Abstract.
Chikungunya virus (CHIKV) is a global emergent arthritogenic alphavirus transmitted by anthropophilic Stegomyia mosquitoes. Chikungunya fever may evolve to chronic arthralgia in 57–80% of infected patients. This study was developed to identify possibly fast, simple low-cost biomarkers to monitor chronic CHIKV-induced articular disease. Between 2017 and 2018, we analyzed clinical data of patients meeting the criteria established by standard protocols to define chronic chikungunya articular disease. Patients were classified according to the disease activity scores, inflammatory biomarkers (erythrocyte sedimentation rate [ESR], ferritin, and C-reactive protein [CRP] serum), positive rheumatoid factor, comorbidities, smoking, and previous use of corticosteroids determined before beginning therapy. Of 106 patients, 98 (92.5%) were women with mean age of 52 ± 13 years, 6.8 ± 4.4 months of illness duration at the first medical appointment, and 6.7 ± 4.5 affected joints. Mean ESR (26 ± 19), CRP (2.6 ± 3.6), and stratified ferritin (144 ± 115) levels were normal according to reference values. There was no significance in comparing the levels of inflammatory biomarkers and the additional variables analyzed in the presence of moderate chronic joint disease in the study population. However, we identified a negative correlation between disease activity measures and duration of disease at the first medical evaluation after initial infection (P < 0.001), corroborating data observed in the literature.
INTRODUCTION
Chikungunya virus (CHIKV) is a global public health emergent arbovirus transmitted by anthropophilic urban Aedes aegypti and Aedes albopictus mosquitoes.1 In 2014, the first cases of CHIKV were documented in Brazil. The Asian and the East, Central, and South African genotypes were almost concomitantly introduced in the cities of Oiapoche, Amapá, and Feira de Santana, Bahia.2
CHIKV is responsible for asymptomatic (3–25% of infected people) to febrile acute disease characterized by a sudden onset of fever and intense arthralgia, which may be accompanied by myalgia, headache, and skin rash.3 These manifestations may progress to resolution within two weeks; however, 88% of these patients may experience subacute and chronic articular forms of the disease.4 Atypical (0.3–1%) and severe atypical cases (0.1%) are associated with 10–30% mortality rates.5
Long-term chronic illness produces an impact on the daily life quality,6 with the most frequent sequelae being persistent arthralgia.7 Risk factors for the development of chronic rheumatologic manifestations and severe disease include female gender, elderly subjects, severity of acute disease, and certain comorbidities, such as obesity, diabetes, cardiac diseases, and asthma.8–10
The pathogenesis of articular disease lasting 12 weeks or more has not been reported. In mice models, viral nucleic acid was detected and has been reported to persist in joint tissues for at least 16 weeks postinfection.11 Based on that, two main hypothesis emerged: persistence of low replicative virus or non-replicative CHIKV debris in the joint and muscle tissue, leading to inflammatory cell chemotaxis and perpetuation of inflammation, and triggering of persistent immune activation.6 However, isolation of the virus in synovial fluid and biopsy has not been observed.12,13 Some pro-inflammatory cytokines such as interleukin-1β (IL-1β), IL-6, tumour necrosis factor alpha [TNF-α], monocyte chemoattractant protein-1 [MCP-1], regulated on activation normal T cell expressed and secreted [RANTES], interferon (IFN)-α, and IFN-γ have been involved in the progression of CHIKV-associated chronic arthralgia.11,14
Clinically, chikungunya arthropathy resembles autoimmune diseases such as rheumatoid arthritis and other hyperferritinemia syndromes, which are accompanied by increased ferritin levels. During infections and inflammatory injuries, ferritin concentrations may increase drastically in the organism.15
The erythrocyte sedimentation rate (ESR) is a simple low-cost clinical parameter characterized by blood cell aggregation, rouleaux, and fast plasma protein-induced erythrocyte sedimentation. The ESR indicates the degree of inflammation in several conditions and is frequently used in rheumatology.16
C-reactive protein (CRP) is an acute-phase pentraxin of hepatocyte origin which increases in response to pro-inflammatory cytokines such as IL-6. C-reactive protein is considered an opsonin involved in the complement cascade activation which may be frequently increased during chronic inflammatory injuries, despite the fact that some viral infections with concurrent high levels of IFN-α present unaltered levels of CRP.16,17
The progress on studies involving immune pathogenesis mechanisms in CHIKV-induced arthropathy associated with the lack of specific therapy protocols with reduced side effects demonstrates the complexity of this chronic lesion. Because there are no current biomarkers to monitor CHIKV-induced arthropathy, this observational study was conducted to investigate if routinely common low-cost inflammatory tests might represent useful tools to monitor CHIKV chronic articular disease along with disease activity score (DAS).
METHODS
This cross-sectional study was approved by the Institutional Ethics Committee (CEP saúde, Universidade Federal de Mato Grosso approval 2.658.648) and followed the Conselho Nacional de Saúde (CNS) resolution 466/2012. Patients who met the criteria established by the Brazilian Rheumatology Society standard protocols to define chikungunya chronic articular disease and who were receiving medical care between June 2017 and December 2018, at the arthropathy outpatient department at Cuiabá University were invited to participate in this study and were included only after having signed a written consent form.
A case was established according to compatible clinical and epidemiological history defined as arthritis in ≥ 4 joints for more than 6 weeks associated with laboratorial confirmation of recent acute infection, for example, CHIKV gene reverse-transcriptase polymerase chain reaction (RT-PCR) until the fifth day of disease or anti-CHIKV IgM and IgG (capture ELISA, Euroimmun, São Caetano do Sul, SP, Brazil) after the fifth day of symptoms, associated with tests to exclude other polyarthritis etiologies.18
During the first medical appointment, the duration of disease at the first medical evaluation after initial infection and demographical and clinical data were obtained, including those characterizing the chronic articular disease during physical examination. Blood samples were taken for ferritin, ESR, and CRP dosage. Only the data obtained before establishing standard therapy were analyzed in this study.
Ferritin levels were determined by chemiluminescence (Abbott Laboratories, Longford, Ireland) according to reference values (11–306 ng/mL). C-reactive protein was dosed by turbidimetry (SENTINEL CH SpA, Milan, Italy) considering > 10 mg/L as reference value. The ESR was determined by using the Westergren modified spontaneous sedimentation method. Reference ESR values were < 15 mm/hour (< 50 years of age), < 20 mm/hour (50–85 years), and < 30 mm/hour (> 85 years) for men and < 20 mm/hour, < 30 mm/hour, and < 40 mm/hour for women in the same age categories.
Clinical Disease Activity Index (CDAI) is a patient and provider composite tool, which adds three components of the provider assessment (28 swollen joint count, 28 tender joint count, and provider global assessment of disease activity) to the patient global assessment of disease activity. Calculation is performed by a simple numerical addition of the component scores, without the need for acute-phase reactant (Table 1).19 The status of activity and respective cutoff points were determined as remission ≤ 2.8, low activity ≤ 10, moderate activity > 10 to ≤ 22, and high activity > 22.20
Table 1.
Composite measures of disease activity used in rheumatoid arthritis
| Component | Clinical Disease Activity Index | Disease activity score (28 joints) (with four variables) |
|---|---|---|
| Number of swollen joints | 0–28 Simple sum | Square root of the simple sum |
| Number of painful joints | 0–28 Simple sum | Square root of the simple sum |
| Acute-phase reagents | – | Erythrocyte sedimentation rate 2–100 mm or C-reactive protein 0.1–10 mg/dL logarithmic transformation |
| Global health assessment (patient) | – | 0–100 mm |
| Assessment of disease activity (patient) | 0–10 cm | – |
| Assessment of disease activity (examiner) | 0–10 cm | – |
| Total index | Simple sum | Calculation formula (requires a calculator) |
| Index variation | 0–76 | 0.49–9.07 |
Modified Mota et al.20
Disease activity score 28 (DAS 28) evaluates 28 articulations for the presence of pain and edema taking into consideration the global health evaluation and the ESR or CRP level.21 In this study, we used both inflammatory biomarkers as evidence to calculate this index (Table 1). Disease score was determined as in remission for values < 2.6 on DAS 28, low activity for values between 2.6 and 3.2, moderate for values between 3.2 and 5.1, and high activity when > 5.1 points (Table 1).20
For the statistical study, continuous variables were described as mean, median, SD, and interquartile range. Categorical variables were described using absolute and relative frequencies. To assess whether the distribution of disease activity indices differed according to the presence of comorbidities, smoking, use of corticosteroids or inflammatory tests, the t-Student22 test was used. We also used the Spearman or Pearson tests to assess the correlation between continuous variables. The analyses were performed with the aid of R 3.5.1 software (R Core Team 2019, R Foundation of Statistical Computing, Vienna, Austria).23 For the hypothesis tests, a significance level of 5% was considered.
RESULTS
A total of 106 patients were included in the study. Ninety-eight of them were women (92.5%) with mean age of 51.63 ± 12.52 years. The median number of affected joints per patient was 6.71 ± 4.52 with 89 (84%) with morning stiffness, consistent with the definition of CHIKV-induced polyarthritis. The mean duration at the first medical evaluation after initial infection was 6.77 ± 4.38 months. To make a diagnosis that differentiates that of rheumatoid arthritis, we measured the rheumatoid factor which resulted positive in three (2.8%) individuals. At the first medical appointment, 66 (62.3%) patients were using corticosteroids regularly, 38 (35.8%) had systemic arterial hypertension, 14 (13.2%) were diabetic, and three (2.8%) smokers. A summary of the data is shown in Table 2.
Table 2.
Initial description of the sample
| Variable | Descriptive |
|---|---|
| Gender | |
| Female | 98/106 (92.5%) |
| Male | 8/106 (7.5%) |
| Age (years) | 51.63 ± 12.52 |
| Time of disease in admission (months) | 6.77 ± 4.38 |
| Number of affected joints | 6.71 ± 4.52 |
| Morning stiffness | |
| No | 17/106 (16.0%) |
| Yes | 89/106 (84.0%) |
| Rheumatoid factor | |
| Negative | 103/106 (97.2%) |
| Positive | 3/106 (2.8%) |
| Corticosteroids | |
| No | 40/106 (37.7%) |
| Yes | 66/106 (62.3%) |
| Systemic arterial hypertension | |
| No | 68/106 (64.2%) |
| Yes | 38/106 (35.8%) |
| Diabetes | |
| No | 92/106 (86.8%) |
| Yes | 14/106 (13.2%) |
| Smoking | |
| No | 103/106 (97.2%) |
| Yes | 3/106 (2.8%) |
The mean value of acute-phase reagent reference for age and gender was 26.18 ± 18.71 mm/hour for the ESR, 2.59 ± 3.62 mg/L for CRP, and 144.48 ± 115.10 ng/dL for ferritin (Table 3). From this, 56 (53.3%) patients had normal ESR levels, 102 (96.2%) had normal CRP levels, and 95 (89.6%) presented normal levels of ferritin (Table 4).
Table 3.
Acute-phase reagents, disease activity measures, and the number of affected joints
| Variable | Mean ± SD | Median (quartis) |
|---|---|---|
| ESR (mm/hour) | 26 ± 19 | 20.00 (13.00; 33.00) |
| CRP (mg/L) | 2.6 ± 3.6 | 1.29 (0.57; 2.71) |
| Ferritin (ng/mL) | 145 ± 115 | 107.00 (62.25; 188.00) |
| DAS 28 ESR | 4.84 ± 1.04 | 4.76 (4.28; 5.32) |
| DAS 28 CRP | 4.04 ± 0.95 | 3.93 (3.49; 4.39) |
| Clinical Disease Activity Index | 21.25 ± 12.24 | 18.00 (13.00; 24.75) |
CRP = C-reactive protein; DAS 28 = disease activity score (28 joints); ESR = erythrocyte sedimentation rate.
Table 4.
Classification of acute-phase reagents and disease activity measures (CDAI and DAS 28)
| Variable | Total |
|---|---|
| Ferritin | |
| Normal | 95/106 (89.6%) |
| High | 9/106 (8.5%) |
| ESR | |
| Normal | 56/105 (53.3%) |
| High | 49/105 (46.7%) |
| CRP | |
| Normal | 102/106 (96.2%) |
| High | 4/106 (3.8%) |
| CDAI | |
| Remission | 0/106 (0.0%) |
| Low | 11/106 (10.4%) |
| Moderate | 65/106 (61.3%) |
| High | 30/106 (28.3%) |
| DAS 28 CRP | |
| Remission | 3/106 (2.8%) |
| Low | 12/106 (11.3%) |
| Moderate | 79/106 (74.5%) |
| High | 12/106 (11.3%) |
| DAS 28 ESR | |
| Remission | 0/106 (0.0%) |
| Low | 6/106 (5.7%) |
| Moderate | 65/106 (61.3%) |
| High | 35/106 (33.0%) |
CDAI = Clinical Disease Activity Index; CRP = C-reactive protein; DAS 28 = disease activity score (28 joints); ESR = erythrocyte sedimentation rate.
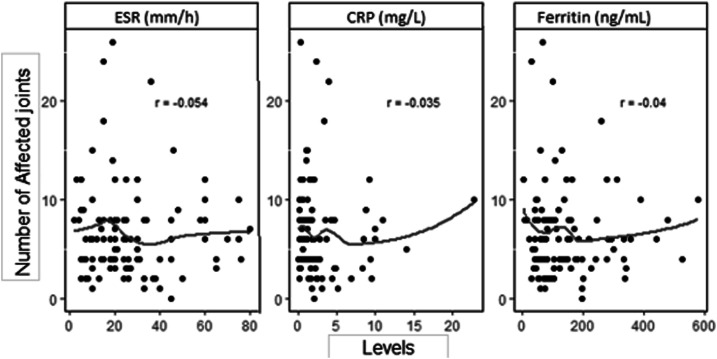
Sixty-five (61, 3%) patients obtained a DAS 28-ESR mean of 4.84 ± 1.04, 79 (74, 5%) patients had a DAS 28-CRP mean of 4.04 ± 0.95, and 65 (61, 3%) had a CDAI mean of 21.25 ± 12.24 (Tables 3 and 4). According to disease activity measures, these patients presented moderate disease activity. Based on the information gathered, we correlated joint manifestations, joint disease activity, and the number of affected joints according to the values of the acute-phase reagents (Figure 1), which were respectively low for all of them.
Figure 1.
Scatter plots and correlation between the number of affected joints and acute-phase inflammatory markers. Erythrocyte sedimentation rate, C-reactive protein, and ferritin. CRP = C-reactive protein; ESR = erythrocyte sedimentation rate.
We also compared the mean values of each of the disease activity indices according to the smoking and use of corticosteroids comorbidities. No statistical differences were found between the increased disease activity measures and the presence of these variables (95% CI).
As there was the possibility of categorizing the acute-phase reagents, we present the comparison between the mean and P-value of each disease activity measure according to the classification of inflammatory tests (Table 5). We chose not to establish the relationship between the ESR and DAS 28-ESR, and CRP and DAS-CRP because these inflammatory parameters were used to calculate these indices. There was no statistical correlation established between acute-phase reagents, which were stratified according to the reference in normal and high values, and the moderate joint activity found in indices calculation (95% CI).
Table 5.
Comparison between the mean and P-value of disease activity measure according to the classification of acute-phase reagents
| Variable | CDAI | DAS 28 CRP | DAS 28 ESR |
|---|---|---|---|
| Ferritin | |||
| Normal | 21.3 ± 12.3 | 4.0 ± 1.0 | 4.8 ± 1.0 |
| High | 19.7 ± 11.2 | 4.0 ± 0.9 | 4.7 ± 1.4 |
| P-value | 0.800 | 0.538 | 0.881 |
| ESR | |||
| Normal | 21.8 ± 13.5 | 4.1 ± 1.0 | – |
| High | 19.8 ± 8.9 | 3.9 ± 0.8 | – |
| P-value | 0.364 | 0.116 | – |
| CRP | |||
| Normal | 21.2 ± 12.5 | – | 4.8 ± 1.1 |
| High | 21.5 ± 5.1 | – | 5.2 ± 0.4 |
| P-value | 0.932 | – | 0.190 |
CDAI = Clinical Disease Activity Index; CRP = C-reactive protein; DAS 28 = disease activity score (28 joints); ESR = erythrocyte sedimentation rate.
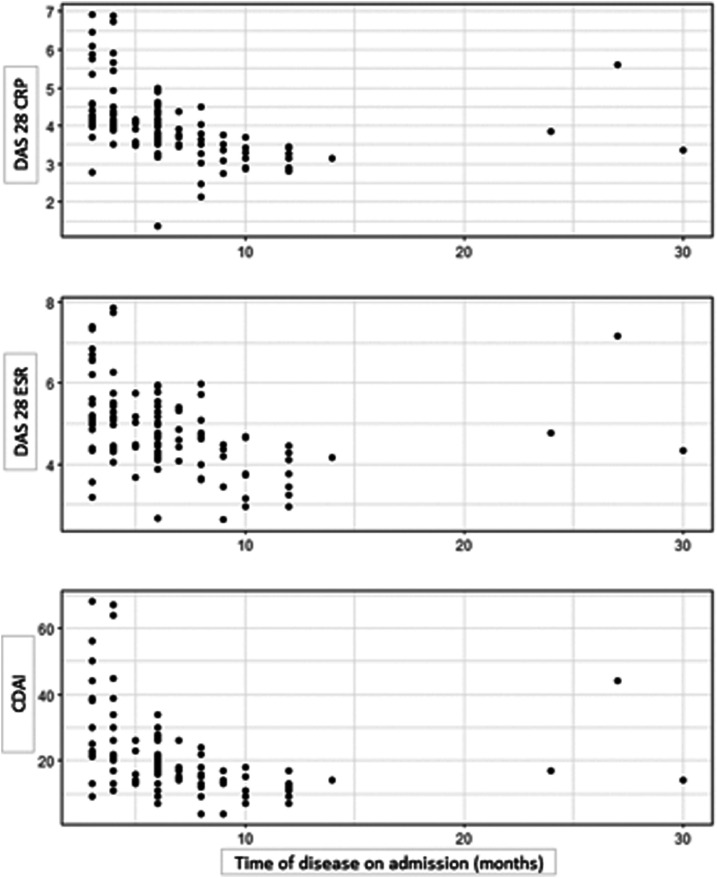
The correlations of disease activity measures and the disease duration at the first medical evaluation after initial infection are shown in the dispersion graphs (Figure 2). The data showed a negative correlation between the indices and the disease duration (Table 6). However, we observed a different behavior in three patients from the rest of the sample (Figure 2). In general, the results do indicate the lower the value in the indices the longer the disease lasts (P < 0.001).
Figure 2.
Dispersion graphs of disease activity in relation to disease duration at the first medical evaluation. CDAI = Clinical Disease Activity Index; CRP = C-reactive protein; DAS 28 = disease activity score (28 joints); ESR = erythrocyte sedimentation rate.
Table 6.
Correlations between disease activity measure and disease duration at the first medical evaluation
| Disease activity measure | Correlation | P-value |
|---|---|---|
| Clinical Disease Activity Index | −0.562 | < 0.001 |
| DAS 28 erythrocyte sedimentation rate | −0.484 | < 0.001 |
| DAS 28 C-reactive protein | −0.621 | < 0.001 |
DAS 28 = disease activity score (28 joints).
DISCUSSION
Clinical and histopathological findings of CHIKV-induced chronic articular patients and those of other inflammatory arthropathies such as rheumatoid arthritis suggest these diseases share similar pathogenic mechanisms.24 Therefore, it is reasonable to speculate that the same biomarkers are expressed in both chronic articular diseases and might be useful to monitor the disease progress. C-reactive protein, ESR, and ferritin are simple, reliable, low-cost tests commonly used in rheumatology routine.16
Anfasa et al.25 demonstrated elevated CRP and ferritin levels in 116 febrile acute CHIKV patients during an epidemic in Curaçao island in 2014–2015. An increase in ferritin levels during the acute phase of the disease predisposed patients to chronic disease progression. In our practice, CRP and ferritin levels did not correlate with disease evolution in most patients. Similarly, clinical manifestations did not correlate with ESR-stratified median values in the study population. These results may be explained by the relatively low number of patients with altered levels of these inflammatory tests when compared with the total number of analyzed patients. Therefore, in this study population, tests cannot be considered in an isolated manner to define a CHIKV chronic disease prognosis. In this matter, Krutikov et al.1 also failed to demonstrate a correlation between the ESR and CRP with CHIKV-induced chronic articular disease severity.
Even without CRP, ESR, and ferritin increased levels in most of these patients, median DAS 28 and CDAI values were compatible with moderate disease activity. These high DAS 28 and CDAI values were derived directly from the high number of compromised articulations associated with the values attributed in the global evaluation of pain intensity. Sepúlveda-Delgado et al.26 confirmed the importance of DAS 28 in a prospective study conducted with 10 patients infected by CHIKV in México. Elevated DAS 28 and WHODAS II scores associated with increased IL-6 levels in the acute phase of the disease predicted the evolution to subacute and chronic forms.
A robust innate immune response against CHIKV mediated mainly by monocytes leads to the expression of IL-1β, IL-6, IL-18, macrophage migration inhibitory factor (MIF), RANTES, C-X-C motif chemokine ligand 9 (CXCL9), MCP-1, and CXCL10, among other cytokines.27 However, their heterogeneity in different CHIKV-infected populations associated with their high dosage cost and the lack of test validation in practice prevent their use as routine disease biomarkers.28
This descriptive study demonstrated some similarities in relation to the risk factor presented in other populations, as seen in diabetes. A study carried out by Amir Tanya found a percentage of diabetic individuals (10.2%) similar to our study but different in the rates of patients with systemic arterial hypertension (12.4%) and smoking (10%).11 Badawi et al.10 observed that hypertension was the most prevalent comorbidity in CHIKV patients (31.3%) as in our sample, followed by diabetes (20.5%). No correlation was found in the association of comorbidities with the risk of chronic CHIKV arthralgia, as the data collected by Heath et al.9 in the city of Grenada, West Indies, have shown where the P values in their population for hypertension (P = 0.7281) and diabetes (P = 0.3894) were not significant.
The reported duration of symptoms is variable in the literature and seems to be reduced the longer the period between disease onset and clinical evaluation lasts. We observed that individuals who reported a longer period between the infection process and admission to our service referred mild symptoms and lower number of affected joints. Crossing these data with the disease activity measures, we obtained a statistical significance value in favor of this statement and the literature. Simon et al.29 informed symptoms persisted in 88%, 86%, 48%, and 4% of patients at 1, 3, 6, and 15 months, respectively, following acute illness. Borgherini et al.30 reported persistent polyarthralgia in 63% of patients in Rèunion Island evaluated a mean of 18 months after confirmation of acute infection. Brighton et al.31 managed to determine 12% of patients with joint symptoms after 3 years of the initial chikungunya infection in a study conducted in South Africa.
In conclusion, in our study, a few patients demonstrated concomitant elevations of all inflammatory tests, indicating that a larger study population or other inflammatory tests might be more suitable to identify biomarkers to monitor the progression of CHIKV-induced chronic polyarthralgia. The presence of risk factors for chronic disease was detected in our sample; however, there was no evidence of correlation with the intensity of musculoskeletal manifestations. And finally, there is a negative association between the disease duration at the first medical evaluation after initial infection and the persistence of joint symptoms in these individuals, indicating the necessity for a follow-up of these cases and a description of the findings.
Acknowledgments:
We are very thankful for patients, colleagues, and laboratory technicians from UNIC and UFMT who contributed to data collection and sample testing.
REFERENCES
- 1.Krutikov M, Manson J, 2016. Chikungunya virus infection: an update on joint manifestations and management. Rambam Maimonides Med J 7: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nunes MR, et al. 2015. Emergence and potential for spread of Chikungunya virus in Brazil. BMC Med 30: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Couderc T, Lecuit M, 2015. Chikungunya virus pathogenesis: bedside to bench. Antiviral Res 121: 120–131. [DOI] [PubMed] [Google Scholar]
- 4.Goupil BA, Mores CN, 2016. A review of Chikungunya virus-induced arthralgia: clinical manifestation, therapeutics, and pathogenesis. Open Rheumatol J 10: 129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cerny T, Schwarz M, Schwarz U, Lemant J, Gérardin P, Keller E, 2017. The range of neurological complications in chikungunya fever. Neurocrit Care 27: 447–457. [DOI] [PubMed] [Google Scholar]
- 6.Paixão ES, Rodrigues LC, Costa MCN, Itaparica M, Barreto F, Gérardin P, Teixeira MG, 2018. Chikungunya chronic disease: a systematic review and meta-analysis. Trans R Soc Trop Med Hyg 112: 301–316. [DOI] [PubMed] [Google Scholar]
- 7.Aalst MV, Nelen CM, Goohuis A, Stijnis C, Grobusch MP, 2017. Long-term sequelae of Chikungunya virus disease: a systematic disease. Travel Med Infect Dis 15: 8–22. [DOI] [PubMed] [Google Scholar]
- 8.Sissoko D, Malvy D, Ezzedine K, Renauld P, Moscetti F, Ledrans M, Pierre V, 2009. Pos-epidemic chikungunya disease on Reunion Island: course of rheumatic manifestations and associated factors over 15-month period. PLoS Negl Trop Dis 3: e389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heath CJ, Lowther J, Noël TP, Mark-George I, Boothroyd DB, Mitchell G, MacPherson C, LaBeaud AD, 2017. The identification of the risk factors for chronic chikungunya arthralgia in Granada, West Indies: a cross-sectional cohort study. Open Forum Infect Dis 5: ofx234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Badawi A, Ryoo SG, Vasileva D, Yaghoubi S, 2018. Prevalence of chronic comorbidities in chikungunya: a systematic review and meta-analysis. Int J Infect Dis 67: 107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanay A, 2017. Chikungunnya virus and autoimmunity. Curr Opin Rheumatol 29: 389–393. [DOI] [PubMed] [Google Scholar]
- 12.Bouquillard E, Fianu A, Bangil M, Charlette N, Ribéra A, Michault A, Favier F, Simon F, Flipo RM, 2018. Rheumatic manifestations associated with Chikungunya virus infection: a study of 307 patients with 32-month follow up. Joint Bone Spine 85: 207. [DOI] [PubMed] [Google Scholar]
- 13.Chang AY, et al. 2018. Chikungunya arthritis mechanisms in the Americas: a cross-sectional analysis of chikungunya arthritis patients twenty-two months after infection demonstrating no detectable viral persistence in synovial fluid. Arthritis Rheumatol 70: 585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hua C, Combe B, 2017. Chikungunya vírus-associated disease. Curr Rheumatol Rep 19: 69. [DOI] [PubMed] [Google Scholar]
- 15.Agmon-Levin N, et al. 2013. Ferritin in the antiphospholipid syndrome and its catastrophic cariant (cAPS). Lupus 22: 1327–1335. [DOI] [PubMed] [Google Scholar]
- 16.Bica BERG, Terreri MT, 2019. Exames laboratoriais: reagentes de fase aguda. Livro Soc Bras Reumatol 1: 46–48. [Google Scholar]
- 17.Enocsson H, Sjöwall C, Skogh T, Eloranta MJ, Ronnblom L, Wettero J, 2009. Interferon-α mediates suppression of C-reactive protein. Arthritis Rheum 60: 3755–3760. [DOI] [PubMed] [Google Scholar]
- 18.Marques CDL, et al. 2017. Recomendações da Sociedade Brasileira de Reumatologia para diagnóstico e tratamento da febre chikungunya. Parte 1: diagnóstico e situações especiais. Rev Bras Reumatol 57: 5421–5437. [Google Scholar]
- 19.Anderson J, Caplan L, Yazdany J, Robbins ML, Neogi T, Michaud K, Saag KG, O’Dell JL, Kazi S, 2012. Rheumatoid arthritis disease activity measures: American College of Rheumatology recommendations for use in clinical practice. Arthritis Care Res 64: 640–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mota LMH, Kakehasi AM, Gomides APM, Duarte ALBP, Cruz BA, Brenol CV, Albuquerque CP, Pinheiro GRC, Laurindo IMM, Pereira IA, 2018. 2017 recommendations of the Brazilian Society of Rheumatology for the pharmacological treatment of rheumatoid arthritis. Adv Rheumatol 58: 1–17. [DOI] [PubMed] [Google Scholar]
- 21.Medeiros MMC, Oliveira BMGB, Cerqueira JVM, Queixada RQS, Oliveira IMAX, 2015. Correlação dos índices de atividade da artrite reumatoide (disease activity score 28 medidos como ERS, PCR, simplified disease activity index e clinical disease activity index) e concordância dos estados de atividade da doença com vários pontos de corte numa população do nordeste brasileiro. Rev Bras Reumatol 55: 477–484.25772662 [Google Scholar]
- 22.Bussab WO, Morettin PA, 2012. Estatística Básica, 7th edition São Paulo, Brazil: Saraiva. [Google Scholar]
- 23.R. Core Team , 2019. A Language and Environment for Statistical Computing . Vienna, Austria: R. Foundation for Statistical Computing; Available at: http://www.R-project.org/. [Google Scholar]
- 24.Miner JJ, Aw-Yeang HX, Fox JM, Taffner S, Malkova ON, Oh ST, Kim AHJ, Diamond MS, Lenschow DJ, Yokoyama WM, 2015. Brief report: chikungunya viral arthritis in the United States: a mimic of seronegative rheumatoid arthritis. Arthritis Rheum 67: 1214–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anfasa F, Provacia L, Geurtsvankessel C, Wever R, Gerstenbluth I, Osterhaus AD, Martina BE, 2017. Hiperferritinemia is a potential marker of chronic chikungunya: a retrospective study on the Island of Curaçao during the 2014–2015 outbreak. J Clin Virol 86: 31–38. [DOI] [PubMed] [Google Scholar]
- 26.Sepúlveda-Delgado J, et al. 2016. Inflammatory biomarkers, disease activity index, and self-reported disability may be predictors of chronic arthritis after chikungunya infection: brief report. Clin Rheumatol 36: 695–699. [DOI] [PubMed] [Google Scholar]
- 27.Assunção-Miranda I, Cruz-Oliveira C, Poian AT, 2013. Molecular mechanisms involved in the pathogenesis of alphavirus-induced arthritis. Biomed Res 2013: 973516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mathew AJ, Ganapati A, Kabeerdoss J, Nair A, Gupta N, Chebbi P, Mandal SK, Danda D, 2017. Chikungunya infection: a global public health menace. Curr Allergy Asthma Rep 17: 13. [DOI] [PubMed] [Google Scholar]
- 29.Simon F, et al. 2007. Chikungunya infection: an emerging rheumatism among travelers returned from Indian Ocean Islands. Report of 47 cases. Medicine (Baltimore) 86: 123. [DOI] [PubMed] [Google Scholar]
- 30.Borgherini G, Poubeau P, Staikowsky F, Lory M, Le Moullec N, Becquart JP, Wengling C, Michault A, Paganin F, 2007. Outbreak of chikungunya on Reunion Island: early clinical and laboratory features in 157 adult patients. Clin Infect Dis 44: 1401. [DOI] [PubMed] [Google Scholar]
- 31.Brigthon SW, Prozesky OW, de la Harpe AL, 1983. Chikungunya virus infection. A retrospective study of 107 cases. S Afr Med J 63: 313. [PubMed] [Google Scholar]