Abstract.
Hand, foot, and mouth disease (HFMD) has brought millions of attacks and a substantial burden in the Asia-Pacific region. Previous studies assessed disease risks around the world, which demonstrated great heterogeneity, and few determined the modification effect of social factors on temperature–disease relationship. We conducted a time-series study to evaluate the temperature-associated HFMD morbidity risk using daily data (from 2011 to 2017) and to identify potential modifiers relating to urban–rural status and aggregation mode of children. By applying a distributed lag nonlinear model (DLNM) and controlling for time-varying factors and other meteorological factors, we found that the relationship between daily mean temperature and the cumulative risk of HFMD was an approximately M-shaped curve. The effects of higher temperature appeared to be greater and more persistent than those of lower temperature. With the reference of −6°C, the cumulative relative risk (RR) values of high temperature (95 percentile) and low temperature (5 percentile) were 3.74 (95% CI: 2.50–5.61) and 1.72 (95% CI: 1.24–2.37) at lag 4–7, respectively. Temperature-associated HFMD morbidity risks were more pronounced among rural children and those attending kindergartens or schools at specific lags and temperatures. Relative risk values for temperature–disease association was highest among the 3- to 6-year group, whereas no gender difference was observed. Studying effect estimates and their modifications using the DLNM on a daily scale helps to identify susceptible groups and guide policy-making and resource allocation according to specific local conditions.
INTRODUCTION
Hand, foot, and mouth disease (HFMD) is an emerging childhood illness caused by various enteroviruses, of which Coxsackievirus A16 and Enterovirus 71 being the dominant circulating serotypes.1,2 Most HFMD cases show self-limiting illness, including fever, skin eruptions on hands and feet, and vesicles in the mouth. However, cases involving progressive neurological and cardiopulmonary complications or even fatalities have also been observed.3
Since first reported in 1957, HFMD has caused millions of attacks and several outbreaks across the world and has become more predominant in the Asia-Pacific region in the past decade.4–7 The disease has led to a substantial burden because children, especially those younger than 5 years, are its major cases. In China, HFMD was designated as a C-class notifiable communicable disease in 2008 and has been the top one of that classification since then. According to the surveillance system, the incidence of HFMD varied from 120.21/100,000 to 203.16/100,000, and the mortality rate ranged from 13.78/100,000 to 51.00/100,000 from 2010 to 2014 in mainland China.8 So far, relatively high asymptomatic rate and lack of specific prevention and treatment have limited the controlling of the disease, which highlight the role of identifying risk factors of HFMD.9
Hand, foot, and mouth disease is endemic in the tropical and subtropical countries, with the tendency of higher number of cases in wet and warm seasons depending on geographical locations.10,11 At the national level, in southern China, the occurrence of HFMD displays a bimodal pattern per year, usually in May and October, whereas in northern China, only one peak is experienced in summer.12 The peculiar spatial distribution and the seasonal pattern of the disease cases may involve a certain meteorological mechanism.
Previously, the risk of HFMD cases was extensively reported to be positively correlated with higher temperature.13–17 Hii and others18 showed that every 1°C increase in maximum temperature greater than 32°C elevated the risk of incidence by 36%. Du et al.19 explored the threshold effects of meteorological factors on HFMD using classification and regression tree models, and showed that when the temperature was greater than 24.03°C and the relative humidity was between 80.6% and 82.6%, the relative risk (RR) of HFMD was 3.49 relative to monthly average incidence. Increasing evidence also suggested that the temperature–HFMD association was nonlinear and manifested delayed effects; thus, the frequently used models to develop the linear coefficients for immediate effects can be largely limited.
In addition, according to spatial autocorrelation analysis by Wang et al.,20 HFMD cases were mainly distributed in the counties of eastern and southern China, as well as provincial capitals and their surrounding counties, which implied the socioeconomic features, such as the population density, can be considered as a confounder in studying the HFMD epidemics. However, few studies have explored those effect modifiers on temperature-related HFMD cases.
This study aims to establish a relationship and analyze the effects of daily mean temperature associated with the risk of HFMD cases in Wuxi, China, by applying a newly developed distributed lag nonlinear model (DLNM) and to further look into the potential modificated factors regarding the urban–rural status and aggregation mode of the population.
METHODS
Population and study area.
Wuxi is a modern metropolis covering an area of 4,627 km2 (of which the area of urban regions is 1,644 km2 and that of water surfaces is 1,342 km2) and population (registered residents) of 4.86 million in 2017. The city is located at 31°07′–32°02′ north latitude and 119°31′–120°36′ east longitude and belongs to southeast of Jiangsu Province and the corridor part of rivers and lakes in the Yangtze River Delta, which is the largest economic zone of China. As a provincial city of Jiangsu, its per capita gross domestic product reaches Renminbi 141,300 (statistical yearbook for Wuxi 2017). The city experiences the subtropical maritime climate, with four distinct seasons, plentiful rainfall, and ample sunshine.
Surveillance data of HFMD.
The surveillance data of HFMD collected as case reports from January 1, 2011 to December 31, 2017 were obtained from the database of the National Center for Public Health Surveillance and Information Services, China CDC. Hand, foot, and mouth disease cases were diagnosed based on fever and papular or vesicular rash on the hands, feet, mouth, or buttocks, according to the National Clinic Guide (version 2009). The cases were reported online via the aforementioned database within 24 hours of diagnosis by using a standardized form including demographic information (gender, date of birth, and current address) and case description (date of symptoms onset, case severity, and classification).
Population data were collected from the database of the Wuxi CDC.
Meteorological data.
We obtained weather condition data on daily mean temperature, relative humidity, rainfall, sunshine hours, and wind speed from Wuxi Municipal Meteorological Monitoring Center. The weather data were measured at a single fixed-site station in Xishan district of Wuxi. Daily mean temperature was calculated by averaging temperature readings at 2 am, 8 am, 2 pm, and 8 pm for each day.
Analytical strategies.
For descriptive analysis, minimum, 25% quartile, median, 75% quartile, maximum, mean, and SD were calculated by stratification factors.
Distributed lag nonlinear models with negative binomial distribution were applied to evaluate the effects of temperature on HFMD, with daily disease cases as the dependent variable and temperature as the predictor. The model used in this research was:
| (1) |
where t is the day of observation, Yt is the daily count of HFMD cases on day t, is the expected HFMD case counts on day t, l is the lag days, is the cross-basis objects used to create two sets of basis functions defining a two-dimensional relationship of the predictor (temperature) and the lag, and is a natural cubic spline. We applied for both temperature and lag in the cross-basis, the degrees of freedom (df) of which are 5 and 4, respectively. The time’s natural spline is set to 8 df. Day of the week and public holidays are set as dummy variables in the model, with and being the regression coefficients. ns(weather confounders, df), represents four independent natural splines fit to relative humidity, rainfall, sunshine hours, and wind speed, all with 3 df, in accordance with previous studies.15,21,22
We then determined the potential delayed effect of temperature in our model and found the effect was negligible for a lag of more than 28 days, so a maximum lag of 4 weeks was used. Also, minimum lag was set to four considering the natural incubation period of the virus (approximately 3–7 days). Spline knots were set at equal intervals within the range of variables depending on df choosing. To learn about the exposure–lag–response relationship on HFMD, 15°C was defined as the reference value for plotting. Pre-evaluation of the temperature–HFMD association revealed smaller RR values at low temperature than at high temperature, the curve of which was approximately M-shaped. Thus, we redefined −6°C (the temperature where lowest cumulative RR occurred) as the reference value to calculate RRs. Different temperature strictures (5th, 25th, 50th, 75th, and 95th percentiles) and lag structures (lags 4, 7, 14, 21, and 28) were listed to determine lag-specific and temperature-specific effects on HFMD, respectively.
Stratified analyses were conducted with regard to different gender, age, urban–rural status, and aggregation mode of children. Although RR values were obtained, the estimates may change with the resetting of the reference value. Thereby, we considered between-group differences with a 2-fold change or more to be noteworthy, regardless of statistical significance.23
To check the robustness of our models, we performed sensitivity analysis by changing the lag structure (from 14 to 30 days) and the df selection for weather variables (from 4 to 8).
This study did not involve informed consent as patient records or information was anonymized and de-identified before we obtained the data.
All statistical analyses were conducted in R software, version 3.2.4 (R Development Core Team, https://www.r-project.org/), using the dlnm package.24,25
RESULTS
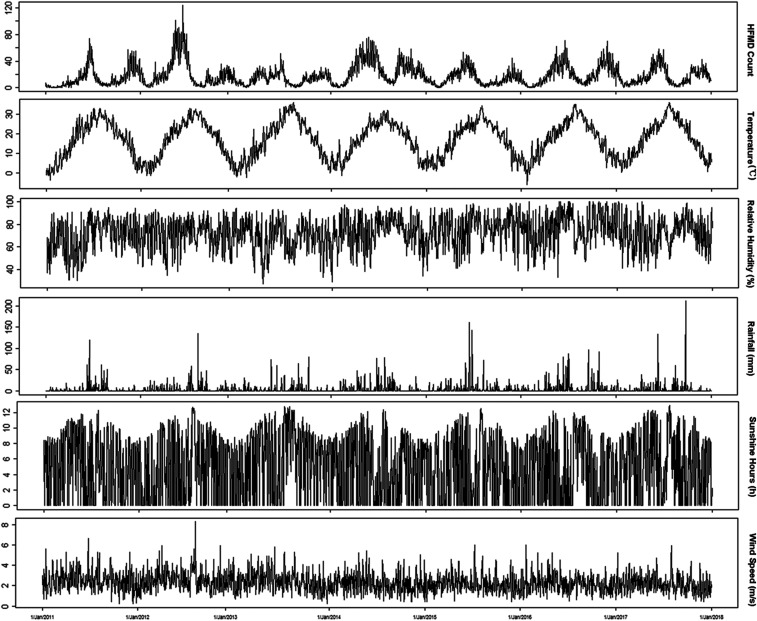
Table 1 showed the summary statistics of HFMD cases and meteorological variables in Wuxi between January 1, 2011 and December 31, 2017. A total of 107,906 cases were reported, 91.9% (99,212 cases) of which were aged 0–6 years. Among the HFMD cases, the male-to-female gender ratio was 1.44:1. The scattered children (those being raised at home or home-schooled, instead of attending nurseries, kindergartens, or schools) were the majority of the cases, accounting for 61.1% of the reported cases, followed by children in kindergartens or schools (38.4%). The daily counts for cases living in urban areas, city-country fringe areas, and rural areas were 15.5, 10.3, and 16.4, respectively. The average level of daily mean temperature, relative humidity, rainfall, sunshine hours, and wind speed were 17.0°C (range: −6.1–36.0°C), 72.1% (range: 27–100%), 3.8 mm (range: 0–211.3 mm), 4.9 hours (range: 0–12.9 hours), and 2.3 m/second (range: 0.2–8.3 m/second), respectively. Figure 1 demonstrated the seasonal fluctuation of the HFMD case counts and weather indicators. Although the prevalence of HFMD varied from year to year, bimodal outbreaks were commonly observed, with the first peak from May to July and the second minor peak from November to December during 2011 to 2017.
Table 1.
Descriptive statistics for daily data on HFMD cases and meteorological variables in Wuxi, 2011–2017
| N | Mean | SD | Minimum | 25% quartile | Median | 75% quartile | Maximum | |
|---|---|---|---|---|---|---|---|---|
| HMFD cases | ||||||||
| Total | 2,557 | 42.2 | 33.0 | 0 | 17 | 33 | 59 | 213 |
| Male | 2,557 | 24.9 | 19.6 | 0 | 10 | 20 | 35 | 118 |
| Female | 2,557 | 17.3 | 14.1 | 0 | 7 | 14 | 24 | 96 |
| < 1 year | 2,557 | 2.5 | 2.8 | 0 | 0 | 2 | 4 | 18 |
| 1–3 years | 2,557 | 18.3 | 14.7 | 0 | 8 | 14 | 25 | 92 |
| 3–6 years | 2,557 | 18.0 | 15.7 | 0 | 6 | 14 | 25 | 124 |
| Scattered children | 2,557 | 25.8 | 21.2 | 0 | 10.5 | 19 | 35 | 123 |
| Children in kindergartens or schools | 2,557 | 16.2 | 15.1 | 0 | 5 | 12 | 24 | 131 |
| Urban | 2,557 | 15.5 | 12.9 | 0 | 6 | 12 | 21 | 92 |
| City-country fringe | 2,557 | 10.3 | 8.9 | 0 | 4 | 8 | 14 | 57 |
| Rural | 2,557 | 16.4 | 14.1 | 0 | 6 | 12 | 24 | 79 |
| Meteorological factors | ||||||||
| Temperature (°C) | 2,557 | 17.0 | 9.2 | −6.1 | 8.8 | 18.0 | 24.5 | 36.0 |
| Relative humidity (%) | 2,557 | 72.1 | 13.8 | 27 | 63 | 73 | 82 | 100 |
| Rainfall (mm) | 2,556* | 3.8 | 12.1 | 0 | 0 | 0 | 1.3 | 211.3 |
| Sunshine hours (hour) | 2,557 | 4.9 | 4.1 | 0 | 0 | 5.3 | 8.6 | 12.9 |
| Wind speed (m/second) | 2,557 | 2.3 | 0.9 | 0.2 | 1.7 | 2.2 | 2.8 | 8.3 |
HFMD = hand, foot, and mouth disease.
One missing value in rainfall data.
Figure 1.
Daily distribution of hand, foot, and mouth disease (HFMD) cases and meteorological variables in Wuxi, 2011–2017.
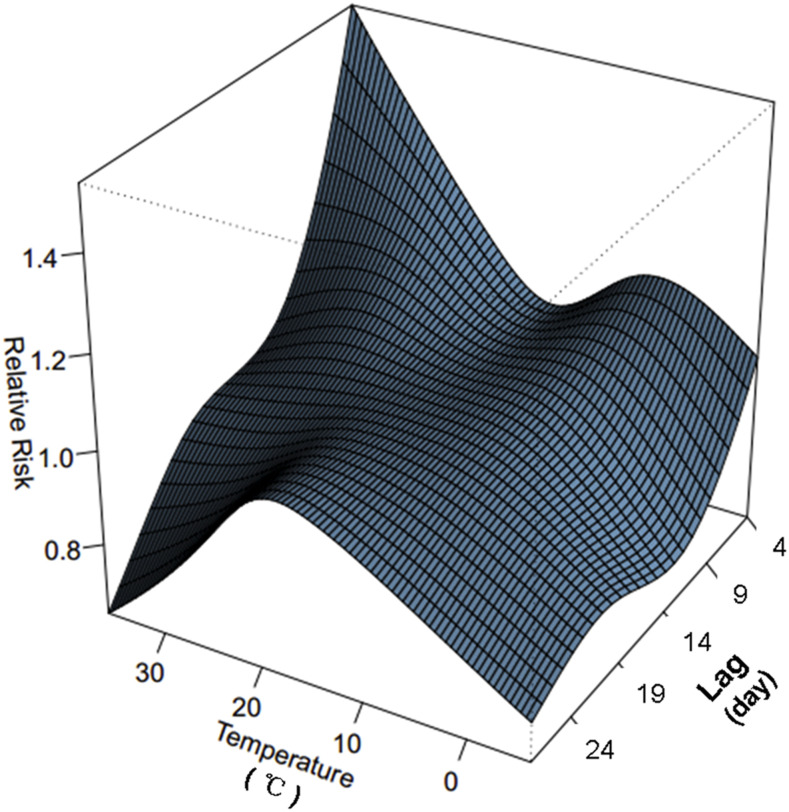
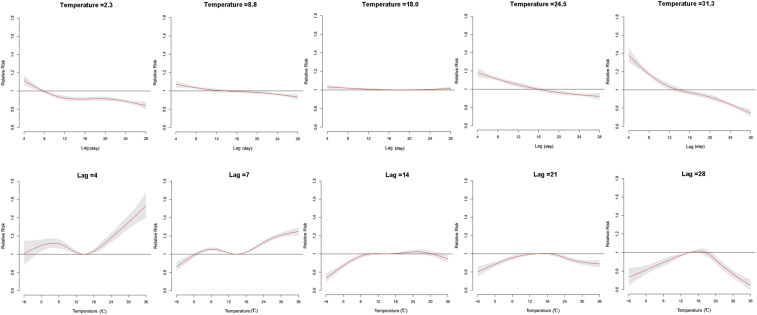
The three-dimensional plot depicted the relationship between daily mean temperature and HFMD cases over 28 days (lag 4–28) (Figure 2). The effects of temperature on HFMD case counts showed a nonlinear pattern at various lag days. Thus, we studied the RR of HFMD by temperature at specific lags (4, 7, 14, 21, and 28) and by lag at specific temperatures (2.3°C, 8.8°C, 18.0°C, 24.5°C, and 31.3°C), corresponding approximately to the 5th, 25th, 50th, 75th, and 95th percentiles of temperature distribution (Figure 3). Compared with the estimated effects at lower temperature (2.3°C and 8.8°C), higher temperature–associated effects appeared to be greater and more persistent during the first 2 weeks, whereas the effects declined significantly for 14–28 days. At specific lag days (lag 4 and lag 7), the RR curves displayed bimodal pattern, with the maximum RR value occurring at 36°C, whereas at lags 14, 21, and 28, the RR curve tended to be mono-peak, with lower RR estimates.
Figure 2.
Three-dimensional plot of the relationship between temperature and hand, foot, and mouth disease cases over 28 lag days. This figure appears in color at www.ajtmh.org.
Figure 3.
The relative risks of temperature on hand, foot, and mouth disease cases at specific temperature values and lags (15°C as reference). This figure appears in color at www.ajtmh.org.
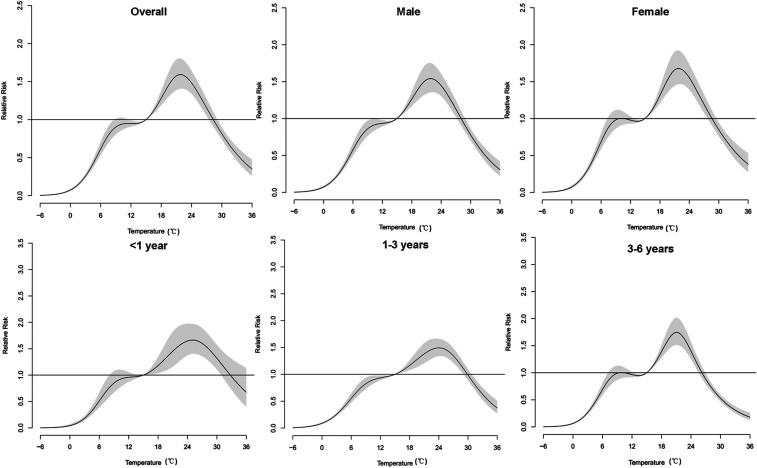
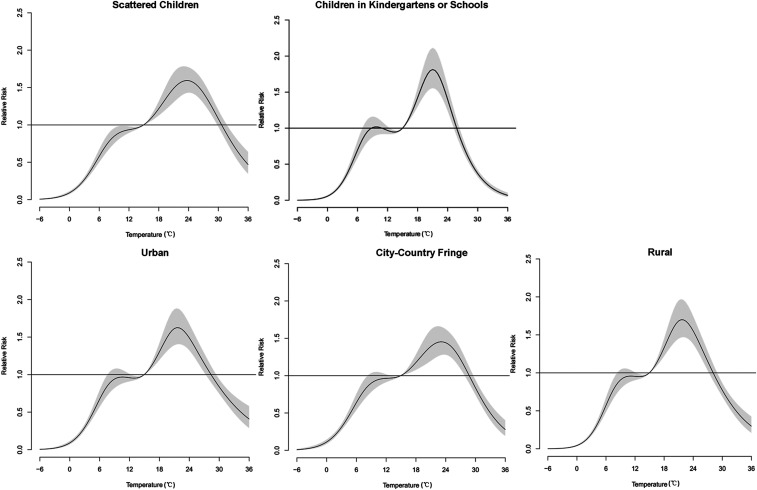
Figure 4 depicted an approximately M-shaped relationship between cumulative RR of HFMD and daily mean temperature over 28 days. The plot showed a significant increase in the RR estimates when temperature was greater than 0°C, and then reached its platform at 10–13°C. Likewise, the risk estimates rose steeply at 13–22°C, whereas greater than that, RRs decreased along with the increasing temperature. The exposure–response curves shared similar patterns in the overall effects between temperature and gender- or age-specific HFMD cases, as well as cases with varied sociological characteristics (Figures 4 and 5). The peak cumulative risk estimates were slightly larger in females than in males. As for different age-groups, peak RRs were higher among the 3- to 6-year group and the < 1-year group. Children living in rural areas gained greater RRs than children living in city-country fringe areas. It was worth noticing that the cumulative RR values of children attending kindergartens or schools were greater than those of scattered children. Moreover, the peak of children attending childcare centers arrived at a lower temperature (21.0°C) than that of scattered children (23.5°C).
Figure 4.
The cumulative relative risks of temperature on gender-specific and age-specific hand, foot, and mouth disease cases over 28 lag days (15°C as reference).
Figure 5.
The cumulative relative risks of temperature on hand, foot, and mouth disease cases with varied sociological characteristics over 28 lag days (15°C as reference).
The RRs of different mean temperatures for gender-specific and age-specific HFMD cases at various lags are summarized in Table 2. According to Schenker et al.,23 we considered between-group differences with a 2-fold change or more to be noteworthy. The risk estimate for females was 1.32 times higher than that for males at 31.3°C on the lag 4–14 days. At single-day lags, the changes of RRs were less than two times between different age-groups. At moving-average lags, however, RRs of the 3- to 6-year group were higher than those of < 3-year groups at lower temperatures (2.3°C and 8.8°C). For example, RRs for the 3- to 6-year group were 2.28 and 2.40 times higher than those of the 1- to 3-year group (for lag 4–7) at corresponding temperatures. At lag 4–14, RRs for the 3- to 6-year group achieved the maximum values at the observed temperatures among the age-groups (except at 31.3°C).
Table 2.
Relative risks of different temperatures for gender-specific and age-specific hand, foot, and mouth disease cases (−6°C as reference)
| Temperature | Total | Male | Female | < 1 year | 1–3 years | 3–6 years | |
|---|---|---|---|---|---|---|---|
| Lag 4 | P5 | 1.11 (0.99,1.24) | 1.04 (0.92,1.18) | 1.21 (1.06,1.38) | 1.27 (0.98,1.65) | 1.06 (0.93,1.21) | 1.13 (0.98,1.29) |
| P25 | 1.07 (0.92,1.23) | 1.00 (0.85,1.16) | 1.17 (0.99,1.39) | 1.24 (0.89,1.73) | 1.02 (0.86,1.19) | 1.09 (0.92,1.29) | |
| P50 | 1.03 (0.9,1.18) | 0.98 (0.84,1.13) | 1.10 (0.94,1.29) | 1.15 (0.84,1.57) | 0.98 (0.84,1.14) | 1.05 (0.89,1.23) | |
| P75 | 1.17 (1.01,1.36) | 1.11 (0.95,1.29) | 1.27 (1.07,1.49) | 1.33 (0.96,1.84) | 1.11 (0.95,1.31) | 1.17 (0.99,1.39) | |
| P95 | 1.37 (1.18,1.59) | 1.29 (1.1,1.51) | 1.48 (1.25,1.75) | 1.56 (1.13,2.16) | 1.31 (1.11,1.54) | 1.33 (1.12,1.58) | |
| Lag 14 | P5 | 1.24 (1.17,1.31) | 1.26 (1.19,1.34) | 1.20 (1.13,1.28) | 1.14 (1.01,1.29) | 1.21 (1.13,1.28) | 1.27 (1.19,1.36) |
| P25 | 1.35 (1.26,1.45) | 1.38 (1.28,1.49) | 1.31 (1.21,1.42) | 1.24 (1.06,1.46) | 1.32 (1.22,1.42) | 1.39 (1.28,1.51) | |
| P50 | 1.36 (1.28,1.46) | 1.38 (1.29,1.49) | 1.33 (1.23,1.43) | 1.27 (1.09,1.47) | 1.34 (1.25,1.44) | 1.39 (1.29,1.51) | |
| P75 | 1.39 (1.29,1.49) | 1.41 (1.31,1.52) | 1.35 (1.25,1.46) | 1.28 (1.10,1.50) | 1.37 (1.27,1.48) | 1.41 (1.3,1.54) | |
| P95 | 1.35 (1.26,1.45) | 1.37 (1.27,1.48) | 1.32 (1.22,1.43) | 1.26 (1.08,1.48) | 1.34 (1.24,1.45) | 1.36 (1.25,1.48) | |
| Lag 4–7 | P5 | 1.72 (1.24,2.37) | 1.46 (1.04,2.05) | 2.17 (1.51,3.14) | 2.22 (1.09,4.55) | 1.44 (1.01,2.05) | 1.92 (1.32,2.79) |
| P25 | 1.66 (1.11,2.48) | 1.39 (0.91,2.12) | 2.17 (1.37,3.43) | 2.21 (0.89,5.49) | 1.37 (0.88,2.14) | 1.89 (1.18,3.02) | |
| P50 | 1.48 (1.02,2.16) | 1.29 (0.87,1.92) | 1.81 (1.18,2.78) | 1.84 (0.79,4.28) | 1.26 (0.83,1.91) | 1.62 (1.04,2.52) | |
| P75 | 2.35 (1.58,3.5) | 2.00 (1.32,3.05) | 2.91 (1.85,4.58) | 2.99 (1.24,7.23) | 1.97 (1.27,3.06) | 2.38 (1.50,3.80) | |
| P95 | 3.74 (2.5,5.61) | 3.15 (2.06,4.82) | 4.63 (2.93,7.32) | 4.90 (2.02,11.88) | 3.22 (2.06,5.02) | 3.44 (2.15,5.51) | |
| Lag 4–14 | P5 | 7.51 (4.76,11.85) | 6.84 (4.22,11.09) | 8.68 (5.17,14.59) | 5.15 (1.89,14.02) | 4.85 (2.93,8.04) | 11.08 (6.49,18.93) |
| P25 | 12.05 (6.85,21.19) | 10.86 (5.95,19.79) | 14.16 (7.42,27.01) | 8.20 (2.31,29.06) | 7.65 (4.08,14.33) | 18.39 (9.45,35.79) | |
| P50 | 10.66 (6.32,17.97) | 9.77 (5.60,17.02) | 12.20 (6.71,22.16) | 7.72 (2.41,24.77) | 7.75 (4.34,13.85) | 14.10 (7.61,26.11) | |
| P75 | 23.98 (13.84,41.57) | 21.57 (12.04,38.66) | 27.95 (14.95,52.27) | 17.24 (5.13,57.98) | 17.47 (9.5,32.11) | 27.92 (14.64,53.26) | |
| P95 | 39.68 (22.90,68.75) | 34.96 (19.52,62.59) | 46.18 (24.72,86.27) | 31.42 (9.39,105.12) | 30.28 (16.49,55.6) | 37.45 (19.62,71.47) | |
| Lag 4–21 | P5 | 23.26 (13.73,39.42) | 21.40 (12.18,37.58) | 25.62 (13.98,46.95) | 21.45 (6.51,70.64) | 14.14 (7.86,25.43) | 34.30 (18.33,64.18) |
| P25 | 64.82 (33.72,124.58) | 59.92 (29.74,120.71) | 69.92 (32.86,148.79) | 65.64 (14.48,297.52) | 37.29 (17.96,77.44) | 99.71 (45.67,217.70) | |
| P50 | 62.50 (34.12,114.49) | 59.12 (30.89,113.12) | 64.76 (32.17,130.37) | 58.17 (14.36,235.75) | 38.79 (19.72,76.32) | 91.84 (44.53,189.42) | |
| P75 | 122.99 (65.47,231.05) | 115.26 (58.72,226.25) | 128.73 (62.27,266.13) | 114.89 (27.14,486.46) | 79.27 (39.24,160.12) | 158.83 (74.95,336.6) | |
| P95 | 156.30 (83.70,291.86) | 141.09 (72.32,275.27) | 166.71 (81.17,342.41) | 177.50 (42.47,741.90) | 112.44 (56.04,225.62) | 144.13 (68.37,303.85) |
Table 3 showed the RRs of different temperatures for HFMD cases with varied sociological characteristics at various lags. Relative risks for children in kindergartens or schools were 2.22–3.43 times larger than those for scattered children at 5th, 25th, 50th, and 75th percentiles of temperature distribution. Likewise, RRs for those attending childcare centers were 2.06–3.76 times higher than those for scattered children at temperatures of 2.3°C, 8.8°C, 18.0°C, and 24.5°C. In addition, as for the regional differences, the RRs of children in rural areas were 2.67–10.81 times and 3.10–11.22 times higher than those of children in urban areas and city-country fringe areas, respectively, at lag 4–14 and lag 4–21.
Table 3.
Relative risks of different temperatures for hand, foot, and mouth disease cases with varied sociological characteristics (−6°C as reference)
| Temperature | Scattered children | Children in kindergartens or schools | Urban | City-country fringe | Rural | |
|---|---|---|---|---|---|---|
| Lag 4 | P5 | 1.05 (0.92,1.18) | 1.21 (1.04,1.4) | 1.08 (0.94,1.24) | 1.12 (0.97,1.29) | 1.12 (0.97,1.30) |
| P25 | 1.00 (0.85,1.16) | 1.18 (0.99,1.42) | 1.02 (0.86,1.21) | 1.07 (0.89,1.29) | 1.10 (0.91,1.32) | |
| P50 | 0.97 (0.84,1.13) | 1.13 (0.95,1.34) | 0.96 (0.81,1.13) | 1.00 (0.84,1.18) | 1.11 (0.93,1.32) | |
| P75 | 1.13 (0.97,1.32) | 1.25 (1.04,1.5) | 1.07 (0.90,1.28) | 1.12 (0.94,1.34) | 1.29 (1.07,1.54) | |
| P95 | 1.35 (1.15,1.58) | 1.35 (1.12,1.63) | 1.27 (1.06,1.51) | 1.31 (1.09,1.58) | 1.47 (1.22,1.76) | |
| Lag 14 | P5 | 1.22 (1.15,1.29) | 1.27 (1.18,1.36) | 1.21 (1.14,1.30) | 1.17 (1.09,1.25) | 1.33 (1.24,1.43) |
| P25 | 1.33 (1.23,1.43) | 1.39 (1.27,1.52) | 1.31 (1.21,1.43) | 1.26 (1.16,1.38) | 1.5 (1.37,1.64) | |
| P50 | 1.35 (1.25,1.44) | 1.39 (1.28,1.51) | 1.31 (1.21,1.42) | 1.29 (1.19,1.40) | 1.5 (1.38,1.64) | |
| P75 | 1.38 (1.28,1.48) | 1.41 (1.29,1.54) | 1.33 (1.22,1.45) | 1.32 (1.21,1.44) | 1.54 (1.41,1.69) | |
| P95 | 1.35 (1.25,1.45) | 1.34 (1.23,1.47) | 1.29 (1.19,1.41) | 1.28 (1.18,1.40) | 1.51 (1.38,1.65) | |
| Lag 4–7 | P5 | 1.38 (0.98,1.95) | 2.39 (1.6,3.56) | 1.53 (1.05,2.24) | 1.65 (1.11,2.45) | 1.98 (1.32,2.96) |
| P25 | 1.29 (0.84,1.98) | 2.46 (1.49,4.07) | 1.40 (0.87,2.26) | 1.57 (0.95,2.58) | 2.07 (1.24,3.43) | |
| P50 | 1.23 (0.83,1.84) | 2.11 (1.32,3.38) | 1.14 (0.73,1.78) | 1.28 (0.80,2.04) | 2.13 (1.33,3.42) | |
| P75 | 2.07 (1.36,3.17) | 2.97 (1.81,4.88) | 1.68 (1.05,2.69) | 1.93 (1.18,3.16) | 3.62 (2.2,5.96) | |
| P95 | 3.50 (2.28,5.37) | 3.71 (2.24,6.14) | 2.74 (1.70,4.43) | 3.11 (1.89,5.12) | 5.34 (3.23,8.85) | |
| Lag 4–14 | P5 | 4.82 (2.97,7.84) | 15.27 (8.63,26.99) | 5.69 (3.31,9.79) | 4.90 (2.78,8.63) | 15.18 (8.50,27.11) |
| P25 | 7.26 (3.97,13.27) | 27.27 (13.42,55.44) | 7.93 (4.04,15.54) | 7.27 (3.59,14.72) | 31.42 (15.24,64.80) | |
| P50 | 7.53 (4.31,13.15) | 21.02 (10.89,40.56) | 5.74 (3.08,10.70) | 6.29 (3.28,12.09) | 34.03 (17.42,66.47) | |
| P75 | 18.96 (10.55,34.06) | 39.02 (19.62,77.62) | 11.22 (5.84,21.55) | 13.47 (6.80,26.67) | 89.92 (44.64,181.16) | |
| P95 | 34.83 (19.41,62.51) | 42.78 (21.46,85.28) | 18.99 (9.90,36.46) | 22.78 (11.51,45.09) | 137.26 (68.25,276.05) | |
| Lag 4–21 | P5 | 15.01 (8.55,26.38) | 43.55 (22.36,84.83) | 16.73 (8.89,31.5) | 12.48 (6.43,24.22) | 57.48 (29.14,113.39) |
| P25 | 38.73 (19.23,77.99) | 133.01 (57.94,305.34) | 38.47 (17.51,84.51) | 28.79 (12.58,65.93) | 232.26 (99.23,543.62) | |
| P50 | 40.83 (21.36,78.08) | 121.66 (56.27,263.06) | 30.71 (14.82,63.65) | 27.48 (12.76,59.20) | 265.47 (120.68,583.97) | |
| P75 | 91.13 (46.43,178.89) | 202.22 (90.96,449.59) | 54.70 (25.65,116.62) | 52.66 (23.76,116.70) | 591.01 (261.05,1337.99) | |
| P95 | 138.73 (71.15,270.48) | 127.26 (57.42,282.05) | 72.16 (34.07,152.82) | 67.2 (30.50,148.04) | 694.34 (309.07,1559.89) |
DISCUSSION
To date, studies document the exposure–response relationship between temperature and HFMD display variations in different regions depending on geographic and climatic conditions,26 and very few have determined the modifying effects of social factors on the temperature–HFMD association. Our findings showed that the overall effect of temperature on HFMD incidence was an approximately M-shaped curve with the cumulative RR value peaked at 22°C in Wuxi. In addition, rural residents and children attending schools or kindergartens might be more vulnerable to HFMD at certain temperatures.
During the observing period, HFMD in Wuxi showed semiannual peaks of activity, including a major peak in spring and early summer followed by a smaller peak in autumn, in accord with the general description of the seasonality in southern China, which also showed HFMD cases were fewer during school holidays when temperatures were extremely low or high. This might imply a nonlinear exposure–response relationship between temperature and HFMD or other modifiers. In our study, this relationship was approximately M-shaped, of which the minor peak occurred in 10°C and a major peak in 22°C, in consistent with studies in Minhang district, Shanghai,15 and in Chengdu.21 Likewise, the threshold temperatures were reported at 25.0–27.5°C in Beijing,22 20°C in Japan,27 and 32°C in Singapore,18 whereas log–linear links were identified in Guangzhou28 and Shenzhen.29 More recently, Xiao et al.26 pooled data from 143 cities in mainland China and demonstrated a nearly inverted V-shaped curve for temperature–HFMD link, which peaked at the 91st percentile of temperature with a risk ratio of 1:30 (95% CI: 1.23–1.37) compared with its 50th percentile. The contradictory mechanisms of HFMD transmission dynamics may partly explain this non-monotonic association.30 On the one hand, evidence affirms higher temperature favors host activity31 and excretion of enterovirus32; thereby, hot weather may increase the chances that susceptible people contact with HFMD cases or contaminated environmental reservoirs. On the other hand, extremely hot weather, accompanied by ultraviolet radiation, may shorten the survival time of enterovirus in the environment and, therefore, reduce the chances of transmission.33 For the lag-specific effects, we also found that the risk due to hot weather occurred earlier and lasted longer than that of cold weather, coinciding with a study in Beijing.22 The pathogen activity and host behavior may be involved in the complex mechanisms; however, further studies are still needed.
As mentioned before, HFMD cases were fewer during school holidays than during school days. Separation of children during summer and winter vacations decreased social contacts; thus, it interrupted transmission in the childcare centers and led HFMD incidence to reduce.18 Conversely, both factors of suitable weather during school days and more frequent contacts of susceptible individuals may interplay, thus compounding the positive effects on HFMD transmission. Moreover, in our study, the cumulative RR estimates on temperature–HFMD associations of children attending childcare centers were higher than those of scattered children at various temperatures, and, also, the peak value of the former arrived earlier (at a lower temperature) than the latter one, suggesting a modifying effect of children’s aggregation mode. A research carried out by Hu et al.34 reported that six climatic factors, including mean temperature, were significantly associated with the morbidity of HFMD, whereas the child population density had a greater influence on morbidity than those meteorological factors.
In terms of interregional distinctions, we found that children in rural areas were more likely to achieve greater RR values than children in urban areas and city-country fringe at specific temperatures. To our knowledge, only one study has reported the impact meteorological factors had on HFMD across social status in time series study and reported differential patterns in exposure–response relationship between developed and less developed regions,28 which may be because of the population density and the proportion of student population.35 Zhu et al.34,36 also reported the regional indicator, numbers of healthcare institution, and annual household income to be associated modifiers in temperature–HFMD relationship. Other than those predictors, in our case, rural regions retain much larger water areas in Wuxi, which are considered as ideal natural reservoirs for enterovirus.37 In the water environment, enterovirus becomes more resistant to various factors leading to virus inactivation, such as temperature, salinity, and pH,38 which might enhance the chances of human contacts with pathogens. However, more evidence is called for to verify this hypothesis. In addition, the age-stratified results indicated children aged 3–6 years appeared to be more vulnerable to temperature, in line with the Zhang et al.13 study. This could result from the fact that children aged < 1 year gained protection from maternal antibodies,39,40 whereas children aged 1–3 years tended to spend less time in outdoor activities, which decreased the odds of being infected.41,42
Besides the aforementioned findings, DLNMs performed in our study facilitated the simultaneous calculation of predictors–HFMD relationships at differential lags without colinearity problems.28 Nevertheless, limitations of our analysis should also be noted. First, under the design of eco-epidemiology, the observed association was captured at the population level, so any further inference should be treated with caution. Second, the absence of asymptomatic and unreported cases is commonly encountered in the infectious disease studies, which could lead to bias of the analysis. Enterovirus 71 vaccine was approved in China in 2016 and became more commonly used in 2017; our study did not determine the potential effect of vaccine. Also, various enterovirus strains are reported to respond distinctively to temperature.43 Better knowledge on the population-representative seroepidemiology research studies would greatly refine understanding of HFMD dynamics and its relationship with meteorological factors.
In summary, in this time-series study, we found a nonlinear association between childhood HFMD and daily mean temperature in Wuxi between 2011 and 2017. Furthermore, our results suggested that age, urban–rural status, and aggregation mode of the population modify the short-term effects of temperature on HFMD. These findings enhance our knowledge of the disease dynamics caused by changing environments, provide information for temperature-based early warning, and facilitate policy-making and resource allocation.
REFERENCES
- 1.Pallansch MA, Oberste MS, Whitton JL, 2013. Enteroviruses: polioviruses, coxsackieviruses, echoviruses, and newer enteroviruses. In Knipe DM, Howley P, editors. Fields Virology 2. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 490–530. [Google Scholar]
- 2.Li Y, et al. 2018. Emerging enteroviruses causing hand, foot and mouth disease, China, 2010–2016. Emerg Infect Dis 24: 1902–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ooi MH, Wong SC, Lewthwaite P, Cardosa MJ, Solomon T, 2010. Clinical features, diagnosis, and management of Enterovirus 71. Lancet Neurol 9: 1097–1105. [DOI] [PubMed] [Google Scholar]
- 4.Chan LG, et al. Outbreak Study Group , 2000. Deaths of children during an outbreak of hand, foot, and mouth disease in Sarawak, Malaysia: clinical and pathological characteristics of the disease. Clin Infect Dis 31: 678–683. [DOI] [PubMed] [Google Scholar]
- 5.Chan KP, Goh KT, Chong CY, Teo ES, Lau G, Ling AL, 2003. Epidemic hand, foot and mouth disease caused by human Enterovirus 71, Singapore. Emerg Infect Dis 9: 78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma E, Chan KC, Cheng P, Wong C, Chuang SK, 2010. The Enterovirus 71 epidemic in 2008–public health implications for Hong Kong. Int J Infect Dis 14: e775–e780. [DOI] [PubMed] [Google Scholar]
- 7.Koh WM, Bogich T, Siegel K, Jin J, Chong EY, Tan CY, Chen MI, Horby P, Cook AR, 2016. The epidemiology of hand, foot and mouth disease in Asia: a systematic review and analysis. Pediatr Infect Dis J 35: e285–e300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhuang ZC, et al. 2015. Epidemiological research on hand, foot, and mouth disease in Mainland China. Viruses 7: 6400–6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koh WM, Badaruddin H, La H, Chen MI, Cook AR, 2018. Severity and burden of hand, foot and mouth disease in Asia: a modelling study. BMJ Glob Health 3: e000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO , 2006. Communicable Disease Surveillance and Response Systems. Lyon, France: World Health Organization. [Google Scholar]
- 11.Wang JF, Guo YS, Christakos G, Yang WZ, Liao YL, Zhong-Jie LI, Xiao-Zhou LI, Lai SJ, Chen HY, 2011. Hand, foot and mouth disease: spatiotemporal transmission and climate. Int J Health Geogr 10: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xing W, et al. 2014. Hand, foot, and mouth disease in China, 2008–12: an epidemiological study. Lancet Infect Dis 14: 308–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang W, Du Z, Zhang D, Yu Shao Y, 2015. Quantifying the adverse effect of excessive heat on children: an elevated risk of hand, foot and mouth disease in hot days. Sci Total Environ 541: 194–199. [DOI] [PubMed] [Google Scholar]
- 14.Yien Ling H, Joacim RV, Nawi N, 2011. Short term effects of weather on hand, foot and mouth disease. PLoS One 6: e16796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qi H, et al. 2018. Impact of meteorological factors on the incidence of childhood hand, foot, and mouth disease (HFMD) analyzed by DLNMs-based time series approach. Infect Dis Poverty 7: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang J, Chen S, Wu Y, Tong Y, Wang L, Zhu M, Hu S, Guan X, Wei S, 2018. Quantifying the influence of temperature on hand, foot and mouth disease incidence in Wuhan, central China. Sci Rep 8: 1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coates SJ, Davis MDP, Andersen LK, 2019. Temperature and humidity affect the incidence of hand, foot, and mouth disease: a systematic review of the literature–a report from the International Society of Dermatology Climate Change Committee. Int J Dermatol 58: 388–399. [DOI] [PubMed] [Google Scholar]
- 18.Hii YL, Rocklov J, Ng N, 2011. Short term effects of weather on hand, foot and mouth disease. PLoS One 6: e16796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du Z, Zhang W, Zhang D, Yu S, Hao Y, 2016. The threshold effects of meteorological factors on hand, foot, and mouth disease (HFMD) in China, 2011. Sci Rep 6: 36351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang C, et al. 2016. Spatiotemporal cluster patterns of hand, foot, and mouth disease at the county level in mainland China, 2008–2012. PLoS One 11: e0147532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yin F, Zhang T, Liu L, Lv Q, Li X, 2016. The association between ambient temperature and childhood hand, foot, and mouth disease in Chengdu, China: a distributed lag non-linear analysis. Sci Rep 6: 27305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu M, Yu W, Tong S, Jia L, Liang F, Pan X, 2015. Non-linear association between exposure to ambient temperature and children’s hand-foot-and-mouth disease in Beijing, China. PLoS One 10: e0126171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schenker N, Gentleman JF, 2001. On judging the significance of differences by examining the overlap between confidence intervals. Am Stat 55: 182–186. [Google Scholar]
- 24.Gasparrini A, 2011. Distributed lag linear and non-linear models in R: the package dlnm. J Stat Softw 43: 1–20. [PMC free article] [PubMed] [Google Scholar]
- 25.Gasparrini A, Armstrong B, Kenward MG, 2010. Distributed lag non‐linear models. Stat Med 29: 2224–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiao X, Gasparrini A, Huang J, Liao Q, Liu F, Yin F, Yu H, Li X, 2017. The exposure-response relationship between temperature and childhood hand, foot and mouth disease: a multicity study from mainland China. Environ Int 100: 102–109. [DOI] [PubMed] [Google Scholar]
- 27.Onozuka D, Hashizume M, 2011. The influence of temperature and humidity on the incidence of hand, foot, and mouth disease in Japan. Sci Total Environ 410: 119–125. [DOI] [PubMed] [Google Scholar]
- 28.Zhang W, Du Z, Zhang D, Yu S, Huang Y, Hao Y, 2016. Assessing the impact of humidex on HFMD in Guangdong province and its variability across social-economic status and age groups. Sci Rep 6: 18965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Z, Xie X, Chen X, Li Y, Lu Y, Mei S, Liao Y, Lin H, 2016. Short-term effects of meteorological factors on hand, foot and mouth disease among children in Shenzhen, China: non-linearity, threshold and interaction. Sci Total Environ 539: 576–582. [DOI] [PubMed] [Google Scholar]
- 30.Chen C, et al. 2014. Short-term effects of meteorological factors on children hand, foot and mouth disease in Guangzhou, China. Int J Biometeorol 58: 1605–1614. [DOI] [PubMed] [Google Scholar]
- 31.Bélanger M, Gray-Donald K, Oloughlin J, Paradis G, Hanley J, 2009. Influence of weather conditions and season on physical activity in adolescents. Ann Epidemiol 19: 180–186. [DOI] [PubMed] [Google Scholar]
- 32.Theng-Theng F, Lipp EK, 2005. Enteric viruses of humans and animals in aquatic environments: health risks, detection, and potential water quality assessment tools. Microbiol Mol Biol Rev 69: 357–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bertrand I, et al. 2012. The impact of temperature on the inactivation of enteric viruses in food and water: a review. J Appl Microbiol 112: 1059–1074. [DOI] [PubMed] [Google Scholar]
- 34.Hu M, Li Z, Wang J, Jia L, Liao Y, Lai S, Guo Y, Zhao D, Yang W, 2012. Determinants of the incidence of hand, foot and mouth disease in China using geographically weighted regression models. PLoS One 7: e38978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bo YC, Song C, Wang J-F, Li X-W, 2014. Using an autologistic regression model to identify spatial risk factors and spatial risk patterns of hand, foot and mouth disease (HFMD) in Mainland China. BMC Public Health 14: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu L, Wang X, Guo Y, Xu J, Xue F, Liu Y, 2016. Assessment of temperature effect on childhood hand, foot and mouth disease incidence (0–5 years) and associated effect modifiers: a 17 cities study in Shandong Province, China, 2007–2012. Sci Total Environ 551–552: 452–459. [DOI] [PubMed] [Google Scholar]
- 37.Rajtar B, Majek M, Polański Ł, Polz-Dacewicz M, 2008. Enteroviruses in water environment–a potential threat to public health. Ann Agric Environ Med 15: 199–203. [PubMed] [Google Scholar]
- 38.Salo RJ, Cliver DO, 1976. Effect of acid pH, salts, and temperature on the infectivity and physical integrity of enteroviruses. Arch Virol 52: 269–282. [DOI] [PubMed] [Google Scholar]
- 39.Feng-Cai Z, et al. 2012. Retrospective study of the incidence of HFMD and seroepidemiology of antibodies against EV71 and CoxA16 in prenatal women and their infants. PLoS One 7: e37206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sheng-Wen H, et al. 2009. Reemergence of Enterovirus 71 in 2008 in Taiwan: dynamics of genetic and antigenic evolution from 1998 to 2008. J Clin Microbiol 47: 3653–3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luan-Yin C, et al. 2002. Risk factors of Enterovirus 71 infection and associated hand, foot, and mouth disease/herpangina in children during an epidemic in Taiwan. Pediatrics 109: e88. [DOI] [PubMed] [Google Scholar]
- 42.Suminski RR, Poston WC, Market P, Hyder M, Sara PA, 2008. Meteorological conditions are associated with physical activities performed in open-air settings. Int J Biometeorol 52: 189–197. [DOI] [PubMed] [Google Scholar]
- 43.Liu W, Wu S, Xiong Y, Li T, Wen Z, Yan M, Qin K, Liu Y, Wu J, 2014. Co-circulation and genomic recombination of Coxsackievirus A16 and Enterovirus 71 during a large outbreak of hand, foot, and mouth disease in central China. PLoS One 9: e96051. [DOI] [PMC free article] [PubMed] [Google Scholar]