Abstract
Hospitalized patients have a high prevalence of prolonged QTc and are a high-risk population for Torsades de Pointes (TdP). One modifiable risk factor for TdP is the use of QT prolonging drugs. Electronically alerting providers who are ordering QT prolonging drugs in at-risk patients may help to achieve safer prescribing practices. Our previous study decreased inappropriate prescription of IV haloperidol by 36% using a targeted “smart” electronic alert. We wanted to assess an approach to expanding this type of electronic alert to commonly used QT prolonging medications and evaluate how this would affect prescribing practice. This retrospective cohort study evaluated the impact of these alerts for 12 frequently prescribed high-risk medications across a major health system. Between October 2016 and June 2017, a total of 6453 alerts fired and resulted in 3020 (46.8%) orders being cancelled by the provider. Our focused electronic alert significantly decreased prescribing of QT prolonging medications in high-risk patients.
Keywords: QTc, QT prolonging medications, Torsades de Pointes, electronic alerts, electrocardiogram
INTRODUCTION
The corrected QT interval (QTc) measured on an electrocardiogram (ECG) represents ventricular repolarization time normalized for a patient’s heart rate. QT prolongation represents a longer vulnerable period of repolarization and is associated with the life-threatening Torsades de Pointes (TdP) arrhythmia.1 Acutely ill hospitalized patients have a high prevalence of prolonged QTc, with a substantial incidence of TdP.2–3 One of the most significant modifiable risk factors for TdP is the use of QT prolonging drugs.4
More than 190 drugs are known to prolong QTc, and 57 of them are known to cause TdP at typical prescribed doses.5 Administering one of these 57 high-risk drugs in a patient with a baseline prolonged QTc places him/her at an increased risk for TdP. The non-cardiac medication best described in the literature for prolonging QTc and increasing the risk of TdP is intravenous (IV) haloperidol. In 2007, the FDA issued a black box warning regarding cardiac arrhythmia in patients on IV haloperidol and a prolonged QTc.6 In 2010, the American College of Cardiology released a scientific statement recommending awareness of risk factors for TdP and ECG monitoring for patients receiving all QT prolonging medications.7 In spite of these measures, there is poor compliance with expert recommendations.8–9
Warning providers about high-risk patients with prolonged QTc at the correct point in their workflow may decrease this dangerous practice. Targeting alerts to only the patients with identified higher risk at the time of drug prescribing has potential to reduce alert fatigue compared to less selective alerts. With most providers in the United States now using electronic health records (EHR) and computerized provider order entry (CPOE), notifying them during the ordering process is a reasonable approach to the problem.10 In a previous study, our group was able to decrease the inappropriate prescribing of IV haloperidol in patients by 36% through the use of a “smart” alert that fired on high-risk patients prior to completion of the order.11 The purpose of this study was to assess an approach to expand this type of electronic alert to the highest-risk commonly used QT prolonging medications and evaluate how this would affect prescribing practice.
METHODS
This descriptive study was conducted at the University of Colorado Health (UCH), a major health system that includes 7 hospitals with 1620 hospital beds and surrounding clinics in the western United States. The evaluation included patients of all ages who were admitted to one of the hospitals or seen at one of the clinics. This quality improvement project was approved by the Colorado Multiple Institutional Review Board.
UCH uses an EHR (EPIC Systems, Verona, Wisconsin, USA) that includes CPOE and an integrated pharmacy module for reconciling and dispensing hospital medications. The electronic alerting system used is an Epic functionality called “best practice advisories” (BPAs).
Based on the success of our IV haloperidol “smart” BPA study, we expanded the scope by including additional QT prolonging medications.11 To minimize alert fatigue, we wanted to create a list of the most frequently prescribed highest-risk drugs. A survey was sent to our inpatient providers and pharmacists in May 2013 asking them to select the most frequently prescribed medications from the CredibleMeds list of drugs with “Known Risk of Torsade de Pointes (TdP)”.5 There were 51 respondents to the survey, including 21 internal medicine clinicians and 30 pharmacists. We also ran a utilization report during this time frame. The top 12 drugs were selected based on the results of the survey and the report (Table 1). Dofetilide and sotalol were excluded, as these are usually ordered by our cardiology or electrophysiology service, and we have a policy on QTc monitoring for both drugs. Amiodarone was also excluded, as chronic administration of this drug prolongs the QT interval but is less likely to be associated with TdP compared to other QT prolonging medications.7
Table 1.
QT prolonging medications with BPAs
| Medication | BPA Fired | Orders Overridden (%) | Orders Cancelled (%) |
|---|---|---|---|
| Azithromycin | 761 | 351 (46) | 410 (54) |
| Chlorpromazine | 12 | 8 (67) | 4 (33) |
| Citalopram | 203 | 167 (82) | 36 (18) |
| Clarithromycin | 30 | 18 (60) | 12 (40) |
| Droperidol | 3 | 0 (0) | 3 (100) |
| Erythromycin | 67 | 34 (51) | 33 (49) |
| Escitalopram | 198 | 156 (79) | 42 (21) |
| Haloperidol (IV) | 537 | 205 (38) | 332 (62) |
| Levofloxacin | 670 | 378 (56) | 292 (44) |
| Methadone | 82 | 51 (62) | 31 (38) |
| Moxifloxacin | 9 | 6 (67) | 3 (33) |
| Ondansetron (IV) | 3881 | 2059 (53) | 1822 (47) |
| Total | 6453 | 3433 (53) | 3020 (47) |
As in our previous study, we designed the BPA to fire when any of the 12 drugs are ordered in a patient with a QTc interval ≥ 500 ms. The BPA used the most recent ECG available and reviewed ECGs up to 2 weeks prior to the drug order. The alert will not fire if no ECG was available during this 2-week period. The BPA provides the date and time of last QTc and QRS duration. When the alert appears, the ordering provider is given the choice either to cancel the order or override the alert with one of the following reasons: QTc falsely prolonged by prolonged QRS, end of life care, benefits outweighs risk, or see comment.
The BPA went into effect in July 2016. No alerts, except for IV haloperidol from our previous study, were available to providers when they ordered QT prolonging medications prior to this roll-out. After implementation of the BPA, information was collected on the number of times the alert fired, the number of orders cancelled as a result of the BPA, the number of times the BPA was overridden, and the reasons selected when the BPA was overridden.
RESULTS
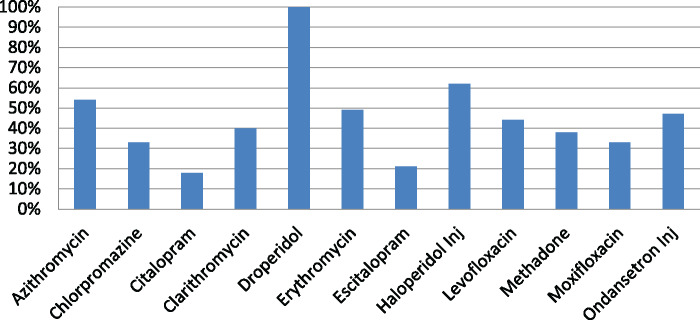
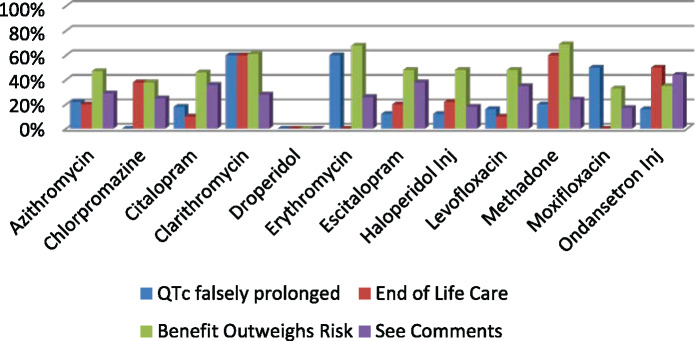
During the period of October 1, 2016, to June 30, 2017, a total of 6453 BPAs fired, of which 3020 (46.8%) orders were cancelled by the provider as a result of the BPA. Table 1 provides information on the total number of BPAs fired, orders overridden, and orders cancelled for each drug. Figure 1 shows the percentage of orders that were cancelled as a result of the BPA. During this 9-month period, the majority (90%) of the BPAs were fired for ondansetron, azithromycin, levofloxacin, and haloperidol. The BPA was successful in getting the providers to cancel orders for these 4 drugs 44% to 62% of the time. Across all 12 medications, overriding the alert and continuing with the original order ranged from 0% to 82%. There was a higher cancellation rate for haloperidol of 62% compared to the other medications. Although we will not be able to elucidate the exact reason for this, one reason may be attributable to providers being more aware of IV haloperidol’s QT prolongation risk from exposure to this BPA since its implementation in 2012 from our previous study. Another reason may be that providers were able to use alternatives, both pharmacologic and/or non-pharmacologic, in lieu of haloperidol. Figure 2 shows the reasons selected by providers when they overrode the BPA for each of these medications. The most common reason selected was “benefit outweighs risk” followed by “see comments.” Since a hard stop was not implemented to force the provider to enter a comment, only about 20% of the time was a comment entered by the provider.
Figure 1.
Percentage of orders cancelled due to BPA.
Figure 2.
Override reasons selected by provider.
DISCUSSION
Implementation of a focused electronic alert for QT prolonging medications in high-risk patients influenced ordering practice 46% of the time. This is similar to the results from our previously described IV haloperidol BPA study and provides further evidence of efficacy of targeted alerts on prescribing behavior.11,12 These results suggest that tailored drug-disease alert focused on high-volume high-risk drugs and can have a large impact on safe prescribing practices.
Our study addresses many of the challenges associated with implementation of EHR alerts for drug disease interactions. Designing a tailored alert that is useful and fires at the right time is important to deter inappropriate overrides by providers13 A study by Tisdale et al.14 showed how a validated QT prolongation risk score paired with a computer alert influenced the prescribing of QT prolonging drugs. We were also challenged with how to design our alert either by choosing to include our most frequently prescribed medications to maximize the number of patients who would benefit, or choosing the highest-risk but infrequently prescribed medications that providers may not be as familiar with. We ultimately selected the medications using a national database and combining our institution-specific prescription data. We also incorporated provider feedback using a survey and used this input to refine the final medication list, which may have contributed to buy-in.
There are several limitations to our study. Since our alert captured only patients with a QTc > 500 ms, we have limited ability to further define what our high-risk population is beyond this value. It is possible that prior to our intervention, some orders could have been caught by nursing or pharmacy prior to administration thus creating the impression of a greater magnitude of impact of the intervention that focused on the order itself. We are unable to draw conclusions as to whether the reduction in ordering of these drugs from the BPA resulted in an actual reduction of risk or adverse events. We did not review what medication(s) were ordered in lieu of the original medication when it was cancelled, so we are unable to determine if clinically appropriate alternatives were selected. Finally, our BPA did not exclude patients who have a prolonged QT due to a prolonged QRS, typically seen with bundle branch blocks or ventricular pacing, so this may result in false positive BPA firings. This was mitigated by allowing providers to proceed with the order by selecting the option “QTc falsely prolonged by prolonged QRS” within the alert.
Determining how alerts for medication-disease interactions should best be delivered remains a question. As more robust analytics become available to manage the expansive data in our EHRs, there will be even more opportunities to calculate risk and develop better tailored clinical decision support tools. This offers an opportunity to develop targeted “smart” alerts to focus attention on the patients at greatest risk, reduce alert fatigue, and leverage the EHR effectively for patient safety.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Contributors
DC and JP worked jointly on the design, data collection, and analysis for the entire project. They also authored the manuscript together, including writing of the background, methods, results, and discussion sections of the paper. DC was also responsible for the critical review, editing, and revisions of the entire manuscript and will serve as the corresponding author for this manuscript. GH was the main technical coordinator for the intervention for the project. He worked on the design, customization, and evaluation of the electronic alert used in the project. GH provided the primary data to the team to be analyzed. EC, with his experience on this subject matter, was instrumental in the critical review and editing of the manuscript. All authors give approval for the final version of the manuscript to be published and agree to be accountable for all aspects of the work ensuring that questions related to the accuracy and/or integrity of any part of the work are appropriately investigated and resolved.
Conflict of interest statement. None declared.
REFERENCES
- 1. Nguyen PT, Scheinman MM, Seger J.. Polymorphous ventricular tachycardia: clinical characterization, therapy, and the QT interval. Circulation 1986; 742: 340–9. [DOI] [PubMed] [Google Scholar]
- 2. Vandael E, Vandenberk B, Vandenberghe J, Pincé H, Willems R, Foulon V.. Incidence of Torsade de Pointes in a tertiary hospital population. Int J Cardiol 2017; 243: 511–5. [DOI] [PubMed] [Google Scholar]
- 3. Pickham D, Helfenbein E, Shinn JA, et al. High prevalence of corrected QT interval prolongation in acutely ill patients is associated with mortality: results of the QT in Practice (QTIP) Study. Crit Care Med 2012; 402: 394–9. [DOI] [PubMed] [Google Scholar]
- 4. Tisdale JE, Jaynes HA, Kingery JR, et al. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes 2013; 64: 479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. AZCERT. CredibleMeds. https://crediblemeds.org/new-drug-list/ Accessed May 23, 2017.
- 6.Information for Healthcare Professionals: Haloperidol. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm085203.htm Accessed June 22, 2013.
- 7. Drew BJ, Ackerman MJ, Funk M, et al. Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. J Am Coll Cardiol 2010; 559: 934–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cheung D, Wolfe B, Wald H, et al. Unsafe use of intravenous haloperidol: evaluation of recommendation-concordant care among hospitalized elderly. J Am Geriatr Soc 2013; 611: 160. [DOI] [PubMed] [Google Scholar]
- 9. Tisdale JE, Wroblewski HA, Overholser BR, Kingery JR, Trujillo TN, Kovacs RJ.. Prevalence of QT interval prolongation in patients admitted to cardiac care units and frequency of subsequent administration of QT interval-prolonging drugs: a prospective, observational study in a large urban academic medical center in the US. Drug Saf 2012; 356: 459–70. [DOI] [PubMed] [Google Scholar]
- 10.Health IT Quick Stats accessed 6/12/17 https://dashboard.healthit.gov/quickstats/quickstats.php.
- 11. Pell JM, Cheung D, Jones MA, Cumbler E.. Don't fuel the fire: decreasing intravenous haloperidol use in high risk patients via a customized electronic alert. J Am Med Inform Assoc 2014; 216: 1109–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sorita A, Bos JM, Morlan BW, Tarrell RF, Ackerman MJ, Caraballo PJ.. Impact of clinical decision support preventing the use of QT-prolonging medications for patients at risk for torsade de pointes. J Am Med Inform Assoc 2015; 22: e21–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nanji KC, Seger DL, Amato MG, et al. Medication-related clinical decision support alert overrides in inpatients. J Am Med Inform Assoc 2017; 255: 476–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tisdale JE, Jaynes HA, Kingery JR, et al. Effectiveness of a clinical decision support system for reducing the risk of QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes 2014; 73: 381–90. [DOI] [PMC free article] [PubMed] [Google Scholar]