Abstract.
The use of antimicrobial growth promoters in chicken farming has been commonly associated with high levels of antimicrobial resistance (AMR) in humans. Most of this work, however, has been focused on intensive large-scale operations. Intensive small-scale farming that regularly uses antibiotics is increasing worldwide and has different exposure pathways compared with large-scale farming, most notably the spatial connection between chickens and households. In these communities, free-ranging backyard chickens (not fed antibiotics) can roam freely, whereas broiler chickens (fed antibiotics) are reared in the same husbandry environment but confined to coops. We conducted an observational field study to better understand the spatial distribution of AMR in communities that conduct small-scale farming in northwestern Ecuador. We analyzed phenotypic resistance of Escherichia coli sampled from humans and backyard chickens to 12 antibiotics in relation to the distance to the nearest small-scale farming operation within their community. We did not find a statistically significant relationship between the distance of a household to small-scale farming and antibiotic-resistant E. coli isolated from chicken or human samples. To help explain this result, we monitored the movement of backyard chickens and found they were on average 17 m (min–max: 0–59 m) from their household at any given time. These backyard chickens on average ranged further than the average distance from any study household to its closest neighbor. This level of connectivity provides a viable mechanism for the spread of antimicrobial-resistant bacteria and genes throughout the community.
INTRODUCTION
The emergence, spread, and persistence of antimicrobial resistance (AMR) present one of the greatest global public health concerns facing us today.1 In particular, increased AMR complicates antibiotic treatment and results in increases in healthcare costs, morbidity, and mortality.1,2 This emergent threat is further exacerbated by the continuous use of subtherapeutic antimicrobial agents for growth promotion in large-scale intensive animal farms.3,4 Such intensive farming operations can produce large amounts of animal protein by maximizing animal growth rates. As food security continues to be a pressing concern among many low- and middle-income countries, there has also been an expansion of small-scale farming in regions with limited infrastructure for antimicrobial surveillance.5,6 It is estimated that by 2030, the global use of antimicrobial agents will increase by 67%, primarily led by rapidly developing nations.5
Our study site, located in villages of the Esmeraldas Province in northwestern Ecuador, represents a model system that can be used to assess the impacts of small-scale agricultural development on human and animal health across many rural communities in the tropics. A recently constructed road in this region has facilitated the introduction of micro-industries such as small-scale chicken farming. Within our study system, small-scale poultry farming of broiler meat chickens (which receive antibiotics as growth promoters) co-occurs with farming of local backyard chicken breeds (which are not fed with antibiotics). Broiler chickens are intensively reared within a large-scale farm setting and purchased as day-old chicks by small-scale farming operations that either are based out of a single household or run by multiple households within a collective hatchery. Whereas the farmers rear the broiler chickens in enclosed coops, the backyard chickens are able to move freely between the household, the broiler coop, and surrounding community landscape. These backyard chickens, therefore, may serve as a reservoir hosts for antimicrobial-resistant bacteria (where broiler chickens function as a primary reservoir host). Our previous analyses suggest that the selection for antimicrobial-resistant bacteria in broilers originates from the intensive large-scale farm environment where they are reared to chicks.7,8 At the village level, we demonstrated the presence of spillover from farmed chickens to backyard chickens,9 and at the household level, we have identified the spillover of extended-spectrum beta-lactamase CTX-M-producing Escherichia coli from broiler chickens to backyard chickens and possibly to children.8 We also have detected greater richness of antimicrobial-resistant genes present in broiler chickens than backyard chickens.10 Despite our previous foundational work, we are still limited in our understanding of the spatial spread of antimicrobial-resistant bacteria.11
Antimicrobial-resistant bacteria have been recorded in the surrounding environment in close proximity to farming activity.12,13 One study detected clustering of ampicillin- and tetracycline-resistant Pasteurella multocida isolated from cattle.14 We hypothesize that AMR levels might also follow a similar clustering pattern: backyard chickens and children of households closer in distance to intensive small-scale broiler farming households could exhibit increased levels of resistance.
The primary aim of this observational field study was to evaluate the spatial relationship between intensive small-scale broiler chicken husbandry and AMR found in surrounding backyard chickens and, ultimately, humans. To better interpret these antimicrobial-resistant bacteria data, we also measured the spatial ecology of free-ranging domestic chickens (Gallus gallus domesticus).
MATERIALS AND METHODS
Field study design.
Between February and May 2017, we collected fecal samples from backyard chickens (n = 439) and children (n = 375) from three villages (Borbón, Colon Eloy, and Timbiré) within the province of Esmeraldas, Ecuador. We collected these samples during the three observational sample periods (S1: February 2–February 6, S2: March 29–April 1, and S3: May 24–May 27). During these sample periods, we sampled all available households that both had backyard chickens and were willing to participate in the study (n = 66). At each household, we collected fecal samples via cloacal swabs from four randomly backyard chickens. If fewer than four chickens were available, we sampled all chickens. Of the 66 households in which we sampled backyard chickens, in 48, we also collected child stool samples. To increase our sample size of children, in additional 34 households, we collected samples only from children. We enrolled all households (n = 100) willing to participate and attempted to collect all child stool samples (between 5 and 18 years) from these households.
Microbial processing of phenotypic resistance.
Samples were first plated on MacConkey lactose agar followed by Chromocult agar for confirmation of E. coli colonies.7,8 We selected up to three E. coli colonies from fecal samples stored in Cary–Blair medium.15 For each isolate, antibiotic sensitivity was assessed using the Kirby–Bauer disc diffusion method16 for 12 antibiotics: amoxicillin–clavulanate (10 μg), ampicillin (10 μg), cefotaxime (30 μg), cephalothin (30 μg), chloramphenicol (30 μg), enrofloxacin (5 μg), gentamicin (10 μg), streptomycin (10 μg), sulfisoxazole (1 mg), tetracycline (30 μg), and trimethoprim–sulfamethoxazole (25 μg). Zones of inhibition were measured after 24 hours of incubation using digital calipers. We classified phenotypic resistance as resistant or sensitive (intermediate isolates were categorized as sensitive) as determined by the Clinical and Laboratory Standards Institute.17,18 We used reference strains (E. coli ATCC 25922, Staphylococcus aureus ATCC 25923, and Pseudomonas aeruginosa ATCC 27853) as controls for each batch of the disc diffusion test.
Chicken farming data.
Beginning December 2016 (2 months before fecal sampling) to May 2017 (the end of fecal sampling), we conducted structured household surveys to households raising either breeds of chicken (n = 60 households farming broiler chickens and n = 99 households). The survey characterized flock dynamics including poultry flock demographics, including the number of symptomatic, dead, and alive each month. All 66 households where backyard chicken fecal samples were collected were included in the 99 households. Throughout the study period, flock sizes varied by breed (broiler chicken: 66 ± 15.6 [SE], min–max: 2–300; backyard chicken: 13 ± 0.8, min–max: 1–137). Broiler chicken coop environments were constructed of bamboo or timber with dirt floors and sawdust used for bedding. Surveys were conducted in Spanish by community partners with tablets. Responses were recorded using Qualtrics software (Qualtrics, Provo, UT, www.qualtrics.com). Households involved in broiler farming activity within 60 days before sampling were categorized as sources of environmental AMR exposure. Latitude and longitude coordinates were recorded at the threshold of every studied household using a Garmin 62 Handheld GPS Navigator.
Chicken spatial movement data collection.
From May to December 2016, chicken movement patterns were collected from five backyard chickens owned by one household. We chose this particular household because it had no permanent fences and represented a typical backyard environment in communities of the region. Sample size was limited because of the number of available GPS units. Sampling days (n = 14) were limited by the availability of the field technician in the community. An i-GOT-U GPS 120 Logger (Mobile Action Technology, Inc., Taiwan) was placed on each chicken to record their coordinates every second (Figure 1). Each day, GPS units were placed on chickens at 0700 before the birds were released from their coops and retrieved at 1700 when they returned to rest for the evening.
Figure 1.
Example of the insertion of the i-Got-U 120 GPS unit under the wing (A) and chicken after the unit is inserted (B). This figure appears in color at www.ajtmh.org.
Samples collection and ethical treatment of animals and humans.
All avian and human samples were placed in Cary–Blair medium and transported to Quito for analysis. Consent to participate was obtained from all households, and all study protocols were reviewed and approved by the University of Michigan Institutional Review Board and the Universidad San Francisco de Quito Bioethics Committee.
Data analysis.
Our outcome variable is at the sample level for chickens and humans. If one or more of the isolates tested were found to be resistant to an antibiotic, that sample was defined as resistant. Euclidian distances to the nearest household farming broiler chickens were calculated for every study household. Logistic regression models for AMR were applied at the household level using the sample period as a covariate. The predictor was the distance to nearest broiler farming household, and the outcome was the presence of phenotypic antimicrobial-resistant bacteria within a sample. We produced maps to display the households in which a backyard chicken or child sample was collected as well as houses engaged in broiler farming using ArcGIS 10.5.1 (ESRI, Redlands, CA) (Supplemental Figures 1–3).
To explore the spatial relationships between antimicrobial-resistant bacteria in backyard chickens and the location of farmed chickens, we used a “broken stick” regression model.19 This piecewise linear regression model assumes that there is a linear decrease in AMR as the distance from farming activity increases up to a threshold distance, after which there is no spatial relationship. We applied the “broken stick” modeling framework within the context of generalized linear mixed models and logistic regressions. We ultimately aggregated all of the data samples from the three communities by sample observation period with conventional logistic regressions.
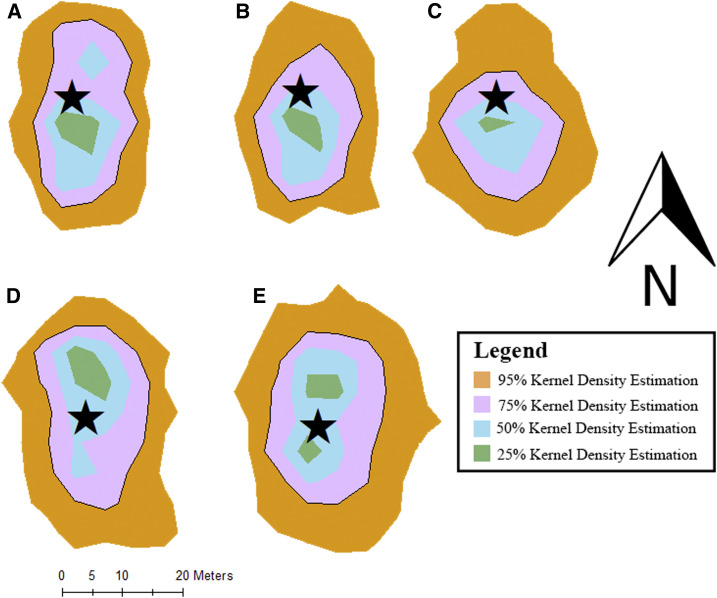
The backyard chicken home range of movement around the household was summarized and described using a kernel density estimate (KDE)20 approach, with which we derived maps describing the areas where it is likely to find the chicken at any given time point with a certain probability. In this article, we present maps showing areas where there is 95%, 75%, 50%, and 25%, probability, respectively, that the chicken will be contained in it, which is termed home range analysis (Figure 1). The KDE approach is one of the most widely accepted techniques for home range analysis because of its robustness as a nonparametric probability estimate.21,22 Home range analysis provides information on the spatial boundaries and frequency of movements within an area. All chicken movement data were analyzed using R Statistical Software version 3.5.2 (2019, R Core Team, Vienna, Austria) and packages rgdal, maptools, and adehabitatHR.
To evaluate phenotypic resistance profile variation between humans and backyard chickens, we applied Spearman’s rank correlation test. To address the fact that the number of samples collected from backyard chickens was greater than that of humans, we used a bootstrap approach taking random subsets of samples from backyard chickens and computing Spearman correlation between the random subsets of backyard chicken samples and human samples. We summarized all these Spearman correlations with the average of all these correlations.
RESULTS
Phenotypic resistance profiles.
We observed many strong similarities between E. coli phenotypic resistance from backyard chickens and humans. In total, we collected 1,323 and 1,144 E. coli isolates from backyard chickens and humans, respectively (Table 1). A high mean percentage of both human and backyard chicken E. coli were resistant to tetracycline (39.3% and 48.0% of isolates, respectively; Spearman: r = 0.001, P-value = 0.53), cephalothin (45.8% and 49.0%, respectively; Spearman: r = −0.001, P-value = 0.49), and streptomycin (59.4% and 58.1%, respectively; Spearman: r = −0.001, P-value = 0.53). Both shared relatively low E. coli phenotypic resistance to gentamycin (5.6% and 5.7%, respectively; Spearman: r = −0.003, P-value = 0.49), chloramphenicol (6.6% and 12.0%, respectively; Spearman: r = 0.006, P-value = 0.52), and cefotaxime (9.1% and 16.0%, respectively; Spearman: r = −0.005, P-value = 0.52).
Table 1.
Number of phenotypic resistance Escherichia coli isolates from backyard chickens and humans sampled from three villages in Ecuador during the total sampling period
| Antibiotic | Human | Backyard chicken |
|---|---|---|
| (n = 1,144) | (n = 1,323) | |
| Gentamicin | 64 (5.6) | 74 (5.6) |
| Streptomycin | 702 (61.4) | 772 (58.4) |
| Amoxicillin–clavulanate | 183 (16.0) | 182 (13.8) |
| Ampicillin | 426 (37.2) | 352 (26.6) |
| Cefotaxime | 105 (9.2) | 70 (5.3) |
| Cephalothin | 523 (45.7) | 649 (49.1) |
| Chloramphenicol | 75 (6.6) | 158 (11.9) |
| Sulfisoxazole | 368 (32.2) | 309 (23.4) |
| Ciprofloxacin | 45 (3.9) | 100 (7.6) |
| Enrofloxacin | 64 (5.6) | 95 (7.2) |
| Trimethoprim–sulfamethoxazole | 310 (27.1) | 256 (19.3) |
| Tetracycline | 449 (39.2) | 634 (47.9) |
The most common E. coli phenotypic resistance profiles for backyard chickens was “S” (n = 21), for broiler chickens was “AMP-G-AMC-CTX-CF-C-CIP-SXT-ENO-TE-S” (n = 3), and for children was “CF-S” (n = 21). In 49 households (n = 49), we collected at least one child and one backyard chicken and quantified the proportion of shared E. coli phenotypic resistance detected in at least one backyard chicken and one child. We observed a high proportion of shared E. coli resistance to G (78%), CF (92%), SXT (70%), and TE (89%). At the same time, we also detected differences, such as consistently higher human sulfisoxazole and ampicillin resistance than backyard chickens (32.1% versus 23.5% and 37.2% versus 26.5%, respectively).
Spatial epidemiological model outcomes.
Using both a broken stick regression model and a logistic regression model with a linear term for distance to broiler farming, we were unable to detect any significant relationships between either backyard chicken or human AMR and distance to broiler farming (Tables 2 and 3 present logistic model results). In addition, when the presence of backyard chicken was added as a binary covariate to the model, the effect was estimated to be not significantly different from 0, indicating no effect on the odds of having antibiotic resistance.
Table 2.
Association between the distance (m) of a household to broiler chicken farming (in the last 60 days) and human phenotypic resistance residing in that household
| Antibiotic | S1 | S2 | S3 | |||
|---|---|---|---|---|---|---|
| Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | |
| Gentamicin | −8.7 | −19.7–2.4 | 0.4 | −2.0–2.6 | 0.2 | −1.9–2.4 |
| Streptomycin | 1.0 | −4.8–6.7 | −4.9 | −9.1–0.8 | 2.1 | −0.7–4.9 |
| Amoxicillin–clavulanate | 1.1 | −2.7–4.9 | −0.5 | −2.5–1.6 | 0.7 | −1.2–2.5 |
| Ampicillin | −0.2 | −1.3–0.9 | −1.7 | −3.7–0.3 | −0.1 | −1.7–1.4 |
| Cefotaxime | −3.2 | −10.3–3.9 | 0.8 | −1.3–2.9 | 0.7 | −1.1–2.6 |
| Cephalothin | 0.5 | −3.8–4.7 | −0.4 | −1.0–2.5 | −1.0 | −2.8–0.7 |
| Chloramphenicol | −0.2 | −4.5–4.2 | 0.1 | −2.0–2.1 | 0.8 | −1.2–2.8 |
| Sulfisoxazole | −3.5 | −7.5–0.5 | −1.0 | 0.1–1.2 | 1.9 | 0.2–3.6 |
| Ciprofloxacin | −3.9 | −10.8–3.0 | 0.5 | −1.8–2.8 | −0.4 | −3.44–2.6 |
| Enrofloxacin | −1.5 | −6.7–3.8 | −0.2 | −2.3–2.0 | −0.8 | −3.1–1.6 |
| Tetracycline | −1.0 | −5.0–3.0 | −0.8 | −2.9–1.3 | 0.3 | −1.4–2.0 |
| Trimethoprim–sulfamethoxazole | −3.4 | −7.7–0.9 | −1.9 | −3.8–0.1 | 1.5 | −0.1–3.2 |
Estimates obtained through logistic regression models for 12 antibiotics during sample period one (S1), two (S2), and three (S3).
Table 3.
Association between the distance (m) of a household to broiler chicken farming (in the last 60 days) and backyard chicken phenotypic resistance residing in that household
| Antibiotic | S1 | S2 | S3 | |||
|---|---|---|---|---|---|---|
| Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | |
| Gentamicin | −3.9 | −11.4–3.5 | 0.0 | −2.3–2.3 | 0.6 | −1.3–2.5 |
| Streptomycin | −0.9 | −6.9–4.3 | 0.7 | −2.5–3.8 | 3.3 | −0.1–6.6 |
| Amoxicillin–clavulanate | 1.0 | −2.7–4.7 | −0.9 | −2.9–1.1 | 2.6 | 0.5–4.6 |
| Ampicillin | 0.2 | −3.5–3.9 | −1.2 | −3.1–0.8 | 0.0 | −1.5–1.6 |
| Cefotaxime | 1.0 | −3.8–5.7 | −0.3 | −2.4–1.9 | 0.7 | −1.6–2.9 |
| Cephalothin | 3.2 | −1.7–8.1 | −1.2 | −3.4–1.3 | −2.4 | −4.1–0.7 |
| Chloramphenicol | −1.9 | −7.0–3.1 | 2.6 | 0.5–4.7 | −0.2 | −2.3–7.4 |
| Sulfisoxazole | 1.8 | −1.9–5.5 | −0.2 | −2.1–1.6 | 0.3 | −1.8–1.3 |
| Ciprofloxacin | 0.9 | −6.5–8.3 | −1.5 | −4.0–1.1 | −3.0 | −8.2–2.2 |
| Enrofloxacin | 0.3 | −5.7–6.3 | 0.3 | −2.1–2.8 | −2.4 | −5.6–0.9 |
| Tetracycline | −0.9 | −4.8–3.0 | 1.5 | −0.6–3.7 | −0.6 | −2.3–1.1 |
| Trimethoprim–sulfamethoxazole | 0.7 | −3.1–4.5 | 0.8 | −1.1–2.7 | −0.3 | −1.9–1.3 |
Estimates obtained through logistic regression models for 12 antibiotics during sample period one (S1), two (S2), and three (S3).
Chicken movement data.
On average, the backyard chickens monitored traveled a mean distance of 17.0 m from their household (min–max: 0.0–59.0 m). Chicken movement ranges around their households were summarized by the mean vertical bisection (40 m; min–max: 36–43 m) and the mean horizontal bisection (29 m; min–max: 23–39 m) (Table 4). Both the horizontal and vertical bisections metrics were substantially greater than the average household distance to nearest neighbor (15 m; min–max: 1.5–156.9 m) and significantly shorter than the average distance to the nearest small-scale broiler farming household (176.1 m; min–max: 0–839.0 m). In addition, the mean total daily distance traveled by a chicken was 8,000 m (4,000–9,660 m) and the average area covered by a chicken in its daily movement was 2,421 m2 (4,853–4,853 m2) (Figure 2). The average shared spatial overlap among the five chickens was 75% (39–98%). The five chickens that we monitored occupied 51.9% of the same point locations during the observational study. Anecdotally, field technicians observed that the location of chickens clustered along a stream system that channeled through most of the community.
Table 4.
Descriptive statistics of the spatial ecology of backyard chickens in rural Ecuador using data collected from GPS units attached to each chicken
| Chicken number | Maximum distance from household (m) | Mean distance from household (m) | Vertical bisection of 95% kernel density estimation (m) | Horizontal bisection of 95% kernel density estimation (m) | Mean distance to the nearest household farming broiler chickens (m) | Mean distance to the nearest neighboring household (m) | Total distance moved (km) |
|---|---|---|---|---|---|---|---|
| 1 | 59.4 | 21.0 | 36.5 | 24.3 | 22.1 | 16.3 | 85.1 |
| 2 | 58.0 | 17.6 | 37.3 | 23.2 | 21.0 | 16.0 | 92.5 |
| 3 | 71.6 | 15.4 | 38.6 | 39.7 | 19.6 | 15.8 | 124.6 |
| 4 | 122.1 | 10.4 | 43.5 | 26.4 | 21.4 | 15.8 | 137.8 |
| 5 | 83.1 | 19.5 | 42.1 | 32.3 | 19.5 | 16.0 | 200.1 |
Figure 2.
Kernel density estimations calculated from movement data of five different chickens (chicken 1 [A], chicken 2 [B], chicken 3 [C], chicken 4 [D], and chicken 5 [E]). In each figure, the black star symbolizes the location of the household. This figure appears in color at www.ajtmh.org.
DISCUSSION
Spatial transmission of antimicrobial-resistant bacteria has been understudied in comparison to pathogens.11,14 In this study, we found that the household distance to the nearest small-scale broiler farming operation was not a significant predictor of the presence of antimicrobial-resistant bacteria in backyard chicken. The spatial ecology of free-ranging domestic chickens provides a possible explanation for this null finding. Lengths of chicken home range bisections, a measure of chicken’s range of movement, were greater on average than the mean household distance to the nearest small-scale broiler farming location, suggesting that free-ranging chickens have the potential to spread antimicrobial-resistant bacteria from one household to a neighboring household, and subsequently, through chains of transmission, throughout the village landscapes. Backyard chickens, therefore, may function as reservoir host, attenuating any spatial pattern of antimicrobial-resistant bacteria.
Reservoir hosts are common in many systems worldwide where free-ranging domestic animals frequently overlap in their environments with both wildlife and humans (e.g., pigs and Japanese encephalitis virus23; horses and Hendra virus24). Currently, there is not an international standard of biosecurity for small-scale broiler farming.25 In Kenya, a study of free-ranging domestic pigs found that pigs spent most of the time outside the homestead, suggesting that pigs can be an important reservoir for the spread of swine pathogens such as African swine fever.26 Another study detected a positive association of methicillin-resistant Staphylococcus aureus (MRSA) in veal calves with free-ranging cats and sheep. Similar to our study, these free-ranging domestic animals may function as important drivers for the spread of antimicrobial-resistant bacteria throughout an agricultural setting.27 Moreover, other work has shown that noncommercial poultry operations can increase the likelihood of disease spread between free-ranging poultry and wild avian species.28 Improved understanding of chicken spatial ecology could better inform risk of antimicrobial transmission in surrounding human populations.
We speculate that the ubiquity of backyard chicken husbandry within the study communities (61% of households enrolled) could make them an important reservoir host for the spread of antimicrobial-resistant bacteria. Intensively raised broiler chickens are raised with commercial feed and water containing antibiotics.7 However, they are confined to their coops. By contrast, backyard chickens typically are fed antibiotic-free diets consisting of table scraps and are left to forage in landscapes surrounding households. The free-ranging and self-sufficient practice of backyard chicken husbandry allows them to be widely accessible throughout community environments. At both the household and community scales, our previous studies have found that backyard chickens have greater antimicrobial-resistant bacteria levels when raised with broiler chickens.8–10 Our previous work has detected virginiamycin, chloramphenicol, lincomycin, and tetracycline present in commercial broiler feed.7 Although farmers from the study communities have self-reported antimicrobial application in the water of broilers, our previous study did not detect any statistical difference between broiler chickens reared with and without antimicrobial supplementation in their water.7 This study, therefore, suggests that backyard chickens could account for significant transmission between households and, ultimately, explains the environmental spread of antimicrobial-resistant bacteria initiated through broiler farming. Anecdotally, during our survey, heads of household commonly reported backyard chickens of their neighbors entering their households.
Many risk factors within the community setting could promote the spread of AMR from broiler to backyard chickens. Our previous work has documented that farmers of small-scale intensive operations discard manure into the river or use it as fertilizer on their property7 where it is directly accessible to foraging backyard chickens. We also observed backyard chickens regularly roosting next to broiler chickens, in a proximity that facilitates the spread of AMR. In support of these data, we detected an increase in E. coli phenotypic cephalosporin resistance in backyard chickens after the initiation of broiler chicken farming at the community level.8 These observations support using free-ranging backyard chickens to monitor the resistome of broiler chickens and the overall environment.
Although, E. coli only represents a small proportion of the total mammalian gut microbiota, it is still an informative indicator species for monitoring bacterial resistance in natural environments. This commensal bacterium, as well as other intestinal facultative anaerobes, is likely the most actively transmitted microbes between humans and chickens.29 Furthermore, fecal E. coli is a model microorganism for monitoring AMR determinants in the ecosystem because of their ability to preserve, acquire, and transmit antibiotic-resistant genes in the intestinal microbiota of animals and humans.29–32 Most the microbiota are strict anaerobes that die after contact with air.
Future studies should analyze the change in the entire antibiotic resistome with respect to the distance between small-scale broiler farming operations as an alternative outcome measure. In addition, our exposure measures could be refined. For example, our spatial predictor of the Euclidian distance for determining exposure to antibiotic resistance might have not fully captured realistic pathways in the environment because of various natural habitats (e.g., marsh, secondary forest, agricultural fields, and pastures) interspersed into the village fabric alongside other free-ranging domestic animals. Other factors that are known to affect the presence of antimicrobial-resistant bacteria in children stool samples and which could confound the conclusions of this analysis include nutrition, sanitation systems, and community-acquired resistance (i.e., school or immediate contact networks). Further analysis could include the development of contact network transmission models between broiler chickens and backyard chickens to improve our understanding of the ecological mechanisms facilitating the spread of resistance across the entire village. In tandem, further research on movement and social connectivity of children within the villages could provide a broader context and explain the null result observed in this study.
Analysis of intensive small-scale broiler farming at a fine temporal and spatial scale can provide an improved mechanistic understanding of antimicrobial-resistant bacterial spread, given that we did not detect a spatial relationship between the prevalence of antimicrobial-resistant bacteria and proximity to small-scale broiler farming activity, which suggests widespread community-level transmission within backyard chickens.
Supplemental figures
Acknowledgments:
We are grateful for the invaluable contributions of the Field Coordinator Jorge Mejía Zamora and the entire “Resistencia Zoonotica” field team members: Eduardo Zamora Castillo, Mauricio Ayovi, Leonar Hurtado, Sulay Borja Perlaza, Evelin Melisa Valdéz, Sulay Borja Perlaza, and Eduardo Zamora Castillo. We thank Sanchitha Meda and Eric Krawczyk for laboratory assistance at Michigan State University. We are grateful for statistical analysis assistance from Michael Clark and Manish Verma.
Note: Supplemental figures and table appear at www.ajtmh.org.
REFERENCES
- 1.Lipsitch M, Singer RS, Levin BR, 2002. Antibiotics in agriculture: when is it time to close the barn door? Proc Natl Acad Sci U S A 99: 5752–5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy CP, Carson C, Smith BA, Chapman B, Marrotte J, McCann M, Primeau C, Sharma P, Parmley EJ, 2018. Factors potentially linked with the occurrence of antimicrobial resistance in selected bacteria from cattle, chickens and pigs: a scoping review of publications for use in modelling of antimicrobial resistance (IAM.AMR Project). Zoonoses Public Health 65: 957–971. [DOI] [PubMed] [Google Scholar]
- 3.Gilchrist MJ, Greko C, Wallinga DB, Beran GW, Riley DG, Thorne PS, 2007. The potential role of concentrated animal feeding operations in infectious disease epidemics and antibiotic resistance. Environ Health Perspec 115: 313–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graham JP, Eisenberg JN, Trueba G, Zhang L, Johnson TJ, 2017. Small-scale food animal production and antimicrobial resistance: mountain, molehill, or something in-between? Environ Health Perspec 125: 313–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Boeckel TP, Brower C, Gilbert M, Grenfell BT, Levin SA, Robinson TP, Teillant A, Laxminarayan R, 2015. Global trends in antimicrobial use in food animals. Proc Natl Acad Sci U S A 112: 5649–5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Boeckel TP, Glennon EE, Chen D, Gilbert M, Robinson TP, Grenfell BT, Levin SA, Bonhoeffer S, Laxminarayan R, 2017. Reducing antimicrobial use in food animals. Science 357: 1350–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braykov NP, et al. 2016. Antibiotic resistance in animal and environmental samples associated with small-scale poultry farming in northwestern Ecuador. mSphere 1: e00021-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hedman HD, Eisenberg JNS, Vasco KA, Blair CN, Trueba G, Berrocal VJ, Zhang L, 2019. High prevalence of extended-spectrum beta-lactamase CTX-M–producing Escherichia coli in small-scale poultry farming in rural Ecuador. Am J Trop Med Hyg 100: 374–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moser KA, et al. 2017. The role of mobile genetic elements in the spread of antimicrobial-resistant Escherichia coli from chickens to humans in small-scale production poultry operations in rural Ecuador. Am J Epidemiol 187: 558–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo X, Stedtfeld RD, Hedman H, Eisenberg JNS, Trueba G, Yin D, Tiedje JM, Zhang L, 2018. Antibiotic resistome associated with small-scale poultry production in rural Ecuador. Environ Sci Tech 52: 8165–8172. [DOI] [PubMed] [Google Scholar]
- 11.Singer RS, Ward MP, Maldonado G, 2007. Can landscape ecology untangle the complexity of antibiotic resistance? Nat Rev 4: 943–952. [DOI] [PubMed] [Google Scholar]
- 12.Rooklidge SJ, 2004. Environmental antimicrobial contamination from terraccumulation and diffuse pollution pathways. Sci Total Environ 325: 1–13. [DOI] [PubMed] [Google Scholar]
- 13.Cuong NV, Padungtod P, Thwaites G, Carrique-Mas J, 2018. Antimicrobial usage in animal production: a review of the literature with a focus on low-and middle-income countries. Antibiotics 7: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singer RS, Case JT, Carpenter TE, Walker RL, Hirsh DC, 1998. Assessment of spatial and temporal clustering of ampicillin- and tetracycline-resistant strains of Pasteurella multocida and P. haemolytica isolated from cattle in California. J Am Vet Med Assoc 212: 1001–1005. [PubMed] [Google Scholar]
- 15.Neumann DA, Benenson MW, Hubster E, Tuan TNT, 1972. Cary-Blair, a transport medium for Vibrio parahemolyticus. Am J Clin Pathol 57: 33–34. [DOI] [PubMed] [Google Scholar]
- 16.Drew WL, Barry AL, O’Toole R, Sherris JC, 1972. Reliability of Kirby-Bauer disc diffusion method for detecting methicillin-resistant strains of Staphylococcus aureus. Appl Microbiol 24: 240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clinical and Laboratory Standards Institute , 2009. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, Vol. 28 Approved Standard M31-A4 Wayne, PA: Clinical and Laboratory Standards Institute, 47. [Google Scholar]
- 18.Clinical and Laboratory Standards Institute , 2012. Performance Standards for Antimicrobial Susceptibility Testing. 22nd Informational Supplement. CLSI M100-S22 Wayne, PA: Clinical and Laboratory Standards Institute. [Google Scholar]
- 19.Frontier S, 1976. Study of the decrease of eigenvalues in principal component analysis: comparison with the broken stick codel. J Exp Mar Biol Ecol 25: 67–75. [Google Scholar]
- 20.Worton BJ, 1989. Kernel methods for estimating the utilization distribution in home‐range studies. Ecology 70: 164–168. [Google Scholar]
- 21.Börger L, Franconi N, De Michele G, Gantz A, Meschi F, Manica A, Lovari S, Coulson T, 2006. Effects of sampling regime on the mean and variance of home range size estimates. J Anim Ecol 75: 1393–1405. [DOI] [PubMed] [Google Scholar]
- 22.Fleming CH, Fagan WF, Mueller T, Olson KA, Leimgruber P, Calabrese JM, 2015. Rigorous home range estimation with movement data: a new autocorrelated kernel density estimator. Ecology 96: 1182–1188. [DOI] [PubMed] [Google Scholar]
- 23.Mansfield KL, Hernández-Triana LM, Banyard AC, Fooks AR, Johnson N, 2017. Japanese encephalitis virus infection, diagnosis and control in domestic animals. Vet Microbiol 201: 85–92. [DOI] [PubMed] [Google Scholar]
- 24.Middleton D, et al. 2014. Hendra virus vaccine, a one health approach to protecting horse, human, and environmental health. Emerg Infect Dis 20: 372–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conan A, Goutard FL, Sorn S, Vong S, 2012. Biosecurity measures for backyard poultry in developing countries: a systematic review. BMC Vet Res 8: 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas LF, Glanville WA, Cook EA, Fèvre EM, 2013. The spatial ecology of free-ranging domestic pigs (Sus scrofa) in western Kenya. BMC Vet Res 9: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dorado-García A, Dohmen W, Bos MEH, Verstappen KM, Houben M, Wagenaar JA, Heederik DJJ, 2015. Dose-response relationship between antimicrobial drugs and livestock-associated MRSA in pig farming. Emerg Infect Dis 21: 950–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Jiang Z, Zhenyu J, Tan H, Xu B, 2013. Risk factors for infectious diseases in backyard poultry farms in the Poyang Lake area, China. PLoS One 8: e67366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alonso CA, Zarazaga M, Ben Sallem R, Jouini A, Ben Slama K, Torres C, 2017. Antibiotic resistance in Escherichia coli in husbandry animals: the African perspective. Lett Appl Microbiol 64: 318–334. [DOI] [PubMed] [Google Scholar]
- 30.Moeller AH, Suzuki TA, Phifer-Rixey M, Nachman MW, 2018. Transmission modes of the mammalian gut microbiota. Science 362: 453–457. [DOI] [PubMed] [Google Scholar]
- 31.Kozak GK, Boerlin P, Janecko N, Reid-Smith RJ, Jardine C, 2009. Antimicrobial resistance in Escherichia coli isolates from swine and wild small mammals in the proximity of swine farms and in natural environments in Ontario, Canada. Appl Environ Microbiol 75: 559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katakweba AA, Muhairwa AP, Lupindu AM, Damborg P, Rosenkrantz JT, Minga UM, Mtambo MM, Olsen JE, 2018. First report on a randomized investigation of antimicrobial resistance in fecal indicator bacteria from livestock, poultry, and humans in Tanzania. Microb Drug Res 24: 260–268. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.