Abstract
Objectives
Seven years after the introduction of direct-acting antivirals (DAAs) for the treatment of hepatitis C, high prices remain a barrier for treatment programs worldwide. This study seeks to describe current prices for originator DAAs in 50 countries and evaluate the relationship between prices and GDP per capita.
Methods
Data on prices of sofosbuvir, daclatasvir, sofosbuvir/ledipasvir, sofosbuvir/velpatasvir, and glecaprevir/pibrentasvir were collected from national databases for 50 countries. Cost-based generic prices were estimated using an established algorithm, which accounts for costs of the active pharmaceutical ingredient (API), excipients, conversion costs of API to finished pharmaceutical product, taxes assuming manufacture in India, and a 10% profit margin. Correlation between current market prices and GDP per capita was assessed by Spearman rank-order correlation.
Results
Median originator prices per standard course were US$40,502 for sofosbuvir, US$26,928 for daclatasvir, US$46,812 for sofosbuvir/ledipasvir, US$34,381 for sofosbuvir/velpatasvir, and US$30,710 for glecaprevir/pibrentasvir (G/P). The estimated cost-based generic prices for a 12-week course were US$28 for sofosbuvir, US$31 for ledipasvir, US$58 for velpatasvir, US$4 for daclatasvir. For fixed-dose combinations, estimated cost-based prices were US$58 for sofosbuvir/ledipasvir, US$85 for sofosbuvir/velpatasvir, and US$31 for sofosbuvir/daclatasvir (API cost data were insufficient to calculate an estimate for G/P). Cumulative originator sales of WHO-recommended DAAs reached US$82 billion by the end of 2019. Across the 50 countries, there was no correlation between GDP per capita and DAA price, nor between estimated viraemic population and DAA price. Sub-analyses within World Bank income groups found a significant negative correlation between price and GDP per capita for all DAAs within the high-income countries group.
Conclusions
Prices of DAAs vary widely across countries. The lack of correlation between DAA price and GDP per capita and viraemic population suggests that prices for DAAs are not adjusted based on country income level or potential patient population. Among high-income countries, DAA prices fall as income levels rise, possibly due to greater negotiating power of wealthier countries. DAA prices in most countries remain many times higher than estimated cost-based generic prices.
Keywords: Hepatitis C, Sofosbuvir, Daclatasvir, Ledipasvir, Velpatasvir
1. Introduction
Treatment for hepatitis C virus (HCV) infection has been transformed by the introduction of direct-acting antivirals (DAAs). WHO estimated in 2015 that 71 million people were infected with HCV and 399,000 died from cirrhosis or hepatocellular carcinoma caused by HCV infection.1 Sofosbuvir, the first DAA to achieve cure rates over 90%, was approved in the USA in 2013 and in the EU in 2014.2,3 Prior to this, the standard of treatment for HCV was interferon-based regimens, with success rates between 40 and 70% and severe side effects.1 WHO treatment guidelines recommend three pangenotypic regimens for HCV treatment: glecaprevir/pibrentasvir, sofosbuvir/daclatasvir, and sofosbuvir/velpatasvir. Depending on treatment history and the severity of cirrhosis, some patients may require up to 24 weeks of treatment, or be eligible for a shorter 8-week regimens. There is currently no WHO-recommended pangenotypic regimen for adolescents aged 12–17.1
While the introduction of DAAs may make hepatitis C elimination possible, new infections continue to outpace the number of people treated. WHO estimates that, of 71 million prevalent cases in 2015 and 2016, only 1.10–1.76 million initiated treatment, while there were an estimated 1.75 million new HCV infections.4 The Global Health Sector Strategy (GHSS) 2016–2021 for viral hepatitis calls for a 90% reduction in new chronic infections and a 65% reduction in mortality.5 To reach these targets, national health programs must be able to access affordable, quality medicines and diagnostics.
In countries where generic DAAs are marketed, prices are far below the $84,000 list price at which sofosbuvir was originally introduced in the United States. However, cost remains a key barrier to access for many patients and for scale-up in many national health programs. A 2018 study found that in at least 22 European countries there were restrictions on DAA reimbursement based on disease stage.6
Local production or importation of generics is only possible where DAAs are not under patent protection, or where voluntary or compulsory licences are in place. Where there is local manufacture of generic medicines, for example in Pakistan, prices have fallen to $34 for a 12-week course of sofosbuvir/daclatasvir and $92 for sofosbuvir/velpatasvir. An estimated 60% of people living with HCV infection globally live in countries that can procure generic DAAs.4 However, several middle-income countries with large HCV burden have not been included in voluntary licensing agreements, notably Brazil, China, Colombia, Kazakhstan, and Mexico.4,7
Some originator companies claim that their pricing takes into account average income levels. For example, the originator company (Gilead) for sofosbuvir, ledipasvir, and velpatasvir states on their website that, for middle-income countries, pricing is “based on GNI per capita and disease burden”.8 The originator company (BMS) for daclatasvir states that their pricing “takes into consideration several factors, including countries’ economic development and burden of disease, as well as the commitment of the government to holistically address hepatitis C, including treatment and care”.9
A limited number of studies have looked generally at the relationship between pricing of originator medicines and country income levels. Danzon and Furukawa (2008) found that pricing is not proportional to differences in per-capita income between high-income countries and middle-income countries.10 Danzon, Mulcahy, and Towse (2015) found that correlation of prices for originator HIV, TB, and malaria drugs to per-capita income was very weak across all countries and non-significant among low- and middle-income countries.11 Watal and Dai (2019) found that, for originator medicines, correlation of prices to GDP per capita was 0.11, across 70 markets and over 500 products.12 Other studies have looked at specific disease categories. Scherer and Watal (2002) found that, for 15 HIV/AIDS medicines, correlation between price and per-capita income was 0.13 across 18 countries.13 Vogler (2016) found high variability of prices for cancer medicines in 18 countries, though they did not formally analyse correlation to income levels.14 Hill and Sim (2018) found no observable relationship between GDP per capita and the price of dolutegravir, within World Bank income categories, across 52 countries.15 For hepatitis C originator DAAs, we are aware of one previous analysis of prices versus income level: Iyengar (2016) found no observable relationship between originator DAA prices and GDP per capita across 30 countries.16
This study aims to add to the literature on HCV pricing by describing current prices across a range of countries, estimating cost-of-production at current API costs, and comparing current costs to GDP per capita, as an indicator of local affordability.
2. Methods
2.1. Study design
We collected current prices of WHO-recommended DAAs – sofosbuvir (SOF), sofosbuvir/ledipasvir (SOF/LED), sofosbuvir/velpatasvir (SOF/VEL), daclatasvir (DAC), and glecaprevir/pibrentasvir (G/P). We compared current prices across countries and compared prices to country income levels expressed as gross domestic product (GDP) per capita. We included all countries for which we were able to identify publicly available drug price databases (50 countries). We also collected annual sales data, where available, from quarterly earnings reports published by the originator companies.
2.2. Data sources
In all cases, publicly accessible databases were used (Appendix). Databases may cite the price of a medicine at various points in the supply chain. For example, one database may cite the price including pharmacy mark-up and value-added tax, while another may cite the ex-factory price (i.e. amount paid to the manufacturer). Some databases give prices at multiple points in the supply chain – in these cases, the price closest to the ex-factory price, that is, net of any retailer, distributor, or hospital mark-ups, and value-added taxes, was used. Where multiple packaging formats were available (e.g. as 21 tablets or 84 tablets), the lowest per-unit price was used. We assumed the standard dosage recommended in WHO guidelines.
In some cases, generic versions were identified. For the main analysis, generics were excluded, in order to assess the relation of originator product pricing to country income level. We present the generic prices found in the survey separately.
In many countries, confidential discounts or rebates are given by pharmaceutical manufacturers to public buyers (e.g. the UK National Health Service). We are unable to consistently adjust for these discounts across the range of countries; this is a well-described limitation to studies comparing pharmaceutical prices internationally.17
We used average exchange rates for March 2019 published by the US Treasury.18 Data on GDP per capita are for 2018, in current US dollars, as reported by the World Bank.
2.3. Estimation of cost-based generic price
We estimated cost-based generic prices for WHO-recommended DAAs using methodology described earlier,19 which accounts for costs of the active pharmaceutical ingredient (API), excipients, conversion costs, tax obligations assuming manufacture in India, and a 10% profit margin.
2.4. Pricing comparisons
To measure the strength of association between DAA price and GDP per capita, we calculated the Spearman rank-order correlation, using R version 1.0.153. We also calculated correlation within World Bank income categories, as this classification is known to be used in pricing decisions and can govern eligibility for access or voluntary licence schemes.
2.5. Sensitivity analysis
There are no public data available on market size. Similar studies (e.g. Iyengar et al.) have used viraemic population as a proxy for market size.16 While there are reasons to be cautious about such an approach (see Discussion), sensitivity analysis was conducted using a regression model evaluating the association between DAA price and GDP per capita, adjusted for viraemic population.
3. Results
Table 1 summarizes originator prices for sofosbuvir, daclatasvir, sofosbuvir/ledipasvir, sofosbuvir/velpatasvir, and glecaprevir/pibrenatsvir in 50 countries where publicly accessible price databases could be found, as well as the ratio of DAA price to GDP per capita. The median originator price of sofosbuvir was US$40,502 per 12-week course, ranging from US$10,730 in Argentina to US$91,461 in Italy. The median price of daclatasvir across all countries was US$26,928 per 12-week course, ranging from US$3144 in Russia to US$100,415 in Italy. The median originator price of sofosbuvir/ledipasvir was US$46,812 per 12-week course, ranging from US$1249 in Morocco to US$73,771 in Latvia. The median price of sofosbuvir/velpatasvir was US$ 34,381 per 12-week course, ranging from US$10,368 in China to US$92,719 in Italy. The median price of glecaprevir/pibrentasvir was US$30,710 per 8-week course, ranging from US$15,628 in Brazil to US$89,485 in Canada. The most expensive drug relative to GDP per capita was sofosbuvir/velpatasvir in Lebanon, which costs 5.9 times the GDP per capita.
Table 1.
Price per course of originator sofosbuvir, daclatasvir, sofosbuvir+daclatasvir, sofosbuvir/ledipasvir, sofosbuvir/velpatasvir, and glecaprevir/pibrentasvir.
| GDP per capita (USD) | Originator price per course (USD) (Ratio of price to GDP per capita) |
||||||
|---|---|---|---|---|---|---|---|
| SOF | DAC | SOF/DAC | SOF/LED | SOF/VEL | G/P | ||
| Estimated cost-based price | N/A | $28 | $4 | $31 | $31 | $58 | – |
| Argentina | $11,653 | $10,730 (0.92) | $19,282 (1.65) | $30,012 (2.58) | $37,499 (3.22) | $37,499 (3.22) | $28,455 (2.44) |
| Australia | $57,305 | $26,615 (0.46) | $16,324 (0.28) | $42,938 (0.75) | – | $26,615 (0.46) | $26,496 (0.46) |
| Austria | $51,513 | – | – | – | – | $28,483 (0.55) | $28,510 (0.55) |
| Azerbaijan | $4721 | – | – | – | – | – | – |
| Bahrain | $24,051 | – | – | – | – | – | – |
| Belgium | $46,556 | $28,584 (0.61) | – | $28,584 (0.61) | $42,876 (0.92) | $28,584 (0.61) | $35,746 (0.77) |
| Brazil | $8921 | $17,268 (1.94) | $8464 (0.95) | $25,732 (2.88) | $28,481 (3.19) | $13,632 (1.53) | $15,268 (1.71) |
| Bulgaria | $9273 | – | – | – | $40,442 (4.36) | $26,961 (2.91) | $27,382 (2.95) |
| Canada | $46,125 | $41,014 (0.89) | $26,846 (0.58) | $67,860 (1.47) | $49,963 (1.08) | $44,743 (0.97) | $89,485 (1.94) |
| Chile | $15,923 | – | – | – | – | – | – |
| China | $9771 | $11,619 (1.19) | – | – | – | $10,368 (1.06) | – |
| Colombia | $6651 | – | – | – | – | – | – |
| Croatia | $14,869 | – | – | – | $56,860 (3.82) | $57,305 (3.85) | $32,449 (2.18) |
| Cyprus | $28,159 | $43,779 (1.55) | – | $43,779 (1.55) | $52,355 (1.86) | $44,316 (1.57) | $32,731 (1.16) |
| Czech Republic | $22,973 | $47,934 (2.09) | $31,005 (1.35) | $78,939 (3.44) | $56,423 (2.46) | $53,711 (2.34) | $30,292 (1.32) |
| Denmark | $60,596 | $48,828 (0.81) | – | $48,828 (0.81) | $56,199 (0.93) | $58,231 (0.96) | $30,365 (0.5) |
| France | $41,464 | $27,972 (0.67) | – | $27,972 (0.67) | $41,738 (1.01) | $27,972 (0.67) | $32,414 (0.78) |
| Germany | $48,196 | – | – | – | – | $33,697 (0.7) | $33,697 (0.7) |
| Greece | $20,324 | – | – | – | – | – | – |
| Hungary | $15,939 | – | – | – | $52,975 (3.32) | $49,182 (3.09) | – |
| Iceland | $73,191 | $43,921 (0.6) | – | – | $44,957 (0.61) | $29,971 (0.41) | – |
| India | $2016 | – | – | – | – | – | – |
| Indonesia | $3894 | – | – | – | – | – | – |
| Israel | $41,614 | $39,989 (0.96) | $28,782 (0.69) | $68,771 (1.65) | $49,203 (1.18) | $44,286 (1.06) | $31,055 (0.75) |
| Italy | $34,318 | $91,461 (2.67) | $100,415 (2.93) | $191,875 (5.59) | – | $92,719 (2.7) | $51,923 (1.51) |
| Japan | $39,287 | – | – | – | – | – | $36,676 (0.93) |
| Latvia | $18,089 | $65,754 (3.64) | – | $65,754 (3.64) | $73,771 (4.08) | $45,695 (2.53) | $43,294 (2.39) |
| Lebanon | $8270 | $31,884 (3.86) | – | $31,884 (3.86) | $48,997 (5.92) | $34,381 (4.16) | $36,796 (4.45) |
| Lithuania | $19,090 | – | – | – | – | – | – |
| Luxembourg | $114,340 | $26,966 (0.24) | $31,038 (0.27) | – | $40,449 (0.35) | $26,966 (0.24) | – |
| Mexico | $9698 | – | $17,411 (1.8) | $17,411 (1.8) | – | – | $26,551 (2.74) |
| Moldova | $3189 | – | – | – | – | – | – |
| Morocco | $3238 | – | – | – | $1249 (0.39) | – | – |
| Netherlands | $52,978 | $35,823 (0.68) | $27,011 (0.51) | $62,834 (1.19) | $45,464 (0.86) | $30,312 (0.57) | $15,746 (0.3) |
| New Zealand | $41,966 | – | – | – | $49,721 (1.18) | – | $33,673 (0.8) |
| Norway | $81,807 | $42,340 (0.52) | $26,045 (0.32) | $68,385 (0.84) | $48,160 (0.59) | $51,805 (0.63) | $30,290 (0.37) |
| Oman | $16,419 | – | $39,847 (2.43) | – | – | – | – |
| Peru | $6947 | – | – | – | – | – | – |
| Russian Federation | $11,289 | – | $3145 (0.28) | – | – | – | – |
| Saudi Arabia | $23,219 | $49,647 (2.14) | $33,252 (1.43) | $82,899 (3.57) | $66,446 (2.86) | $37,711 (1.62) | $25,141 (1.08) |
| Serbia | $7234 | $32,407 (4.48) | – | – | $33,105 (4.58) | – | – |
| Slovenia | $26,234 | – | – | – | $40,542 (1.55) | $27,059 (1.03) | – |
| South Africa | $6340 | – | – | – | – | – | – |
| Switzerland | $82,839 | – | – | – | $42,272 (0.51) | $29,596 (0.36) | $29,596 (0.36) |
| Thailand | $7274 | – | $6751 (0.93) | – | – | – | – |
| Turkey | $9311 | $22,830 (2.45) | $5412 (0.58) | – | $33,897 (3.64) | – | – |
| Ukraine | $3095 | – | – | – | – | – | – |
| United Arab Emirates | $43,005 | $51,835 (1.21) | $35,989 (0.84) | – | $63,101 (1.47) | $70,414 (1.64) | – |
| United Kingdom | $42,491 | $45,789 (1.08) | – | $45,789 (1.08) | $51,021 (1.2) | $51,021 (1.2) | $34,015 (0.8) |
| United States (VA) | $62,641 | $64,693 (1.03) | $46,966 (0.75) | $111,659 (1.78) | $26,948 (0.43) | $17,965 (0.29) | $19,015 (0.3) |
No prices for DAAs recorded in price databases for Azerbaijan, Bahrain, Chile, Colombia, Greece, India, Indonesia, Lithuania, Moldova, Peru, and South Africa. Assumed duration of treatment course: 8 weeks for G/P, 12 weeks for other DAAs.
No originator prices for sofosbuvir were recorded in 25 countries, daclatasvir in 31 countries, sofosbuvir/ledipasvir in 22 countries, sofosbuvir/velpatasvir in 20 countries, and glecaprevir/pibrentasvir in 24 countries. No prices for originator versions of any WHO-recommended DAAs were recorded in price databases in 11 countries: Azerbaijan, Bahrain, Chile, Colombia, Greece, India, Indonesia, Lithuania, Moldova, Peru, and South Africa. Some countries where no prices were found in national databases are included in the territories of voluntary licences for DAAs. Key examples include South Africa, Thailand, Ukraine, India, and Indonesia.
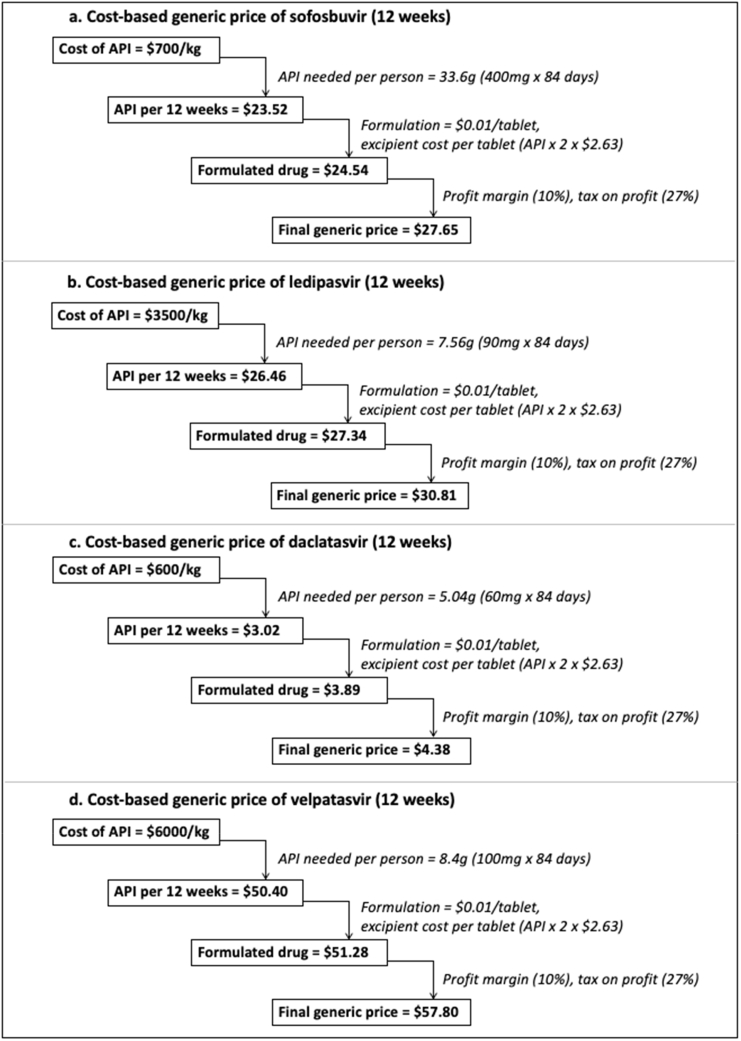
The estimated manufacturing cost-based prices for a 12-week course were US$28 for sofosbuvir, US$31 for ledipasvir, US$58 for velpatasvir, and US$4 for daclatasvir (Fig. 1 and Table 1). For fixed-dose combinations, estimated cost-based prices were US$58 for sofosbuvir/ledipasvir, US$85 for sofosbuvir/velpatasvir, and US$31 for sofosbuvir/daclatasvir (Table 1). Data on API costs were not available to estimate the cost of production for glecaprevir and pibrentasvir.
Fig. 1.
Estimated cost-based generic prices for sofosbuvir, ledipasvir, daclatasvir, and velpatasvir.
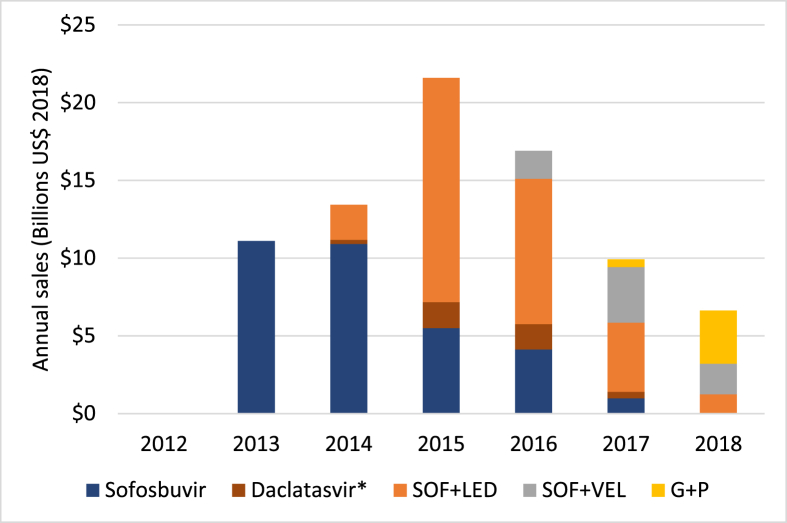
Cumulative originator sales of WHO-recommended DAAs reached US$80 billion by the end of 2018 (Fig. 2), giving an average of US$16 billion annually over 2013–2018. Originator sales peaked at US$21.6 billion in 2015.
Fig. 2.
Annual sales of HCV DAAs by year.
∗Regulatory filings for BMS report daclatasvir sales along with sales for asunaprevir
and beclabuvir.
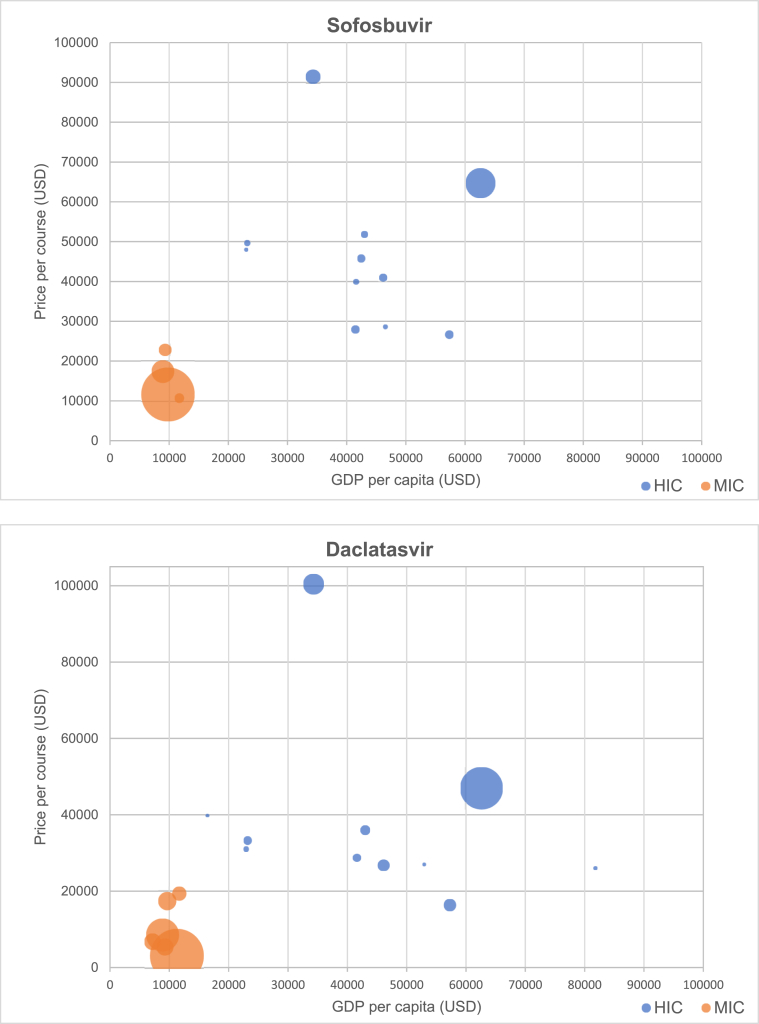
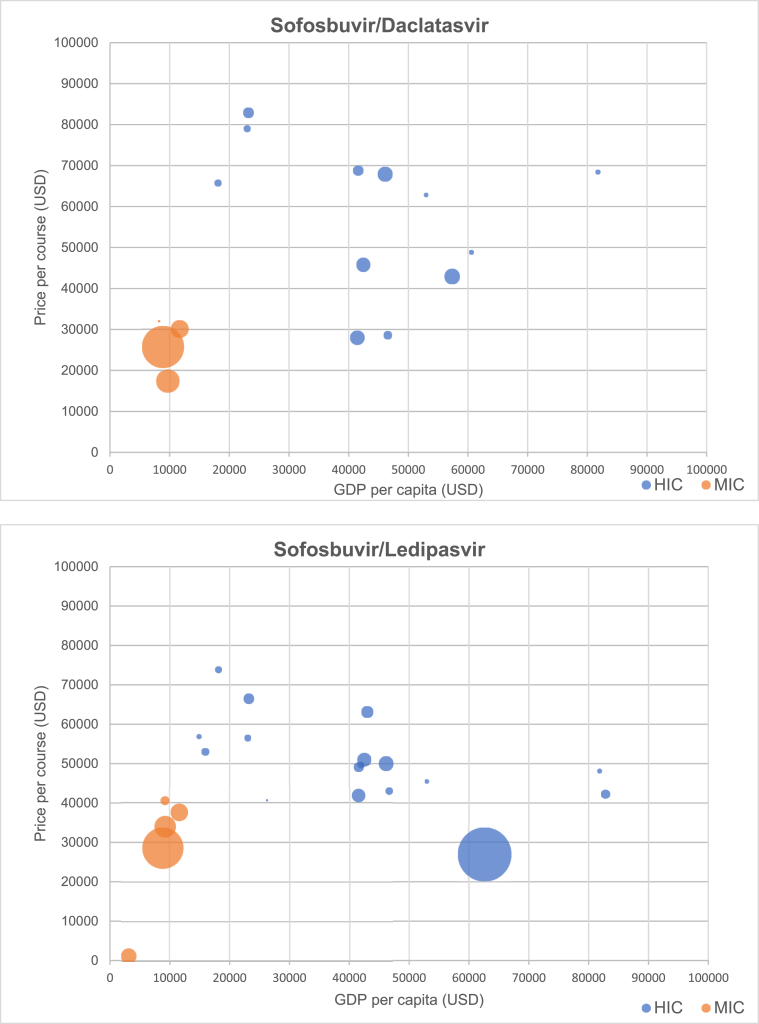
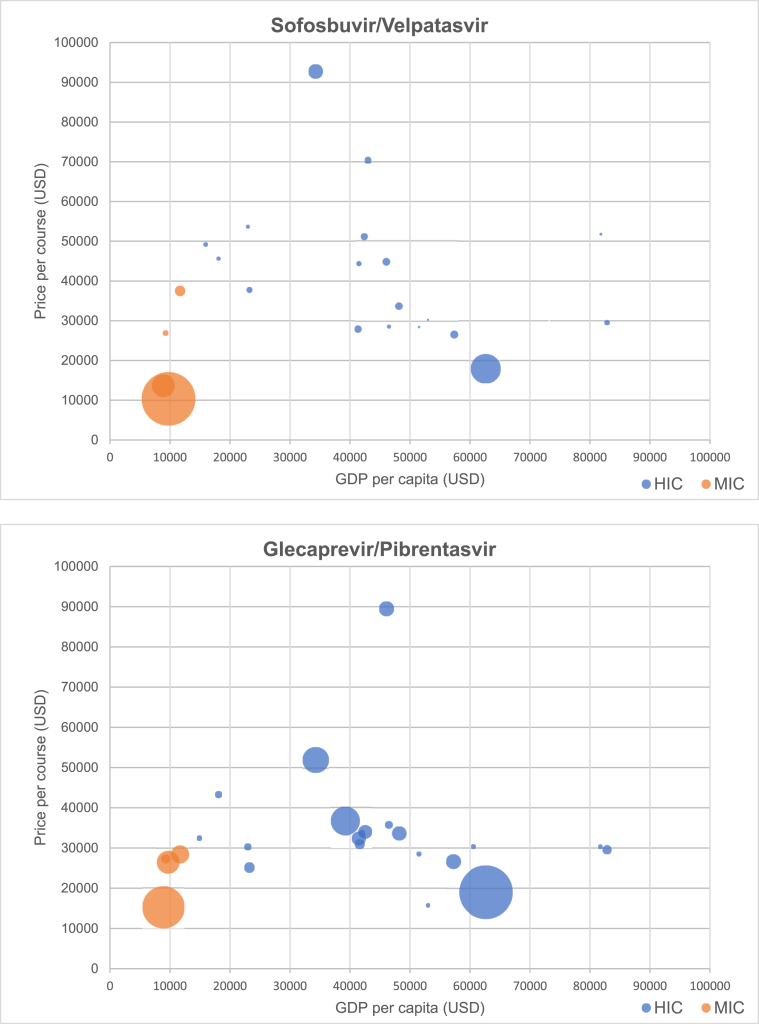
Fig. 3 presents scatterplots of originator DAA price versus GDP per capita, by World Bank income category. Prices of generic DAAs identified in our survey are given in Table 2.
Fig. 3.
Relationship between price of DAAs and GDP per capita (by income group), with bubble size scaled to estimated number of prevalent infections by country Relationship.
Table 2.
Generic prices for DAAs.
| Country | SOF | DAC | SOF/DAC | SOF/LED | SOF/VEL | G/P |
|---|---|---|---|---|---|---|
| Argentina | $74 | |||||
| Azerbaijan | $275 | |||||
| India | $40 | $93 | ||||
| Indonesia | $160 | |||||
| Kazakhstan | $84 | |||||
| Lebanon | $17 | |||||
| Moldova | $532 | $240 | $1050 | |||
| Pakistan | $34 | $92 | ||||
| Peru | $485 | |||||
| Russian Federation | $5831a | |||||
| Thailand | $270 | $327 | ||||
| Ukraine | $60 | $18 | $90 |
Local manufacturer under licence from Gilead).
Table 3 presents Spearman's rank-order correlation coefficients for each DAA and GDP per capita across all countries, among high-income countries, and among middle-income countries (no low-income countries were included in the study). The correlation across all countries between DAA price and GDP per capita was weak across all DAAs except daclatasvir. Among high-income countries, correlation between GDP and DAA price was negative for all DAAs, with higher GDP per capita associated with lower DAA price, and was significant at 95% confidence for sofosbuvir/ledipasvir (r = -0.612; p < 0.01), sofosbuvir/velpatasvir (r = -0.420; p < 0.05), and glecaprevir/pibrentasvir (r = -0.444; p < 0.05). Among middle-income countries, correlation was weak for all DAAs except sofosbuvir, which had a strong negative correlation (r = -0.943; p < 0.01).
Table 3.
Spearman's rank-order correlation between GDP and DAA price.
| DAA | Across high-income countries (p-value) | Across middle-income countries (p-value) |
|---|---|---|
| Sofosbuvir | -0.395 (0.105) | -0.943 (0.005) |
| Daclatasvir | -0.392 (0.208) | 0.257 (0.623) |
| Sofosbuvir/ledipasvir | -0.612 (0.003) | 0.464 (0.293) |
| Sofosbuvir/velpatasvir | -0.420 (0.041) | 0.100 (0.873) |
| Glecaprevir/pibrentasvir |
-0.444 (0.044) |
-0.100 (0.873) |
Sensitivity analysis exploring the role of viraemic population (as an indicator for market size) as a confounder in the relationship between price and GDP per capita found that GDP per capita, but not viraemic population, was significantly associated (p < 0.05) with DAA price.
4. Discussion
Originator prices for WHO-recommended DAA regimens remain high relative to GDP per capita. Across 50 countries, the pricing of originator DAAs has no apparent correlation to income level. Surprisingly, among high-income countries, pricing of all originator DAAs showed a moderately strong and statistically significant inverse correlation – countries with higher incomes have lower prices.
Taken broadly, a lack of correlation to income suggests either that originators do not adjust prices based on country income, or that such adjustments are obscured in overall trends by other price-setting factors. This finding is consistent with more general research demonstrating that pricing is in general not adjusted to country income levels20 and is also consistent with the hypothesis that DAA originator companies simply set prices with a view to maximize revenues.21
Within the high-income country set, the moderate inverse correlation may be explained by wealthier countries having greater negotiating power and/or more effective price control mechanisms.
As outlined in the Introduction, earlier studies of correlation of originator drug prices to country income levels have found very limited or no correlation.10, 11, 12, 13, 14, 15, 16 One possible explanation for these results is DAA price is primarily a function of market size, rather than GDP per capita. For originator DAAs, Iyengar et al. found no relationship between originator DAA pricing and market size, across 30 countries.16 However, Iyengar et al. estimated market size based on estimated prevalence. There are reasons to believe that national prevalence data might be weak proxies for market size and/or demand. Demand and market size are linked to prevalence, but critical mediators and confounders include the proportion of people with known HCV status, financing and insurance structures (who pays, and how, and by what incentives), and procurement institutions in-country (i.e. the strength and ability of national bodies to negotiate and the size and distribution of private health providers). Nevertheless, in our results, we arrive at the same result as Iyengar et al. in finding that viraemic population is not significantly associated with DAA price.
Since sofosbuvir was first approved in 2013, revolutionizing the treatment of hepatitis C, the pricing of, and global access to, patented DAAs has been the subject of intense debate, and has triggered a range of policy responses. In some countries sofosbuvir and other DAAs were not protected by intellectual property, allowing local generics manufacturers to bring less expensive versions to market. Compulsory licensing – a legal mechanism to enable generic manufacture or importation without authorization from the patent-holder – has been explored in numerous countries,22 and employed as an access strategy in Malaysia.23 In Australia, the government has negotiated an ‘all you can eat’ (or ‘Netflix’) agreement for certain DAA combinations, wherein the government pays a fixed annual fee and is provided with as many treatment courses as needed.24
All of the included DAAs are covered by voluntary licences (VLs) in some countries. VLs are agreements offered by originator companies to generic manufacturers in certain low- and middle-income countries, which allow the manufacturers to market generic versions within a defined territory, normally comprising low-income and lower-middle income countries. Daclatasvir and glecaprevir/pibrentasvir are covered by VLs agreed between their respective originator companies and the Medicines Patent Pool, a UN-backed non-profit initiative that manages VLs.25,26 Sofosbuvir, sofosbuvir/ledipasvir, and sofosbuvir/velpatasvir are covered by bilateral VLs between the originator company and a number of mainly Indian generics manufacturers.27 However, these VLs do not include many upper-middle-income countries with high hepatitis C burden, such as Thailand, Brazil, and China and Russia. The originator for daclatasvir has discontinued the product in the US, and has announced that it will discontinue the product in European countries “where alternative treatments are available.”
In the US, the originator company for sofosbuvir, sofosbuvir/ledipasvir, and sofosbuvir/velpatasvir launched in September 2018 lower priced versions of the originator medicines, marketed by a subsidiary of the originator (sometimes referred to as ‘authorised generics’).28 As the originator has not done the same in other countries, the result is that now the lowest price for originator sofosbuvir/ledipasvir captured in our survey is in fact seen in the United States. Before the launch of the lower-priced version, the US in general had the highest prices for originator DAAs.16
Prices of originator DAAs remain high, with costs for a treatment course in some countries reaching as high as 6 times GDP per capita for sofosbuvir/ledipasvir, 4 times for sofosbuvir/velpatasvir, 3 times for daclatasvir, and twice for glecaprevir/pibrentasvir (Table 1). The cost of sofosbuvir, sofosbuvir/ledipasvir, and sofosbuvir/velpatasvir exceeded GDP per capita in over half of cases where data were comparable. The cost of daclatasvir exceeded GDP per capita in one third of cases, and of glecaprevir/pibrentasvir in one quarter of cases. Prices are in many cases hundreds to thousands of times higher than prices for generic versions currently available, for example, in Egypt and India (Table 2).
We estimated that DAAs can now be profitably manufactured for as little as US$31 for a sofosbuvir/daclatasvir fixed-dose combination (there is no originator version of such a combination, but generic versions are available).4 These estimates of cost-based generic prices have steadily fallen since 2016, when we estimated that generic sofosbuvir could be profitably sold for US$178 per course and daclatasvir for US$22 per course (although a higher profit margin was assumed in those estimates, price has fallen about three-fold even after adjusting for this).29 At our estimated generic price of $31 per course for sofosbuvir+daclatasvir, the $80 billion in originator sales revenue since 2013 would be sufficient to pay for treatment of the 71 million people currently living with hepatitis C 36 times over.
Declaration of interests
MJB, DG, and GK declare no competing interests. AH has received a consultancy payment from Merck for clinical trial review, not connected with this project.
Funding
MJB and AH received funding from the International Treatment Preparedness Coalition (ITPC) as part of the Unitaid-supported project “Affordable medicines for developing countries".
Footnotes
The recommended regimen for adolescents 12–17 with chronic HCV infection is sofosbuvir/ledipasvir (12 weeks) for genotypes 1, 4, 5 and 6 sofosbuvir/ribavirin (12 weeks) for genotype 2, and sofosbuvir/ribavirin (24 weeks) for genotype 3.
Appendix. National price databases.
References
- 1.WHO . WHO; Geneva: 2018 Jul. Guidelines for the Care and Treatment of Persons Diagnosed with Chronic Hepatitis C Virus Infection [Internet]https://apps.who.int/iris/bitstream/handle/10665/273174/9789241550345-eng.pdf Available from: [PubMed] [Google Scholar]
- 2.EMA Sovaldi [Internet] https://www.ema.europa.eu/en/medicines/human/EPAR/sovaldi Available from:
- 3.FDA FDA letter to Gilead Sciences, Inc [Internet] 2013. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2013/204671Orig1s000ltr.pdf Available from:
- 4.WHO . WHO; Geneva: 2018. Progress Report on Access to Hepatitis C Treatment: Focus on Overcoming Barriers in Low- and Middle-Income Countries [Internet]https://apps.who.int/iris/bitstream/handle/10665/260445/WHO-CDS-HIV-18.4-eng.pdf?sequence=1 Mar. Available from: [Google Scholar]
- 5.WHO . WHO; Geneva: 2016 Jun. Global Health Sector Strategy on Viral Hepatitis 2016-2021.https://www.who.int/hepatitis/strategy2016-2021/ghss-hep/en/ [Internet] Available from: [Google Scholar]
- 6.Marshall A.D., Cunningham E.B., Nielsen S. Restrictions for reimbursement of interferon-free direct-acting antiviral drugs for HCV infection in Europe. Lancet Gastroenterol. Hepatol. 2018;3:125–133. doi: 10.1016/S2468-1253(17)30284-4. World Health Organization. [DOI] [PubMed] [Google Scholar]
- 7.Londeix P. Médecins du Monde; 2014. New Treatments for Hepatitis C Virus: Strategies for Achieving Universal Access.http://www.hepcoalition.org/IMG/pdf/web_daas_ strategies_for_achieving_universal_access_en.pdf [Internet] Mar. Available from: [Google Scholar]
- 8.Gilead Expanding chronic hepatitis C treatment in low- and middle-income countries [Internet] https://www.gilead.com/-/media/files/pdfs/other/hcv-access-fact-sheet-061219.pdf?la=en&hash=ECF6F2AE73040656D7313C2D1BB27812 Available from:
- 9.BMS HCV developing World strategy [Internet]. BMS. https://www.bms.com/about-us/responsibility/access-to-medicines-in-the-developing-world/hcv-developing-world-strategy.html Available from:
- 10.Danzon P.M., Furukawa M.F. International prices and availability of pharmaceuticals in 2005. Health Aff. 2008 Jan;27(1):221–233. doi: 10.1377/hlthaff.27.1.221. [DOI] [PubMed] [Google Scholar]
- 11.Danzon P.M., Mulcahy A.W., Towse A.K. Pharmaceutical pricing in emerging markets: effects of income, competition, and procurement: pharmaceutical pricing in emerging markets. Health Econ. 2015 Feb;24(2):238–252. doi: 10.1002/hec.3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watal J., Dai R. Product patents and access to innovative medicines in a post-TRIPS-era. Staff working paper ERSD-2019-05. [Internet] https://www.wto.org/english/res_e/reser_e/ersd201905_e.pdf World Trade Organizaion. [cited 2020 Feb 26]. Available from:
- 13.Scherer F.M., Watal J. Post-TRIPS options for access to patented medicines in developing nations. J. Int. Econ. Law. 2002 Dec 1;5(4):913–939. [Google Scholar]
- 14.Vogler S., Vitry A., Babar Z.-U.-D. Cancer drugs in 16 European countries, Australia, and New Zealand: a cross-country price comparison study. Lancet Oncol. 2016 Jan;17(1):39–47. doi: 10.1016/S1470-2045(15)00449-0. [DOI] [PubMed] [Google Scholar]
- 15.Sim J., Hill A. Is pricing of dolutegravir equitable? A comparative analysis of price and country income level in 52 countries. J Virus Erad. 2018 Oct 1;4(4):230–237. doi: 10.1016/S2055-6640(20)30311-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iyengar S., Tay-Teo K., Vogler S., Beyer P., Wiktor S., de Joncheere K. Prices, costs, and affordability of new medicines for hepatitis C in 30 countries: an economic analysis. PLoS Med. 2016;13(5) doi: 10.1371/journal.pmed.1002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vogler S., Zimmermann N., Habl C., Piessnegger J., Bucsics A. Discounts and rebates granted to public payers for medicines in European countries. South Med Rev. 2012;5(1):38. [PMC free article] [PubMed] [Google Scholar]
- 18.US Treasury Treasury reporting rates of exchange as of March 31, 2019 [internet] https://fiscal.treasury.gov/files/reports-statements/treasury-reporting-rates-exchange/itin-03-31-2019.pdf Available from:
- 19.Hill A.M., Barber M.J., Gotham D. Estimated costs of production and potential prices for the WHO Essential Medicines List. BMJ Glob Health. 2018;3(1) doi: 10.1136/bmjgh-2017-000571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flynn S., Hollis A., Palmedo M. An economic justification for open access to essential medicine patents in developing countries. J. Law Med. Ethics. 2009;37(2):184–208. doi: 10.1111/j.1748-720X.2009.00365.x. [DOI] [PubMed] [Google Scholar]
- 21.United States Senate Committee on Finance Wyden-grassley sovaldi investigation finds revenue-driven pricing strategy behind $84,000 hepatitis drug [internet] https://www.finance.senate.gov/ranking-members-news/wyden-grassley-sovaldi-investigation-finds-revenue-driven-pricing-strategy-behind-84-000-hepatitis-drug Available from:
- 22.Johnson C. Louisiana considers radical step to counter high drug prices. The Washington Post [Internet]. 2017 Jul 3. https://www.washingtonpost.com/business/economy/louisiana-considers-radical-step-to-counter-high-drug-prices-federal-intervention/2017/07/03/456b99f6-4a59-11e7-a186-60c031eab644_story.html?utm_term=.00bb41886e67 Available from:
- 23.Brennan Z. Regulatory Affairs Professionals Society; 2017. Malaysia Issues Compulsory License for Gilead Hepatitis C Drug [Internet] Available from: https://www.raps.org/regulatory-focusTM/news-articles/2017/9/malaysia-issues-compulsory-license-for-gilead-hepatitis-c-drug. [Google Scholar]
- 24.Moon S., Erickson E. Universal medicine access through lump-sum remuneration—Australia’s approach to hepatitis C. N. Engl. J. Med. 2019;380(7):607–610. doi: 10.1056/NEJMp1813728. [DOI] [PubMed] [Google Scholar]
- 25.Medicines Patent Pool Glecaprevir/pibrentasvir (G/P) licence [Internet] https://medicinespatentpool.org/licence-post/glecaprevirpibrentasvir-gp/ Available from:
- 26.Medicines Patent Pool Daclatasvir (DAC) licence [Internet] https://medicinespatentpool.org/licence-post/daclatasvir-dcv/ Available from:
- 27.Gilead Sciences Amended and restated license agreement [Internet] https://www.gilead.com/-/media/files/pdfs/other/form%20ar%20hcv%20license%20agmt%20gild%2011202017.pdf?la=en Available from:
- 28.Gilead Gilead subsidiary to launch authorized generics of Epclusa® (Sofosbuvir/Velpatasvir) and Harvoni® (Ledipasvir/Sofosbuvir) for the treatment of chronic hepatitis C [Internet] 2018. https://www.gilead.com/news-and-press/press-room/press-releases/2018/9/gilead-subsidiary-to-launch-authorized-generics-of-epclusa-sofosbuvirvelpatasvir-and-harvoni-ledipasvirsofosbuvir-for-the-treatment-of-chronic Available from:
- 29.Hill A., Simmons B., Gotham D., Fortunak J. Rapid reductions in prices for generic sofosbuvir and daclatasvir to treat hepatitis C. J Virus Erad. 2016;2(1):28. doi: 10.1016/S2055-6640(20)30691-9. [DOI] [PMC free article] [PubMed] [Google Scholar]