Abstract.
Vector control methods that mobilize and impact rapidly during dengue, Zika, and chikungunya outbreaks are urgently needed in urban contexts. We investigated whether one person using a handheld aerosolized insecticide could achieve efficacy levels comparable to targeted indoor residual spraying (TIRS), using pyrethroid-resistant Aedes aegypti in a semi-field setting with experimental houses in Mexico. The insecticide product (H24, a carbamate and pyrethroid mixture), available over-the-counter locally, was sprayed only on known Ae. aegypti–resting surfaces, for example, walls less than 1.5 m and dark hidden areas. In six identical houses with paired bedrooms, one bedroom was treated, and the other remained an untreated control. Each week for 8 weeks, 100 female pyrethroid-resistant Ae. aegypti were released in each bedroom and followed up daily. Mortality rates in treated bedrooms exceeded 90% for at least 2 weeks, and more than 80% (89.2; 95% CI: 79.98–98.35) for 3 weeks or more. Mortality rates in control houses were zero. Results demonstrate that the immediate impact of TIRS can be delivered by one person using existing products, at an estimated cost for the average household in Mexico of under US$3 per month. Triggered by early outbreak signs, dissemination via community hubs and mass/social media of instructions to treat the home immediately, with monthly re-treatment thereafter, provides a simple means to engage and empower householders. Compatible with integrated vector management strategies, it enables self-protection even if existing agencies falter, a situation exemplified by the potential impact on vector control of the restrictions imposed during the 2020 COVID-19 pandemic.
INTRODUCTION
The mosquito Aedes aegypti is the primary vector worldwide of the arboviruses causing dengue, chikungunya, and Zika.1–3 After decades of vector control programs based on larvicide treatment or elimination of breeding sites, and control of adult mosquitoes by outdoor space spraying (e.g., truck-mounted ultralow-volume spraying), the highly endophilic Ae. aegypti adult mosquito population can also now be targeted.4 Indoor residual spraying (IRS) of resting sites of Aedes spp. mosquitoes was recommended by the Vector Control Advisory Group of the WHO5 in 2016, to improve vector control and a primary intervention for immediate response in the context of Zika transmission.5
Typically, IRS aimed at controlling Ae. aegypti is termed targeted indoor residual spraying (TIRS) because it is applied by focusing on the vector’s known intra-domiciliary resting sites. These are interior lower walls (< 1.5 m), surfaces behind/under furniture, inside wardrobes/closets, etc., and other dark or shaded objects/areas in dormitories.6–9 Trials in experimental houses in Mexico have shown that TIRS using an appropriate insecticide achieved and maintained more than 80% mortality for 4 months, but with shorter spraying time and 30% insecticide in comparison with “classic” IRS, where all wall surfaces are treated.6 As a result, the “Manual for IRS in Urban Areas for Aedes aegypti Control” was developed by the Pan American Health Organization to guide institutional implementation of TIRS by vector control programs.10
Previously, the use of handheld commercial aerosolized insecticides (CAIs) applied as a residue by householders has been encouraged as part of strategies to transfer control to communities.11,12 The routine use of household aerosols for mosquito control is common, particularly during outbreaks of dengue, chikungunya, and Zika. In recent studies in the Mexican state of Yucatan, nearly 94% of households reported using insecticide aerosols regularly, 70–90% of which were CAIs, at an estimated annual cost of approximately US$25 per house.13–15 Here, we report an evaluation of the residual efficacy of over-the-counter household insecticide aerosols applied as TIRS against pyrethroid-resistant Ae. aegypti within experimental houses in Merida, Mexico.
MATERIALS AND METHODS
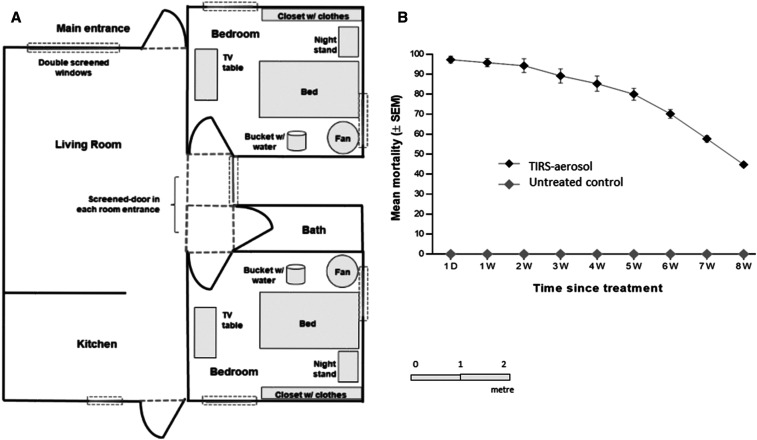
The study was carried out in six identical houses at the Ciudad Caucel experimental house site in Merida, Yucatan, Mexico, between July and September 2017, the rainy season in Yucatán when more than 80% of dengue transmission occurs.16–18 These houses had been used previously for studies on TIRS and Ae. aegypti.6 Each single storey concrete house had two bedrooms, and all 12 bedrooms were fitted with identical furniture and other contents, typifying that seen in local houses (Figure 1A). Detailed specifications of the experimental houses and contents are given in Dunbar.6 To prevent any mosquitoes from escaping from the bedrooms, all windows and doors were screened on both the outside and inside of each dormitory before the study began. In addition, a screened door was built into the main entrance of each bedroom to allow personnel to enter and exit while preventing mosquitoes from escaping (Figure 1). Ant baits (Antex Gel, Allister de México, S.A. de C.V., Guadalajara, Jalisco, México) were placed next to door or at any location where ants might have entered.
Figure 1.
(A) Layout of an experimental house, showing the positions and sizes of furniture in the identical bedrooms. All external and internal windows and doors were screened to prevent mosquito passage. A bucket (0.5 L water) and a 40-cm-diam. oscillating fan stabilized relative humidity and temperature in each bedroom. The interiors of all six experimental houses were identical. (B) Mortality of pyrethroid-resistant Aedes aegypti by targeted indoor residual spraying (TIRS) with a household aerosolized insecticide over time. Symbols denote sample means, and error bars are the standard error of the mean. D = day; W = weeks.
The insecticide aerosol used was H24® Poder Fulminante Ultra Eficaz® (propoxur 4.60 g/kg, tetramethrin 1.03 g/kg, and fenvalerate 4.55 g/kg; Industrias H24 SA de CV, Naucalpan, México), sprayed in 10-second bursts, 30 cm from the treated surface, as instructed by the label. Insecticide was applied uniformly and evenly with horizontal strips with a flow rate for the formulation of 1.9 g/second (SD = 0.15), to deposit an estimated 0.01 g of insecticide per square meter. Application was made by the same individual at each of the experimental houses.
The treatment was randomly allocated to one bedroom inside each experimental house, and the second bedroom was a control (no treatment). The treated surfaces were lower walls (< 1.5 m), curtains, under the bed, on and behind the nightstand and television table, and the closet (Figure 1A). The insecticide was allowed to dry for 24 hours before the first mosquitoes were released.
Adult female Ae. aegypti from a colony of the San Lorenzo strain at UADY (Unidad Colaborativa para Bioensayos Entomológicos, UADY, Merida, Mexico) were used in all tests. This strain was recently colonized from local populations and is resistant to pyrethroids, but fully susceptible to carbamates.19,20
In all bedrooms, 100 sugar-fed female mosquitoes (no blood meal), aged 3–7 days post-eclosion, were released at 24 hours posttreatment, and every week thereafter for 8 weeks, until the average mortality reached 50%. At 24 hours after release, each room was inspected by a team of four field technicians who retrieved any live mosquitoes using a Prokopack aspirator and dead mosquitoes by hand.
For each sampling period, mortality per room was calculated by dividing the number of dead individuals by the number of individuals released. Mean mortality rates between arms were compared using a Student’s t-test, with significance expressed at the 5% level. A cutoff efficacy criterion of 80% mortality at 24 hours was established as the mean value for rejecting or accepting the null hypothesis, following the criteria established for biological efficacy tests by the WHO.21
Ethics statement.
This was an experimental study where laboratory-reared unfed mosquitoes were released into uninhabited houses, and hence did not require an institutional review board approval.
RESULTS
A total of 5,400 Ae. aegypti females per arm were released within the experimental houses during the trial. Almost all released mosquitoes in bedrooms treated with TIRS/CAIs were killed during the first 24 hours post-application for 2 weeks post-application (ranging from 94.3% to 97.3%; Figure 1B, Table 1). At 3 weeks post-application, the mortality had fallen but remained significantly higher than 80% (89.2; 95% CI: 79.98–98.35, t = 2.6, degrees of freedom [df] = 5). By the fourth week, the average mortality was statistically lower than 80% (85.3; 95% CI: 75.59–95.08, t = 1.4, df = 5), and from the fifth week onward, the desired efficacy of 80% was not reached and continued to decrease until week 8, the lowest level recorded (< 50%).
Table 1.
Mean (±standard error of the mean [SEM]) and 95% CIs of 24 hours post-exposition mortality of a pyrethroid-resistant Aedes aegypti strain
| Time since treatment | Mean (±SEM) | 95% CI | P-value |
|---|---|---|---|
| 1 day | 97.3 (±1.71) | 92.95–101.72 | < 0.001* |
| 1 week | 95.8 (±2.07) | 90.51–101.16 | < 0.001* |
| 2 weeks | 94.3 (±3.45) | 85.46–103.20 | 0.004* |
| 3 weeks | 89.2 (±3.57) | 79.98–98.35 | 0.025* |
| 4 weeks | 85.3 (±3.79) | 75.59–95.08 | 0.109 |
| 5 weeks | 80.0 (±2.92) | 72.49–87.51 | 0.500 |
| 6 weeks | 70.2 (±2.24) | 64.40–75.93 | 0.996 |
| 7 weeks | 57.7 (±1.60) | 53.54–61.79 | 1.000 |
| 8 weeks | 44.8 (±1.17) | 41.83–47.83 | 1.000 |
Average mortality is not statistically higher than 80%, t-test for a sample (α = 0.05), n − 1 degrees of freedom (df).
DISCUSSION
Previously, we reported that IRS targeting mosquito resting sites with an appropriate insecticide can impact Ae. aegypti infestation rates and dengue transmission.22 Although it is now a WHO-recommended procedure for control of Aedes spp.,5 with numerous time-saving and cost advantages, TIRS still requires considerable investment to implement and sustain,6,17,20 and is not always accepted by at-risk communities.22,23 Here, we show that inhabitants of those communities could use over-the-counter insecticide aerosols to deliver TIRS within their own homes, with a single treatment providing 80–95% efficacy for 3 or more weeks thereafter. Moreover, with a carbamate-based insecticide mixture effective against the target population,24 this is possible even where the vectors are resistant to pyrethroids.
Our data suggest that householder- or occupant-delivered TIRS could work as a complement to professional TIRS in situations where widespread insecticide coverage and relatively low residual efficacy (∼ 1 month) are acceptable, for example, during dengue, chikungunya, or Zika outbreaks. With some guidance, particularly with non-pyrethroid ingredients, communities could be mobilized to safely apply effective and persistent CAI residues far more rapidly than TIRS, and provide an alternative to interventions that require the entry of ministry of health or independent vector control personnel into households.
The use of CAIs for the control of Ae. aegypti may induce insecticide tolerance in the mosquito populations and select insecticide resistance, mainly for pyrethroids,13 considering that the use of household aerosol insecticides for mosquito control is highly prevalent in the Merida.13,14,25 It is important to consider that the 100% of CAIs available in the local market of Yucatan contain at least one pyrethroid as active ingredient.26 The selection of non–pyrethroid-based formulations is a good option to complement current control strategies, resistance management, and to overtake the negative impact of pyrethroid resistance on the efficacy of pyrethroid-based CAIs.13 Measuring the resistance of local Ae. aegypti populations must be taken into consideration and providing baseline data for program planning and pesticide selection before the start of control strategies. At least for Merida, the complete susceptibility to carbamates in the pyrethroid-resistant local populations is well documented.20,27,28
Whereas further research is merited, recent events have increased the value of self-administered vector control. The 2016 Zika pandemic highlighted the urgent need for simple affordable effective measures, in addition to the use of topical skin or clothing repellents, that those in exposed populations can adopt to protect themselves against Ae. aegypti. That situation is worse in 2020 as many countries in Latin America simultaneously face outbreaks of dengue29 and the COVID-19 pandemic. As the restrictions on human movement imposed to reduce the spread of the COVID-19 are likely to seriously impede dengue vector control, the impact of the dengue outbreak is likely to be higher. Here is an exemplary situation where householder-led TIRS could empower those under threat from dengue, to not only protect their families from vector mosquitoes but also to do so without the additional risk of contracting COVID-19 infection from dengue control staff.
Nearly 94% of households reported using commercial insecticides on a regular basis (Gray et al.13), from which CAIs were the most common (70–90%),14,15 with an estimated annual average expense per house of approximately $570.00 Mexican pesos14,15 indicating an annual market in excess of $75 million Mexican pesos (> $5.7 million USD) for Merida alone, the state capital.25 Regrettably, most householders of Yucatan have, as preferred practice, to spray insecticide in the air and with formulations containing pyrethroids, which are the most common products available in the retail market.13,24 This is a misuse (not so obvious for people) because air spraying of CAIs is poorly effective and does not confer sustainable protection, and it is even less effective when levels of insecticide of resistance to pryrethoids are elevated in local Ae. aegypti populations.27,30
Some householders from Yucatan spray CAIs over specific household surfaces to kill mosquitoes (still formulations containing the pyrethroids cypermethrin, cyfluthrin, and imiprothrin), although such formulations are marketed for ants, scorpions, and cockroaches (Gray et al.13, Dzib-Florez et al.24). A recent study with WHO cones evaluated locally commercially available residual surface sprays and found that the product here evaluated provided residual efficacy ≥ 80% of mortalities for up to 2 months on local pyrethroid-resistant Ae. aegypti populations when applied in different substrates typically present in urban residential premises (Dzib-Florez et al.24). Results of this study demonstrate that the immediate impact of TIRS can be delivered by one person using existing products, at an estimated cost for the average household in Mexico of under US$3 per month. This has the potential to provide a lasting residual effect indoors compatible with the need for rapid and lasting mosquito control during arbovirus outbreaks and may be suitable for community-based TIRS. Triggered by early outbreak signs, dissemination via community hubs and mass/social media of instructions to treat the home immediately, with monthly re-treatment thereafter, provides a simple means to engage and empower householders. Compatible with integrated vector management strategies, it enables self-protection even if existing agencies falter, a situation exemplified by the potential impact on vector control of the restrictions imposed during the 2020 COVID-19 pandemic.
Acknowledgment:
We thank the team at Unidad Colaborativa para Bioensayos Entomologicos, Universidad Autonoma de Yucatan for their assistance.
REFERENCES
- 1.Brady OJ, Hay SI, 2020. The global expansion of dengue: how Aedes aegypti mosquitoes enabled the first pandemic arbovirus. Ann Rev Entomol 65: 191–208. [DOI] [PubMed] [Google Scholar]
- 2.Messina JP, et al. 2019. The current and future global distribution and population at risk of dengue. Nat Microbiol 4: 1508–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weaver SC, Charlier C, Vasilakis N, Lecuit M, 2018. Zika, chikungunya, and other emerging vector-borne viral diseases. Annu Rev Med 69: 395–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson AL, Courtenay O, Kelly-Hope LA, Scott TW, Takken W, Torr SJ, Lindsay SW, 2020. The importance of vector control for the control and elimination of vector-borne diseases. PLoS Negl Trop Dis 14: e0007831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization , 2016. Mosquito (Vector) Control Emergency Response and Preparedness for Zika Virus. Geneva, Switzerland: WHO; Available at: https://www.who.int/neglected_diseases/news/mosquito_vector_control_response/en/. Accessed May 9, 2020. [Google Scholar]
- 6.Dunbar MW, et al. 2019. Efficacy of novel indoor residual spraying methods targeting pyrethroid-resistant Aedes aegypti within experimental houses. PLoS Negl Trop Dis 13: e0007203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dzul-Manzanilla F, Ibarra-Lopez J, Bibiano-Marin W, Martini-Jaimes A, Leyva JT, Correa-Morales F, Huerta H, Manrique-Saide P, Vazquez-Prokopec GM, 2017. Indoor resting behavior of Aedes aegypti (Diptera: Culicidae) in Acapulco, Mexico. J Med Entomol 54: 501–504. [DOI] [PubMed] [Google Scholar]
- 8.Perich MJ, Davila G, Turner A, Garcia A, Nelson M, 2000. Behavior of resting Aedes aegypti (Culicidae: Diptera) and its relation to ultra-low volume adulticide efficacy in Panama city, Panama. J Med Entomol 37: 541–546. [DOI] [PubMed] [Google Scholar]
- 9.Vazquez-Prokopec G, Montgomery MBL, Horne P, Clennon JA, Ritchie SA, 2017. Combining contact tracing with targeted indoor residual spraying significantly reduces dengue transmission. Sci Adv 3: e1602024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan American Health Organization , 2019. Manual for Indoor Residual Spraying in Urban Areas for Aedes aegypti Control. Washington, DC: PAHO; Available at: https://iris.paho.org/handle/10665.2/51637. Accessed May 19, 2020. [Google Scholar]
- 11.Gartner C, Ritchie S, Capra M, 2001. Laboratory evaluation of an aerosol insecticide surface spray against the mosquito Aedes aegypti. J Environ Health 1: 61–66. [Google Scholar]
- 12.Pai HH, Hsu EL, 2014. Effectiveness and acceptance of total release insecticidal aerosol cans as a control measure in reducing dengue vectors. J Environ Health 76: 68–74. [PubMed] [Google Scholar]
- 13.Gray L, Dzib-Florez S, Medina A, Vadillo-Sánchez J, González-Olvera G, Lenhart A, Manrique-Saide P, Vazquez-Prokopec GM, 2018. Experimental evaluation of the impact of household aerosolized insecticides on pyrethroid resistant Aedes aegypti. Sci Rep 8: 125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loroño-Pino MA, et al. 2013. Towards a Casa Segura: a consumer product study of the effect of insecticide-treated curtains on Aedes aegypti and dengue virus infections in the home. Am J Trop Med Hyg 89: 385–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosecrans K, Cruz-Martin G, King A, Dumonteil E, 2014. Opportunities for improved Chagas disease vector control based on knowledge, attitudes and practices of communities in the Yucatan peninsula, Mexico. PLoS Negl Trop Dis 8: e2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hladish TJ, Pearson CA, Chao DL, Rojas DP, Recchia GL, Gomez-Dantes H, Halloran ME, Pulliam JRC, Longini IM, 2016. Projected impact of dengue vaccination in Yucatan, Mexico. PLoS Negl Trop Dis 10: e0004661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hladish TJ, Pearson CAB, Rojas P, Gomez-Dantes H, Halloran ME, Vazquez-Prokopec GM, Logini IM, 2018. Forecasting the effectiveness of indoor residual spraying for reducing dengue burden. PLoS Negl Trop Dis 12: e0006570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bisanzio D, et al. 2018. Spatiotemporal coherence of dengue, chikungunya and Zika outbreaks in Merida, Mexico. PLoS Negl Trop Dis 12: e0006298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deming R, Manrique-Saide P, Medina Barreiro A, Cardeña EUK, Che-Mendoza A, Jones B, Liebman K, Vizcaino L, Vazquez-Prokopec G, Lenhart A, 2016. Spatial variation of insecticide resistance in the dengue vector Aedes aegypti presents unique vector control challenges. Parasites Vectors 9: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vazquez-Prokopec GM, et al. 2017. Deltamethrin resistance in Aedes aegypti results in treatment failure in Merida, Mexico. PLoS Negl Trop Dis 11: e0005656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization , 2006. Guidelines for Testing Mosquito Adulticides for Indoor Residual Spraying and Treatment of Mosquito Nets. Geneva, Switzerland: WHO; Available at: https://apps.who.int/iris/handle/10665/69296. Accessed May 11, 2020. [Google Scholar]
- 22.Vazquez-Prokopec GM, Kitron U, Montgomery B, Horne P, Ritchie SA, 2010. Quantifying the spatial dimension of dengue virus epidemic spread within a tropical urban environment. PLoS Negl Trop Dis 4: e920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paz-Soldán VA, et al. 2018. To spray or not to spray? Understanding participation in an indoor residual spray campaign in Arequipa, Peru. Glob Public Health 13: 65–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dzib-Florez S, et al. 2020. Bio-efficacy of commercially available residual insecticides for the control of Aedes aegypti in Mexico. J Am Mosquito Control Assoc 36: 16–21. [DOI] [PubMed] [Google Scholar]
- 25.Loroño-Pino MA, Chan-Dzul YN, Zapata-Gil R, Carrillo-Solís C, Uitz-Mena A, García-Rejón JE, Keefe TK, Beaty BJ, Eisen L, 2014. Household use of insecticide consumer products in a dengue-endemic area in México. Trop Med Int Health 19: 1267–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Che-Mendoza A, 2016. Evaluation of Impact of Long-Lasting Insecticidal House Screening (LLIS) on Pyrethroid Resistant Population of the Dengue Vector Aedes aegypti in Mexico. PhD Thesis, University of Liverpool, Liverpool, UK. [Google Scholar]
- 27.Kuri-Morales PA, Correa-Morales F, González-Acosta C, Moreno-Garcia M, Santos-Luna R, Román-Pérez S, Salazar-Penagos F, Lombera-González M, Sánchez-Tejeda G, González-Roldán JF, 2018. Insecticide susceptibility status in Mexican populations of Stegomyia aegypti (= Aedes aegypti): a nationwide assessment. Med Vet Entomol 32: 162–174. [DOI] [PubMed] [Google Scholar]
- 28.Saavedra-Rodriguez K, et al. 2015. Local evolution of pyrethroid resistance offsets gene flow among Aedes aegypti collections in Yucatan state, Mexico. Am J Trop Med Hyg 92: 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomson Reuters , 2020. ‘Dengue Kills Too’ - Latin America Faces Two Epidemics at Once. New York: Available at: https://www.reuters.com/article/us-health-coronavirus-latam-dengue-featu/dengue-kills-too-latin-america-faces-two-epidemics-at-once-idUSKBN22O1W2. Accessed May 17, 2020. [Google Scholar]
- 30.Kuri-Morales P, Correa-Morales AF, González-Acosta C, Moreno-Garcia M, Dávalos-Becerril E, Benitez-Alva JI, Peralta-Rodriguez J, Salazar-Bueyes V, González-Roldán JF, 2018. Efficacy of 13 commercial household aerosol insecticides against Aedes aegypti (Diptera: Culicidae) from Morelos, Mexico. J Med Entomol 55: 417–422. [DOI] [PubMed] [Google Scholar]