Abstract.
We describe six cases of healthcare professionals in Brazil who recovered but again presented symptoms consistent with COVID-19, with new positive reverse transcription (RT)-PCR test results. The cases reported herein presented symptom onset between March 16, 2020 and April 9, 2020. All were health professionals (four medical doctors), five were female, with a median age of 43.5 years, and three had comorbidities. All patients were confirmed for SARS-CoV-2 detection by RT-PCR in naso and/or oropharyngeal swab samples. Among the reported cases, three (50%) underwent RT-PCR testing in the period between the two symptomatic episodes, with negative results. The time elapsed between the onset of symptoms in the two episodes ranged from 53 to 70 days (median, 56.5 days). In the first episode, the main symptoms described were fever (4/6), myalgia (3/6), sore throat (3/6), and cough (3/6). Meanwhile, during the second episode, fever (4/6) and weakness (3/6) predominated. Most of the cases progressed without complications, although one individual presented hypoxemia (minimum SatO2 of 90%) in both episodes, and two, only in the second, one of which required intensive care unit admission, progressing with improvement after medication and receiving noninvasive ventilatory support. We report cases with recurrence of symptoms compatible with COVID-19, with positive RT-PCR results, that could represent the occurrence of viral reactivation or reinfection. The true nature of this phenomenon should be better clarified in future studies.
INTRODUCTION
The new coronavirus SARS-CoV-2, the etiological agent of COVID-19, first emerged in Wuhan, China, in December 2019 and quickly spread throughout the world,1,2 with Brazil being one of the countries with the highest number of cases.3
Most cases evolve in a benign and self-limited manner. There is little definitive evidence for the development of immune protection after natural infection. In February 2020, four confirmed patients were reported, who were discharged after two negative reverse transcription (RT)-PCR tests, but reverted to being positive by RT-PCR 5–13 days after hospital discharge.4 Soon after that, several groups reported a similar phenomenon, which has received variable designations in the literature (recurrence, possible human reactivation, or reinfection by SARS-CoV-2).1,5–7
A distinct situation seems to happen when COVID-19 relapses with clinical manifestations of the disease associated with viral RNA detection on molecular testing, characterizing a condition compatible with the recurrence of COVID-19. A retrospective cohort of 55 individuals identified five cases (9%) that required readmission because of symptom recurrence between 4 and 17 days after discharge. Clinical progression was benign, with no differences observed from the acute phase.8 More recently, a case series was described involving 11 individuals in a similar situation, with reappearance of symptoms between 24 and 49 days after the beginning of the first episode. In this series, three deaths were reported, and viral detection was possible in cultured samples from one of the two recurrent cases tested.9 However, so far, there is no consensus on the possibility of recurrence, much less the distinction between possible human reactivation and reinfection by SARS-CoV-2.5–7
In Ceará, northeastern Brazil, more than 140,000 cases of COVID-19 have been reported, with more than 7,000 deaths,10,11 which has led the health system to collapse. Here, we describe six cases of healthcare professionals who recovered but again presented symptoms consistent with COVID-19, with new positive RT-PCR test results.
CASE REPORTS
The cases reported herein presented symptom onset between March 16, 2020 and April 9, 2020. All were health professionals (four medical doctors), five were female, with a median age of 43.5 years, and three had comorbidities (Table 1). The cases presented were officially notified by the state epidemiological surveillance service of Ceará government. After information gathering, all patients agreed with the use of their anonymous medical data.
Table 1.
Demographic aspects, signs, and symptoms of the cases of COVID-19 in Ceará, Brazil
| Case | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Age (gender) | 29 (male) | 63 (male) | 40 (female) | 67 (male) | 47 (male) | 31 (male) |
| Comorbidity | – | SAH | Ankylosing spondylitis and asthma | Obesity, SAH, obstructive sleep apnea syndrome, and rhinitis | – | – |
| DOS of first infection | March 16 | March 16 | March 18 | March 20 | March 23 | April 9 |
| Date of first RT-PCR | March 27 | March 27 | March 18 | March 24 | March 23 | April 15 |
| DOS of second infection | May 8 | May 13 | May 27 | May 13 | May 18 | June 5 |
| Date of second RT-PCR | May 13 | May 18 | June 1 | May 16 | May 22 | June 8 |
| Time between episodes | 53 | 58 | 70 | 54 | 56 | 57 |
| Time test: symptom 1 | 11 | 11 | 0 | 4 | 0 | 6 |
| Time test: symptom 2 | 5 | 5 | 5 | 3 | 4 | 3 |
| Adynamia | Yes | Yes | – | – | – | – |
| Chills | Yes | Yes | – | – | – | – |
| Myalgia | Yes | – | Yes | – | Yes | Yes |
| Arthralgia | – | – | Yes | Yes | – | – |
| Sore throat | Yes | Yes | – | – | – | – |
| Cough | Yes | – | Yes | – | Yes | – |
| Coryza | Yes | – | – | Yes | – | – |
| Headache | Yes | – | – | – | – | Yes |
| Asthenia | – | – | – | – | – | – |
| Anorexia | – | – | – | Yes | – | – |
| Diarrhea | – | – | – | Yes | – | – |
| Dyspnea | – | Yes | – | – | – | Yes |
| Fever | Yes | Yes | Yes | – | Yes | Yes |
| Oxygen saturation < 95% | – | – | – | – | – | Yes |
| Loss of sense of taste | Yes | – | – | – | – | – |
| Loss of sense of smell | Yes | – | Yes | – | – | – |
| Complications in the first episode | No | No | No | No | No | Hypoxemia (SatO2 92%) |
| Complications in the second episode | No | Hypoxemia | No | Hypoxemia (intensive care unit) | No | Hypoxemia (SatO2 90%) |
DOS = date of onset of symptoms; RT-PCR = reverse transcription-PCR; SAH = systemic arterial hypertension.
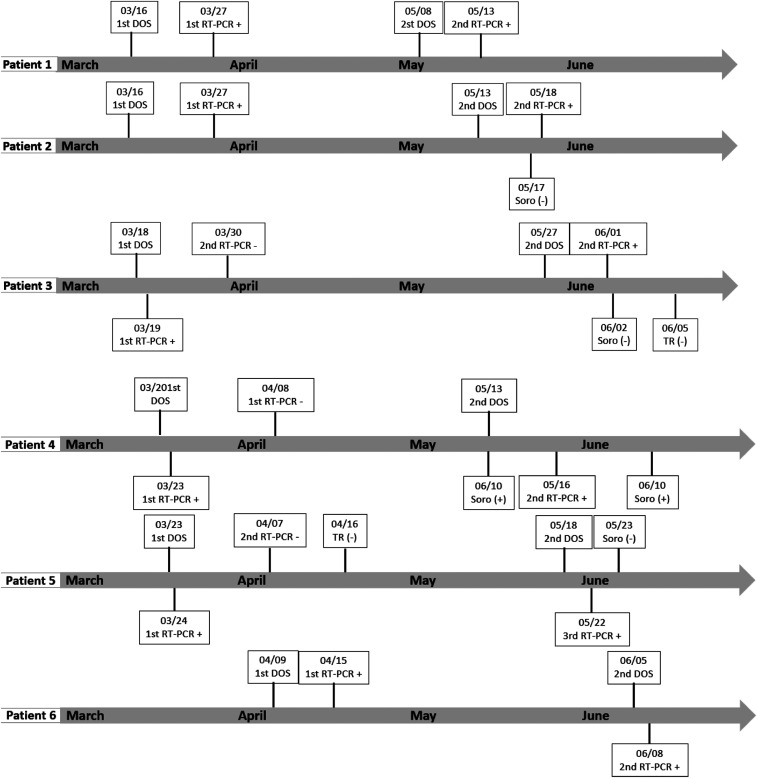
All patients were confirmed for SARS-CoV-2 detection by RT-PCR in naso and/or oropharyngeal swab samples, which were collected between 0 and 11 days after the first symptoms. Among the reported cases, 50% (3) underwent RT-PCR testing in the period between the two symptomatic episodes, with negative results. Only one of the patients underwent serological rapid testing in the same interval, which was also negative in two separate samples. In four cases, serological testing was also performed, but only after the second clinical episode, with negative initial results. Three cases later seroconverted positive by chemiluminescence microparticle immunoassay serology, collected on the 11th, the 28th, and the 37th day after the onset of symptoms in the second episode (Figure 1).
Figure 1.
Time line of the confirmed cases in Ceará, Brazil. DOS = date of onset of symptoms; RT-PCR = Reverse transcription-PCR; + = positive; − = negative.
The time elapsed between the onset of symptoms in both episodes ranged from 53 to 70 days (median, 56.5 days). In the first episode, the main symptoms described were fever (4/6), myalgia (3/6), sore throat (3/6), and cough (3/6). Meanwhile, during the second episode, fever (4/6) and weakness (3/6) predominated. Myalgia and diarrhea were reported only in the first episode, but the highest number of symptoms was described during the second episode. In two cases, anosmia was observed in the second episode. Most of the cases progressed without complications, although one individual presented hypoxemia (minimum SatO2 of 90%) in both episodes, and two, only in the second, one of which required intensive care unit (ICU) admission, progressing with improvement after medication and receiving noninvasive ventilatory support.
During the first episode, patient 1 received oseltamivir for 10 days, and in the second, symptomatic medications, azithromycin and hydroxychloroquine, were prescribed, in addition to oseltamivir. Patient 2 was treated with symptomatic medication and oseltamivir in the first episode, whereas in the second, only symptomatic medication was prescribed. Patient 3 presented a high-resolution chest tomography (HRCT) with slight scattered and bilateral ground-glass pulmonary opacities, more evident in peripheral regions (less than 50% extension) and was treated with prednisone and azithromycin only in the second episode. Patient 4 required hospitalization only in the second episode, when he used hydroxychloroquine (800 mg on the first day, and then 400 mg/day for another 6 days), azithromycin (500 mg/day) for 5 days, and levofloxacin (750 mg/day) for 7 days. He evolved without improvement, being transferred to the ICU to receive high-flow oxygen therapy. He had HRCT with pulmonary involvement of 50–75% in extension. A schedule was planned for piperacillin–tazobactam (8 days ago) and methylprednisolone (1 mg/kg/day for 5 days, with progressive reduction) and prophylactic anticoagulation. Patient 6, who exhibited hypoxemia in both episodes, used hydroxychloroquine 400 mg, azithromycin 500 mg for 5 days, and levofloxacin 750 mg from the 6th to the 10th day. On the second visit, the patient was prescribed ivermectin 6 mg (two tablets, 24/24 hours) for 3 days, azithromycin 500 mg/day for 5 days, and prednisone 80 mg/day from the 6th to the 10th day.
DISCUSSION
We report cases with recurrence of symptoms compatible with COVID-19, with positive RT-PCR results, that could represent the occurrence of viral reactivation or reinfection. The interpretation of this phenomenon is subject to questioning because the detection of the viral genome by RT-PCR has persisted in some cases for up to 6 weeks.6 The long asymptomatic period and characteristics of the symptoms reinforce the possibility of recurrence. However, our data are not sufficient to prove the occurrence of such phenomena, nor to characterize their nature.
Despite efforts to produce a vaccine, a licensed one remains unavailable.12 It is likely that health professionals with COVID-19 will require post-discharge and post-recovery surveillance of signs and symptoms, with retesting for SARS-CoV-2 for those who present recurrent clinical manifestations of the disease. We emphasize the importance of maintaining the use of personal protective equipment and hand hygiene even after curing COVID-19 when returning for assistance. Determining the possibility of reinfection or reactivation of the virus and immunological protection after natural infection has important implications for coping with the COVID-19 pandemic in the future.
One limitation found in the present investigation was the absence of cultures and sequencing of the virus, which were not possible. After the occurrence of these cases, the local government implemented surveillance by electronic means, with the objective of detecting earlier new suspected cases with this profile. In addition, longitudinal cohort studies will assist in better understanding the prognosis of the disease.
Acknowledgment:
Publication charges for this article were waived due to the ongoing pandemic of COVID-19.
REFERENCES
- 1.Zhu N, et al. China Novel Coronavirus Investigating and Research Team , 2020. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382: 727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holshue ML, et al. Washington State 2019-nCoV Case Investigation Team , 2020. First case of 2019 novel coronavirus in the United States. N Engl J Med 382: 929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brazilian Ministry of Health , 2020. Coronavirus (COVID-19). Available at: https://covid.saude.gov.br/. Accessed April 24, 2020. [Google Scholar]
- 4.Lan L, Xu D, Ye G, Xia C, Wang S, Li Y, Xu H, 2020. Positive RT-PCR test results in patients recovered from COVID-19. JAMA 323: 1502–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xing Y, Mo P, Xiao Y, Zhao O, Zhang Y, Wang F, 2020. Post-discharge surveillance and positive virus detection in two medical staff recovered from Coronavirus disease 2019 (COVID-19), China, January to February 2020. Euro Surveill 25: 2000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao AT, Tong YX, Zhang S, 2020. False-negative of RT-PCR and prolonged nucleic acid conversion in COVID-19: rather than recurrence. J Med Virol, 10.1002/jmv.25855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young BE, et al. Singapore 2019 Novel Coronavirus Outbreak Research Team , 2020. Epidemiologic features and clinical course of patients with SARS-CoV-2 in Singapore. JAMA 232: 1488–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ye G, Pan Z, Pan Y, Deng Q, Chen L, Li J, Li Y, Wang X, 2020. Clinical characteristics of severe acute respiratory syndrome coronavirus 2 reactivation. J Infect 80: e14–e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Batisse D, et al. 2020. Clinical recurrences of COVID-19 symptoms after recovery: viral relapse, reinfection or inflammatory rebound? J Infect, 10.1016/j.jinf.2020.06.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lemos DRQ, et al. 2020. Health system collapse 45 days after the detection of COVID-19 in Ceará, northeast Brazil: a preliminary analysis. Rev Soc Bras Med Trop 53: e20200354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ceará State Government , 2020. IntegraSUS. Available at: https://integrasus.saude.ce.gov.br/. Accessed July 11, 2020. [Google Scholar]
- 12.Graepel KW, Kochhar S, Clayton EW, Edwards KE, 2020. Balancing expediency and scientific rigor in severe acute respiratory syndrome coronavirus 2 vaccine development. J Infect Dis 222: 180–182. [DOI] [PMC free article] [PubMed] [Google Scholar]