHM + ART experienced serious adverse events. Fourteen participants underwent TI; all experienced VL rebound with no difference in time between arms: VHM + ART (n = 9) median: 4 weeks and ART only (n = 5) median: 5 weeks. VHM induced a 2.2-fold increase in VL (p = 0.008) by single-copy HIV RNA assay after the first cycle. Neopterin levels increased significantly following the first two cycles. After VHM treatment, the frequencies of peripheral blood mononuclear cells harboring total HIV DNA and cell-associated RNA were unchanged. All participants achieved VL suppression following ART re-initiation.
Conclusions
Administration of VHM increased HIV VL in plasma, but this was not sustained. VHM did not impact time to viral rebound following TI and had no impact on the size of the HIV reservoir, suggesting that HIV reservoir elimination will require alternative treatment strategies.
Keywords: Acute HIV infection, Vorinostat, Maraviroc, HIV remission, Latency reversal
1. Introduction
Antiretroviral therapy (ART) can control but not eliminate human immunodeficiency virus (HIV).1 The “shock-and-kill” strategy, whereby HIV is activated from latently infected cells and killed by viral-induced cytopathicity and/or the immune system has been proposed as a cure strategy.2 Histone deacetylase inhibitors (HDACi) have been used for cancer therapy and evaluated as HIV latency reversing agents (LRA) in clinical trials of individuals with chronic HIV infection following promising in vitro and ex vivo results.3,4 HIV clinical trials of HDACi have included valproic acid.5 vorinostat 3,6,7 panobinostat 8, and romidepsin.9 However, while latency reversal was reported, a reduction in the size of the HIV reservoir in vivo was not observed, with the exception of three of four participants in the valproic acid trial, 5 implying that virus activation was incomplete, did not result in death of infected cells and/or the HIV-specific immune response was inadequate to clear infected cells.
One mechanism for HIV persistence could be immune activation leading to new rounds of infection in activated CD4+ T cells, at sites where ART penetration may be sub-optimal.10 Hydroxychloroquine (HCQ) has been proposed as an anti-viral and anti-inflammatory agent in HIV disease.11, 12, 13 One study in HIV-infected immunological non-responders on ART noted that HCQ led to a reduction in innate and adaptive immune activation, including a reduction in T-cell activation markers expressed on both CD4+ and CD8+ T cells and an increase in the frequency of plasmacytoid dendritic cells (pDC) with decreased interferon-alpha (IFN-α). However, the role of HCQ and its analogue chloroquine on IFN-α is unclear with studies reporting inhibitory,14 enhanced, 15 or no effect on induction.16 Reports of the impact of HCQ or its analogue chloroquine on reduced expression of markers of immune activation (CD38 and/or HLA-DR) on CD4+ and CD8+ T cells have shown either no effect 11,15,16 or down-regulation. 17 However, the markers of immune activation measured and treatment time varied between these studies as did administration with 15,17 or without ART. 11,16
The occurrence of ongoing viral replication in the presence of ART has been controversial.10,18 Maraviroc is a potent entry inhibitor of CCR5-tropic viruses.19 Since HIV latency reversal could induce viral replication, the addition of a different class of ART to the pre-existing regimen was thought beneficial in terms of ensuring that new rounds of HIV infection following potential latency reversal by vorinostat were blocked to prevent possible expansion of the HIV reservoir. The HIV reservoir in this study is defined as any cell harboring total DNA as this marker has been found to correlate with induction of HIV virions.20,21
At the time of this trial’s conceptualization and initiation, previous and then ongoing trials of HDACi as HIV LRA were conducted in individuals who started ART in chronic infection.3, 6, 7, 8, 9 Since then, a study of the impact of vorinostat as a “shock” agent has been performed in individuals with recent HIV infection.22 We assessed the combination of vorinostat, hydroxychloroquine and maraviroc (VHM) in Thai individuals who initiated ART during acute HIV infection (AHI). We hypothesized that these participants would respond better to strategies aimed at eliminating latency as they have fewer latently infected cells, a relatively intact immune system and viruses predominantly using CCR-5 with less genetic diversity relative to chronic infection23 Vorinostat was selected as the LRA because at the time of study design, the drug had the largest published safety data profile, facilitating more ready approval for importation by the Thai FDA and had the best published evidence of HIV transcription.3,7 The rationale for the combination was that vorinostat would induce latency reversal and infected cell death through either virological or immunological mechanisms. Activated CD4+ T cells are primary targets of HIV infection,24 hence HCQ was included due to its potential dampening effect on CD4+ T cell but not CD8+ T cell activation,15 in the event that there was immune activation following latency reversal leading to additional target cells for infection. Maraviroc was added as intensification therapy to potentially prevent new rounds of infection, as participants in this AHI cohort are primarily infected with CCR-5 tropic viruses.23 The primary objective of the study was to compare the proportion of patients between VHM plus ART versus ART only maintaining plasma HIV RNA below detection (<50 copies/ml) during 24 weeks of TI. Secondary objectives of the study were: (i) time to HIV RNA detection and rebound after TI; (ii) change in HIV DNA and cell-associated RNA; (iii) change in histone acetylation (H3); (iv) adverse events both related and unrelated to the combination of VHM; and (v) the occurrence and severity of acute retroviral syndrome following TI.
A study with macaques infected with simian immunodeficiency virus and treated with a combination of latency reversing agents including vorinostat raised concerns of detrimental neurotoxic effects.25 Therefore, participants were given the option to enroll in a neurologic sub-study to monitor the effects of VHM on neurotoxicity and the central nervous system (CNS) HIV reservoir. Since prevention of neuronal inflammation or injury is imperative, the neurologic sub-study aimed to monitor inflammatory markers in cerebrospinal fluid (CSF) and impact on neuropsychological testing (NPT).
2. Methods
2.1. Eligibility
SEARCH019 (clinicaltrials.gov identification number: NCT02475915) recruited 15 participants from the ongoing RV254/SEARCH 010 AHI cohort in Thailand (clinicaltrials.gov identification numbers: NCT00796146 and NCT00796263) from January through March 2015 23,26 Participants were eligible if they were 18–60 years old, at Fiebig III-V stages of AHI at ART initiation - i.e. prior to a Western blot positive but incomplete pattern,27 maintained viral suppression (HIV RNA <50 copies/mL) for ≥ 28 weeks prior to enrolment, and had a CD4 count of >450 cells/μL. Additional entry criteria included normal EKG and no retinal disease. Individuals positive for HBsAg or with malignancy were excluded. In addition, female participants were required to use contraception throughout the course of the trial. Participants were randomized 2:1 to VHM plus ART or ART alone. The study was approved by the Institutional Review Board of Chulalongkorn University, Thailand. All participants provided written informed consent. The investigators adhered to the policies for protection of human subjects as prescribed in AR-70, a US Department of Army policy on the protection of human subjects, and the Declaration of Helsinki. A data safety monitoring board was appointed and met twice during the course of the study: (1) Following completion of the VHM regimen (week 10) for the first 5 subjects and (2) Completion of the study by 50% of participants. Individuals who enrolled into the neurologic sub-study (clinicaltrials.gov identification number: NCT02470351) underwent lumbar puncture (LP), unless contraindicated, and NPT.
2.2. Intervention
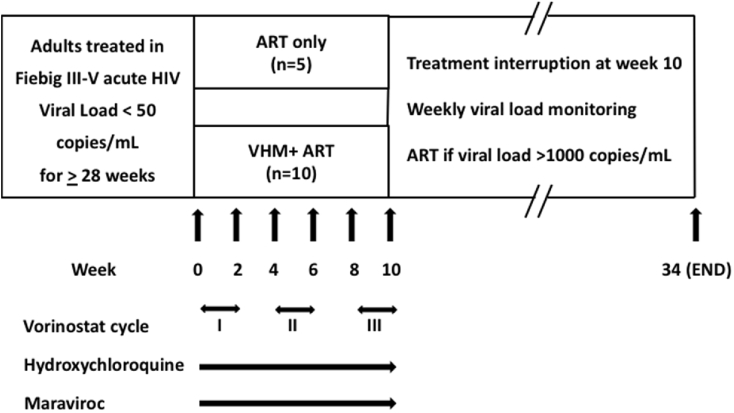
The treatment phase of the trial spanned ten weeks (Fig. 1). Participants in the VHM plus ART arm received vorinostat at 400 mg/day for three two-week cycles with two-week rest periods between cycles. The dose was based on the safety profile from oncology studies28 and induces HIV expression from resting CD4+T cells in vivo.3 HCQ was administered at 400 mg/day and maraviroc at 300 mg/day or 1,200 mg/day for participants on protease-inhibitor or non-nucleoside reverse transcriptase (NNRTI) containing ART regimens, respectively, throughout the treatment phase. Those on NNRTI based ART regimens had the NNRTI switched to darunavir/ritonavir at week eight, two weeks prior to TI to prevent the risk of development of NNRTI-resistance during ATI due to ART cessation. VHM was discontinued and ART interrupted at week ten; ART was reinitiated when plasma viremia exceeded 1,000 copies RNA/mL and/or the CD4 count was <350 cells/μL on two consecutive occasions.
Fig. 1.
Protocol schema for multiple cycles of vorinostat/hydroxychloroquine/maraviroc (VHM).
Vorinostat was administered at 400 mg/day, hydroxychloroquine at 400 mg/day and maraviroc at 300–1,200mg/day. Antiretroviral therapy (ART) was interrupted at week 10.
2.3. Safety laboratory tests
Safety laboratory tests, including pregnancy, clinical chemistries and complete blood count (CBC) were performed at weeks 0, 2, 4, 6, 8, 10, 12, 16, 22 and 34 with additional CBC at weeks 11, 13, 14, 18, 26, 28, 30 and 32.
2.4. HIV genotyping and co-receptor usage
Genotyping for HIV reverse-transcriptase and protease inhibitor resistance mutations by a previously published in-house assay was performed at RV254/SEARCH 010 study entry and at the first time point with plasma viral load (VL) > 1,000 copies/mL after TI.29 Viruses were tested for co-receptor usage at the same two time points using the Trofile phenotypic viral RNA assay (Monogram Biosciences, San Francisco, CA, USA).
2.5. Plasma and CSF HIV-1 RNA measurement
VL was monitored at weeks 0, 2, 4, 6, 8 and 10 during the treatment phase of the trial, weekly until week 12 following TI and every two weeks thereafter until the end of the trial using the COBAS® Taqman HIV-1 Test (Roche Diagnostics, Branchburg, NJ, USA) with a lower limit of detection of 20 HIV RNA copies/mL. VL was monitored at weeks 0, 2, 6, 10 and 34 for samples <20 copies/mL by single-copy assay (SCA) with a limit of detection as low as 0.44 HIV RNA copies/mL for the volume of plasma tested, as previously described.30 CSF VL was monitored at weeks 0 and 10 by both viral load assays, with a lower limit of detection of 0.26 HIV RNA copies/mL for the CSF SCA. Although the clinical viral load assay used in this study had a lower limit of quantitation of <20 copies/mL, initial measurements in the parent protocol, RV254/SEARCH 010, were performed with an assay with a lower limit of quantitation of <50 copies/mL, hence this value was used to define HIV suppression for the purpose of longitudinal comparisons.
2.6. CD4 count
CD4 counts were measured at weeks 0, 2, 4, 6, 8, 10, time of viral rebound, and week 34.
2.7. Analysis of histone acetylation
Histone acetylation was measured using 1 × 106 cryopreserved peripheral blood mononuclear cells (PBMC) collected prior to, 8 h and 24 h after administration of vorinostat, and at two-week intervals thereafter through week 10. PBMC were permeabilised and fixed with methanol. Acetylated (Ac) histone (H)3 was measured by intracellular flow cytometry using antibodies to AcH3 and Ac lysine (Millipore, Billerica, MA, USA) as described6 Data were expressed as mean fluorescence intensity (MFI) above the associated antibody isotype control. Fold-changes in MFI relative to baseline were calculated for each time-point.
2.8. Cell-associated unspliced (CA-US) HIV RNA and HIV DNA (CA-DNA)
CA-US HIV RNA and total HIV DNA were quantified by PCR from cryopreserved PBMC using a minimum sample input of 1 × 104 and 3 × 104 CD4+ T cells and PBMC, respectively, collected within 28 days prior to trial entry and at weeks 0, 2, 6, 10, time of plasma virus rebound, and week 34.
CA-US HIV RNA was measured using a semi-nested real-time quantitative PCR as previously described for HIV subtype B with minor modifications in primer design (Supplementary Table 1) for CRF01_AE, the predominant circulating subtype in Thailand. HIV RNA copy numbers were standardized to cellular equivalents using an 18s RNA real time standard as previously described.7 Total DNA was measured using primers specific for the LTR and gag regions which detect multiple HIV subtypes as previously described.31
2.9. Activation markers in plasma and CSF
Four proteins associated with monocyte activation were measured at study weeks 0, 2, 6 and 10, time of viral rebound, and at the end of the study: soluble CD163 (sCD163), sCD14, monocyte chemoattractant protein (MCP)-1, and neopterin. Interferon γ–induced protein (IP-10) and interferon-alpha (IFN-α) were also measured. MCP-1, sCD14, sCD163 and IP-10 were quantified using Human Magnetic Luminex Screening Assay (R&D Systems, Minneapolis, MN, USA). Neopterin was measured by enzyme-linked immunoassay (B.R.A.H.M.S., Hennigsdorf, Germany) and IFN-α with the Simoa Human IFN-α kit (Quanterix, Billerica, MA, USA).
2.10. Neuropsychological assessment
The NPT battery consisted of thirteen tests, summarized as NPZ Global as previously described at weeks 0, 10, during ATI, and after resuming ART.32 Testing was completed by blinded, certified nurse-psychometrists. Data were compared to a normative set of 500 HIV-negative, healthy Thais, stratified by age and educational attainment to define standardized z scores.
2.11. Statistical analysis
Time-to-event analysis was performed for time from treatment interruption to the first viral load detection (≥20 copies/mL) and rebound (>1,000 copies/mL). Time from viral load detection to ART resumption and time from ART resumption to plasma viremia suppression were also evaluated. Survival functions between study arms were compared using log-rank test. Demographic and HIV-disease related characteristics are described as median (minimum–maximum range) or frequencies. The Wilcoxon signed-rank and Mann-Whitney tests were used to compare variables within and between groups.
The Friedman test was used to compare multiple repeated measures. Analyses were performed with Stata Statistical Software version 13 (StataCorp, College Station, TX, USA) and Prism version 6.0e (GraphPad Software, San Diego, CA, USA).
This was an exploratory study of a unique group of early-treated individuals with low HIV reservoir size.26 The sample size was based on feasibility with regards to the complexity of clinic visits and budget. The assumption was that the proportion of subjects achieving drug-free HIV remission would be higher than in the VISCONTI study (15%),33 because participants had initiated ART earlier and exhibited lower HIV DNA. The power calculation was based on proportion with drug-free HIV remission being 15% and showed that there was no adequate statistical power to test differences in post-treatment viremic controllers between the arms. Nevertheless, the study was considered important in informing the effect of VHM on HIV remission and reservoir size and to provide data on the safety of VHM in Thais with the aim to expand to a larger trial if there was any significant difference in time to viral load rebound between the two study arms.
3. Results
Sixteen individuals were screened, as one individual withdrew consent after enrollment and randomization, but prior to assigned treatment (Supplementary Fig. 1). Fourteen individuals were infected with HIV CRF01_AE and one (S019-120) with subtype B. The majority were men who have sex with men (Table 1). Participants had been on ART for a median of 178 weeks (79–259). Two participants experienced adverse events that despite being grade 2 (moderate), were regarded as serious adverse events based on leading to hospitalization and, in one case, treatment discontinuation: one participant discontinued VHM during the second vorinostat cycle for renal insufficiency (grade 2, previously normal) treated with IV hydration, and thrombocytopenia (grade 2, previously normal). Both renal function and platelets normalized following VHM discontinuation. Another participant was hospitalized for diarrhea grade 2 which was deemed possibly related to VHM, but he made a full recovery without interrupting study intervention. There were 118 clinical adverse events reported: 81 in the VHM + ART participants and 37 in the ART only participants (Supplementary Table 2). There were 40 mild to moderate laboratory adverse events, with 34 of these occurring in the VHM + ART group with thrombocytopenia the most common (Supplementary Table 3). During treatment, all participants maintained HIV VL < 20 copies/mL, with one participant (S019-700) showing a viral load of 38 copies/mL at the end of the first VHM rest period (week 4).
Table 1.
Baseline characteristics of participants with acute HIV infection stratified by treatment arm.
| Characteristics | VHMa + ARTb (N = 10) | ARTb only (N = 5) |
|---|---|---|
| Acute HIV infection stage at ART initiation |
8 Fiebig III/2 Fiebig IV |
5 Fiebig III |
| Gender (Male: Female) |
9:1 |
4:1 |
| Viral load at ART initiation, log10 copies/mL |
6.1 (4.7–7.5) |
5.6 (3.1–7.1) |
| CD4+ T cells/μL at ART initiation |
397 (132–574) |
532 (213–740) |
| CD4: CD8 ratio at ART initiation |
0.4 (0.3–2.1) |
0.8 (0.6–1.0) |
| Total HIV DNA at ART initiation, copies/106 PBMC |
837 (0.8–2,323) |
594 (19–1,878) |
| Length of time on ART prior to trial entry, weeks |
224 (79–294) |
155 (100–295) |
| ART at trial entry | ||
| EFVc/TDFd/FTCe | 5 | 4 |
| EFV/TDF/3 TCf | 1 | 0 |
| LPV-rg/TDF/FTC | 3 | 0 |
| RALh/TDF/3 TC |
1 |
1 |
| Age at trial entry, years |
28 (22–51) |
26 (24–34) |
| Plasma HIV RNA at trial entry, log10 copies/mL |
<1.3 |
<1.3 |
| CD4+ T cells/μL at trial entry |
634 (501–1,106) |
1079 (537–1,612) |
| CD4: CD8 ratio at trial entry |
1.2 (0.7–2.6) |
1.2 (0.8–1.4) |
| Total HIV DNA at trial entry, copies/106 PBMC | 5.5 (0.8–93.0) | 27.0 (3.0–86.0) |
Data are presented as Median (Minimum-Maximum) unless otherwise specified.
VHM: vorinostat/hydroxychloroquine/maraviroc.
ART: antiretroviral therapy.
EFV: efavirenz.
TDF: tenofovir.
FTC: emtricitabine.
3TC: lamivudine.
LPV-r: ritonavir boosted lopinavir.
RAL: raltegravir.
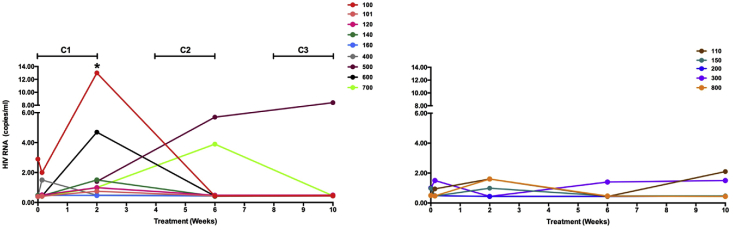
SCA measurements during VHM demonstrated HIV RNA expression at the end of the first vorinostat cycle relative to baseline (p = 0.008), but not subsequent cycles with wide inter-individual variability. Only one participant (S019-160) in the VHM + ART group did not have measurable VL by SCA during treatment (Fig. 2). No significant increases in viral load relative to baseline were seen in the ART only group during treatment.
Fig. 2.
Plasma viral load during 10 weeks of treatment with vorinostat/hydroxychloroquine/maraviroc (VHM) + ART (left panel) and ART only (right panel) measured by the single-copy HIV RNA assay.
The horizontal bars above the left panel show the times of administration of vorinostat. ∗: p = 0.008 relative to study entry. C: Cycle.
VL rebound (confirmed HIV RNA >1,000 copies/mL) occurred as early as two weeks following TI in one individual in the VHM + ART group (Supplementary Fig. 2). There was no difference in time to initial virus detection (≥20 HIV RNA copies/mL) between the two arms following TI (Table 2). Similarly, there was no difference in time to viral rebound following TI: 28 days in the VHM + ART arm versus 35 in the ART only arm (p = 0.425). Combining the arms, the median times from TI to first viral load detection and rebound were 21 and 32 days, respectively.
Table 2.
Virologic and immunologic characteristics of participants following treatment interruption, stratified by study arm.
| Characteristics | VHMa + ARTb (N = 9) | ARTb only (N = 5) | p |
|---|---|---|---|
| Weeks from treatment interruption to viral load detection, ≥ 20 copies/mL |
3 (2–5) |
3.1 (3–11) |
0.61 |
| Viral load at detection, copies/mL |
222 (33–41,822) |
156 (52–395) |
0.39 |
| Weeks from treatment interruption to viral load rebound, >1,000 copies/mL |
4 (2–7) |
5 (4–16) |
0.36 |
| Peak viral load prior to ART resumption, copies/mL |
10,797 (2,823–75,084) |
4,717 (1,614–31,264) |
0.21 |
| Weeks from viral load detection, ≥20 copies/mL, to ART resumption |
1 (0.1–4.1) |
2 (1–5.3) |
0.22 |
| Weeks from ART resumption to VL suppression, <20 copies/mL |
2.9 (0.9–10.9) |
2 (1.9–3.9) |
0.29 |
| CD4+ T cells/μL change from study entry to ARTb resumption | 2 (−376 to 549) | 16 (−284 to 474) | 0.74 |
Data are presented as Median (Minimum-Maximum).
VHM: vorinostat/hydroxychloroquine/maraviroc.
ART: antiretroviral therapy.
Four participants, all in the VHM + ART arm, had concurrent first VL ≥ 20 copies/mL and rebound (>1000 copies/mL). One ART only participant (S019-300) showed a prolonged delay from first VL detection to rebound of 35 days and remained off ART for 16 weeks. This female participant enrolled in the parent protocol at Fiebig stage III and had been on a NRTI/NNRTI regimen for over two years and was found to express the HLA B∗58:01 allele, which is associated with HIV-1 subtype C protection.34 All participants reinitiated ART and had suppressed viremia at the end of the study. The median time to viral load suppression following ART re-initiation was 2.4 weeks (0.9–10.9) with no significant difference between the two arms (data not shown). Neither acute retroviral syndrome, nor novel significant ART-resistance mutations occurred in any participant and there was no change in co-receptor usage (data not shown).
CD4+ T cell counts remained unchanged from baseline to end of treatment in both arms - median: 701 cells/μL (501–1106) at week 0 versus 643 (443–1357) at week 10 for the VHM + ART arm (p = 0.734) and 1079 cells/μL (537–1612) versus 955 (536–1125) for the ART only arm (p = 0.062). (Supplementary Fig. 3). One participant in the VHM + ART arm had a CD4+ T cell count of 312 cells/μL at the end of the trial, which increased to 746 cells/μL two weeks later.
Histone acetylation measurements were performed for only six study participants (Supplementary Fig. 4) due to limited availability of PBMC following issues with one international shipment.
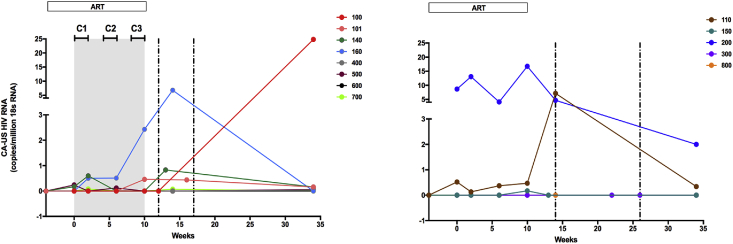
CA-US HIV RNA was detected in two of eight VHM + ART participants assessed (the participant infected with HIV subtype B was not assessed as the primers were specific for CRF01_AE) and two of five ART only participants at baseline (Fig. 3). Overall, CA-US HIV RNA was detected in 5/8 VHM + ART versus 3/5 ART only participants during the 10 weeks prior to TI. Following viral rebound and prior to ART re-initiation, CA-US HIV RNA was detected in 4/8 VHM + ART versus 2/5 ART only participants, all of whom showed measurable CA-US HIV RNA prior to TI. CA-US HIV RNA showed no significant difference at any time point between the VHM + ART and ART only arms, or relative to baseline.
Fig. 3.
Change in CA-US HIV RNA in the vorinostat/hydroxychloroquine/maraviroc (VHM) + ART treatment arm (left panel) versus ART only (right panel).
Grey shaded area represents the VHM treatment period. Treatment in both study arms was interrupted at week 10. The vertical dashed lines represent the minimum and maximum times following treatment interruption at which viral rebound occurred (2–7 weeks in the VHM + ART group and 4–16 weeks in the ART only group). Horizontal bars at the top of the left panel show the timing of vorinostat administration. The color schema used in Fig. 2 for each participant is retained. C: Cycle.
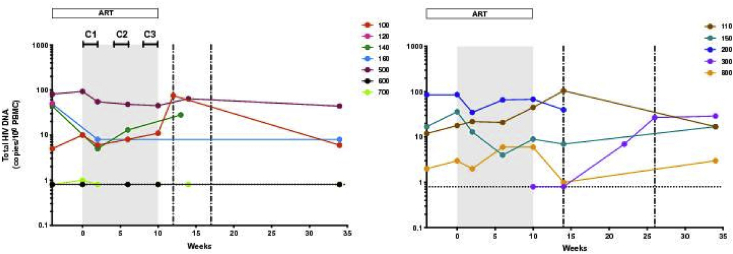
HIV DNA was measured for four VHM + ART participants and four in the ART only group at week 0, with DNA detected in 3/4 and 4/4 participants, respectively (Fig. 4). The median concentrations of total DNA in PBMC were similar between the arms (p = 0.49; Table 1). There was no significant change at the end of VHM treatment (5.9 copies/106 PBMC; 0.8–45.0) relative to week 0 (p = 0.75), with DNA detected in 2 out of 4 participants after treatment. In the ART only arm at the end of treatment, DNA was detected in four out of five participants and the concentration was similar to the VHM + ART arm (9.0 copies/106 PBMC; 0.8–68.0; p = 0.62). Total HIV DNA at time of viral rebound was detected in eight out of ten participants measured (median: 34.0 copies/106 PBMC; 1.0–105.0).
Fig. 4.
Total HIV DNA during the 34-week study period in the vorinostat/hydroxychloroquine/maraviroc treatment arm (left panel) versus ART only (right panel).
Grey shaded area represents the VHM treatment period. Treatment in both arms was interrupted at week 10. The vertical dashed lines represent the minimum and maximum times following treatment interruption at which viral rebound occurred (2–7 weeks in the VHM + ART group and 4–16 weeks in the ART only group). Horizontal bars at the top of the left panel show the timing of vorinostat administration. Horizontal dashed line shows the limit of detection of the assay. The color schema used in Fig. 2 for each participant is retained. C: Cycle.
Neopterin showed a significant increase after the first and second vorinostat cycles, returning to pre-treatment levels at the end of the study (Supplementary Fig. 5). Interestingly, the neopterin peak concentration during VHM + ART treatment was greater than the concentration observed at time of viral rebound: 8.18 nmol/L (2.12–11.96) versus 5.18 nmol/L (3.25–5.86), respectively (p = 0.012). IP-10 showed a similar trend as neopterin but was not significantly elevated (Supplementary Fig. 6). However, neopterin and IP-10 levels were positively correlated (r = 0.268; p = 0.008). Neither neopterin nor IP-10 was correlated with viral load: r = −0.121; p = 0.468 and r = 0.250; p = 0.130, respectively. Other markers of immune activation, including IFN-α showed no significant changes (Supplementary Fig. 6).
CSF HIV RNA was below the limit of detection by clinical and single-copy viral load assays at weeks 0 and 10 in all participants in the neurologic sub-study. Inflammatory markers—CSF protein, IP-10, MCP-1, neopterin, sCD14, and sCD163—did not significantly change among the weeks 0, 10 and viral rebound time-points.
Neuropsychological performance improved with repeated testing in all but one VHM participant whose z-scores declined at the week 10 VHM visit.
4. Discussion
This was the first clinical trial to assess the impact of a combination intervention including an LRA in participants who initiated therapy during AHI. There were more grade 2 adverse events and more laboratory events overall reported in VHM recipients, with one participant in this arm discontinuing study treatment for grade 2 renal insufficiency and thrombocytopenia, regarded as a serious adverse event based on hospitalization for IV hydration. Another individual with grade 2 diarrhea constituted a second serious adverse event due to overnight hospitalization as well. All other related events were grade 1/2 and resolved upon completion of treatment, demonstrating that the regimen was safe and well tolerated by the majority of participants.
Each component of VHM was previously tested in combination with ART for safety. A 14-day trial of vorinostat in HIV infected participants on ART demonstrated a safety profile similar to the current trial.7 Two other single arm trials in HIV-infected participants on ART used up to 22 doses of vorinostat and reported similar safety profiles.6,35 These trials used the same dose of vorinostat as the current study and also reported thrombocytopenia, but were conducted in participants who had started ART in chronic HIV infection.
There was no difference in either the proportion of participants maintaining viral suppression or the time to viral rebound following TI between the study arms. However, the time to viral rebound was highly variable between individuals. Previous and subsequent trials with vorinostat have not included TI.3,6,7,22,36 However, a study with the HDACi, panobinostat, in HIV-infected individuals on ART, also reported rapid viral rebound following a TI.9 We did not observe any deleterious effect of TI on reservoir size, emergence of ART-resistance, co-receptor switching, or immune activation following viral rebound and ART re-initiation.
The detection of plasma viremia during administration of VHM while receiving ART is novel. A previous trial reported no significant increase in plasma viremia using clinical or SCA after a single course of vorinostat.7 A smaller trial using multiple cycles of vorinostat plus ART noted no increase in plasma RNA despite pre-selecting participants based on vorinostat inducing HIV expression from their resting CD4+ T cells.3 The increase in plasma viremia by SCA at the end of the first vorinostat cycle but not later agrees with the observation of a previous trial of multiple cycles of vorinostat where resting CD4+ T cell-associated HIV RNA was dampened by multiple doses relative to a single dose and may be due to a cellular negative feed-back mechanism to compensate vorinostat-induced acetylation.6 Neither HCQ nor maraviroc intensification has been reported to induce HIV plasma viremia, although less frequent and sensitive viral load measurements were used.16, 17, 18 However, a previous trial of maraviroc intensification reported a transient increase in HIV 2-LTR DNA circles, suggesting that the drug induced perturbations in the HIV reservoir.36 Following completion of SEARCH 019, an in vitro study reported that maraviroc reversed latency of both CXCR4-and CCR5-tropic HIV in resting and central memory CD4+ T cells via agonist activity of the CCR5 receptor and activation of nuclear factor kappa B.37 Additionally, reports of pDC expansion and enhanced plasma IFN-α levels in clinical trials of HCQ, 15,17 suggested that HCQ could also function as a LRA. 38,39 Our results do not support the treatment regimen inducing IFN-α.
The absence of any increase in CA-US RNA after two weeks of vorinostat was surprising given previous trials, where most participants had detectable CA-US RNA before vorinostat administration.3,6,7,35 However, those trials were performed in individuals who initiated ART during chronic HIV infection. Also, detection of CA-US HIV RNA prior to vorinostat in the current study was infrequent. One study in Swiss participants initiating ART following AHI reported undetectable CA-US RNA in 14/24 subjects despite total DNA being detected in all.40 The current study had a marginally lower frequency of detection of CA-US HIV RNA, but participants had been treated for longer. Given the low frequency of CA-US HIV RNA detection prior to vorinostat, our study was underpowered to detect changes in CA-US HIV RNA following vorinostat. In addition, changes in CA-US HIV RNA were measured after 14 days of vorinostat, while previous studies demonstrated that initial changes in CA-US HIV RNA occur within 8 h of the first dose.3,7,35 However, the recent RIVER trial of vorinostat did report high frequencies of CA-US HIV RNA prior to and 4–8 weeks following vorinostat administration but also reported no difference in CA-US HIV RNA at 2 h or other time points assessed following vorinostat administration either relative to pre-vorinostat or the ART only group.22
The finding of plasma viremia, in the absence of CA-US RNA was also reported in the Swiss study of ART initiation during primary infection (within 3–15 weeks of infection) which detected HIV plasma viremia, and CA-US RNA at median times of 4.2 and 8 weeks, respectively, following TI, suggesting that the observation is not related to HIV subtype or ethnicity. It may be that the source of plasma viremia was not PBMC.
Measurement of total HIV DNA in PBMC revealed no impact of VHM on HIV reservoir size. The marginal increase seen in the reservoir during viral rebound and its return to baseline is a promising preliminary finding for future treatment interruption trials as there is no evidence that expansion of the reservoir occurred.
Elevated levels of neopterin are associated with plasma viremia and decline with ART.41 The increased neopterin level following the first and second vorinostat cycles but not during viral rebound suggests that the effect was not due to HIV. While treatment intensification with maraviroc has been reported to have no impact on plasma neopterin levels,42 the effect of HCQ or vorinostat or the VHM combination is unknown and their role in the observed elevation cannot be excluded.
No impact of VHM on CNS inflammatory markers or NPT performance was observed, in agreement with the finding by Archin et al.6
A limitation of the current trial was the inability to perform endpoint CA-US RNA, CA-DNA and histone acetylation measurements on all participants due to a PBMC shipment problem. We also did not have multiple study arms with the individual drugs.
The failure of the VHM combination to reduce the HIV reservoir size is similar to other studies with HDACi LRA.5, 6, 7, 8, 9,22,35 In vitro and ex vivo studies have demonstrated that combinations of LRA with different targets and timing of administration will be important in reducing the size of the HIV reservoir.43,44
The only HDACi study that showed a reduction in reservoir size as measured by total HIV DNA/106 CD4+ T cells used romidepsin following therapeutic immunization with a vaccine known to stimulate HIV-specific cell-mediated immune responses.45 However, following TI all participants experienced viral rebound. Our preliminary findings suggest that HIV remission strategies for participants with AHI, despite their relatively reduced reservoir size, will require alternative strategies. Importantly, this pilot study showed that TI in individuals initiating ART during AHI did not result in clinically adverse outcomes, with no acute retroviral syndrome or drug-resistant viruses observed and rapidly suppressed plasma viremia following ART resumption.
Disclaimer
The views expressed are those of the authors and should not be construed to represent the positions of the U.S. Army, the Department of Defense, HJF, or the Thai Red Cross. The data in this manuscript were partially presented at the 21st International AIDS Conference (AIDS 2016), Durban, South Africa, July 18–22, 2016; Abstract 10535.
Declaration of competing interest
NC has served on the scientific advisory board of Theravectys. JA has participated in advisory meetings for ViiV Healthcare, Merck, AbbVie, Gilead, and Roche. All other authors declare no competing interests.
Acknowledgements and Funding
We would like to thank the study participants who committed so much of their time for this study. The participants were from the RV254 acute HIV infection study, which is supported by cooperative agreements (W81XWH-07-2-0067, W81XWH-11-2-0174) between the Henry M Jackson Foundation for the Advancement of Military Medicine, Inc., and the US Department of the Army and by an intramural grant from the Thai Red Cross AIDS Research Centre. The US Army Medical Research Acquisition Activity (820 Chandler Street, Fort Detrick, MD 21702–5014, USA) is the awarding and administering acquisition office for the cooperative agreement. Antiretroviral therapy for RV254 participants was supported by the Thai Government Pharmaceutical Organization, Gilead, Merck and ViiV Healthcare. This work was also funded in part by 1R01NS084911-01 from the National Institute of Neurologic Disorders and Stroke and with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E and by 1R01AI108433 from the National Institute of Allergy and Infectious Diseases. SRL, JJC, AR, AD and ST are supported by National Health and Medical Research Council of Australia (NHMRC Program Grant GNT1052979) and the National Institutes for Health Delaney AIDS Research Enterprise (DARE AI096109 and AI126611), and the American Foundation for AIDS Research. SRL is an NHMRC practitioner fellow.
We thank Brandie A. Fullmer for work on the single-copy HIV RNA assay. We thank the staff from SEARCH and the HIV-NAT laboratory at the Thai Red Cross AIDS Research Centre for their valuable contributions to this study. We also thank Cooper Human Systems, Nashua, NH, USA for providing funding for vorinostat.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jve.2020.100004.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Finzi D., Hermankova M., Pierson T. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 2.Hamer D.H. Can HIV be Cured? Mechanisms of HIV persistence and strategies to combat it. Curr. HIV Res. 2004;2:99–111. doi: 10.2174/1570162043484915. [DOI] [PubMed] [Google Scholar]
- 3.Archin N.M., Liberty A.L., Kashuba A.D. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012;487:482–485. doi: 10.1038/nature11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falkenberg K.J., Johnstone R.W. Histone deacetylases and their inhibitors in cancer, neurological diseases and immune disorders. Nat. Rev. Drug Discov. 2014;13:673–691. doi: 10.1038/nrd4360. [DOI] [PubMed] [Google Scholar]
- 5.Lehrman G., Hogue I.B., Palmer S. Depletion of latent HIV-1 infection in vivo: a proof-of-concept study. Lancet. 2005;366:549–555. doi: 10.1016/S0140-6736(05)67098-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Archin N.M., Bateson R., Tripathy M.K. HIV-1 expression within resting CD4+ T cells after multiple doses of vorinostat. J. Infect. Dis. 2014;210:728–735. doi: 10.1093/infdis/jiu155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elliott J.H., Wightman F., Solomon A. Activation of HIV transcription with short-course vorinostat in HIV-infected patients on suppressive antiretroviral therapy. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rasmussen T.A., Tolstrup M., Brinkmann C.R. Panobinostat, a histone deacetylase inhibitor, for latent-virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: a phase 1/2, single group, clinical trial. Lancet HIV. 2014;1:e13–21. doi: 10.1016/S2352-3018(14)70014-1. [DOI] [PubMed] [Google Scholar]
- 9.Sogaard O.S., Graversen M.E., Leth S. The depsipeptide romidepsin reverses HIV-1 latency in vivo. PLoS Pathog. 2015;11 doi: 10.1371/journal.ppat.1005142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McManus W.R., Bale M.J., Spindler J. HIV-1 in lymph nodes is maintained by cellular proliferation during antiretroviral therapy. J. Clin. Invest. 2019 doi: 10.1172/JCI126714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paton N.I., Goodall R.L., Dunn D.T. Effects of hydroxychloroquine on immune activation and disease progression among HIV-infected patients not receiving antiretroviral therapy: a randomized controlled trial. J. Am. Med. Assoc. 2012;308:353–361. doi: 10.1001/jama.2012.6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paton N.I., Aboulhab J., Karim F. Hydroxychloroquine, hydroxycarbamide, and didanosine as economic treatment for HIV-1. Lancet. 2002;359:1667–1668. doi: 10.1016/S0140-6736(02)08557-4. [DOI] [PubMed] [Google Scholar]
- 13.Paton N.I., Hydroxychloroquine Aboulhab J. Hydroxyurea and didanosine as initial therapy for HIV-infected patients with low viral load: safety, efficacy and resistance profile after 144 weeks. HIV Med. 2005;6:13–20. doi: 10.1111/j.1468-1293.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- 14.Martinson J.A., Montoya C.J., Usuga X., Ronquillo R., Landay A.L., Desai S.N. Chloroquine modulates HIV-1-induced plasmacytoid dendritic cell alpha interferon: implication for T-cell activation. Antimicrob. Agents Chemother. 2010;54:871–881. doi: 10.1128/AAC.01246-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Routy J.P., Angel J.B., Patel M. Assessment of chloroquine as a modulator of immune activation to improve CD4 recovery in immune nonresponding HIV-infected patients receiving antiretroviral therapy. HIV Med. 2015;16:48–56. doi: 10.1111/hiv.12171. [DOI] [PubMed] [Google Scholar]
- 16.Jacobsen J.M., Bosinger S.E., Kang M. The effect of chloroquine on immune activation and interferon signatures associated with HIV-1. AIDS Res. Hum. Retrovir. 2016;32:636–647. doi: 10.1089/aid.2015.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piconi S., Parisotto S., Rizzardini G. Hydroxychloroquine drastically reduces immune activation in HIV-infected, antiretroviral therapy-treated immunologic nonresponders. Blood. 2011;118:3263–3272. doi: 10.1182/blood-2011-01-329060. [DOI] [PubMed] [Google Scholar]
- 18.Puertas M.C., Massanella M., Llibre J.M. Intensification of a raltegravir-based regimen with maraviroc in early HIV-1 infection. AIDS. 2014;28:325–334. doi: 10.1097/QAD.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 19.Woollard S.M., Kanmogne G.D. Maraviroc: a review of its use in HIV infection and beyond. Drug Des. Dev. Ther. 2015;9:5447–5468. doi: 10.2147/DDDT.S90580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avettand-Fènoël V., Hocqueloux L., Ghosn J. Total HIV-1 DNA, a marker of viral reservoir dynamics with clinical implications. Clin. Microbiol. Rev. 2016;29 doi: 10.1128/CMR.00015-16. 859-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cillo A.R., Hong F., Tsai A. Blood biomarkers of expressed and inducible HIV-1. AIDS. 2018;32:699–708. doi: 10.1097/QAD.0000000000001748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fidler S., Stöhr W., Pace M. Antiretroviral therapy alone versus antiretroviral therapy with a kick and kill approach, on measures of the HIV reservoir in participants with recent HIV infection (the RIVER trial): a phase 2, randomised trial. Lancet. 2020;395:888–898. doi: 10.1016/S0140-6736(19)32990-3. [DOI] [PubMed] [Google Scholar]
- 23.Ananworanich J., Schuetz A., Vandergeeten C. Impact of multi-targeted antiretroviral treatment on gut T cell depletion and HIV reservoir seeding during acute HIV infection. PloS One. 2012;7 doi: 10.1371/journal.pone.0033948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDougal J.S., Mawle A., Cort S.P. Cellular tropism of the human retrovirus HTLV-III/LAV. I. Role of T cell activation and expression of the T4 antigen. J. Immunol. 1985;135:3151–3162. [PubMed] [Google Scholar]
- 25.Gama L., Abreu C., Shirk E. Reactivation of simian immunodeficiency virusreservoirs in the brain of virally suppressed macaques. AIDS. 2017;31:5–14. doi: 10.1097/QAD.0000000000001267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ananworanich J., Sacdalan C.P., Pinyakorn S. Virological and immunological characteristics of HIV-infected individuals at the earliest stage of infection. J. Virus Erad. 2016;2:43–48. [PMC free article] [PubMed] [Google Scholar]
- 27.Fiebig E.W., Wright D.J., Rawal B.D. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS. 2003;17:1871–1879. doi: 10.1097/00002030-200309050-00005. [DOI] [PubMed] [Google Scholar]
- 28.Duvic M., Olsen E.A., Breneman D. Evaluation of the long-term tolerability and clinical benefit of vorinostat in patients with advanced cutaneous T-cell lymphoma. Clin. Lymphoma, Myeloma & Leukemia. 2009;9:412–416. doi: 10.3816/CLM.2009.n.082. [DOI] [PubMed] [Google Scholar]
- 29.Sirivichayakul S., Phanuphak P., Pankam T., O-Charoen R., Sutherland D., Ruxrungtham K. HIV drug resistance transmission threshold survey in Bangkok, Thailand. Antivir. Ther. 2008;13(Suppl 2):109–113. [PubMed] [Google Scholar]
- 30.Somsouk M., Dunham R.M., Cohen M. The immunologic effects of mesalamine in treated HIV-infected individuals with incomplete CD4+ T cell recovery: a randomized crossover trial. PloS One. 2014;9 doi: 10.1371/journal.pone.0116306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vandergeeten C., Fromentin R., Merlini E. Cross-clade ultrasensitive PCR-based assays to measure HIV persistence in large-cohort studies. J. Virol. 2014;88:12385–12396. doi: 10.1128/JVI.00609-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heaps J., Valcour V., Chalermchai T. Development of normative neuropsychological performance in Thailand for the assessment of HIV-associated neurocognitive disorders. J. Clin. Exp. Neuropsychol. 2013;35:1–8. doi: 10.1080/13803395.2012.733682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saez-Cirion A., Bacchus C., Hocqueloux L. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kloverpris H.N., Stryhn A., Harndahl M. HLA-B∗57 Micropolymorphism shapes HLA allele-specific epitope immunogenicity, selection pressure, and HIV immune control. J. Virol. 2012;86:919–929. doi: 10.1128/JVI.06150-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Archin N.M., Kirchherr J.L., Sung J.A. Interval dosing with the HDAC inhibitor vorinostat effectively reverses HIV latency. J. Clin. Invest. 2017;127:3126–3135. doi: 10.1172/JCI92684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gutierrez C., Diaz L., Vallejo A. Intensification of antiretroviral therapy with a CCR5 antagonist in patients with chronic HIV-1 infection: effect on T cells latently infected. PloS One. 2011;6 doi: 10.1371/journal.pone.0027864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lopez-Huertas M.R., Jimenez-Tormo L., Madrid-Elena N. The CCR5-antagonist Maraviroc reverses HIV-1 latency in vitro alone or in combination with the PKC-agonist Bryostatin-1. Sci. Rep. 2017;7:2385. doi: 10.1038/s41598-017-02634-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsai A., Irrinki A., Kaur J. Toll-like receptor 7 agonist GS-9620 induces HIV expression and HIV-specific immunity in cells from HIV-infected individuals on suppressive antiretroviral therapy. J. Virol. 2017;91 doi: 10.1128/JVI.02166-16. e02166–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vibholm L., Schleimann M.H., Højen J.F. Short-course toll-like receptor 9 agonist treatment impacts innate immunity and plasma viremia in individuals with human immunodeficiency virus infection. Clin. Infect. Dis. 2017;64:1686–1695. doi: 10.1093/cid/cix201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmid A., Gianella S., von Wyl V. Profound depletion of HIV-1 transcription in patients initiating antiretroviral therapy during acute infection. PloS One. 2010;5 doi: 10.1371/journal.pone.0013310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amirayan-Chevillard N., Tissot-Dupont H., Obadia Y., Gallais H., Mege J.L., Capo C. Highly acive antiretroviral therapy (HAART) and circulating markers of immune activation: specific effect of HAART on neopterin. Clin. Diagn. Lab. Immunol. 2000;7:832–834. doi: 10.1128/cdli.7.5.832-834.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yilmaz A., Verhofstede C., D’Avolio A. Treatment intensification has no effect on the HIV-1 central nervous system infection in patients on suppressive antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 2010;55:590–596. doi: 10.1097/QAI.0b013e3181f5b3d1. [DOI] [PubMed] [Google Scholar]
- 43.Pardons M., Fromentin R., Pagliuzza A., Routy J.P., Chomont N. Latency-reversing agents induce differential responses in distinct memory CD4 T cell subsets in individuals on antiretroviral therapy. Cell Rep. 2019;29:2783–2795. doi: 10.1016/j.celrep.2019.10.101. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwartz C., Bouchat S., Marban C. On the way to find a cure: purging latent HIV-1 reservoirs. Biochem. Pharmacol. 2017;146:10–22. doi: 10.1016/j.bcp.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 45.Leth S., Schleimann M.H., Nissen S.K. Combined effect of Vacc-4x, recombinant human granulocyte macrophage colony-stimulating factor vaccination, and romidepsin on the HIV-1 reservoir (REDUC): a single-arm, phase 1B/2A trial. Lancet HIV. 2016;3:e463–472. doi: 10.1016/S2352-3018(16)30055-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.