Abstract.
In malaria-endemic countries, rapid diagnostic tests (RDTs) targeting Plasmodium falciparum histidine-rich protein 2 (PfHRP2) and lactate dehydrogenase (PfLDH) have been widely used. However, little is known regarding the diagnostic performances of these RDTs in the Assosa zone of northwest Ethiopia. The objective of this study was to determine the diagnostic performances of PfHRP2 and PfLDH RDTs using microscopy and quantitative PCR (qPCR) as a reference test. A health facility–based cross-sectional study design was conducted from malaria-suspected study participants at selected health centers from November to December 2018. Finger-prick blood samples were collected for microscopy, RDTs, and qPCR method. The prevalence of P. falciparum was 26.4%, 30.3%, and 24.1% as determined by microscopy, PfHRP2 RDT, and PfLDH RDT, respectively. Compared with microscopy, the sensitivity and specificity of the PfHRP2 RDT were 96% and 93%, respectively, and those of the PfLDH RDT were 89% and 99%, respectively. Compared with qPCR, the specificity of the PfHRP2 RDT (93%) and PfLDH RDT (98%) was high, but the sensitivity of the PfHRP2 RDT (77%) and PfLDH RDT (70%) was relatively low. These malaria RDTs and reference microscopy methods showed reasonable agreement with a kappa value above 0.85 and provided accurate diagnosis of P. falciparum malaria. Thus, the current malaria RDT in the Ministry of Health program can be used in the Assosa zone of Ethiopia. However, continuous monitoring of the performance of PfHRP2 RDT is important to support control and elimination of malaria in Ethiopia.
INTRODUCTION
Malaria is still a major public health problem in several developing countries.1 In Ethiopia, Plasmodium falciparum and Plasmodium vivax account for 60% and 40%, respectively, of the malaria infections.2 In the northwestern part of Ethiopia, Assosa zone, P. falciparum is the dominant parasite species causing most of the complicated malaria cases.3 Accurate diagnosis and effective treatment are key to reducing morbidity and mortality in malaria-endemic countries.4
Based on the WHO recommendation, all individuals suspected of malaria should receive laboratory diagnosis before antimalarial drug treatment to improve malaria case control and management.5 The diagnosis of malaria can be made by microscopy, rapid diagnostic test (RDT), and molecular methods. Microscopy and RDTs have been widely used in clinical settings for many years, whereas molecular techniques are rarely used for several reasons. Indeed, microscopy is the reference test, and RDTs are recommended in areas where microscopic examination cannot be performed.6,7
Among all malaria RDTs, histidine-rich protein 2 (HRP2) and lactate dehydrogenase (LDH) are two commonly used antigen-based tests for malaria diagnosis.6 In areas where P. falciparum is dominant, the Plasmodium falciparum HRP2 (PfHRP2) RDT is preferred because HRP2 antigen is only produced by P. falciparum, and cannot be used for the detection of P. vivax or other human malarial parasites.4,8 By contrast, the LDH antigen is commonly expressed in all Plasmodium species.9 The amino acid sequences of LDH vary between P. falciparum and other non–P. falciparum species, which allows species-specific LDH to be designed and used for diagnosis of different malaria parasites. For example, the Plasmodium falciparum LDH (PfLDH) RDT is used for the diagnosis of P. falciparum, whereas the Pan-LDH RDT for detection of mixed malaria infection.10 Approximately 10 million PfHRP2 RDTs are used for diagnosis of malaria in Ethiopia per year.2 Because of their widespread use, it is vital to closely monitor the sensitivity and specificity of these malaria RDTs across different malaria-endemic settings and localities to provide prompt treatment and effective case control.11
Studies on the performance of PfHRP2 and PfLDH RDTs have been well documented in different malaria-endemic countries.12–17 Several factors affect the performance of these malaria RDTs which include technical factors such as improper storage and packaging, poor product design, and operating error.18 Biological factors such as immunogenic response of the hosts and genetic changes in the parasites could also result in false-negative and false-positive results and detrimentally impact the performance of malaria RDTs.19,20 Plasmodium falciparum LDH antigen in clinical malaria is used for early detection of P. falciparum and monitoring parasite responses to treatment,21,22 whereas PfHRP2 antigen can persist in blood for more than 3 weeks after antimalaria treatment and could result in false-positive detection by the PfHRP2 RDT.23 However, the PfLDH RDT has been shown with low sensitivity and could give false-negative results in infections with low parasitemia.8,10,20 Regardless of the lower sensitivity of the PfLDH RDT, the use of this RDT could be recommended as an alternative malaria RDT to reduce the false-negative results associated with pfhrp2 gene deletion.19,24
Although previous research has documented the performance of the malaria RDT in Ethiopia,15,17,25 to the best of our knowledge, the performance of the PfLDH RDT has never been investigated in rural and semi-urban areas of Assosa zone. Thus, this study aimed to evaluate the performance of PfHRP2 and PfLDH RDTs for diagnosis of P. falciparum malaria against microscopy and quantitative PCR (qPCR) in the Assosa zone in Ethiopia.
MATERIALS AND METHODS
Study design and area.
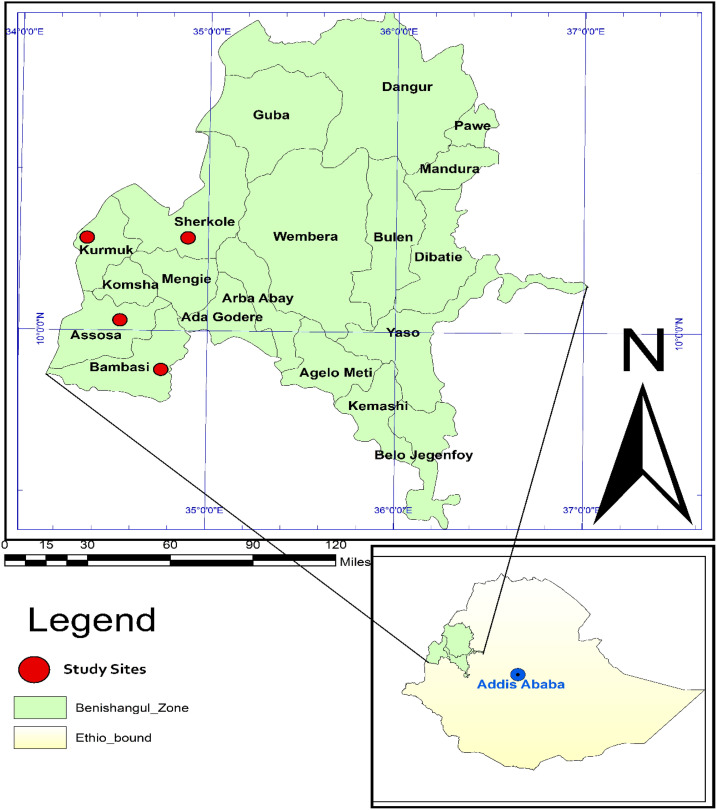
A cross-sectional health facility–based study was conducted in the Assosa zone, northwest Ethiopia, from November to December 2018. Assosa zone is one of the four Zonal administrations in Benishangul-Gumuz regional state and is located 687 km west of Addis Ababa, Ethiopia. It consists of eight woredas, and most of the woreda have high malaria transmission intensity based on the 2016/2017 report by the Health Information Management System from the Benishangul-Gumuz regional state. The present study was performed at four selected health facilities: Assosa, Bambasi, Kurmuk, and Sherkole health centers. The location of the study site was taken by handheld GPS (Garmin GPS 73, Olathe, KS) and the map generated using ArcGIS version 10.0 software (Figure 1).
Figure 1.
Map showing the study area in Assosa zone. The map was generated using ArcGIS version 10.0 software. This figure appears in color at www.ajtmh.org.
Sample size and sampling technique.
The sample size was calculated based on a single population proportion formula ,26 where n is the sample size, z is 1.96 at 95% CI, d is the margin of error, p is the expected malaria prevalence rate in the locality which is 40% based on the assumption of a microscopy-confirmed prevalence of malaria among symptomatic patients according to the 2015 study,3 and d is the margin of error at 5% (standard value of 0.05). A total of 406 study participants were enrolled in this study.
Four health facilities, including Assosa Health Centre, Bambasi Health Centre, Kurmuk Health Centre, and Sherkole Health Centre, were selected using a simple random sampling technique among eight health centers in the Assosa zone. The number of study participants recruited from each selected health center was calculated based on the proportion of confirmed malaria cases in each selected woreda and the annual parasite incidence.2
Demographic data of study participants and blood sample collection.
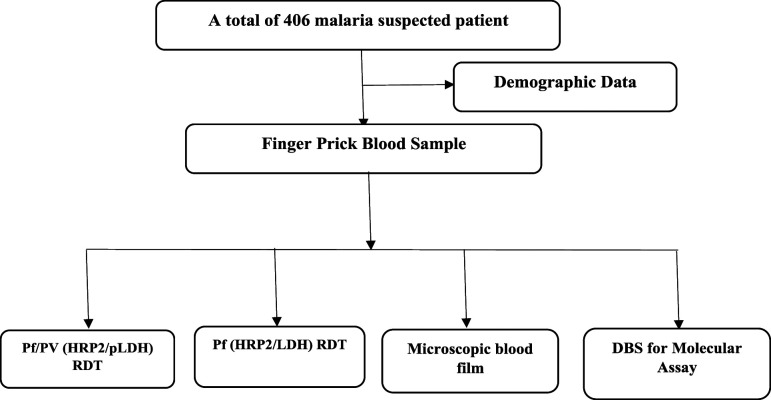
The study participants were all self-presenting clinically suspected malaria patients, whose ages were older than or equal to 5 years, who were attending at four selected health facilities. Individuals with a history of antimalarial chemotherapy in the last month with severe illness were excluded. Demographic data were collected using an interview-based structured questionnaire. Blood samples were collected based on the methodological workflow described in Figure 2.
Figure 2.
Study flowchart for malaria rapid diagnostic test (RDT), microscopy, and molecular assay.
Microscopic blood film examination.
Both thick and thin blood smears were prepared on the same slide for each study participant and were stained with 10% buffer-diluted Giemsa stain working solution for 10 minutes and examined by ×100 oil immersion objective microscopy. The thick smear was examined to detect and measure the density of the parasite. Asexual parasite densities were determined against 200 white blood cells (WBCs), assuming a mean WBC count of 8,000/μL, as per the WHO recommendations.27 The thin smear was examined to identify malaria parasites species. Quality control was maintained during specimen collection, processing, and testing. Each blood smear was read by two independent medical laboratory technicians, and there were no discrepancies in the result.
Malaria RDTs.
The CareStart™ malaria RDTs (Pf/Plasmodium vivax [PV] [HRP2/Plasmodium lactate dehydrogenase (PLDH)] Ag Combo RDT with product catalogue number RMVM-02591 [lot code MV 18C64], and Pf [HRP2/LDH] Ag Combo RDT with product catalogue number RMSM-02591 [lot code MS 18H61] from Access Bio Ethiopia) were used to evaluate their performance against microscopy and qPCR as reference test. The Pf/PV RDT in our study is currently used by the Ministry of the Health program in Ethiopia for malaria diagnosis. For the Pf/PV (HRP2/PLDH) Ag Combo RDT (product code RMVM-02591), LDH is specific for P. vivax and HRP2 for P. falciparum. The PfLDH RDT with RMSM-02591 product code is not currently used in Ethiopia. This PfLDH RDT had a separate line for PfHRP2 and PfLDH. We were evaluating this PfLDH RDT by reading only at PfLDH line for the specific diagnosis of P. falciparum. These malaria RDTs use immunochromatographic methods to detect parasite-specific antigens produced by malaria parasites. Each test was interpreted based on the manufacturer’s instructions in the package insert.
Molecular assay.
Malaria parasite DNA was extracted from dried blood spots using the Chelex-saponin method as described previously.28 SYBR Green qPCR assays were performed to amplify the 18S rRNA gene for confirmation of P. falciparum using of a pair of forward and reverse primer sequences (P. falciparum–specific primers): FAL-18S-F:AGTCATCTTTCGAGGTGACTTTTAGATTGCT and PLASMO-R2:GCCGCAAGCTCCACGCCTGGTGGTGC.29,30 For the quality control, DNA from P. falciparum isolates 7G8 (MRA-926) and HB3 (MRA-155) were used as positive controls, and water and uninfected samples were used as negative controls in all amplifications. In brief, qPCR amplification was carried out in a total reaction volume of 20 μL containing 7 μL of nuclease free water, 10 μL of SYBR Green master mix, 0.5 μL each of the forward and reverse primers, and 2 μL of extracted DNA under the following PCR cycling conditions: initial denaturation at 95°C for 3 minutes, followed by 45 cycles of amplification at 94°C for 30 seconds, 55°C for 30 seconds, and 68°C for 1 minute each.
Data collectors were trained, and close supervision was conducted during the data collection period. Standard operating procedures were used for specimen collection, processing, and testing for maintaining good quality data. Ten percent of the samples were randomly selected and repeated to ensure reproducibility.
Data analysis.
The data were analyzed using Statistical Package for Social Sciences (SPSS) version 20 (IBM Corp., Armonk, NY). The sensitivity, specificity, and predictive values of each test were computed. Kappa values were used to assess the agreement between these malaria diagnostic methods.
Ethics statement.
Ethical clearance was obtained from the Institutional Review Board of the Aklilu Lemma Institute of Pathobiology, Addis Ababa University, before data collection (Ref number ALIPB/IRB006/2017/18). The project was also reviewed and approved by the Institutional Review Board of the University of North Carolina at Charlotte (IRB number 18-0451). Permission was obtained from Benishangul-Gumuz Regional Health Bureau. Information about the study, the objective of the study, the possible risks, and benefits of the study were explained to the participants or their guardians using the local language. Finger-prick blood samples were taken after obtaining written informed consent and assent from parents or guardians in the case of children. Malaria-positive patients were treated by health workers based on national treatment guidelines. Confidentiality was maintained throughout the study.
RESULTS
Study participants.
Among a total of 406 malaria-suspected self-presenting febrile patients, 57.9% (235/406) were females and the remaining were males. The mean age of the study participants was 24.1 years. Sixty-four percent of the study participants were from rural areas, whereas 36% were from semi-urban areas (Table 1).
Table 1.
Plasmodium falciparum positivity rate using different laboratory methods among study participants
| Variable | Study participants (No) | Microscopy (No Pos, %) | Malaria rapid diagnostic test | †qPCR (No Pos, %) | |
|---|---|---|---|---|---|
| PfHRP2 (No Pos, %) | PfLDH (No Pos, %) | ||||
| Gender | |||||
| Male | 171 | 52 (30.4) | 64 (37.4) | 50 (29.2) | 65 (38) |
| Female | 235 | 55 (23.4) | 59 (25.1) | 48 (20.4) | 71 (30.3) |
| P-value* | – | < 0.114 | < 0.008 | < 0.04 | < 0.106 |
| Age-group (years) | |||||
| 5–14 | 90 | 24 (26.7) | 28 (31.1) | 22 (24.4) | 26 (28.9) |
| 15–24 | 161 | 46 (28.6) | 50 (31.1) | 43 (26.7) | 61 (37.9) |
| 25–34 | 93 | 22 (23.7) | 27 (29.0) | 21 (22.6) | 28 (30.1) |
| > 34 | 62 | 15 (24.2) | 18 (29.0) | 12 (19.4) | 21 (34.4) |
| P-value | – | < 0.823 | < 0.979 | < 0.686 | < 0.432 |
| Study site | |||||
| Sherkole | 186 | 54 (29.0) | 57 (30.6) | 44 (23.7) | 55 (29.6) |
| Bambasi | 101 | 33 (32.7) | 40 (39.6) | 34 (33.7) | 44 (44.0) |
| Kurmuk | 74 | 17 (23.0) | 23 (31.1) | 17 (23.0) | 28 (37.8) |
| Assosa | 45 | 3 (6.7) | 3 (6.7) | 3 (6.7) | 9 (20.0) |
| P-value | – | < 0.007 | < 0.001 | < 0.006 | < 0.015 |
No = number; No Pos = number positive.
* Statistically significant when the P-value for a chi-square test is less than 0.05.
Microscopy, RDT, and qPCR results.
The P. falciparum positivity rate was 26.4% (107/406) using microscopy, whereas 30.3% (123/406) and 24.1% (98/406) by PfHRP2 and PfLDH RDTs, respectively (Table 1). Higher numbers of P. falciparum, 33.5% (136/405), were detected by qPCR than microscopy and the malaria RDTs. The infection rate of P. falciparum was higher in males than females in both PfHRP2 and PfLDH-based RDTs (Table 1). Participants aged 15–24 years showed the highest infection rate compared with those younger than 15 and older than 25 years by all diagnostic methods. Also, the incidence of P. falciparum was the highest in Bambasi compared with other study sites using all diagnostic methods (Table 1).
Microscopic parasite density ranged from 201 to 10,000 parasite/µL which was highest in the study participants living in rural areas 59.8% (64/107) compared with participants in semi-urban areas 28.9% (31/107). The level of parasitemia varied with age-groups and gender of the study participants. However, there was no statistically significant association of the living area (P < 0.500), gender (P < 0.339), and age-group (P < 0.780) of the study participants with microscopic parasite density (Table 2).
Table 2.
Distribution of parasite density by gender, age-group, and living area
| Study participants, No (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gender | Age-group (years) | Living area | |||||||
| Parasite (density/µL) | N | Male | Female | 5–14 | 15–24 | 25–34 | > 34 | Rural | Semi-urban |
| 50–200 | 12 | 7 (58.3) | 5 (41.7) | 4 (33.3) | 7 (58.3) | 0 (0.0) | 1 (8.3) | 7 (58.3) | 5 (41.7) |
| 201–500 | 13 | 4 (30.8) | 9 (69.2) | 1 (7.7) | 5 (38.5) | 4 (30.8) | 3 (23.1) | 10 (76.9) | 3 (23.1) |
| 501–2,000 | 16 | 8 (50) | 8 (50) | 3 (18.8) | 7 (43.8) | 4 (25.0) | 2 (12.5) | 13 (81.3) | 3 (18.8) |
| 2,001–10,000 | 42 | 24 (57.1) | 18 (42.9) | 9 (21.4) | 17 (40.5) | 10 (23.8) | 6 (14.36) | 27 (64.3) | 15 (35.7) |
| > 10,000 | 24 | 9 (37.5) | 15 (62.5) | 7 (29.2) | 10 (41.7) | 4 (16.7) | 3 (12.5) | 14 (58.3) | 10 (41.7) |
| P < 0.339 | P < 0.780 | P < 0.500 | |||||||
N = total number examined; No = total number; rural = Sherkole + Kumruk; semi-urban = Bambasi + Assosa.
Sensitivity and specificity of malaria RDTs.
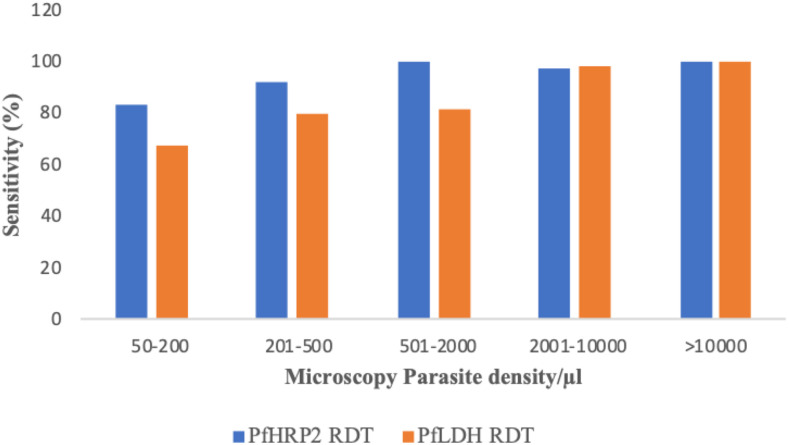
Using microscopy as a gold standard, the sensitivity of PfHRP2 and PfLDH RDTs was 96% (95% CI: 91–99) and 89% (95% CI: 81–93), respectively (Table 3). The corresponding specificity rates were 93% (95% CI: 90–96) and 99% (95% CI: 97–100), respectively. There was a good agreement between the two RDTs (PfHRP2 RDT and PfLDH RDT) and reference microscopy method with kappa values above 0.85 (Table 3). When qPCR was used as a reference test, lower sensitivity was detected in both PfHRP2 RDT, 77% (95% CI: 70–83), and PfLDH RDT, 70% (95% CI: 62–77). However, higher specificity was observed in both PfHRP2 RDT, 93% (95% CI: 89–95), and PfLDH RDT, 98% (95% CI: 91–99). The two RDTs (PfHRP2 RDT and PfLDH RDT) and qPCR also showed a good measure of agreement with a kappa value of 0.70 (Table 3). The sensitivity of PfLDH and PfHRP2 antigen band was improved with the increasing level of parasitemia. The sensitivity of the PfHRP2 RDT was observed to be higher than that of the PfLDH RDT at almost all levels of parasitemia (Figure 3).
Table 3.
Diagnostic performance of PfHRP2 and PfLDH RDTs using microscopy and qPCR as gold standard
| Microscopy as gold standard | qPCR as gold standard | |||
|---|---|---|---|---|
| Performance RDT | PfHRP2 RDT | PfLDH RDT | PfHRP2 RDT | PfLDH RDT |
| Sensitivity (95% CI) | 96 (91, 99) | 89 (81, 93) | 77 (70, 83) | 70 (62, 77) |
| Specificity (95% CI) | 93 (90, 96) | 99 (97, 100) | 93 (89, 95) | 98 (96, 99) |
| Positive predictive value (95% CI) | 84 (76, 89) | 97 (91, 99) | 85 (77, 89) | 97 (91, 99) |
| Negative predictive value (95% CI) | 99 (96, 100) | 96 (93, 98) | 89 (85, 92) | 66 (60, 70) |
| Kappa (95% CI) | 0.86 (0.80, 91) | 0.90 (0.85, 0.95) | 0.71 (0.64, 0.79) | 0.74 (0.67, 0.81) |
PfHRP2 = Plasmodium falciparum histidine-rich protein 2; PfLDH = Plasmodium falciparum lactate dehydrogenase; qPCR = quantitative PCR; RDT = rapid diagnostic test.
Figure 3.
Sensitivity of PfHRP2 and PfLDH RDTs by the level of Plasmodium falciparum parasitemia. PfHRP2 = Plasmodium falciparum histidine-rich protein 2; PfLDH = Plasmodium falciparum lactate dehydrogenase; RDT = rapid diagnostic tests. This figure appears in color at www.ajtmh.org.
False-positive and negative RDTs.
Using microscopy as the gold standard, a higher number of false-positive rates of the PfHRP2 RDT, 6.7% (20/299), were detected than the PfLDH RDT, 1% (3/299), whereas the number of samples missed by the PfLDH RDT, PfLDH false-negative rate of 11.2% (12/107), was three times higher than the PfHRP2 RDT (Supplemental Table 1). When qPCR was used as a gold standard, a higher false-negative rate was observed in both PfHRP2 RDT, 23.5% (32/136), and PfLDH RDT, 30.1 (41/136), whereas lower false-positive rate was shown in both PfLDH RDT, 1.1% (3/269), and PfHRP2 RDT, 7.1% (19/269) (Supplemental Table 2).
Sensitivity and specificity of RDT by study sites.
Using microscopy as a gold standard, both the sensitivity and specificity of the PfHRP2 RDT were slightly higher in rural than semi-urban areas, whereas the sensitivity of the PfLDH RDT was lower in rural (82%) than the semi-urban areas (97%). The specificity of the PfLDH RDT (98%) was identical in both urban and rural areas. When qPCR was used as a gold standard, the sensitivity of the PfHRP2 RDT was within the range of 93–95% in both rural and semi-urban areas; similarly, the specificity of PfHRP2 and PfLDH RDTs was greater than 95% in both rural and semi-urban areas. On the other hand, the sensitivity of the PfLDH RDT was lower to 67% in the rural area and further lower to 61% in semi-urban areas (Table 4).
Table 4.
Sensitivity and specificity of RDT between study sites using microscopy and qPCR as gold standard, Assosa zone, northwest Ethiopia, November to December 2018
| PfHRP2 RDT against microscopy | PfLDH RDT against microscopy | PfHRP2 RDT against qPCR | PfLDH RDT against qPCR | |||||
|---|---|---|---|---|---|---|---|---|
| Value | 95% CI | Value | 95% CI | Value | 95% CI | Value | 95% CI | |
| Rural (n = 260) | ||||||||
| Sensitivity | 96 | (88, 99) | 82 | (71, 89) | 95 | (88, 98) | 67 | (57, 77) |
| Specificity | 94 | (89, 96) | 98 | (95, 99) | 99 | (97, 100) | 97 | (94, 99) |
| Semi-urban (n = 146) | ||||||||
| Sensitivity | 94 | (82, 98) | 97 | (86, 99) | 93 | (82, 98) | 61 | (48, 73) |
| Specificity | 92 | (85, 96) | 98 | (94, 100) | 97 | (91, 99) | 96 | (89, 98) |
PfHRP2 = Plasmodium falciparum histidine-rich protein 2; PfLDH = Plasmodium falciparum lactate dehydrogenase; qPCR = quantitative PCR; RDT = rapid diagnostic test.
Sensitivity and specificity of RDT by age-group.
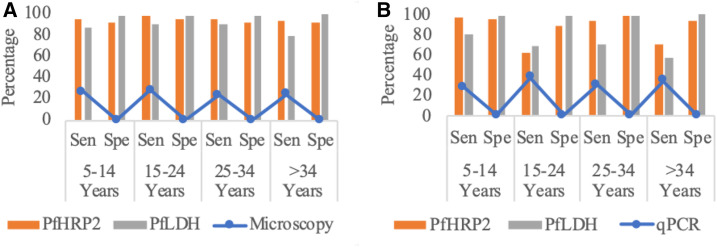
Using microscopy as the gold standard (Supplemental Table 3), the sensitivity of the PfHRP2 RDT was high among participants younger than 34 years (95–98%) and older than 34 years (93%). The specificity of the PfHRP2 RDT was 96% among participants at the age of 5–14 years and 92% for all other age groups. The sensitivity of the PfLDH RDT was 88–91% for most of the age-groups and was slightly lower at the age greater than 34 years (80%); the specificity of the PfLDH RDT was higher than 97% at all age-groups (Figure 4).
Figure 4.
Sensitivity and specificity of RDT by age-groups. (A) Taking microscopy as the gold standard. (B) Taking qPCR as the gold standard. The blue line indicates the positivity rate of microscopy and qPCR. The orange and gray bars indicate sensitivity and specificity for both PfHRP 2 and PfLDH at different age-groups, respectively. PfHRP2 = Plasmodium falciparum histidine-rich protein 2; PfLDH = Plasmodium falciparum lactate dehydrogenase; qPCR = quantitative PCR; RDT = rapid diagnostic test; Sen = sensitivity; Spe = specificity. This figure appears in color at www.ajtmh.org.
When using qPCR as the gold standard (Supplemental Table 4), the sensitivity of the PfHRP2 RDT was high among participants aged 5–14 (96%) and 25–34 years (93%). The sensitivity was lower among participants older than 34 years (71%) and those aged 15–25 years (62%). The specificity of the PfHRP2 RDT was higher at the age of 25–34 years (98%) than specificity of those aged 5–14 (95%), those older than 34 (93%), and those aged 15–24 years (88%). Similarly, the sensitivity and specificity of the PfLDH RDT varied among different age-groups. The sensitivity of the PfLDH RDT was 81% at 5–14 years of age, and it decreased to 57% in adults older than 34 years. The specificity of the PfLDH RDT was high (98–100%) at all age-groups. The specificity of both PfHRP2 and PfLDH RDTs increased with a higher positivity rate of P. falciparum at different age-groups when using microscopy as the gold standard (Figure 4).
DISCUSSION
This study determined the performance of PfHRP2 and PfLDH RDTs for P. falciparum infections in a clinical setting. Continuously monitoring the performance of the malaria RDT in malaria-endemic areas is essential to support the global effort of malaria control and elimination program.4 Our findings on the performance of PfHRP2 and PfLDH RDTs for P. falciparum infections in the Assosa zone of Ethiopia were within the WHO detection limit of sensitivity (> 95%) and specificity (> 90%). Thus, PfHRP2 and PfLDH RDTs could be used for malaria diagnosis at the health post and other public health facility levels in the absence of microscopy.31
In this study, the PfHRP2 RDT showed a high positive rate of P. falciparum (30.3%) compared with the PfLDH RDT (24.1%) and microscopy (26.4%) but lower than qPCR (33.5%). This finding using the malaria RDT was comparable with the report from southwest Ethiopia (31%)32 and northwest Ethiopia (23.8%).33 By contrast, this finding was relatively low compared with Pawe district in northwest Ethiopia (51.5%)34 and Wondo Genet district in southern Ethiopia (49.6%).35 This difference might be due to the current wide implementation of intervention measures throughout the malaria-endemic region in Ethiopia.2 However, the results of this study were higher than those from the Tigray region in north Ethiopia (13.4%)25 and Butajira in south central Ethiopia (20.4%)17 using the malaria RDT. High incidence of P. falciparum in this study area might be due to the geographical difference as the study area is located at the border area of Ethiopia where malaria could be imported and exported between Sudan and Ethiopia. Besides, the highest incidence rate of P. falciparum was observed in the Bambasi district compared with other study sites. This might be due to intensive and broad-scale outdoor agricultural activities in the Bambasi district.
In this study, the infection rate of P. falciparum was higher in males (37.4%, 64/171) than females (25%, 59/235) using the PfHRP2 RDT (P < 0.008). This result was consistent with a previous study conducted in the Pawe district in northwest Ethiopia.36 One possible explanation why males are more affected than females might be due to the fact that males may spend more time outdoors for agricultural and mining activities, and thus have a higher exposure to infected mosquitoes than females. There was no significant association of gender, age-group, and living area (rural and semi-urban settings) with microscopy-based parasite density. This finding is consistent with previous studies.37 However, it is not in line with a study at Uganda.38 This might be due to the difference in the age-group and study participants, clinical malaria–suspected individuals, involved in the study.
Using microscopy as the gold standard, high negative predictive value (NPV) (99%) and positive predictive value (PPV) (84%) of the malaria RDT were detected in this study by the PfHRP2 RDT. This result is in line with a study conducted in Tigray region in north Ethiopia,25 Kenya,39 and Ghana.40 These high NPV and PPV revealed a low false-negative and -positive rates by the PfHRP2 RDT, respectively. These results confirmed that the PfHRP2 RDT has reasonable diagnostic performance at peripheral health centers to correctly identify malaria-free individuals as true negative and individuals with malaria as true positive.41
The sensitivity of the PfHRP2 RDT was high (96%) compared with the PfLDH RDT (89%) and showed a slightly lower specificity (93%) than the PfLDH RDT (99%) against microscopy in our clinical samples. The PfHRP2 RDT also showed a good agreement with reference microscopy, with kappa values above 0.86 in this study. The high sensitivity and specificity of the PfHRP2 RDT in the current study were comparable with reports from Pawe district in northwest Ethiopia,34 Tigray in north Ethiopia,25 Ghana,42 and western Kenya.39 By contrast, slightly higher values of sensitivity and specificity of the PfHRP2 RDT were found in comparison with other studies.12 These differences might be due to the fact that most of the study participants in this study had detectable microscopic parasite density (> 200 parasite/µL) based on the detection limit of the PfHRP2 RDT.11
The high specificity of the PfLDH RDT (99%) in this study was in line with the manufacturers’ report.11 A similar finding was also observed in previous studies.20,43 Such a high specificity of the PfLDH RDT in reference to microscopy could minimize false-positive results in clinical malaria detection.44–46 The sensitivity of the PfLDH RDT (89%) in this study was much lower than that shown in the WHO product testing of malaria RDT report (98%).11 This might be due to less stability of the LDH antigen at high temperatures during the transportation and storage of the PfLDH RDT as the Assosa zone has relatively hot weather.47 The lower sensitivity of the PfLDH RDT in this study, as well as in several other studies,10,20 merits more investigations on how to improve its sensitivity.
Using qPCR as the gold standard, a high specificity of the PfLDH RDT (98%) was achieved in this study. This result was similar to the previous report.10 However, a lower sensitivity of both PfHRP2 (77%) and PfLDH (70%) RDTs was observed in the present study. As a result, a relatively high number of false-negative results were detected by PfHRP2 and PfLDH RDTs (23.5%, 32/136; 30.1%, 41/136, respectively). These false-negative results indicate that a considerable number of malaria-infected patients were misdiagnosed. If these false-negative individuals remain undetected and untreated, it is not only bad for their health but also the patients can serve as malaria reservoirs and may fuel onward malaria transmission in the community.48,49 The low sensitivity of these malaria RDTs compared with qPCR could be explained partly by qPCR that can detect as low as 0.002 parasite/μL including submicroscopic infection.50,51 Also, the sensitivity of the PfHRP2 RDT could be potentially affected by the prozone effect of PfHRP2 antigen and by genetic variation of the pfhrp2 gene, as reported in other studies.13,52
In the Assosa zone of Ethiopia, PfHRP2 and PfLDH RDTs were shown to have high sensitivity and specificity with slight variation among study sites and age-groups using microscopy as the gold standard. There was also no significant difference between rural and semi-urban areas, although most of the participants living in rural areas had parasitemia greater than 200 parasites/µL, higher than those living in semi-urban areas. Our findings did not agree with the previous study that showed malaria RDT performance may vary by malaria prevalence, rural and urban settings, and age-groups.53–55 This discrepancy might be explained partly by the fact that all our study sites in the Assosa zone are high-transmission areas, and malaria-susceptible age-groups (i.e., infants and children younger than 5 years) were not included in this study. Other factors included differences in the infection status of the study participants, the type and batch of test products, and the reference method used to compare the diagnostic methods. In addition, there was no noticeable variation in the socioeconomic status and living conditions between rural and semi-urban areas in our study areas.
LIMITATIONS
In the present study, we did not examine the accuracy of PfHRP2 and PfLDH RDTs among asymptomatic individuals in the community. The performance of these RDTs on low-density asymptomatic infections is yet unclear. Second, the potential cause of false-positive results such as cross-reactivity with rheumatoid factor56 and thermal stability during transportation and storage was not assessed.31 Third, pfhrp2/3 gene deletion,57,58 the cause of a considerable number of PfHRP2 RDT false-negative results, was not assessed in this study.
In conclusion, the sensitivity and specificity of the CareStart™ PfHRP2 and PfLDH RDTs in this study comply with the WHO limit of detection for routine diagnosis of clinical malaria. There was good agreement between these malaria RDTs and the reference microscopy for the diagnosis of clinical malaria in this study. Hence, this currently used Pf/PV (HRP2/PLDH) Ag Combo RDT could be used as an alternative diagnostic tool in the absence of microscopy. Furthermore, implementing both highly sensitive PfHRP2 RDT and highly specific PfLDH RDT will improve the accuracy of malaria diagnosis and case management. Continuous monitoring of the performance of the malaria RDT at local and national scales as well as genetic changes in the parasites is important when considering the PfLDH RDT as an alternative test in support of the control and elimination of malaria in Ethiopia.
Supplemental Tables
Acknowledgments:
We acknowledge the support of Addis Ababa University, Aklilu Lemma Institute of pathobiology, the University of North Carolina at Charlotte (Department of Bioinformatics and Genomics, Department of Biological Science), and Access Bio, Ethiopia. We would also like to thank Benishangul-Gumuz Regional Health Bureau, Assosa Zonal Health Department, and the health centers for provision of the necessary information, facilities, and technical supports. We are indebted to data collectors and supervisors for their cooperation during the fieldwork. We also thank the study participants for voluntarily supplying blood samples and for responding to the questionnaire. The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
Note: Supplemental tables appear at www.ajtmh.org.
REFERENCES
- 1.WHO , 2019. World Malaria Report. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 2.FMOH , 2017. National Malaria Strategic Plan 2017–2020. Addis Ababa, Ethiopia: Federal Ministry of Health. [Google Scholar]
- 3.Geleta G, Ketema T, 2016. Severe malaria associated with Plasmodium falciparum and P. vivax among children in Pawe Hospital, northwest Ethiopia. Mala Res and Treat 2016: 1240962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO , 2016. Global Technical Strategy for Malaria 2016–2030. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 5.WHO , 2015. Guidelines for the Treatment of Malaria. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 6.WHO , 2011. Universal Access to Malaria Diagnostic Testing: An Operational Manual. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 7.EPHI , 2011. Malaria Laboratory Diagnosis External Quality Assessment Scheme Guideline. Addis Ababa, Ethiopia: Ethiopian Public Health Institute. [Google Scholar]
- 8.Jain P, Chakma B, Patra S, Goswami P, 2014. Potential biomarkers and their applications for rapid and reliable detection of malaria. BioMed Res Int 2014: 852645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makler MT, Hinrichs DJ, 1993. Measurement of the lactate dehydrogenase activity of Plasmodium falciparum as an assessment of parasitemia. Am J Trop Med Hyg 48: 205–210. [DOI] [PubMed] [Google Scholar]
- 10.Li B, Sun Z, Li X, Li X, Wang H, Chen W, Chen P, Qiao M, Mao Y, 2107. Performance of pfHRP2 versus pLDH antigen rapid diagnostic tests for the detection of Plasmodium falciparum: a systematic review and meta-analysis. Arch Med Sci 13: 541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO , 2018. Malaria Rapid Diagnostic Test Performance: Results of WHO Product Testing of Malaria RDTs: Round 8 (2016–2018). Geneva, Switzerland: World Health Organization. [Google Scholar]
- 12.Djallé D, Gody JC, Moyen JM, Tekpa G, Ipero J, Madji N, Breurec S, Manirakiza A, 2014. Performance of paracheck™-Pf, SD Bioline malaria Ag-Pf and SD Bioline malaria Ag-Pf/pan for diagnosis of falciparum malaria in the Central African Republic. BMC Infect Dis 14: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gillet P, et al. 2011. Prozone in malaria rapid diagnostics tests: how many cases are missed? Malar J 10: 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meseret C, Berhanu E, Abebe A, Mengistu L, 2011. Performance of CareStart™ malaria Pf/Pv combo test for the diagnosis of Plasmodium falciparum and Plasmodium vivax infections in the Afar region, north east Ethiopia. Ethiop J Health Dev 25: 206–211. [Google Scholar]
- 15.Moges B, Amare B, Belyhun Y, Tekeste Z, Gizachew M, Workineh M, Gebrehiwot A, Woldeyohannes D, Mulu A, Kassu A, 2012. Comparison of CareStart™ HRP2/pLDH COMBO rapid malaria test with light microscopy in north-west Ethiopia. Malar J 11: 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tadesse E, Workalemahu B, Shimelis T, 2016. Diagnostic performance evaluation of the SD Bioline malaria antigen Ag Pf/Pan test (05FK60) in a malaria endemic area of southern Ethiopia. Rev Inst Med Trop São Paulo 58: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woyessa A, Deressa W, Ali A, Lindtjørn B, 2013. Evaluation of CareStart™ malaria Pf/Pv combo test for Plasmodium falciparum and Plasmodium vivax malaria diagnosis in Butajira area, south-central Ethiopia. Malar J 12: 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO , 2017. False-Negative RDT Results and Implications of New Reports of P. falciparum Histidine-Rich Protein 2/3 Gene Deletions. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 19.Cheng Q, Gatton ML, Barnwell J, Chiodini P, McCarthy J, Bell D, Cunningham J, 2014. Plasmodium falciparum parasites lacking histidine-rich protein 2 and 3: a review and recommendations for accurate reporting. Malar J 13: 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maltha J, Guiraud I, Lompo P, Kaboré B, Gillet P, Van Geet C, Tinto H, Jacobs J, 2014. Accuracy of Pf HRP2 versus Pf-pLDH antigen detection by malaria rapid diagnostic tests in hospitalized children in a seasonal hyperendemic malaria transmission area in Burkina Faso. Malar J 13: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houzé S, Boly MD, Le Bras J, Deloron P, Faucher JF, 2009. Pf HRP2 and Pf LDH antigen detection for monitoring the efficacy of artemisinin-based combination therapy (ACT) in the treatment of uncomplicated falciparum malaria. Malar J 8: 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fogg C, Twesigye R, Batwala V, Piola P, Nabasumba C, Kiguli J, Mutebi F, Hook C, Guillerm M, Moody A, 2008. Assessment of three new parasite lactate dehydrogenase (pan-pLDH) tests for diagnosis of uncomplicated malaria. Trans R Soc Trop Med Hyg 102: 25–31. [DOI] [PubMed] [Google Scholar]
- 23.Mouatcho JC, Goldring JPD, 2013. Malaria rapid diagnostic tests: challenges and prospects. J Med Microbiol 62: 1491–1505. [DOI] [PubMed] [Google Scholar]
- 24.WHO , 2018. Protocol for Estimating the Prevalence of Pfhrp2/pfhrp3 Gene Deletions Among Symptomatic Falciparum Patients with False-Negative RDT Results. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 25.Feleke DG, Tarko S, Hadush H, 2017. Performance comparison of CareStart™ HRP2/pLDH combo rapid malaria test with light microscopy in north-western Tigray, Ethiopia: a cross-sectional study. BMC Infect Dis 17: 399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daniel WW, Cross CL, 1995. Biostatistics: A Foundation for Analysis in the Health Sciences. Hoboken, NJ: Wiley, 131–138. [Google Scholar]
- 27.WHO, Research Malaria Microscopy Standards Working Group , 2015. Microscopy for the Detection, Identification and Quantification of Malaria Parasites on Stained Thick and Thin Films. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 28.Baidjoe A, et al. 2013. Combined DNA extraction and antibody elution from filter papers for the assessment of malaria transmission intensity in epidemiological studies. Malar J 12: 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rougemont M, Van Saanen M, Sahli R, Hinrikson HP, Bille J, Jaton K, 2004. Detection of four Plasmodium species in blood from humans by 18S rRNA gene subunit-based and species-specific real-time PCR assays. J Clin Microbiol 42: 5636–5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lo E, Zhou G, Oo W, Afrane Y, Githeko A, Yan G, 2015. Low parasitemia in submicroscopic infections significantly impacts malaria diagnostic sensitivity in the highlands of western Kenya. PLoS One 10: e0121763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.WHO , 2017. Malaria Rapid Diagnostic Test Performance: Results of WHO Product Testing of Malaria RDTs: Round 7 (2015–2016). Geneva, Switzerland: World Health Organization. [Google Scholar]
- 32.Hussein M, Kassa M, Kebede A, Endeshaw T, 2012. Paracheck-pf® test versus microscopy in the diagnosis of falciparum malaria in Arbaminch Zuria Woreda of south Ethiopia. Ethiop J Health Sci 22: 93–98. [PMC free article] [PubMed] [Google Scholar]
- 33.Endeshaw T, et al. 2012. Performance of local light microscopy and the ParaScreen Pan/Pf rapid diagnostic test to detect malaria in health centers in northwest Ethiopia. PLoS One 7: e33014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hailu T, Kebede T, 2014. Assessing the performance of CareStart malaria Pf/Pv combo test against thick blood film in the diagnosis of malaria in northwest Ethiopia. Am J Trop Med Hyg 90: 1109–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharew BLM, Animut A, Jima D, Medhin G, Erko B, 2009. Evaluation of the performance of CareStart™ malaria Pf/Pv combo and paracheck Pf tests for the diagnosis of malaria in Wondo Genet, southern Ethiopia. Acta Tropica 111: 321–324. [DOI] [PubMed] [Google Scholar]
- 36.Mohammed H, Kassa M, Assefa A, Tadesse M, Kebede A, 2017. Genetic polymorphism of merozoite surface protein-2 (MSP-2) in Plasmodium falciparum isolates from Pawe district, north west Ethiopia. PLoS One 12: e0177559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwenti TE, Njunda LA, Tsamul B, Nsagha SD, Assob NJ, Tufon KA, Meriki DH, Orock EG, 2017. Comparative evaluation of a rapid diagnostic test, an antibody ELISA, and a pLDH ELISA in detecting asymptomatic malaria parasitaemia in blood donors in Buea, Cameroon. Infect Dis Poverty 6: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nankabirwa JI, Yeka A, Arinaitwe E, Kigozi R, Drakeley C, Kamya MR, Greenhouse B, Rosenthal PJ, Dorsey G, Staedke SG, 2015. Estimating malaria parasite prevalence from community surveys in Uganda: a comparison of microscopy, rapid diagnostic tests and polymerase chain reaction. Malar J 14: 528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wanja EW, Kuya N, Moranga C, Hickman M, Johnson JD, Moseti C, Anova L, Ogutu B, Ohrt C, 2016. Field evaluation of diagnostic performance of malaria rapid diagnostic tests in western Kenya. Malar J 15: 456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adu-Gyasi D, et al. 2018. Assessing the performance of only HRP2 and HRP2 with pLDH based rapid diagnostic tests for the diagnosis of malaria in middle Ghana, Africa. PLoS One 13: e0203524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh N, Shukla MM, Shukla MK, Mehra RK, Sharma S, Bharti PK, Singh MP, Singh A, Gunasekar A, 2010. Field and laboratory comparative evaluation of rapid malaria diagnostic tests versus traditional and molecular techniques in India. Malar J 9: 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baiden F, et al. 2012. Accuracy of rapid tests for malaria and treatment outcomes for malaria and non-malaria cases among under-five children in rural Ghana. PLoS One 7: e34073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diallo MA, Diongue K, Ndiaye M, Gaye A, Deme A, Badiane AS, Ndiaye D, 2017. Evaluation of CareStart™ malaria HRP2/pLDH (Pf/pan) combo test in a malaria low transmission region of Senegal. Malar J 16: 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maltha J, Gillet P, Bottieau E, Cnops L, van Esbroeck M, Jacobs J, 2010. Evaluation of a rapid diagnostic test (CareStart™ malaria HRP-2/pLDH (Pf/pan) combo test) for the diagnosis of malaria in a reference setting. Malar J 9: 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laban NM, Kobayashi T, Hamapumbu H, Sullivan D, Mharakurwa S, Thuma PE, Shiff CJ, Moss WJ; Southern Africa International Centers of Excellence for Malaria Research , 2015. Comparison of a PfHRP2-based rapid diagnostic test and PCR for malaria in a low prevalence setting in rural southern Zambia: implications for elimination. Malar J 14: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahmed R, Levy EI, Maratina SS, de Jong JJ, Asih PB, Rozi IE, Hawley W, Syafruddin D, ter Kuile F, 2015. Performance of four HRP-2/pLDH combination rapid diagnostic tests and field microscopy as screening tests for malaria in pregnancy in Indonesia: a cross-sectional study. Malar J 14: 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chiodini PL, Bowers K, Jorgensen P, Barnwell JW, Grady KK, Luchavez J, Moody AH, Cenizal A, Bell D, 2007. The heat stability of Plasmodium lactate dehydrogenase-based and histidine-rich protein 2-based malaria rapid diagnostic tests. Trans R Soc Trop Med Hyg 101: 331–337. [DOI] [PubMed] [Google Scholar]
- 48.Okell LC, Ghani AC, Lyons E, Drakeley CJ, 2009. Submicroscopic infection in Plasmodium falciparum-endemic populations: a systematic review and meta-analysis. J Infect Dis 200: 1509–1517. [DOI] [PubMed] [Google Scholar]
- 49.Golassa L, Enweji N, Erko B, Aseffa A, Swedberg G, 2013. Detection of a substantial number of sub-microscopic Plasmodium falciparum infections by polymerase chain reaction: a potential threat to malaria control and diagnosis in Ethiopia. Malar J 12: 352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harris I, et al. 2010. A large proportion of asymptomatic Plasmodium infections with low and sub-microscopic parasite densities in the low transmission setting of Temotu Province, Solomon Islands: challenges for malaria diagnostics in an elimination setting. Malar J 9: 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.WHO , 2014. Policy Brief on Malaria Diagnostics in Low-Transmission Settings. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 52.Atroosh WM, et al. 2015. Genetic variation of pfhrp2 in Plasmodium falciparum isolates from Yemen and the performance of HRP2-based malaria rapid diagnostic test. Parasite Vector 8: 388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laurent A, Schellenberg J, Shirima K, Ketende SC, Alonso PL, Mshinda H, Tanner M, Schellenberg D, 2010. Performance of HRP-2 based rapid diagnostic test for malaria and its variation with age in an area of intense malaria transmission in southern Tanzania. Malar J 9: 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Siahaan L, Panggabean M, Panggabean YC, 2018. RDT accuracy based on age group in hyperendemic malaria. IOP Conf Ser Earth Env Sci 125: 012018. [Google Scholar]
- 55.Berzosa P, et al. 2018. Comparison of three diagnostic methods (microscopy, RDT, and PCR) for the detection of malaria parasites in representative samples from equatorial Guinea. Malar J 17: 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee JH, Jang JW, Cho CH, Kim JY, Han ET, Yun SG, Lim CS, 2014. False-positive results for rapid diagnostic tests for malaria in patients with rheumatoid factor. J Clin Microbiol 52: 3784–3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Girma S, Cheaveau J, Mohon AN, Marasinghe D, Legese R, Balasingam N, Abera A, Feleke SM, Golassa L, Pillai DR, 2019. Prevalence and epidemiological characteristics of asymptomatic malaria based on ultrasensitive diagnostics: a cross-sectional study. Clin Infect Dis 69: 1003–1010. [DOI] [PubMed] [Google Scholar]
- 58.Golassa L, Messele A, Amambua-Ngwa A, Swedberg G, 2020. Extensive deletion and sequence variation of Plasmodium falciparum histidine rich protein 2/3 (pfhrp2/3) genes in Ethiopia: implication for RDT-based malaria diagnosis and control. Malar J (under review). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.