Abstract
It is challenging to tune the response of biosensors to a set of ligands, for example, cross-reactivity to a given target family while maintaining high specificity against interferents, due to the lack of suitable bioreceptors. We present a novel approach for controlling the cross-reactivity of biosensors by employing defined mixtures of aptamers that have differing binding properties. As a demonstration, we develop assays for the specific detection of a family of illicit designer drugs, the synthetic cathinones, with customized responses to each target ligand and interferent. We first use a colorimetric dye-displacement assay to show that the binding spectra of dual-aptamer mixtures can be tuned by altering the molar ratio of these bioreceptors. Optimized assays achieve broad detection of synthetic cathinones with minimal response toward interferents and generally demonstrate better sensing performance than assays utilizing either aptamer alone. The generality of this strategy is demonstrated with a dual-aptamer electrochemical sensor. Our approach enables customization of biosensor responsiveness to an extent that has yet to be achieved through any previously reported aptamer engineering techniques such as sequence mutation or truncation. Since multiple aptamers for the designated target family can routinely be identified via high-throughput sequencing, we believe our strategy offers a generally applicable method for generating near-ideal aptamer biosensors for various analytical applications, including medical diagnostics, environmental monitoring, and drug detection.
Graphical Abstract

The detection of structurally related small molecules such as toxins, antibiotics, and illicit drugs is important for analytical applications in which determining the presence of any member of a designated molecular family, rather than any individual representative of that family, is the priority.1–3 Currently, this is typically achieved using immunoassays based on polyclonal antibodies, which comprise of a mixture of antibodies produced by target-immunized animals.4 Polyclonal antibodies are well-suited for such applications, because the combination of multiple antibodies binding to different epitopes contained by members in the target family greatly expands the target spectrum.5 However, the in vivo nature of the antibody generation process provides no control over the binding properties or composition of the resulting reagent mixture.6 This can result in false negatives, since it is impossible to guarantee that polyclonal antibodies will bind to all members of a designated target family with similar binding affinities or at all.4,7 On the other hand, polyclonal antibodies can also be too promiscuous, producing false positives due to recognition of structurally similar nontarget compounds.5 The binding profiles of polyclonal antibodies can also greatly vary from batch to batch, as they are generated from different animals.5 One can potentially overcome these limitations by mixing multiple monoclonal antibodies with differing binding spectra at an optimized ratio, but the costly and time-consuming process of monoclonal antibody development discourages such efforts.8
Aptamers offer an excellent alternative. These nucleic acid-based biorecognition elements are isolated in vitro via systematic evolution of ligands by exponential enrichment (SELEX)9 from randomized oligonucleotide libraries to recognize various targets and can be chemically synthesized at low cost with no batch-to-batch variation.10 Importantly, the binding profile of an aptamer can be precisely controlled by designing the selection process to favor candidates that bind to either a specific target or a family of structurally related targets.11–13 Moreover, the analytical performance of aptamer-based assays can be tailored for specific applications. For example, the sensitivity and dynamic range of assays can be altered by using a mixture of different aptamers14 or an aptamer and its truncated or mutated derivatives that bind the same target but with different affinities.15,16 Nevertheless, little effort has been made to control the cross-reactivity of aptamer-based assays toward a set of ligands, both targets and interfering compounds.
In this work, we for the first time have demonstrated that the cross-reactivity of aptamer-based sensors can be manipulated to achieve near-ideal sensing performance with unprecedented control by mixing multiple aptamers with differing ligand specificity. This has not yet been achieved with conventional aptamer engineering techniques. As a test bed, we targeted the synthetic cathinones, a large family of designer drugs whose members share the beta-keto phenethylamine core structure but have divergent side chain substituents.17 Our goal was to develop a sensor that can give a yes-or-no response for the presence of drugs in this family while remaining non-responsive to interferent molecules relevant to illicit drug detection. Thus, assay cross-reactivity at a specific cutoff (e.g., ligand concentration) is a more appropriate metric to evaluate the analytical performance of our sensors. We have recently isolated two synthetic cathinone-binding DNA aptamers, SCA1.1 and SCA2.1,13 via SELEX. SCA1.1 shows high cross-reactivity to synthetic cathinones but responds to several compounds not in this family (hereon specified as “interferents”), while SCA2.1 is highly specific to the target family but has lower cross-reactivity to some members. As a result, neither aptamer is ideal for the broad and specific detection of synthetic cathinones. However, by employing an optimized mixture of the two, we were able to maintain advantageous features of both aptamers while minimizing their disadvantages, simultaneously achieving high cross-reactivity to 12 synthetic cathinones and excellent specificity against 17 interferents. Our concept is first demonstrated using SCA1.1 and SCA2.1 in a dye-displacement assay. We found that the target-binding profile of aptamer mixtures at any given molar ratio could be precisely predicted using a mathematical model, which allows for fine-tuning of the sensor’s responsiveness. We subsequently utilized this same concept to develop a dual-aptamer-modified electrochemical aptamer-based (E-AB) sensor. Once again, the sensor demonstrated equally impressive performance as the dye-displacement assay and enabled specific detection of analytes in interferent-ridden binary mixtures. Unlike conventional aptamer engineering approaches, such as sequence mutation and truncation, ours is a generalizable strategy that enables precise control of the cross-reactivity of aptamer-based sensors toward both targets and interferents, and should enable development of near-ideal biosensors for a diversity of targets.
MATERIALS AND METHODS
Cy7 Dye-Displacement Assay.
The diethylthiatricarbocyanine (Cy7)-displacement assay was performed as previously reported.13 Briefly, a 70 μL mixture of SCA1.1 and SCA2.1 (final total aptamer concentration = 3 μM) was first mixed with 2 μL Cy7 (final concentration 2 μM) in reaction buffer (final concentration 10 mM Tris-HCl, 0.5 mM MgCl2, 20 mM NaCl, 0.01% Tween 20, 1% DMSO, pH 7.4) to form Cy7-aptamer complex. The molar ratio of SCA2.1 varied from 0 (i.e., SCA1.1 only) to 1 (i.e., SCA2.1 only) in different experiments. An 8 μL volume of synthetic cathinones (final concentration 10 μM) or interferent compounds (final concentration 100 μM) were mixed with the freshly prepared Cy7-aptamer complexes, and 75 μL of the mixture was loaded into a transparent flat-bottomed 384-well plate. The absorbance at 670 and 775 nm was immediately measured using a Tecan M1000 plate reader, and the absorbance ratio A670/A775 was calculated for each sample. Signal gain was calculated as (R − R0)/R0, where R0 and R are A670/A775 values in the absence and presence of the analyte, respectively. Cross-reactivity was calculated based on signal gain, where the signal gain of 10 μM methylenedioxypyrovalerone (MDPV) was defined as 100% cross-reactivity for each condition. For the synthetic cathinone mixture experiments, a 72 μL solution containing 1.5 μM SCA2.1, 1.5 μM SCA1.1, and 2 μM Cy7 (final concentrations) in reaction buffer was mixed with 8 μL of a synthetic cathinone mixture (either MDPV and methylone or 4-fluoromethcathinone (4-FMC) and cathinone) with molar ratios of 0:10, 2:8, 4:6, 8:2, 10:0 with a total ligand concentration of 10 μM. A 75 μL quantity of the mixture was loaded into a transparent flat-bottomed 384-well plate, and the absorbance at 670 and 775 nm was determined as mentioned previously.
Exonuclease Digestion of Aptamers.
Briefly, 1 μL of 50 μM aptamer was added into a PCR tube and diluted with 4.5 μL of 100 mM Tris-HCl buffer (pH 7.4) and 29.05 μL of deionized water. The mixture was heated at 95 °C for 10 min and cooled down in ice immediately to disrupt aptamer secondary structure. Next, 5 μL of a 200 mM NaCl/5 mM MgCl2 solution was added into the PCR tube, followed by 0.45 μL of 10 mg/mL BSA. 5 μL of deionized water or 2.5 mM MDPV was then added to the solution and the mixture was incubated for 1 h. Then, 5 μL of 0.1 U/μL Exonuclease III (Exo III) alone, 0.5 U/μL Exonuclease I (Exo I) alone, or a mixture of 0.1 U/μL Exo III and 0.5 U/μL Exo I was added to initiate the digestion. A total of 5 μL of each sample was collected at different reaction times and mixed with 10 μL of loading buffer (71.25% formamide, 10% glycerol, 0.125% SDS, 25 mM EDTA, and 0.15% (w/v) bromophenol blue and xylene cyanol) to stop the digestion. The sample was analyzed by 15% denaturing polyacrylamide gel electrophoresis (PAGE), stained with 1× SYBR gold dye, and imaged using a ChemiDoc MP imaging system. The length and concentration of digestion products were characterized using a homemade DNA ladder comprising either SCA2.1 or SCA1.1 and truncated derivatives measuring 43-, 41-, 40-, 39-, 35-, 32-, and 29-nt, where the ladder was matched to the aptamer being analyzed.
Electrochemical Aptamer-Based Sensor Fabrication and Detection.
Aptamer-modified electrodes were prepared as previously reported.18 Briefly, 3 mm gold disk electrodes (CHI) were polished consecutively by 1 μM diamond suspension and 0.05 μM Gamma Alumina suspension (BASi), followed by electrochemical cleaning. 5′-thiolated and 3′-methylene blue-modified aptamers were incubated with 100 mM Tris(2-carboxyethyl)phosphine in PBS buffer (10 mM phosphate, 1 M NaCl, 1 mM MgCl2, pH 7.1) for 2 h at room temperature to reduce disulfide bonds. The cleaned electrodes were immediately immersed into aptamer solutions containing either SCA2.1–34-MB, SCA1.1–34-MB, or both aptamers at various concentrations (10–30 nM) overnight at room temperature. The aptamer-modified electrodes were then incubated with 3 mM 6-mercapto-1-hexanol solution for 2 h at room temperature to backfill vacant surfaces on the gold electrodes. Finally, the electrodes were washed with deionized water and stored in 10 mM Tris-HCl (pH 7.4) before use. Chronocoulometry was applied to measure surface coverage using the method reported by Tarlov et al.19 Detection was performed using a CHI 670D Electrochemical Station with squarewave voltammetry and a three-electrode system, including a platinum counter electrode, an Ag/AgCl reference electrode, and the aptamer-modified working electrode. The peak currents in the absence (I0) and presence of analyte (I) were measured for 12 synthetic cathinones (10 μM), 17 interferents (100 μM), and binary mixtures, including 10 μM MDPV with 100 μM of individual interferents. Signal gain was calculated using the equation: (I − I0)/I0 × 100%. The cross-reactivity of each analyte was calculated by its signal gain, where a cross-reactivity of 100% was defined based on the signal gain achieved with 10 μM MDPV. Each experiment was repeated three times using independently modified electrodes.
RESULTS AND DISCUSSION
We have recently employed a parallel-and-serial selection strategy to isolate two synthetic cathinone-binding DNA aptamers, SCA1.1 and SCA2.1, via library-immobilized SELEX.13 To determine the target-binding profile of these aptamers, we performed a label-free dye-displacement assay that utilizes the dye diethylthiatricarbocyanine (Cy7) as a signal reporter (Figure 1A). Cy7 exists as both a monomer and dimer in aqueous solution, and these forms exhibit absorption maxima of 775 and 670 nm, respectively.20 In the absence of target, Cy7 monomers bind to the aptamer (Figure 1A, left), resulting in enhanced absorbance at 775 nm. Target binding to the aptamer displaces Cy7 monomers, which rapidly dimerize in solution (Figure 1A, right), causing decreased absorbance at 775 nm and increased absorbance at 670 nm (Supporting Information, SI, Figure S1).13 The absorbance ratio A670/A775 can thus be used to measure target binding for these aptamers. We first employed this assay to examine the cross-reactivity of these two aptamers toward 12 synthetic cathinones and their specificity against 17 interferents (SI, Figure S2), some of which are structurally related to the synthetic cathinone targets. Since both SCA1.1 (KD = 0.23 ± 0.02 μM for (−)-MDPV and KD = 30.4 ± 1.0 μM for (+)-MDPV) (SI, Figure S3) and SCA2.113 bind to methylenedioxypyrovalerone (MDPV) strongly with submicromolar binding affinity, we used the signal produced by this analyte as a benchmark to calculate cross-reactivity (see SI for details). We observed that the aptamers had different binding profiles in the Cy7-displacement assay. SCA2.1 displayed >20% cross-reactivity to eight synthetic cathinones at a concentration of 10 μM and minimal response to all interferents at concentrations of 100 μM, with cross-reactivity <8.6%. However, this aptamer exhibited poor binding (<20% cross-reactivity) to methcathione, 3-fluoromethcathinone (3-FMC), cathinone, and 4-fluoromethcathinone (4-FMC; SI, Figure S4A). In contrast, SCA1.1 showed >20% cross-reactivity for 11 synthetic cathinones, including those that SCA2.1 was poorly crossreactive to, and only exhibited low cross-reactivity (13%) to α-pyrrolidinopentiophenone (α-PVP). However, SCA1.1 was much less specific, with cross-reactivities toward interferents as high as 108% (SI, Figure S4B). Thus, SCA2.1 boasts excellent specificity against interferents at the expense of limited cross-reactivity toward the synthetic cathinone targets, while the opposite is true for SCA1.1.
Figure 1.

Target-binding spectra of SCA1.1, SCA2.1, and their mixtures in a Cy7-displacement assay. (A) Schematic of the dual-aptamer Cy7-displacement assay. (B) Target cross-reactivity (Tmax/ Tmin) and specificity (Imax/Tmin) for mixtures of SCA1.1 and SCA2.1 at various molar ratios with a fixed total aptamer concentration of 3 μM. Predicted values (dashed lines) were obtained from simulated binding spectra generated from results in Figure S3. Experimental values (scattered points) were calculated based on the results from Figure S5. Error bars show standard deviation from three measurements. The optimal aptamer ratio achieving both high target cross-reactivity (low Tmax/Tmin) and specificity (low Imax/Tmin) is shaded blue. (C) Binding profiles of SCA1.1, SCA2.1, and a 1:1 mixture of the two aptamers. Each point corresponds to cross-reactivity toward 12 synthetic cathinones (10 μM; black) or 17 interferents (100 μM; red) relative to 10 μM MDPV. The cutoff for 20% cross-reactivity is marked in blue.
We hypothesized that the strengths of both aptamers could be leveraged, and their weaknesses likewise mitigated, by using a mixture of the two. Since target binding to each aptamer is independent, we anticipated that the global response of the aptamer mixture can be mathematically predicted if the target binding profile and molar fraction of each aptamer are known (see SI for mathematical model). When the total concentration of a binary aptamer mixture is fixed, there should be a linear relationship between the signal gain observed for a particular ligand and the molar fraction of either aptamer. To verify this, we mixed SCA2.1 and SCA1.1 at various molar ratios with a fixed total aptamer concentration, and tested their response to six synthetic cathinones (pentylone, MDPV, methylone, 3-FMC, methcathinone, and 4-FMC) and six interferents (methylenedioxymethamphetamine (MDMA), procaine, pseudoephedrine, quinine, levamisole, and paracetamol) in the Cy7-displacement assay. The results confirmed our hypothesis, as almost all analytes showed a linear relationship (SI, Figure S5). MDMA and procaine displayed a nonlinear trend, but since the response was monotonic, the signal response could still be estimated.
An ideal cross-reactive sensor should respond similarly to all targets, such that the ratio of signal gain between the targets with the highest and lowest response (Tmax/Tmin) is minimal. In addition, the sensor should have minimal response to all interferents, such that the ratio of the signal gain between the interferent with the highest response and the target with the lowest response (Imax/Tmin) is minimal. We mathematically predicted that the optimum SCA2.1:SCA1.1 ratio is approximately 1:1 (Figure 1B). We confirmed this prediction by mixing these aptamers at different ratios and determining their binding profile with the aforementioned targets and interferents. As expected, the equimolar 1:1 ratio demonstrated the best cross-reactivity and specificity (Figure 1C). Notably, in contrast with either aptamer alone, this 1:1 mixture exhibited >20% cross-reactivity to all synthetic cathinone targets at a 10 μM concentration (Figure 1C and SI, Figure S6A). Thus, this combination approach overcame SCA2.1’s poor cross-reactivity toward methcathione, cathinone, 3-FMC, and 4-FMC, and SCA1.1’s poor response to α-PVP. Meanwhile, the assay also demonstrated excellent specificity, with cross-reactivities <17% for all interferents at a concentration of 100 μM. Additionally, the experimentally determined cross-reactivity profile of the 1:1 aptamer mixture correlated well with the mathematical prediction calculated from the signal gain obtained with either aptamer alone (SI, Figure S6B). The predicted cross-reactivity is calculated by using the average of the signal gain from each aptamer toward a ligand, where the average signal gain produced by 10 μM MDPV was defined as 100% cross-reactivity. It should be noted that the experimental values for MDMA and procaine were slightly lower than the predicted values due to their nonlinear responses. This demonstrates that the response profile of an aptamer assay in terms of both Tmax/Tmin and Imax/Tmin can be precisely controlled by using multiple aptamers with differing binding profiles at specific molar fractions.
We then determined the response of the Cy7-displacement assay to mixtures of synthetic cathinones with varying amounts of each constituent. As a demonstration, we prepared two pairs of target mixtures (4-FMC + cathinone and methylone + MDPV) at various molar ratios at a fixed total drug concentration of 10 μM and tested their response at a constant aptamer concentration. We found that the response of the assays based on SCA1.1 or SCA2.1 alone were sensitive to the molar fraction of constituents for both synthetic cathinone mixtures. However, the aptamer mixture was relatively insensitive to this parameter (SI, Figure S7), because the mixture has a similar cross-reactivity to both targets. The signal gains obtained for the target mixture are not necessarily an arithmetic mean. This may be due to the aptamer having differing binding affinities for the enantiomers of these drugs and the fact that each aptamer has a different affinity and binding stoichiometry for Cy7.
We next explored whether the same multiaptamer strategy can be generally applied to different sensing platforms such as E-AB sensors. E-AB sensors have been successfully employed for sensitive and specific detection of small molecules in complex samples such as soil, foodstuffs, urine, and whole blood.21–23 This sensing platform utilizes redox-tag-labeled structure-switching aptamers, which undergo a target-induced conformational change to produce an electrochemical signal that indicates the presence and quantity of the target.1,24 SCA1.1 and SCA2.1 are both 46-nt fully folded stem-loop-structured aptamers that lack structure-switching functionality. We therefore used our previously developed exonuclease III (Exo III)-based assay25 to introduce this functionality into these two aptamers. Exo III is a 3′-to-5′ exonuclease that digests aptamers with duplexed ends into short single-stranded products in the absence of target. For target-bound aptamers, this digestion is halted a few bases prior to the target-binding domain, resulting in truncated aptamers with structure-switching functionality. We found that after 6 h of Exo III digestion, both aptamers were digested into 40-nt major products in the presence of target (SI, Figure S8). In the absence of target, Exo III generated shortened products, but digestion was incomplete, possibly due to the low activity of this enzyme on single-stranded DNA (Figure 2, Exo III and SI, Figure S8). To remedy this problem, we combined Exo III with exonuclease I (Exo I), a single-strand 3′-to-5′ exonuclease,26 to perform aptamer digestion. Using this mixture, both aptamers were completely digested in the absence of target, whereas 40-nt major products were retained in the presence of target (Figure 2, Exo M and SI, Figure S9).
Figure 2.

Polyacrylamide gel electrophoresis analysis of exonuclease digestion of (A) SCA1.1 and (B) SCA2.1 by Exo III or Exo M (the mixture of Exo III and Exo I) after 4 h in the absence (−) or presence (+) of MDPV. Structures (based on mfold27) and sequences for major digestion products are shown at right of each gel.
To confirm that the truncated aptamers had structure-switching functionality, we synthesized the 40-nt products (SCA1.1–40 and SCA2.1–40) and digested them with Exo I.26 Both aptamers were completely digested within 2 h in the absence of target, indicating that they are single-stranded (SI, Figure S10). In contrast, 89% and 92% of SCA1.1–40 and SCA2.1–40, respectively, remained intact in the presence of target due to the formation of a folded target-aptamer complex that resisted Exo I digestion. The structure-switching functionality of these two truncated aptamers was further confirmed by the dramatic change of their circular dichroism spectra28 upon the addition of MDPV (SI, Figure S11). Isothermal titration calorimetry experiments were then performed to determine the affinity of the truncated aptamers for both enantiomers of MDPV, (+)-MDPV, and (−)-MDPV. SCA2.1–40 binds to (−)-MDPV and (+)-MDPV with equilibrium dissociation constants (KD) of 0.65 ± 0.03 μM and 292 ± 14 μM, respectively (SI, Figure S12). SCA1.1–40 exhibits a similar pattern of binding preferentially to (−)-MDPV, with a KD of 1.05 ± 0.05 μM versus 181 ± 2 μM for (+)-MDPV (SI, Figure S13). This weaker affinity for (+)-MDPV should not be of concern for analytical purposes, as synthetic cathinones always exist as racemic mixtures in drug samples.29 We therefore concluded that SCA2.1–40 and SCA1.1–40 retain strong binding to synthetic cathinones and were suitable for fabricating E-AB sensors due to their structure-switching functionality.
To achieve efficient electron transfer rates for E-AB sensing,30 we further removed the 6-nt overhangs at the 5′ terminus of SCA2.1–40 and SCA1.1–40 to generate SCA2.1–34 (KD = 0.30 ± 0.04 μM for (−)-MDPV and KD = 96 ± 4.0 μM for (+)-MDPV) (SI, Figure S14) and SCA1.1–34 (KD = 1.46 ± 0.03 μM for (−)-MDPV and KD = 185 ± 3.0 μM for (+)-MDPV) (SI, Figure S15) and tested whether such truncation influenced their target binding spectra in a Cy7-displacement assay. The truncated aptamers alone as well as the aptamer mixture yielded lower signal gains relative to the parent aptamer mixture (SI, Figures S16 and S17), indicating that Cy7 and the analytes bind more weakly to the truncated aptamers. Nonetheless, the cross-reactivity profile of the truncated aptamer mixture for both targets and interferents remained unchanged relative to the original aptamers (Figure 3).
Figure 3.
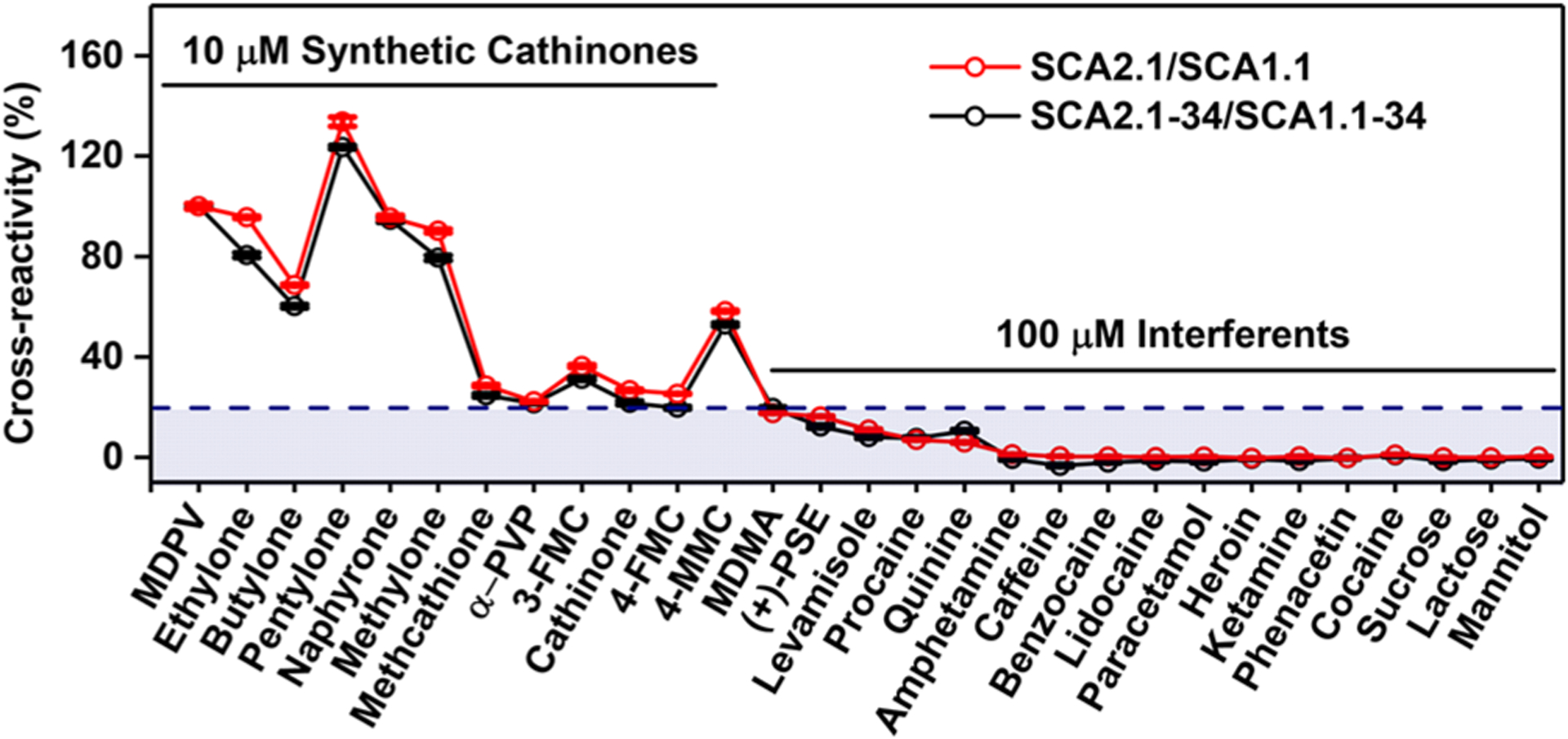
Cross-reactivity of SCA2.1 and SCA1.1 (red) and SCA2.1–34 and SCA1.1–34 (black) mixtures in Cy7-displacement assays with 12 synthetic cathinones and 17 interferents at concentrations of 10 μM and 100 μM, respectively. The cutoff for 20% cross-reactivity is marked in blue. Error bars represent the standard deviation of three individual experiments.
We then fabricated E-AB sensors using each individual aptamer modified with 5′ thiol and 3′ methylene blue (MB; SCA2.1–34-MB and SCA1.1–34-MB). Both sensors produced increasing current with increasing concentrations of target, with linear ranges of 0–1000 nM and limits of detection of 100 nM for MDPV (SI, Figures S18A,B and S19A,B). We then challenged the sensors with 12 synthetic cathinones at a concentration of 10 μM and 17 interferents at 100 μM. Both sensors had similar target-binding profiles to those seen with the Cy7-displacement assay. Specifically, the SCA2.1-based sensor showed cross-reactivity >20% for the 12 targets and strong discrimination against all interferents (≤20% cross-reactivity; SI, Figure S18C). However, the response to each target was quite variable, and cross-reactivity toward methcathinone, cathinone, and 4-FMC were low. The SCA1.1-based sensor responded to all synthetic cathinones with cross-reactivity >20%, but exhibited poor specificity toward some interferents, particularly MDMA, (+)-pseudoephedrine, levamisole, procaine, and quinine, with cross-reactivity ranging from 36–79% (SI, Figure S19C). ITC confirmed that SCA1.1 binds to these interferents weakly (SI, Figure S20). Clearly, neither aptamer alone could achieve optimal detection of synthetic cathinones.
We therefore fabricated an E-AB sensor with a 1:1 mixture of SCA1.1–34-MB and SCA2.1–34-MB (Figure 4A). Given that the electrode surface-modification efficiency of SCA1.1–34-MB was slightly higher than that of SCA2.1–34-MB (SI, Figure S21), we used an aptamer ratio of 5:6 (SCA1.1–34-MB:SCA2.1–34-MB) to achieve an approximate surface density ratio of 1:1 during the electrode modification step. The dual-aptamer-modified E-AB sensor had a limit of detection of 50 nM for MDPV, with a linear range of 50–500 nM (Figure 4B), slightly better than the E-AB sensors fabricated with either aptamer alone. As expected, this sensor exhibited high cross-reactivity (>50%) to all synthetic cathinones at a concentration of 10 μM while having excellent specificity (≤20% cross-reactivity) against all tested interferents at 100 μM (Figure 4C and SI, Figures S22 and S23). We observed similar predicted and experimental cross-reactivity (SI, Figure S24). These results confirm that the dual-aptamer-modified E-AB sensor is amenable for synthetic cathinone detection in interferent-ridden samples. To demonstrate this, we challenged the sensor with 17 binary mixtures of 10 μM MDPV with 100 μM interferent. We found that the sensor yielded similar responses to all binary mixtures relative to MDPV alone, except for the mixture containing quinine, which exhibited low levels of signal suppression (SI, Figures S25 and S26). This may occur because quinine has been reported to inhibit electron transfer from methylene blue to electrode surfaces.31
Figure 4.
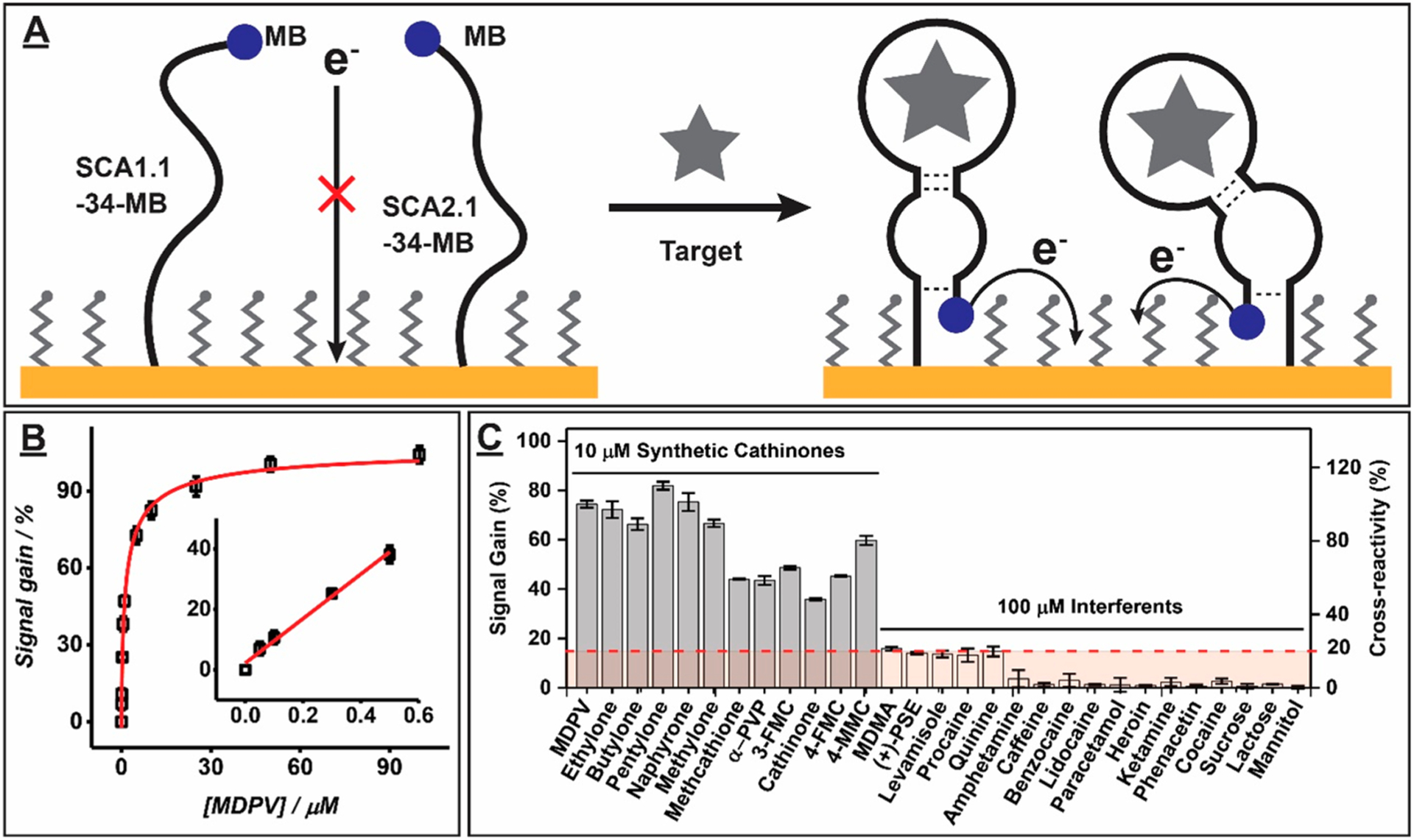
Dual-aptamer-based E-AB sensor. (A) Schematic of the E-AB sensor. Aptamers are initially unfolded in the absence of target (left), situating the MB tag far from the electrode and producing minimal current. Target binding causes the aptamers to fold, resulting in enhanced electron transfer and an increase in current. (B) Calibration curve of MDPV on our 1:1 SCA1.1–34-MB and SCA2.1–34-MB E-AB sensor, obtained via square-wave voltammetry. Inset shows the linear range. (C) The 1:1 dual-aptamer sensor shows high cross-reactivity for 12 synthetic cathinones at a concentration of 10 μM and low cross-reactivity for 17 interferents at a concentration of 100 μM. The dashed red line and box indicate a threshold value of 20% cross-reactivity. Error bars represent the standard deviation of measurements with three different electrodes.
The cross-reactivity profile of the Cy7-displacement assay using the truncated aptamer mixture is practically the same as that of the E-AB sensor (SI, Figure S27). However, the E-AB sensor is generally more cross-reactive to synthetic cathinones as well as interferents like MDMA, procaine, and quinine. There are several potential reasons for this discrepancy including the different signal transduction mechanism between two biosensors as well as different physicochemical conditions (e.g. salt concentrations) or the surface-immobilized aptamers have different binding affinities than free aptamers or surface coimmobilization of different aptamers may affect their binding behavior. Since our E-AB sensor exhibits strong cross-reactivity to all of the tested synthetic cathinones, our sensor can be used as a general screening assay to sensitively determine the concentration of these drugs in a sample regardless of whether there is a mixture. In this context, it is not necessary for our E-AB sensor to differentiate among different synthetic cathinones, as almost all members of this family are considered drugs of abuse. As such, a semiquantitative “yes-or-no” answer is sufficient for on-site drug testing. Likewise, in a medical context, the identity of the synthetic cathinone(s) does not need to be known, as drugs in this family have similar toxicological effects and are treated in the same fashion.
CONCLUSION
The detection of a group of structurally related compounds requires a sensor that yields maximal response to its intended targets and minimal response to interferents. To this end, much work has been dedicated to developing biorecognition elements with these ideal binding properties, with limited success to date. We have identified an alternative approach. Rather than searching for one “perfect” bioreceptor, in this work we have employed a mixture of aptamers, each with their own favorable and unfavorable binding properties, that can be used together to develop high-performance sensors that enable broad and specific detection of a molecular family. We first used a Cy7-displacement assay to illustrate the divergent binding profiles of two different synthetic cathinone-binding aptamers. One aptamer (SCA1.1) displays excellent target cross-reactivity but poor specificity, while the other (SCA2.1) has lower target cross-reactivity but higher specificity. We demonstrated that the strengths of both aptamers could be leveraged by mixing them at an optimized ratio. As a result, up to 12 synthetic cathinones with diverse substituents could be detected with high cross-reactivity with minimal response to 17 interferents, which was not possible using either aptamer alone. The generality of this approach was demonstrated by converting these two aptamers into structure-switching aptamers that possess the same binding profile as their parent aptamers and then incorporating these into an E-AB sensor at an optimized ratio. As with the Cy7-displacement assay, this dual-aptamer sensor demonstrated high cross-reactivity and specificity, achieving interference-free target detection in binary mixtures containing 90% interferent.
Our results demonstrate for the first time that the cross-reactivity of biosensors can be precisely fine-tuned with the right combination of aptamers. This is extremely valuable, as it can be challenging to identify individual bioreceptors that enable optimal sensor performance. For our purposes, two aptamers were sufficient to enable detection of the current most common synthetic cathinones. However, in future applications, as many aptamers as needed should be employed to provide target coverage that is sufficient for the application at hand. Increasing the number of aptamers greatens target coverage and sensor flexibility. The multireceptor approach is particularly well-suited for aptamers, especially considering that high-throughput sequencing technologies can easily identify multiple aptamers for a given target or class of targets with varying binding characteristics.32 The aptamers need not have similar target-binding affinities, although they should not vary by more than 1000-fold, and, of course, should have minimal binding toward any relevant interferents. For scenarios where families of compounds need to be detected, such as with antibiotics, environmental toxins, explosives, or illicit drugs, to name a few, we believe our multiaptamer method will be a viable option to enable such goal.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institute of Justice, Grant Office of Justice Programs, U.S. Department of Justice Award 2016-DN-BX0167X and the National Institutes of Health - National Institute on Drug Abuse [R15DA036821-01A1, R21DA045334-01A1]. H.Y. and O.A. acknowledge support from the Dissertation Year Fellowship and the Presidential Fellowship, respectively. Both fellowships are awarded by the University Graduate School of Florida International University.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.analchem.9b05339.
Additional details of materials and methods for circular dichroism, isothermal titration calorimetry (ITC), and mathematical prediction of signal gain using a mixture of two aptamers; DNA sequences used in this work; aptamer, ligand, and ligand concentration used for each ITC experiment; Cy7-displacement assay for MDPV detection; chemical structures of different synthetic cathinones and interferents used in this work; characterization of the target-binding affinity of SCA1.1 using ITC; signal gain and cross-reactivity from colorimetric Cy7-displacement assays performed with SCA2.1 or SCA1.1; the relationship between the fraction of SCA2.1/SCA1.1 in the aptamer mixture and signal gain obtained for various synthetic cathinones or interferents; target cross-reactivity and specificity of the Cy7-displacement dual-aptamer assay; PAGE analysis of time-course digestion of SCA1.1 and SCA2.1 incubated with Exo III; PAGE analysis of time-course digestion of SCA1.1 and SCA2.1 incubated with a mixture of Exo III and Exo I; PAGE analysis of time-course digestion of SCA1.1–40 and SCA2.1–40 by Exo I; CD spectra of SCA1.1–40 and SCA2.1–40; characterization of the target-binding affinity of SCA2.1–40, SCA2.1–34, SCA1.1–40, and SCA1.1–34 using ITC; colorimetric signal gain of the 1:1 aptamer mixtures SCA2.1 and SCA1.1 and SCA2.1–34 and SCA1.1–34; signal gain and cross-reactivity from colorimetric Cy7-displacement assays performed with SCA2.1–34 alone, SCA1.1–34 alone or the mixture of both aptamers; performance of an E-AB sensor modified with SCA2.1–34-MB and SCA1.1–34-MB alone; characterization of the interferent-binding affinity of SCA1.1–34 using ITC; comparison of surface coverages of electrodes modified with SCA2.1–34-MB or SCA1.1–34-MB alone; squarewave voltammetry spectra collected in the absence and presence of 12 synthetic cathinones, interferents, or binary mixtures; signal gain and cross-reactivity from binary mixtures; experimental and predicted cross-reactivity of SCA2.1–34 and SCA1.1–34 mixtures using E-AB sensors; Cross-reactivity of the truncated mixture of SCA2.1–34 and SCA1.1–34 in the Cy7-displacement assay and an E-AB sensor with 12 synthetic cathinones and 17 interferents (PDF)
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.analchem.9b05339
The authors declare no competing financial interest.
REFERENCES
- (1).Verma N; Bhardwaj A Appl. Biochem. Biotechnol 2015, 175, 3093–3119. [DOI] [PubMed] [Google Scholar]
- (2).Huet A-C; Fodey T; Haughey SA; Weigel S; Elliott C; Delahaut P TrAC, Trends Anal. Chem 2010, 29, 1281–1294. [Google Scholar]
- (3).Gandhi S; Suman P; Kumar A; Sharma P; Capalash N; Suri CR BioImpacts 2015, 5, 207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Spinks CA Trends Food Sci. Technol 2000, 11, 210–217. [Google Scholar]
- (5).Ascoli CA; Aggeler B BioTechniques 2018, 65, 127–136. [DOI] [PubMed] [Google Scholar]
- (6).Leenaars M; Hendriksen CFM ILAR J. 2005, 46, 269–279. [DOI] [PubMed] [Google Scholar]
- (7).Zhang H; Wang SJ Immunol. Methods 2009, 350, 1–13. [DOI] [PubMed] [Google Scholar]
- (8).Yokoyama WM; Christensen M; Dos Santos G; Miller D; Ho J; Wu T; Dziegelewski M; Neethling FA Curr. Protoc. Immunol 2013, 102, 2.5.1–2.5.29. [DOI] [PubMed] [Google Scholar]
- (9).Tuerk C; Gold L Science 1990, 249, 505–510. [DOI] [PubMed] [Google Scholar]
- (10).Dunn MR; Jimenez RM; Chaput JC Nat. Rev. Chem 2017, 1, 76. [Google Scholar]
- (11).Jenison RD; Gill SC; Pardi A; Polisky B Science 1994, 263, 1425–1429. [DOI] [PubMed] [Google Scholar]
- (12).White R; Rusconi C; Scardino E; Wolberg A; Lawson J; Hoffman M; Sullenger B Mol. Ther 2001, 4, 567–573. [DOI] [PubMed] [Google Scholar]
- (13).Yang W; Yu H; Alkhamis O; Liu Y; Canoura J; Fu F; Xiao Y Nucleic Acids Res. 2019, 47, No. e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Drabovich AP; Okhonin V; Berezovski M; Krylov SN J. Am. Chem. Soc 2007, 129, 7260–7261. [DOI] [PubMed] [Google Scholar]
- (15).Porchetta A; Vallee-Belisle A; Plaxco KW; Ricci FJ Am. Chem. Soc 2012, 134, 20601–20604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Schoukroun-Barnes LR; Glaser EP; White RJ Langmuir 2015, 31, 6563–6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).German CL; Fleckenstein AE; Hanson GR Life Sci 2014, 97, 2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Xiao Y; Lai RY; Plaxco KW Nat. Protoc 2007, 2, 2875–2880. [DOI] [PubMed] [Google Scholar]
- (19).Herne TM; Tarlov MJ J. Am. Chem. Soc 1997, 119, 8916–8920. [Google Scholar]
- (20).Garoff RA; Litzinger EA; Connor RE; Fishman I; Armitage BA Langmuir 2002, 18, 6330–6337. [Google Scholar]
- (21).Baker BR; Lai RY; Wood MS; Doctor EH; Heeger AJ; Plaxco KW J. Am. Chem. Soc 2006, 128, 3138–3139. [DOI] [PubMed] [Google Scholar]
- (22).Somerson J; Plaxco KW Molecules 2018, 23, 912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Xiao Y; Rowe AA; Plaxco KW J. Am. Chem. Soc 2007, 129, 262–263. [DOI] [PubMed] [Google Scholar]
- (24).Xiao Y; Lubin AA; Heeger AJ; Plaxco KW Angew. Chem., Int. Ed 2005, 44, 5456–5459. [DOI] [PubMed] [Google Scholar]
- (25).Wang Z; Yu H; Canoura J; Liu Y; Alkhamis O; Fu F; Xiao Y Nucleic Acids Res. 2018, 46, No. e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Lehman IR; Nussbaum AL J. Biol. Chem 1964, 239, 2628–2636. [PubMed] [Google Scholar]
- (27).Zuker M Nucleic Acids Res. 2003, 31, 3406–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Kypr J; Kejnovská I; Renčiuk D; Vorlíčková M Nucleic Acids Res. 2009, 37, 1713–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Silva B; Fernandes C; Tiritan ME; Pinto MMM; Valente MJ; Carvalho M; de Pinho PG; Remião F Forensic Toxicol. 2016, 34, 372–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).White RJ; Rowe AA; Plaxco KW Analyst 2010, 135, 589–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Zhang Q; Huang Y; Guo L; Chen C; Guo D; Chen Y; Fu Y New J. Chem 2014, 38, 4600–4606. [Google Scholar]
- (32).Nguyen Quang N; Perret G; Duconge F Pharmaceuticals 2016, 9, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


