Abstract.
The number of Asian migrants working in sub-Saharan developing countries like Angola has been increasing. Their malaria risk, prevention, and care-seeking practices have not been characterized. A cross-sectional survey was conducted in 733 Chinese and Southeast Asian migrants in Angola. Respondents were interviewed and provided blood samples. Samples were analyzed to detect Plasmodium antigen and characterize host anti-Plasmodium response. Positive samples were genotyped using the pfs47 marker. Most respondents (72%; 95% CI: 68–75) reported using bed nets, but less than 1% reported using chemoprophylaxis. Depending on the assay, 1–4% of respondents had evidence of active malaria infection. By contrast, 55% (95% CI: 52–59) were seropositive for Plasmodium antibodies. Most infections were Plasmodium falciparum, but infection and/or exposure to Plasmodium vivax and Plasmodium malariae was also detected. Seroprevalence by time in Angola showed most exposure occurred locally. One respondent had sufficiently high parasitemia for pfs47 genotyping, which showed that the infection was likely locally acquired despite recent travel to home country. Asian migrants to Angola are at substantial risk of malaria. Employers should consider enhanced malaria prevention programs, including chemoprophylaxis; embassies should encourage prevention practices. Angolan healthcare workers should be aware of high malaria exposure in Asian migrants.
INTRODUCTION
With 3–4% annual growth forecasted for 2020 and 2021, Africa continues to experience a period of economic expansion.1 Foreign direct investments increased on the continent by 11% in 2018 during a period when investments were decreasing globally. These investments encompass a wide variety of sectors, such as agriculture, construction, and mining, which often rely on work provided by international migrants.2
International migrants are persons outside the state of which they are citizens, including those who intend to move permanently or temporarily.3 Rates of international immigration continue to increase yearly, accounting for 3.5% of the world’s population (approximately 272 million people) in 2019.4 Although some international migration is driven by duress—war, natural disaster, and persecution—the vast majority is related to family, education, or economic opportunity.
In Africa and elsewhere, international migrant workers may encounter numerous infectious risks, including agents for which they are immunologically naive. Migrant workers may not be aware of local diseases or have access to appropriate health care or prevention methods in their new setting. Work environment—whether a mosquito-infested construction site, cramped factory, or urban market—may introduce additional transmission risk. Furthermore, migrant workers may not possess the immunologic status, protective strategies, or financial means to mitigate those threats. In addition to danger to themselves from endemic diseases, migrant workers may also introduce new infectious diseases to their new community—or take back new diseases to their country of origin.
History demonstrates this is more than a theoretical concern. A Chinese study from 2014 to 2016 showed that migrant workers returning from abroad were diagnosed with higher rates of tuberculosis, hepatitis A, hepatitis C, HIV, syphilis, and yellow fever than other returning travelers.5 Emerging vector-borne diseases such as Zika virus and Rift Valley fever virus have been introduced to non-endemic regions by migrant workers.6,7 The role of migrant workers in the spread of multidrug-resistant tuberculosis is also well established.8,9
Intracontinental spread of chloroquine-resistant Plasmodium falciparum malaria in the mid-twentieth century has been linked to foreign laborers in western Cambodian gem mines, where permissive mosquito-breeding conditions combined with highly mobile and naive populations amplified the epidemic throughout Asia.10 Resistance to chloroquine, and also sulfadoxine–pyrimethamine, later spread to sub-Saharan Africa, resulting in considerable malaria morbidity and mortality.11,12
Today, migrant worker introduction of malaria to non-endemic areas and antimalarial resistance to areas without resistance remain a persistent risk. Although endemic malaria reached historic lows in China over the last decade, a large proportion of malaria morbidity and mortality in the country is now attributable to migrant workers returning to China from Africa.13,14 Mutations associated with antimalarial resistance have been noted in this population.15,16 In Venezuela, a shattered economy and skyrocketing malaria rates combined with workers leaving to find work abroad threaten malaria control gains in the region.17 Sri Lanka, a country which was certified by the WHO as malaria free in 2016, reported a case of Plasmodium vivax infection in an Indian migrant worker and subsequently in a local resident in 2018.18 In Nigeria, a cluster of malaria cases in Thai migrant mine workers revealed that none had received proper malaria prophylaxis or prevention strategies,19 an issue also noted in other studies.20,21
Sub-Saharan Africa is the destination for approximately 23.6 million international migrants, with South Africa, Ivory Coast, and Uganda being the top three country destinations.4 In 2019, Angola was estimated to have 669 thousand international migrants, twice as many compared with 2010 and more than 10 times the total in 2005.22 Despite the increasing numbers of migrants, many of whom come to Angola from Asia to work, including in large resource extraction and infrastructure projects, scant malaria prevention and care-seeking data exist for this population. In practical terms, the Angolan National Malaria Control Program (NMCP) possesses limited information for a substantial, concentrated, nonimmune subgroup of the population that is at high risk for importing and spreading resistant malaria parasites. This study was undertaken to better understand the risk factors, prevalence, and types of malaria in this unique population, with the aim to enable the Angolan NMCP—as well as other African NMCPs with comparable workforces—to optimize malaria control strategies for international migrant workers.
METHODS
A cross-sectional survey of Asian migrants in Angola was conducted to characterize malaria prevention practices, malaria knowledge and care-seeking behavior, prevalence of malaria infection, and past history of malaria exposure.
Study population.
The target population comprised adult (aged ≥ 18 years) Asian migrants living in Angola at the time of the survey. Sampling was stratified into Chinese and Southeast Asians. Data collection took place consecutively in three purposefully selected provinces in Angola during the dry season: Luanda (May–June 2019), Lunda Sul (June 2019), and Zaire (July–September 2019). Luanda Province is largely urban and comprises the areas in and around the eponymous capital city, with generally low malaria transmission. Lunda Sul is a high-transmission province in the east of the country bordering the Democratic Republic of Congo (DRC) and Zambia, and a site of multiple large infrastructure and mineral extraction projects. Zaire is a high-transmission province in the north of the country also bordering the DRC and is the site of a large petroleum extraction industry.
Participants were conveniently sampled using a variety of channels: companies including mining and petroleum sites known to hire Asian workers were requested to provide contacts for workers; the Angolan Ministry of Foreign Affairs and the Chinese, Vietnamese, and Filipino embassies in Angola provided additional contacts; social media were used to post recruitment messages; and respondents also nominated contacts for potential inclusion in the survey. The target was to identify 150 Chinese and 150 Southeast Asians in each province, for a total target sample size of 300 individuals per province. This was powered to yield a precision of at most ±8 percentage-points per site for outcomes on malaria exposure and prevention.
Data collection.
Trained interviewers administered standardized questionnaires using survey forms programmed onto Open Data Kit (ODK)-based tablet software (KoBoToolbox, Harvard Humanitarian Initiative, Cambridge, MA). Interpreters were used for interviews with respondents preferring a language other than Portuguese. Surveyors collected data on demographic characteristics, history of travel, malaria knowledge, malaria prevention practices, care-seeking behaviors for febrile illness, and past history of malaria infection. Following administration of the questionnaire, interviewers performed a CareStart combination P. falciparum/P. vivax rapid diagnostic test (RDT) (Access Bio, Somerset, NJ) for all consenting participants. Subsequently, interviewers collected up to five drops of blood on Whatman 903 filter paper (GE Healthcare, Chicago, IL), which were dried overnight and subsequently packaged in individual Ziploc bags with desiccant. Any survey respondents testing positive by RDT were treated in accordance with Angolan national treatment guidelines.
Laboratory analysis.
Dried blood spots were stored at room temperature and shipped to CDC laboratories in Atlanta, GA. A 6-mm dried blood spot punch was eluted in buffer as described previously,23 stored at 4°C, and later consecutively assayed for the presence of malaria antigens and IgG antibodies to 24 malaria antigens using a bead-based multiplex immunoassay.24 Presence and concentration of P. falciparum–specific HRP2, pan-Plasmodium aldolase (pAldo), pan-Plasmodium lactate dehydrogenase (LDH), and P. vivax–specific LDH were assayed using previously described protocols.23,25 Next, using the same eluate, antibody response to a panel of malaria antigens was measured.26 The panel included 21 P. falciparum antigens, two P. vivax antigens, and one Plasmodium malariae antigen (Supplemental Table S1). Samples with antigen (positive RDT result or any Plasmodium antigen detected in the laboratory) or antibody response (median fluorescence intensity - background [MFI-bg] ≥ 5,000 for any antigen and seropositivity to ≥ 5 antigens) indicative of active Plasmodium infection were then screened with genus- and species-specific photo-induced electron transfer (PET)-PCR.26 Samples with sufficient Plasmodium DNA were then sequenced using a previously described next-generation sequencing targeted amplicon sequencing workflow for a panel of resistance markers,27 modified to also include pfs47, a marker of P. falciparum geographic population structure.28 To provide a background of the pfs47 gene in Angolan samples, 59 samples from the 2019 Angola therapeutic efficacy study in Zaire, Lunda Sul, and Benguela provinces were sequenced.
Data analysis.
Demographic characteristics, reported knowledge and use of malaria prevention practices, and healthcare-seeking practices were recorded and tabulated, stratifying by province for the primary analysis. Further sub-stratifications by country of origin were also calculated and are presented in the Supplemental Tables.
Curves representing the association between seropositivity and Plasmodium antigens by total time spent in Angola were plotted. Overall seropositivity to the antigens included in the antibody assay was calculated individually for all antigens, and the absolute response, in mean fluorescence intensity-background units, was plotted for positive samples. Breadth of response for P. falciparum, defined as the total number of P. falciparum antigens for which an antibody was detected, was calculated for each sample, and its empiric distribution was characterized stratifying by province. The origin of parasites from P. falciparum DNA + samples was inferred from phylogenetic analysis using publicly available pfs47 sequences from Asia, Latin America, Africa, and the background 2019 Angolan samples newly sequenced for this study. A minimum spanning haplotype network29 was built to show the relatedness between the pfs47 sequences.
A logistic regression model was used to assess the association between respondent characteristics and RDT, antigen, and antibody positivity. Analysis was performed in R version 3.6.0 (R Foundation for Statistical Computing, Austria, Vienna).
Ethical considerations.
Respondents provided written informed consent before participation in the study. The study was reviewed and approved by human subjects review boards at the CDC (protocol number CGH2018469) and the Angola Ministry of Health.
RESULTS
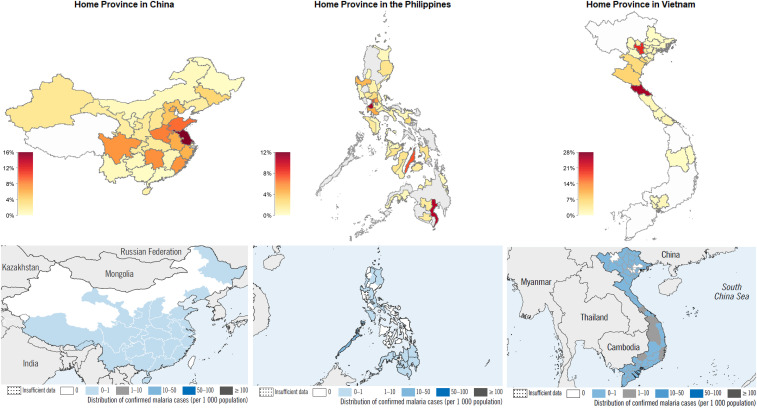
A total of 733 Asian migrants were enrolled, including 341 Chinese and 392 Southeast Asian (188 Filipinos and 204 Vietnamese). They were predominantly male (81%) and had a median age of 40 years (interquartile range [IQR]: 32–49 years) (Table 1). Although a majority (81%) reported being married, only 32% were accompanied by a family member in Angola. Most (81%) reported speaking at least some Portuguese. Construction or machine operation was the most common occupation (39%), followed by retail or wholesale (33%). Most participants had been in Angola for several years, with a median 5 (IQR: 2–8) years of the total time spent in Angola. Participants’ home provinces in their place of origin were widely dispersed and included areas historically endemic for malaria (Figure 1). The most common home provinces were Jiangsu in China (16%, 53/341), Ha Tinh in Vietnam (27%, 56/204), and Cavite in the Philippines (11%, 21/188).
Table 1.
Demographic characteristics of surveyed Asian migrants in Angola, 2019
| Luanda | Lunda Sul | Zaire | Total (N = 733) | ||||
|---|---|---|---|---|---|---|---|
| Chinese (N = 155) | Southeast Asian (N = 144) | Chinese (N = 90) | Southeast Asian (N = 219) | Chinese (N = 96) | Southeast Asian (N = 29) | ||
| Male | 124 (80) | 88 (61) | 80 (89) | 191 (87) | 86 (90) | 24 (83) | 593 (81) |
| Age (years), median (IQR) | 39 (32–49) | 40 (33–50) | 46 (39–52) | 37 (31–46) | 42 (33–49) | 34 (27–43) | 40 (32–49) |
| Married | 120 (77) | 114 (79) | 82 (91) | 175 (80) | 82 (85) | 23 (79) | 596 (81) |
| Accompanied by family member | 34 (22) | 55 (38) | 27 (30) | 71 (32) | 26 (27) | 18 (62) | 231 (32) |
| Speaks Portuguese* | 94 (61) | 135 (94) | 66 (73) | 211 (96) | 64 (67) | 25 (86) | 595 (81) |
| Employment status | |||||||
| Employed | 115 (74) | 104 (72) | 77 (86) | 192 (88) | 89 (93) | 26 (90) | 603 (82) |
| Self-employed/family employed | 38 (25) | 34 (24) | 13 (14) | 23 (11) | 5 (5) | 3 (10) | 116 (16) |
| Unemployed/other | 2 (1) | 6 (4) | 0 (0) | 4 (2) | 2 (2) | 0 (0) | 14 (2) |
| Occupation | |||||||
| Construction/machine operation | 41 (26) | 32 (22) | 56 (62) | 94 (43) | 53 (55) | 8 (28) | 284 (39) |
| Retail/wholesale | 66 (43) | 61 (42) | 24 (27) | 68 (31) | 11 (11) | 11 (38) | 241 (33) |
| Administration/finance | 8 (5) | 23 (16) | 2 (2) | 10 (5) | 2 (2) | 4 (14) | 49 (7) |
| Restaurant | 19 (12) | 12 (8) | 1 (1) | 2 (0.9) | 5 (5) | 1 (3) | 40 (5) |
| Logistics/transport | 5 (3) | 2 (1) | 4 (4) | 15 (7) | 11 (11) | 0 (0) | 37 (5) |
| Mining | 1 (0.6) | 0 (0) | 1 (1) | 22 (10) | 1 (1) | 0 (0) | 25 (3) |
| Agriculture | 2 (1) | 1 (0.7) | 2 (2) | 0 (0) | 1 (1) | 0 (0) | 6 (0.8) |
| Other/missing | 13 (8) | 13 (9) | 0 (0) | 8 (4) | 12 (12) | 5 (17) | 51 (7) |
| Total years spent in Angola, median (IQR) | 3 (1–7) | 7 (4–10) | 5 (2–7) | 6 (3–8) | 4 (2–6) | 5 (2–9) | 5 (2–8) |
IQR = interquartile range. Cells represent n (%) unless otherwise specified.
Some or well.
Figure 1.
Home province in country of origin for surveyed Asian migrants in Angola (top panels). Malaria incidence by province, WHO estimates 2011 (bottom panels). This figure appears in color at www.ajtmh.org.
Travel to other countries in Africa was rarely reported; Ethiopia was the most commonly visited country on the continent, but only 1.4% of participants reported travel there (Supplemental Figure S1A). By contrast, travel outside the province of residence in Angola was more common (29%, 210/733) and widely distributed across all of Angola’s provinces (Supplemental Figure S1B). Travel back to home country was also common, with 395/733 (54%) respondents traveling to their home country at least once a year. This figure was higher in Southeast Asians (61%) than in Chinese (46%) (chi-square P-value < 0.01).
More than 90% of respondents reported knowing that malaria existed in Angola, and 73% felt at risk of acquiring malaria in Angola (Table 2). A large majority, 86%, knew malaria is transmitted by mosquitoes. Less than half had heard a communication message about malaria in the preceding 6 months, and friends and family were the most common source of information on malaria. Those reporting speaking at least some Portuguese were more likely to report having heard a communication message about malaria (chi-square P-value < 0.05). Most respondents (70%) had received some information about malaria from their employer, and more than half (54%) had received information on chemoprophylaxis from their employer.
Table 2.
Malaria knowledge and prevention practices reported by Asian migrants surveyed in Angola, 2019
| n | N | % (95% CI) | |
|---|---|---|---|
| Knows malaria exists in Angola | 649 | 716 | 91 (88–93) |
| Feels at risk of malaria | 521 | 714 | 73 (70–76) |
| Knows malaria spread by mosquitoes | 626 | 730 | 86 (83–88) |
| Heard communication about malaria in last 6 months | 326 | 703 | 46 (43–50) |
| Source of information on malaria | |||
| Friends/family | 196 | 326 | 60 (55–65) |
| TV/radio | 114 | 326 | 35 (30–40) |
| Poster/leaflet | 65 | 326 | 20 (16–25) |
| Employer | 109 | 326 | 33 (28–39) |
| Other | 125 | 326 | 38 (33–44) |
| Received information about malaria from employer | 453 | 647 | 70 (66–73) |
| Received information about chemoprophylaxis from employer | 340 | 632 | 54 (50–58) |
| Uses bed net | 526 | 733 | 72 (68–75) |
| Long-lasting insecticide treated net | 412 | 526 | 78 (75–82) |
| Source of net | |||
| Brought net from home | 245 | 526 | 47 (42–51) |
| Bought net in Angola | 187 | 526 | 36 (31–40) |
| Other | 94 | 526 | 18 (15–21) |
| Slept under net last night | 508 | 526 | 97 (95–98) |
| Travels with bed net | 121 | 372 | 33 (28–38) |
| Uses other forms of malaria prevention | |||
| Insecticide spray | 380 | 733 | 52 (48–56) |
| Fan/air conditioning | 292 | 733 | 40 (36–43) |
| Repellent | 237 | 733 | 32 (29–36) |
| Coil | 85 | 733 | 12 (9–14) |
| Screens | 59 | 733 | 8 (6–10) |
| Indoor residual spraying | 46 | 733 | 6 (5–8) |
| Electric fly swatter | 8 | 733 | 1 (0.5–2) |
| Long-sleeved clothing/socks | 5 | 733 | 0.7 (0.3–2) |
| Chemoprophylaxis* | 3 | 733 | 0.4 (0.1–1) |
| No malaria prevention reported | 4 | 733 | 0.5 (0.2–1) |
Two do not know and one artemisinin.
Despite the high rates of knowledge on chemoprophylaxis, chemoprophylaxis use was very rarely reported by participants (3/733, 0.4%) (Table 2, Supplemental Table S2). Bed nets were the most commonly used form of malaria prevention, reported by 72% of participants. Most of the nets (78%) were long-lasting insecticidal nets, and a plurality (47%) had been brought from participant home country. Nearly all (97%) respondents using a bed net reported using it the night before. However, only 33% reported bringing a bed net when travelling to other provinces in Angola. Other common forms of malaria prevention included use of insecticide sprays (52%), fans or air conditioning (40%), and insect repellent (32%). Overall, only 4 (0.5%) participants reported not practicing any form of malaria prevention.
A total of 53 respondents reported having had a febrile illness in the 3 months preceding the survey (Table 3, Supplemental Table S3). Of these, 14 (26%) self-medicated, whereas 34 (64%) sought health care, most commonly (62%) in the private healthcare sector. Thirty-three (62%) of the 53 respondents with febrile illness reported being tested for malaria. Twenty-six (49%) ultimately took an antimalarial drug to treat the fever; most of these (73%) had reported having a positive test. Of the 22 recalling the kind of antimalarial, only 6 (27%) had taken an artemisinin-based combination therapy (ACT).
Table 3.
Healthcare-seeking practices for febrile illness reported by Asian migrants surveyed in Angola, 2019
| n | N | % (95% CI) | |
|---|---|---|---|
| Had fever in last 3 months | 53 | 728 | 7 (6–9) |
| Self-medicated | 14 | 53 | 26 (16–41) |
| Sought health care | 34 | 53 | 64 (50–77) |
| Setting for healthcare seeking | |||
| Public health facility | 10 | 34 | 29 (16–48) |
| Private health facility/pharmacy | 21 | 34 | 62 (44–77) |
| Employer health facility | 3 | 34 | 9 (2–25) |
| Tested for malaria | 33 | 53 | 62 (48–75) |
| Took antimalarial drug | 26 | 53 | 49 (35–63) |
| Following positive test | 19 | 26 | 73 (52–88) |
| Antimalarial drug taken | |||
| Artemisinin-based combination therapy | 6 | 22 | 27 (12–50) |
| Other | 16 | 22 | 73 (50–88) |
Prevalence of malaria infection at the time of the survey was low. A total of 17 respondents (2%) were RDT positive, including 11 P. falciparum–only, four P. vivax–only, and two mixed P. falciparum/P. vivax infections (Table 4, Supplemental Table S4). The bead-based laboratory assay detected Plasmodium antigen in 26 (4%) individuals, including 19 individuals with antigen profile consistent with active or recent P. falciparum monoinfection, three mixed P. falciparum/P. vivax infections, and four non–falciparum and non–vivax Plasmodium infections (Supplemental Table S5).
Table 4.
Prevalence of current or past malaria infection in Asian migrants surveyed in Angola, 2019
| n | N | % (95% CI) | |
|---|---|---|---|
| Current/recent infection | |||
| Rapid diagnostic test at time of survey | |||
| Any Plasmodium+ | 17 | 725 | 2 (1–4) |
| Pf+ | 11 | 725 | 2 (0.8–3) |
| Pv+ | 4 | 725 | 0.6 (0.2–2) |
| Pf+/Pv+ | 2 | 725 | 0.3 (0.05–1) |
| Negative | 708 | 725 | 98 (96–99) |
| Laboratory antigen detection | |||
| Any Plasmodium+ | 26 | 731 | 4 (2–5) |
| Pf+ | 19 | 731 | 3 (2–4) |
| Pv+ | 0 | 731 | 0 (0–0.7) |
| Pf+/Pv+ | 3 | 731 | 0.4 (0.1–1) |
| Pm + or Plasmodium ovale+ | 4 | 731 | 0.5 (0.2–1) |
| Negative | 705 | 731 | 96 (95–98) |
| Past infection | |||
| Self-reported past malaria infection | 311 | 733 | 42 (39–46) |
| In home country | 44 | 733 | 6 (4–8) |
| In Angola | 296 | 732 | 40 (37–44) |
| Laboratory antibody detection | |||
| Any Plasmodium+ | 396 | 714 | 55 (52–59) |
| Pf+ | 355 | 714 | 50 (46–53) |
| Pv+ | 8 | 714 | 1 (0.5–2) |
| Pf+/Pv+ | 30 | 714 | 4 (3–6) |
| Pm+ | 3 | 714 | 0.4 (0.1–1) |
| Negative | 318 | 714 | 45 (41–48) |
Pf = Plasmodium falciparum; Pm = Plasmodium malariae; Pv = Plasmodium vivax.
Many participants had evidence of past exposure to Plasmodium infection. A total of 396 (55%) had the presence of any antibodies to the panel of Plasmodium antigens, with 355 (50%) seropositive to P. falciparum antigens only, 8 (1%) to P. vivax antigens only, 30 (4%) to both P. falciparum and P. vivax antigens, and 3 (0.4%) to P. malariae antigens only.
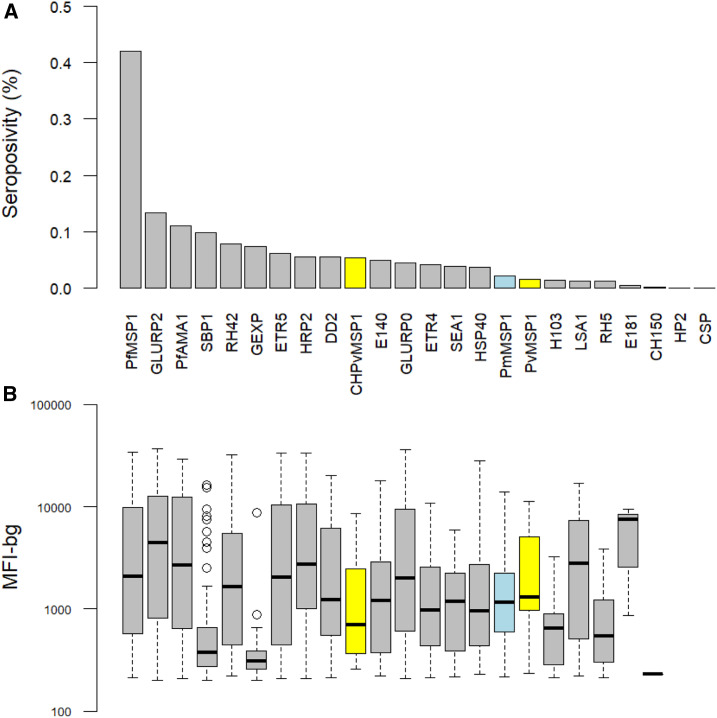
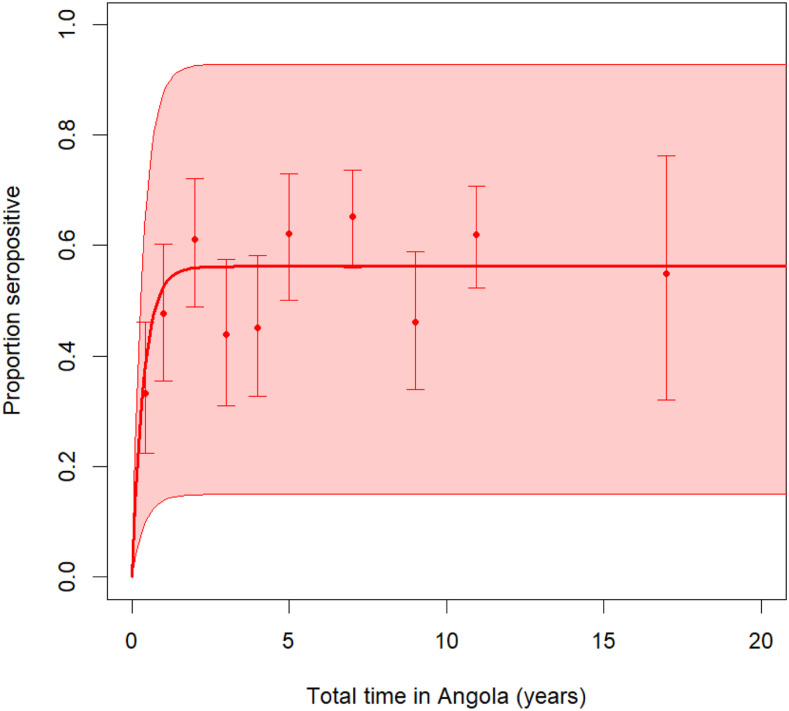
Seropositivity for antibodies to P. falciparum merozoite surface protein 1 (PfMSP1) was the most common (42%), followed by glutamate-rich protein R2 (GLURP2) (13%) and P. falciparum apical membrane antigen 1 (PfAMA1) (11%) (Figure 2A). Measured antibody responses were high in those who were seropositive, with median MFI-bg values greater than 1,000 for most antigens (Figure 2B). Breadth of response (seropositivity to multiple P. falciparum antigens) was highest in Lunda Sul and Zaire and lowest in Luanda, a trend also reflected in rates of seroprevalence (Supplemental Figures S2 and S3). Antibody seropositivity sharply increased with time spent in Angola before reaching a plateau around 5 years of time spent in Angola (Figure 3).
Figure 2.
Prevalence of antibody seropositivity (A) and strength of antibody response in those positive (B) using a panel of Plasmodium antigens for Asian migrants to Angola, 2019. MFI-bg: mean fluorescence intensity-background. This figure appears in color at www.ajtmh.org.
Figure 3.
Seropositivity to any Plasmodium falciparum antigen vs. time spent in Angola in Asian migrants sampled in Angola, 2019. This figure appears in color at www.ajtmh.org.
Overall, rates of serological evidence of past exposure matched responses during the questionnaire, in which 311 (42%) of respondents reported having past infection, primarily incurred while in Angola. Respondents who reported having past infection had statistically higher rates of seropositivity for many antigens (Supplemental Table S6).
Individuals with other or missing occupation had higher odds of testing RDT positive than those working in construction or machine operation jobs as the reference group (odds ratio [OR]: 6.76, P-value < 0.01) (Table 5). Individuals working in administration or office jobs had lower odds of serological evidence of past Plasmodium (OR: 0.44, P-value 0.02). Participants from Lunda Sul had higher odds (OR: 1.87, P-value < 0.01) of antibody seropositivity than those in Luanda. The total time spent in Angola (OR: 1.06/year, P-value < 0.01) and bed net use (OR: 2.02, P-value < 0.01) were also associated with higher risks of antibody positivity.
Table 5.
Risk factors for current or past Plasmodium infection in Asian migrants to Angola, 2019
| Rapid diagnostic test+ | Antigen+ | Antibody+ | ||||
|---|---|---|---|---|---|---|
| OR | P-value | OR | P-value | OR | P-value | |
| Age (years) | ||||||
| < 30 | Ref | – | Ref | – | Ref | – |
| 30–39 | 1.12 | 0.91 | 1.30 | 0.72 | 0.71 | 0.16 |
| 40–49 | 1.42 | 0.70 | 1.50 | 0.59 | 0.74 | 0.25 |
| 50–59 | 0.63 | 0.68 | 2.12 | 0.34 | 0.82 | 0.49 |
| 60+ | 1.12 | 0.94 | 0.00 | 0.99 | 0.65 | 0.35 |
| Gender | ||||||
| Male | Ref | – | Ref | – | Ref | – |
| Female | 0.00 | 0.99 | 1.47 | 0.53 | 0.68 | 0.10 |
| Province | ||||||
| Luanda | Ref | – | Ref | – | Ref | – |
| Lunda Sul | 0.49 | 0.35 | 1.04 | 0.94 | 1.87 | < 0.01 |
| Zaire | 1.91 | 0.33 | 2.57 | 0.12 | 1.04 | 0.88 |
| Total years spent in Angola | 1.06 | 0.31 | 1.01 | 0.85 | 1.06 | < 0.01 |
| Home country | ||||||
| China | Ref | – | Ref | – | Ref | – |
| Southeast Asia | 1.74 | 0.39 | 2.46 | 0.07 | 0.75 | 0.12 |
| Occupation | ||||||
| Construction/machine operation | Ref | – | Ref | – | Ref | – |
| Retail/wholesale | 0.46 | 0.36 | 0.62 | 0.39 | 0.66 | 0.04 |
| Administration/finance | 0.00 | 0.99 | 0.84 | 0.84 | 0.44 | 0.02 |
| Restaurant | 3.97 | 0.14 | 0.59 | 0.65 | 0.95 | 0.90 |
| Logistics/transport | 2.22 | 0.35 | 1.69 | 0.44 | 1.01 | 0.99 |
| Mining | 0.00 | 1.00 | 0.00 | 0.99 | 0.52 | 0.14 |
| Agriculture | 0.00 | 1.00 | 0.00 | 1.00 | 0.37 | 0.27 |
| Other/missing | 6.76 | < 0.01 | 0.41 | 0.43 | 1.25 | 0.56 |
| Uses bed net | 1.20 | 0.84 | 1.30 | 0.70 | 2.02 | < 0.01 |
| Uses other form of malaria prevention | 1.02 | 0.99 | 0.92 | 0.91 | 0.67 | 0.14 |
OR = odds ratio. Bold values represents significant associations (P-value < 0.01).
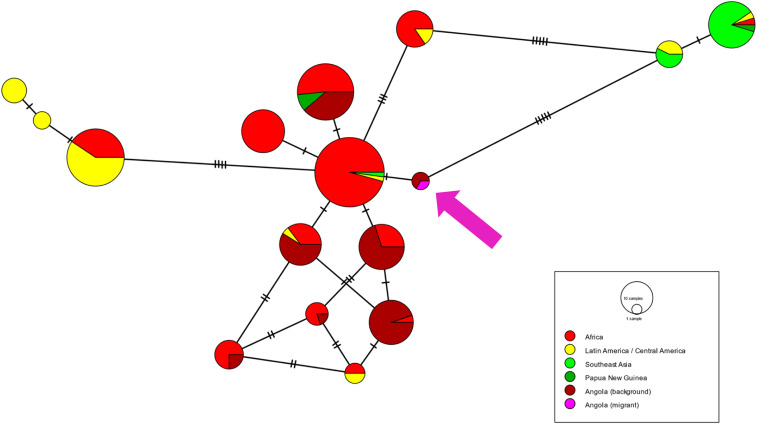
Samples from 70 participants who were positive by RDT, positive for any antigen in the bead-based antigen assay, or with antibody response indicative of recent infection were screened for Plasmodium DNA by PET-PCR. Of these, 7 (10% of PET-PCR-tested samples and 1% of all samples) were malaria DNA positive (three P. falciparum, one P. falciparum/Plasmodium ovale, one P. vivax, one P. malariae, and one Plasmodium genus). Of these seven, one was successfully sequenced using the targeted amplicon sequencing panel. The sample was from a P. falciparum infection in an RDT + Filipino male from Luanda, who had reported last being in the Philippines in April 2019. The parasite, however, was wild type for the artemisinin resistance marker pfk13. Its pfs47 sequence had not been previously reported but was identical to sequences from two samples from children enrolled in the Zaire arms of the 2019 therapeutic efficacy study in Angola and clustered together with other African isolates (Figure 4).
Figure 4.
Minimum spanning haplotype network showing relatedness between global pfs47 sequences. Purple arrow indicates location of sample from Filipino migrant in Angola. Dashes on lines represent number of nucleotide differences between haplotypes.
DISCUSSION
In this survey of Asian migrant workers residing in Angola, respondents had low rates of active infection, between 1% and 4% depending on the assay, as expected in asymptomatic populations sampled during the dry season. However, reported past histories of malaria infection and measured antibody responses suggest substantial exposure to malaria infection in respondents, with roughly half showing serological evidence of past malaria infection. Predominance of P. falciparum seropositivity, higher rates of reported malaria infection in Angola rather than in home country, and an association between seropositivity and time spent in Angola all point to exposure in Angola as the primary source for malaria infection in this population.
The rapid increase in seroprevalence followed by a plateau around 5 years spent in Angola is consistent with heterogeneity of risk, where naive individuals in high exposure settings rapidly acquire antibodies shortly after arrival, whereas another subset never acquires antibodies, even after decades spent in Angola because of low levels of exposure. Risk factor analysis revealed that respondents working in office jobs were less likely to have acquired antibodies to malaria than those in the construction or machine operation sector. Exposure was higher in respondents living in the high-transmission interior provinces than in those in Luanda. This differential in risk was reflected both in overall rates of seropositivity to any Plasmodium antigen and higher rates of seropositivity to individual antigens, as well as the breadth/robustness of observed responses. However, even in Luanda, an urban setting with historically low rates of malaria transmission,30 39% of respondents had serological evidence of past malaria infection, possibly tied to high rates of reported travel to the interior provinces. Findings of high rates of seropositivity match a previous study of Brazilian soldiers deployed to Angola for 6 months during the high-transmission season that found 35% of the returning soldiers had elevated anti–P. falciparum IgG titers.31
Chemoprophylaxis is a mainstay of malaria prevention in Western travelers to endemic areas. Even though more than half of respondents reported receiving information on malaria chemoprophylaxis from their employers, less than 1% of respondents reported using chemoprophylaxis. Instead, nearly three-quarters of respondents reported using bed nets for malaria prevention, and nearly all of these reported using a bed net the night preceding the interview. Bed net use was associated with higher odds of antibody positivity, possibly reflecting better adherence to malaria prevention practices in individuals with past experience with malaria disease and higher perceived risk or those living and working in environments with higher mosquito densities. Overall, there was a wide range of malaria prevention practices reported by respondents, and nearly all respondents reported using at least one form of malaria prevention. Respondents with febrile illness in the preceding 3 months were 2.5 times more likely to seek care in the private sector or through their employer than in the public health sector; in comparison, this ratio was reversed for febrile illness in children in the most recent nationwide household survey in Angola, where respondents were 7.5 times more likely to use the public sector.32 Among Asian migrants who took an antimalarial, only a minority (27%) took an ACT. By contrast, in the nationwide Angola household survey, 77% of those receiving any antimalarial took an ACT, and health facility surveys in Angola have shown that prescription of antimalarials other than ACTs is rare in the public health sector.33
Artemisinin resistance is currently widespread in Southeast Asia,34 whereas P. falciparum parasites in Africa are still largely susceptible to ACTs.35 Importation of artemisinin-resistant parasites into Africa from Asia, similar to the case of chloroquine and sulfadoxine–pyrimethamine, would have drastic consequences on malaria morbidity and mortality on the continent, which accounts for the vast majority of the global malaria burden. The risk of this importation was drawn into focus with the case report of a Vietnamese patient from Lunda Sul, with P. falciparum infection that did not respond to artemisinins.36 Although the cause of the observed nonresponse was never fully resolved,37,38 Lunda Sul was added to the list of therapeutic efficacy monitoring sites in Angola. Notably, none of Angola’s routine biennial therapeutic efficacy studies39–41 or nonroutine efficacy studies42 have shown genotypic or phenotypic evidence of artemisinin resistance. However, Asian migrants surveyed here came from a diverse group of geographic locations in their country of origin, including malaria-endemic areas. Combined with frequent travel back to home country, with more than half reporting visiting their home country at least once a year, importation of artemisinin-resistant P. falciparum to Angola remains a risk. The one participant in this current study who had an infection with sufficiently high parasite density to allow genotyping of the parasite had travelled back to the Philippines. However, analysis of the parasite’s pfs47 sequence showed evidence that this infection was likely acquired in Angola, underlining the utility of novel genotyping approaches to characterize parasite origin.
Unlike P. falciparum, P. vivax can relapse years after the initial infection. Thus, it is difficult to distinguish locally acquired infections from relapse of infections acquired in the home country. None of the respondents with evidence of P. vivax infection carried enough parasite DNA to allow molecular confirmation of origin. Parasite densities in persons with asymptomatic infections are generally low in highly endemic areas, often hindering amplification of enough DNA to genotype infections, either for origin or molecular markers of resistance. In cross-sectional surveys targeting largely asymptomatic populations, other measures of malaria risk, such as serological assays using panels with multiple antigens,43 can be more informative than routinely used smear microscopy and RDTs.
Respondents to the survey represented a convenience sample, and thus the results cannot be extrapolated to the entire Asian migrant population in Angola, including migrants from countries other than the three included here. Moreover, migrants without legal immigration status and those in remote areas in the interior of the country were likely under-sampled. The lack of a P. ovale antigen in the panel meant that exposure to this species, prevalent in Angola, was not able to be measured. A cross-sectional study design limited the ability to directly measure malaria incidence, with point prevalence of infection and serology used as indirect measures of incidence. Recall bias could have affected the validity of the reported rate of malaria prevention and healthcare-seeking behaviors. However, this survey was implemented because routine surveillance in this population is made complicated by the same factors. Low rates of utilization of the public health sector mean that the true burden of malaria morbidity in this population is not readily accessible through routine health information systems.
Further elucidation of malaria burden in Asian migrants, identification of risk of importation, and its contribution to the overall malaria situation in Angola will require targeted surveillance activities and engagement with the private healthcare sector. Management of malaria risk in this diverse and dispersed population will also require different strategies that capitalize on the existing social networks of this well-established population.
Employee malaria prevention programs run by large companies44 and government agencies45 that place staff in highly malaria endemic areas rely substantially on chemoprophylaxis. These programs involve both providing access to the chemoprophylaxis drugs and including compliance monitoring. Education around other malaria prevention practices such as the use of bed nets is also often included. Asian companies sending staff to malaria-endemic countries could consider adopting similar measures. However, for self-employed or family-employed migrants, roughly a quarter of respondents in this survey, other avenues of intervention, are needed. To address this, embassies representing large numbers of workers could consider outreach activities to encourage and facilitate malaria prevention practices and care-seeking, taking advantage of the same social networks used to recruit participants to this survey. Finally, the Angolan Ministry of Health could intervene to improve the quality of malaria case management at private health facilities, focusing on educating healthcare workers about the high risk of malaria exposure in Asian migrants.
The COVID-19 pandemic has highlighted the connectedness of the global population. A deeper understanding of human movement patterns, including between Asia and Africa, and their influence on global spread of infectious disease will aid in developing more effective disease control interventions for malaria as well as emerging diseases.
Supplemental information, tables, and figures
Acknowledgments:
We thank all participants; staff at the Ministry of Health and Ministry of Foreign Affairs in Angola; China, the Philippines, and Vietnam embassies; and provincial authorities in Luanda, Lunda Sul, and Zaire. We acknowledge the contribution of Albert Kilian in developing the study protocol and data collection instruments.
Note: Supplemental tables and figures appear at www.ajtmh.org.
REFERENCES
- 1.African Development Bank , 2019. African Economic Outlook 2020: Developing Africa’s Workforce for the Future. Abidjan, Côte d’Ivoire: African Development Bank–Building Today, A Better Africa Tomorrow; Available at: https://www.afdb.org/en/knowledge/publications/african-economic-outlook. Accessed March 20, 2020. [Google Scholar]
- 2.International Labour Organization , 2019. Labour Migration in Africa. Geneva, Switzerland: International Labour Organization. Available at: http://www.ilo.org/africa/areas-of-work/labour-migration/WCMS_670561/lang--en/index.htm. Accessed March 20, 2020. [Google Scholar]
- 3.International Organization for Migration , 2016. Glossary on Migration (2019). Grand-Saconnex, Switzerland: International Organization for Migration; Available at: https://www.iom.int/glossary-migration-2019. Accessed March 20, 2020. [Google Scholar]
- 4.International Organization for Migration , 2020. World Migration Report 2020. Grand-Saconnex, Switzerland: IOM Online Bookstore; Available at: https://publications.iom.int/books/world-migration-report-2020. Accessed March 20, 2020. [Google Scholar]
- 5.Fang LQ, et al. 2018. Travel-related infections in mainland China, 2014–2016: an active surveillance study. Lancet Public Health 3: e385–e394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu W, Sun FJ, Tong YG, Zhang SQ, Cao WC, 2016. Rift valley fever virus imported into China from Angola. Lancet Infect Dis 16: 1226. [DOI] [PubMed] [Google Scholar]
- 7.Leo Y-S, Chow A, 2016. Zika virus has arrived in Singapore. Lancet Infect Dis 16: 1317–1319. [DOI] [PubMed] [Google Scholar]
- 8.Truzyan N, Crape B, Grigoryan R, Martirosyan H, Petrosyan V, 2015. Increased risk for multidrug-resistant tuberculosis in migratory workers, Armenia. Emerg Infect Dis 21: 474–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Satti H, Seung K, Keshavjee S, Furin J, 2008. Extensively drug-resistant tuberculosis, Lesotho. Emerg Infect Dis 14: 992–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Packard RM, 2014. The origins of antimalarial-drug resistance. N Engl J Med 371: 397–399. [DOI] [PubMed] [Google Scholar]
- 11.Roper C, Pearce R, Nair S, Sharp B, Nosten F, Anderson T, 2004. Intercontinental spread of pyrimethamine-resistant malaria. Science 305: 1124. [DOI] [PubMed] [Google Scholar]
- 12.Blasco B, Leroy D, Fidock DA, 2017. Antimalarial drug resistance: linking Plasmodium falciparum parasite biology to the clinic. Nat Med 23: 917–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lai S, et al. 2016. Plasmodium falciparum malaria importation from Africa to China and its mortality: an analysis of driving factors. Sci Rep 6: 39524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu H, Fang Z, Zhao D, Chen Y, Liu C, Liang X, 2017. A study on the epidemiological characteristics and infectious forecast model of malaria at Guangzhou airport among Chinese returnees from Africa. Malar J 16: 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng J, Li J, Yan H, Feng X, Xia Z, 2015. Evaluation of antimalarial resistance marker polymorphism in returned migrant workers in China. Antimicrob Agents Chemother 59: 326–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang C, Zhang H, Zhou R, Qian D, Liu Y, Zhao Y, Li S, Xu B, 2017. Polymorphisms of Plasmodium falciparum k13-propeller gene among migrant workers returning to Henan province, China from Africa. BMC Infect Dis 17: 560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tuite AR, Thomas-Bachli A, Acosta H, Bhatia D, Huber C, Petrasek K, Watts A, Yong JHE, Bogoch II, Khan K, 2018. Infectious disease implications of large-scale migration of Venezuelan nationals. J Travel Med 25: tay077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karunasena VM, 2019. The first introduced malaria case reported from Sri Lanka after elimination: implications for preventing the re-introduction of malaria in recently eliminated countries. Malar J 18: 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsee W, Chotivanich K, Chatapat L, Piyaphanee W, 2018. Case report: a cluster of Plasmodium falciparum malaria cases among Thai workers in Gembu, Nigeria. Am J Trop Med Hyg 99: 623–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Z, et al. 2016. Epidemiologic features of overseas imported malaria in the People’s Republic of China. Malar J 15: 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang M, Liu Z, He H, Luo L, Wang S, Bu H, Zhou X, 2011. Knowledge, attitudes, and practices on malaria prevention among Chinese international travelers. J Travel Med 18: 173–177. [DOI] [PubMed] [Google Scholar]
- 22.United Nations Population Division , Department of Economic and Social Affairs , 2020. New York, NY: United Nations Department of Economic and Social Affairs. Available at: https://www.un.org/en/development/desa/population/migration/data/estimates2/estimates17.asp. Accessed March 20, 2020.
- 23.Plucinski MM, et al. 2019. Screening for Pfhrp2/3-deleted Plasmodium falciparum, non-falciparum, and low-density malaria infections by a multiplex antigen assay. J Infect Dis 219: 437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rogier E, et al. 2019. High-throughput malaria serosurveillance using a one-step multiplex bead assay. Malar J 18: 402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rogier E, et al. 2017. Bead-based immunoassay allows sub-picogram detection of histidine-rich protein 2 from Plasmodium falciparum and estimates reliability of malaria rapid diagnostic tests. PLoS One 12: e0172139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lucchi NW, Narayanan J, Karell MA, Xayavong M, Kariuki S, DaSilva AJ, Hill V, Udhayakumar V, 2013. Molecular diagnosis of malaria by photo-induced electron transfer fluorogenic primers: PET-PCR. PLoS One 8: e56677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Talundzic E, et al. 2018. Next-generation sequencing and bioinformatics protocol for malaria drug resistance marker surveillance. Antimicrob Agents Chemother 62: e02474-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molina-Cruz A, et al. 2020. Plasmodium falciparum evades immunity of anopheline mosquitoes by interacting with a Pfs47 midgut receptor. Proc Natl Acad Sci U S A 117: 2597–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leigh JW, Bryant D, 2015. popart: full-feature software for haplotype network construction. Methods Ecol Evol 6: 1110–1116. [Google Scholar]
- 30.Thwing JI, Mihigo J, Fernandes AP, Saute F, Ferreira C, Fortes F, de Oliveira AM, Newman RD, 2009. How much malaria occurs in urban Luanda, Angola? A health facility-based assessment. Am J Trop Med Hyg 80: 487–491. [PubMed] [Google Scholar]
- 31.Sanchez JL, Bendet I, Grogl M, Lima JB, Pang LW, Guimaraes MF, Guedes CM, Milhous WK, Green MD, Todd GD, 2000. Malaria in Brazilian military personnel deployed to Angola. J Travel Med 7: 275–282. [DOI] [PubMed] [Google Scholar]
- 32.INE , MINSA , The DHS Program , ICF , 2017. Angola Inquérito de Indicadores Múltiplos e de Saúde (IIMS) 2015–2016. Available at: https://dhsprogram.com/publications/publication-FR327-DHS-Final-Reports.cfm. Accessed April 8, 2020. [Google Scholar]
- 33.Plucinski MM, et al. 2017. Evaluating malaria case management at public health facilities in two provinces in Angola. Malar J 16: 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fairhurst RM, Dondorp AM, 2016. Artemisinin-resistant Plasmodium falciparum malaria. Microbiol Spectr 4: EI10-0013-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conrad MD, Rosenthal PJ, 2019. Antimalarial drug resistance in Africa: the calm before the storm? Lancet Infect Dis 19: e338–e351. [DOI] [PubMed] [Google Scholar]
- 36.Van Hong N, et al. 2014. Severe malaria not responsive to artemisinin derivatives in man returning from Angola to Vietnam. Emerg Infect Dis 20: 1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ringwald P, Dondorp A, 2015. Severe malaria not responsive to artemisinin derivatives in man returning from Angola to Vietnam [letter]. Emerg Infect Dis 21: 1264–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Hong N, et al. 2015. Severe malaria not responsive to artemisinin derivatives in man returning from Angola to Vietnam [letter]. Emerg Infect Dis 21: 1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plucinski MM, et al. 2015. Efficacy of artemether-lumefantrine and dihydroartemisinin-piperaquine for treatment of uncomplicated malaria in children in Zaire and Uíge provinces, Angola. Antimicrob Agents Chemother 59: 437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Plucinski MM, et al. 2017. Efficacy of artemether–lumefantrine, artesunate–amodiaquine, and dihydroartemisinin–piperaquine for treatment of uncomplicated Plasmodium falciparum malaria in Angola, 2015. Malar J 16: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davlantes E, et al. 2018. Efficacy and safety of artemether–lumefantrine, artesunate–amodiaquine, and dihydroartemisinin–piperaquine for the treatment of uncomplicated Plasmodium falciparum malaria in three provinces in Angola, 2017. Malar J 17: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fançony C, Brito M, Gil JP, 2016. Plasmodium falciparum drug resistance in Angola. Malar J 15: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Plucinski MM, et al. 2018. Multiplex serology for impact evaluation of bed net distribution on burden of lymphatic filariasis and four species of human malaria in northern Mozambique. PLoS Negl Trop Dis 12: e0006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Diara M, Ngunjiri S, 2014. Malaria chemoprophylaxis compliance program: thinking inside the box. Travel Med Infect Dis 12: 303–304. [DOI] [PubMed] [Google Scholar]
- 45.Landman KZ, Tan KR, Arguin PM, 2014. Knowledge, attitudes, and practices regarding antimalarial chemoprophylaxis in U.S. Peace corps volunteers—Africa, 2013. MMWR Morb Mortal Wkly Rep 63: 516–517. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.