Abstract
Atherosclerosis is responsible for a large percentage of all-cause mortality worldwide, but it is only now beginning to be understood as a complex disease process involving metabolic insult, chronic inflammation, and multiple immune mechanisms. Abs targeting apolipoprotein A-I (ApoA-I) have been found in patients with cardiovascular disease, autoimmune conditions, as well as those with no documented history of either. However, relatively little is known about how these Abs are generated and their relationship to diet and sex. In the current study, we modeled this aspect of autoimmunity using anti–ApoA-I immunization of male and female C57BL/6 mice. Unexpectedly, we found that autoantibodies directed against a single, previously unknown, epitope within the ApoA-I protein developed irrespective of immunization status or dyslipidemia in mice. When total IgG subclasses were analyzed over the course of time, we observed that rather than driving an increase in inflammatory IgG subclasses, consumption of Western diet suppressed age-dependent increases in IgG2b and IgG2c in male mice only. The lack of change observed in female mice suggested that diet and sex might play a combined role in Th1/Th2 balance and, ultimately, in immunity to pathogen challenge. This report demonstrates the need for inclusion of both sexes in studies pertaining to diet and aging and suggests that further study of immunogenic epitopes present in ApoA-I is warranted.
INTRODUCTION
Atherosclerosis, a disease process characterized by the buildup of fatty plaques in the vasculature, involves chronic inflammation and targeting of modified self-antigens, including oxidized low-density lipoprotein (oxLDL) (1, 2). The autoantibody response to these modified self-antigens and possible connections between diet, aberrant adaptive immunity, and atherosclerosis have come under increasing scrutiny in recent years. A number of atherogenesis-promoting Ags, including oxLDL, heat shock protein 60 (HSP60), and high mobility group box-1 (HMGB-1) protein have been identified, and studies have confirmed the development of autoantibodies to these in mice fed a Western diet (WD; high-fat, high cholesterol) for as little as 2 mo (1, 3–5).
High-density lipoprotein (HDL) participates in reverse cholesterol transport primarily through apolipoprotein A-I (ApoA-I), which binds macrophage ATP-binding cassette transporter ABCA1 to facilitate cholesterol efflux (2, 6, 7). Because of this role in removing excess cholesterol, both HDL and ApoA-I are considered atheroprotective. As with oxLDL, ApoA-I can become immunogenic when modified by mechanisms such as neutrophil myeloperoxidase, leading to destabilization of atherosclerotic plaques (8, 9). Anti–ApoA-I Abs have been shown to play pathologic roles in both humans and mice with autoimmune conditions including systemic lupus erythematosus, a condition commonly characterized by altered lipid profiles and an increased incidence of atherosclerosis (10–12). ApoA-I autoantibodies have also been found in human patients with cardiovascular disease and no established autoimmune conditions (13, 14). However, there is little consensus on their function or their relationship to age, sex, and diet.
Most studies involving B cell function and diet have been conducted in the context of diet-induced obesity and insulin resistance, albeit over short periods of time. For example, Winer et al. (15) observed significant increases in proinflammatory IgG2c in visceral adipose tissue and serum in C57BL/6 males fed a high-fat diet for 6–12 wk and suggested a pathogenic role for B cells in the development of insulin resistance. Another study of atherosclerotic progression found that MHC class II expression on B cells was required for oxLDL-specific IgM and IgG1 responses in mice given a high-fat diet for 8 wk (16). However, this study concluded that oxLDL-specific IgG1 responses were not required for atherosclerotic development and additionally suggested that IgM and IgG titers may exist in a regulatory balance. To the best of our knowledge, no other study has examined how IgG subclasses change over the course of more than 9 mo and how aging, sex, and diet might have confluent effects on both normal subclass development and abnormal anti–self-responses. In the current study, we show that anti–ApoA-I responses develop early in life and that diet may influence IgG subclass levels in a sex-dependent manner.
MATERIALS AND METHODS
Mice
Six-week-old male and female C57BL/6J mice (stock no. 000664) and male B6.129S7-Ldlrtm1Her/J (low-density lipoprotein receptor knockout [LDLr−/−]; stock no. 002207) were purchased from The Jackson Laboratory (Bar Harbor, ME) and housed in a specific pathogen-free facility at the University of Kentucky. Mice were sedated for blood collection and immunization using isoflurane gas. Blood was collected by superficial temporal vein puncture using a small animal lancet (Medipoint) into a microcentrifuge tube and centrifuged for 10 min at 2000 × g after standing at 4°C for 1 h. Serum was stored at −80°C for later Ab and cholesterol detection. Study diet, consisting of ad-libitum normal chow (Fig. 1 only; Teklad no. 2918), WD (no. D12079B; Research Diets), or controldiet, (CD; no. D14042701; Research Diets) was commenced following initial blood collection. All procedures were approved by the University of Kentucky Institutional Animal Care and Use Committee.
FIGURE 1. Anti–ApoA-I immunization is not required to break tolerance in dyslipidemic mice.
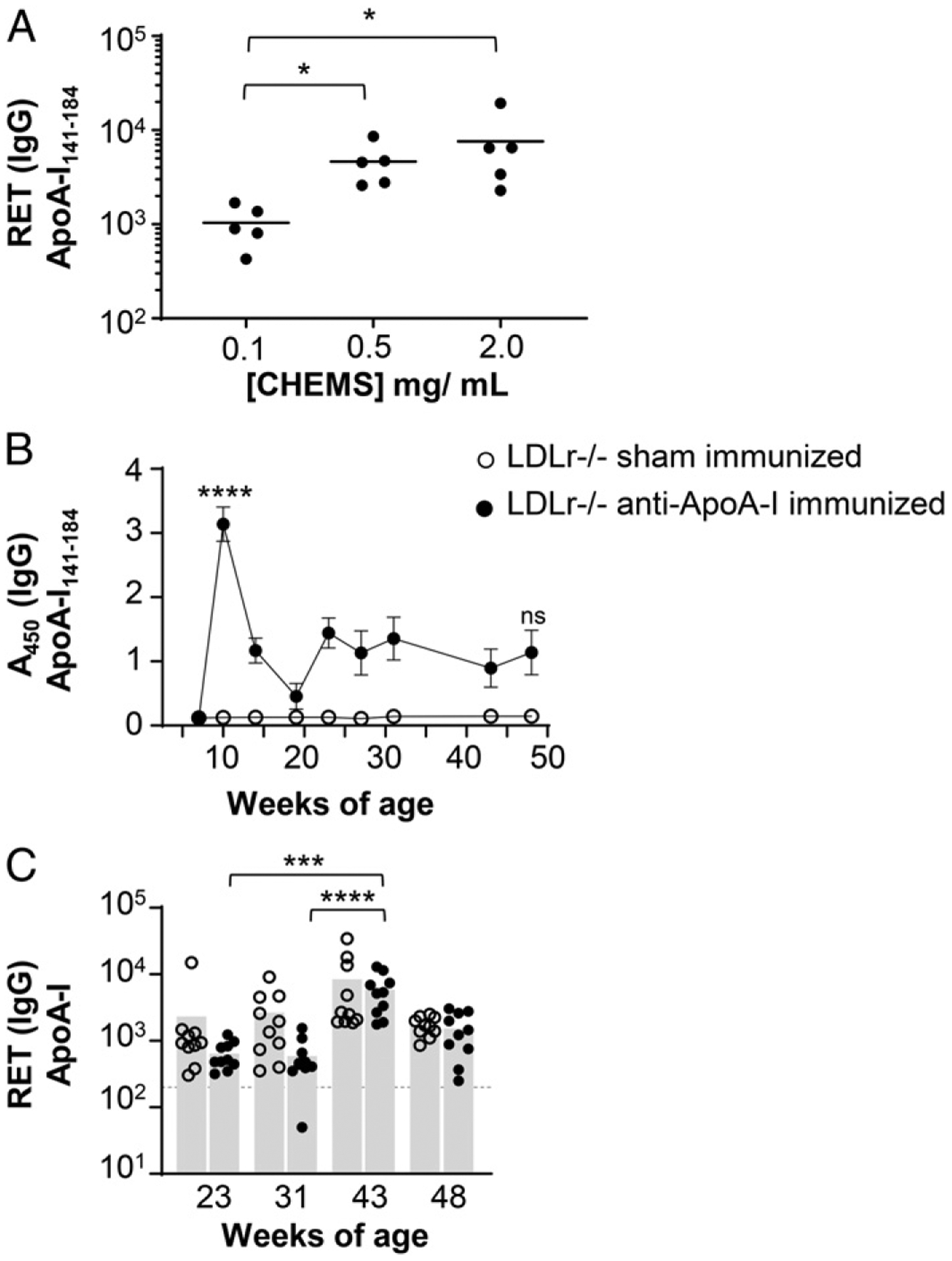
(A) Female C57BL/6 mice were immunized at 9 and 11 wk of age with a liposomal formulation containing increasing concentrations of CHEMS-conjugated ApoA-I peptide subunit (L-ApoA-I). RET for anti–ApoA-I141–184 IgG were determined 1 wk after the second immunization and results analyzed by one-way ANOVA with Dunn multiple comparisons test. Horizontal lines indicate mean; n = 5 per treatment. (B and C) Male LDLr−/− mice on a normal chow diet were immunized at 7, 9, and 22 wk of age with liposomes containing L-ApoA-I and 1.5 mol% MPL, or 1.5 mol% MPL alone (sham), and monitored regularly until 48 wk of age. (B) Average absorbance values ± SEM for IgG responses to the immunized Ag ApoA-I141–184 are shown. Significance determined by two-way ANOVA with Tukey multiple comparisons test; comparisons are shown for sham versus immunized at 10 and 48 wk. (C) RET for anti– full-length ApoA-I IgG were determined through serial dilution. Bars indicate mean; symbols correspond to individual mice in each treatment group. Significance determined by one-way ANOVA with Dunn multiple comparisons test; n = 10 mice per treatment. *p < 0.05, ***p < 0.001, ****p < 0.0001.
One cage of (5) sham-immunized female mice on CD was removed from study because of development of a skin condition unrelated to the study. Thus, Figs. 2 and 3 use five mice in that group and 10 in all others.
FIGURE 2. Chronic anti–ApoA-I responses develop in wild-type mice over time.

Male and female C57BL/6 mice on CD or WD were given a sham immunization at 8 and 10 wk of age; n = 10 mice per group except for female CD, where n = 5. (A) Total serum cholesterol values were determined by colorimetric assay; symbols indicate mean values ± SEM. (B) Serum IL-6 results are shown at 9 and 11 wk. (C) Absorbance values at 450 nm are shown for anti–full-length ApoA-I IgG; dashed line indicates limit of detection. (A) Significance determined by two-way ANOVA with Tukey multiple comparisons test. (B and C) Significance determined by one-way ANOVA with Dunn multiple comparison test; bars indicate mean and symbols correspond to individual mice. *p < 0.05, **p < 0.01, ****p < 0.0001. ns, not significant.
FIGURE 3. WD depresses IgG2b and IgG2c secretion in male mice.

Male and female C57BL/6 mice were sham-immunized and placed on diet as detailed in Fig. 2. Total serum IgG1, IgG2b, IgG2c, and IgG3 were analyzed by ELISA. Mean values ± SEM for 7, 11, and 41 wk of age are shown; results analyzed by two-way ANOVA with Tukey multiple comparisons test. Significant comparisons only are shown for the terminal bleed. *p < 0.05, **p < 0.01. ns, not significant.
Immunizations
Mice were immunized s.c. three times at 7, 9, and 22 wk of age (LDLr−/−mice) or twice at 8 and 10 wk (C57BL/6 mice)with 50 μl of a 20 mM liposomal formulation prepared as follows. A mixture of 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC; Avanti Polar Lipids), 1,2-dimyristoyl-sn-glycero-3-phosphoglycerol (DMPG; Avanti Polar Lipids), cholesterol, and monophosphoryl lipid A (MPL; Sigma-Aldrich) were mixed at a 15:2:3:0.3 molar ratio and dried by rotary evaporation. The mixture was dissolved in equal parts chloroform and methanol and a portion was removed for preparation of peptide-free injections (sham immunization). The remaining lipids were combined with cholesteryl hemisuccinate (CHEMS)–conjugated ApoA-I peptide (ApoA-I141–184; Elim Biopharm) at a concentration of 1 mg/ml in chloroform and the lipids were dried down slowly to form a thin lipid film. On the day of immunization, lipids were rehydrated in sterile PBS and sonicated at room temperature until translucent. The resulting liposome solution was extruded 11 times through a 0.2-μm filter (Avanti Polar Lipids) before injection.
ApoA-I peptide biotinylation
Biotinylation of a peptide sequence spanning residues 141–184 of the mouse ApoA-I protein (ApoA-I141–184) was carried out by adding 50 mg of crude peptide on resin (sequence βAGGLS PVAEEFRDRMRTHVDSLRTQLAPHSEQMRESLAQRLAELK SN) (Elim Biopharm) to a peptide synthesis vessel containing 0.28 mmol biotin (Sigma-Aldrich), 0.17 mmol hydroxybenzo-triazole (HOBT; TCI America), 0.24 mmol hexafluorophosphate benzotriazole tetramethyl uranium (HBTU; TCI America) and 15 μl of diisopropylethylamine (DIPEA; TCI America) in 2 ml of N-methyl-2-pyrrolidone (VWR Chemicals). This mixture was charged with nitrogen gas and allowed to react for 7 d at room temperature.
After 7 d the reaction mixture was removed, and the resin beads were washed of excess biotin three times using methanol and chloroform, and dried. The resin beads were then transferred to a stirring solution of 4.7 ml of trifluoracetic acid, 125 μl of ethanedithiol, 125 μl of water, and 50 μl of triisopropylsilane. After 30 min, this solution was pipetted through a glass wool filter into a conical vial containing cold ether (−20°C) and left overnight at −20°C. The following morning, the conical vial was centrifuged at 4000 rpm for 5 min and the peptide pellet was resuspended in cold ether and allowed to sit for overnight at −20°C. Centrifugation was repeated, and the resulting pellet was dried, weighed, and resuspended in a 1:1 mixture of water and tetrahydrofuran at 2 mg/ml. Concentration was confirmed by absorbance at 205 nm.
Circulating Ab ELISA
Serum samples were diluted in PBS containing 0.05% casein (PBS-C; 124240; Beantown Chemical), as noted in figure legends, and kept frozen at −80°C until use. In all cases, plates were washed six times with 200 μl of PBS containing 0.1% Tween-20 (Sigma-Aldrich) between steps. Samples and HRP-conjugated detection Abs were incubated for 30 min at 37°C using 100 μl of each. Binding was quantified by incubating the samples with 100 μl of tetramethylbenzidine (Rockland) for 30 min at room temperature and then quenched with 0.5 M H2SO4. Absorbance at 450 nm was recorded using a BioTek Synergy H1 microplate reader.
For anti–full-length ApoA-I ELISA, 96-well high-binding plates (Greiner Bio-One) were coated with 100 μl of mouse ApoA-I (MBS206112; MyBioSource) diluted to 1 μg/ml in carbonate buffer (0.53% sodium carbonate [pH 9.7]; VWR LifeScience) and incubated for 2 h at 37°C. Plates were blocked with 100 μl of PBS-C for 30 min. and diluted samples were subsequently applied in duplicate for an additional 30 min.Binding was detected using goat anti-mouse IgG-HRP (16066; Invitrogen) diluted 1:2000 in PBS-C.
For anti–ApoA-I141–184 (peptide) ELISA, 96-well streptavidin-coated plates (05124; Thermo Fisher Scientific) were coated with 100 μl of biotinylated mouse ApoA-I141–184 diluted to 2 μg/ml in PBS containing 0.1% Tween-20 and incubated for 2 h at 37°C. Diluted samples were applied in duplicate and incubated for an additional 30 min. Binding was detected using goat anti-mouse IgG-HRP, as above.
Circulating Ab reciprocal end point titers (RET) were quantified by serial dilution beginning at 1:200 using capture and detection strategies as outlined above. The slope of the linear portion of the curve was calculated and divided by two times the average of the serum-free control wells to generate a RET.
For total subclass-specific ELISA, high-binding plates were coated with goat anti-mouse subclass-specific Abs diluted to 2 μg/ml in carbonate buffer (IgG1 [115-005-205; Jackson ImmunoResearch], IgG2b [115-005-207; Jackson ImmunoResearch], IgG2c [115-005-208; Jackson ImmunoResearch], IgG3 [115-005-209; Jackson ImmunoResearch]) and incubated for 2 h at 37°C. Plates were blocked with PBS-C for 30 min. Isotype controls (IgG1 [02-6100; Invitrogen], IgG2b [02-6300; Invitrogen], IgG2c [MBS 679122; MyBioSource], IgG3 [14-4742-82; eBiosciences]) were used to create a standard curve and samples were diluted 1:2000-1:120,000, dependent on age and immunization status, before being added to plates in duplicate for an additional 30 min. Binding was detected with subclass-specific HRP Abs (IgG1 [ab98693, 1 μg/40 ml; Abcam], IgG2b [ab98703, 1 μg/40 ml; Abcam], IgG2c [ab98722, 1 μg/10 ml; Abcam], IgG3 [115-035-209, 1 μg/12.5 ml; Jackson]).
Epitope mapping by peptide array
Serum samples from the mice shown in Figs. 2 and 3 with an anti–full-length ApoA-I absorbance (A450) >1.0 at study termination were selected for epitope mapping by peptide array. Where possible, a matched sample from 11 wk of age was also included. Serum samples were analyzed by JPT Peptide Technologies (Berlin, Germany) PepStar peptide microarrays using a series of 58 synthetic 15-mer peptides with 4-aa offsets spanning the entire 243-aa sequence of murine ApoA-I. Serum samples were incubated with the microarray slide for 1 h at 30°C. After washing the microarray, fluorescently labeled anti-mouse IgG secondary Ab at 1 μg/ml was added and incubated for 1 h. Control wells lacking mouse serum were used to assess nonspecific binding of the secondary Ab toward each peptide. After washing, the array was scanned at 635 nm to obtain fluorescence intensity profiles and resulting images were quantified to yield a mean pixel value for each peptide, which is represented as a heatmap.
Cytokine ELISA
IL-6 and IFN-γ ELISA Max kits were purchased from BioLegend (no. 431305 and 430805) and assays were performed according to manufacturer instructions. Because of time considerations, serum samples were analyzed after being frozen overnight.
Cholesterol quantification
Serum was diluted in 0.9% saline and quantified at 492 nm using an enzymatic cholesterol reagent (C7510; Pointe Sci) and its accompanying standard (C7509).
Statistics
Statistical comparisons were performed using Graph Pad Prism version 8. Fig. 1A and 1B had n = 5 mice per group and Figs. 1C, 2, and 3 had n = 10 mice per group except for sham-immunized female mice on CD, for which five females on CD were removed from consideration as stated in the Materials and Methods section on Mice. Statistical methods are detailed in each figure: *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
RESULTS
Liposomal ApoA-I immunization generates a rapid Ab response
Autoantibodies to ApoA-I have been reported in human patients with cardiovascular disease (14, 17, 18). These Abs have also been observed in patients with various immunopathologies and correlate with increased levels of inflammatory biomarkers (14, 18, 19). To investigate the link between inflammation and potentially atherogenic autoantibodies, we immunized female C57BL/6 mice against the domain of ApoA-I spanning the lecithin:cholesterol acyltransferase (LCAT) binding region (ApoA-I141–184), which has been reported as a target of these Abs in humans (17). The liposomal vaccine (L-ApoA-I) consisted of natural lipids, the adjuvant monophosphoryl lipid A (MPL), and ApoA-I141–184, which was anchored to the liposome via CHEMS. The immunization strategy was tested at various concentrations of peptide (Fig. 1A) and demonstrated a robust antipeptide response in C57BL/6 females following two immunizations with peptide concentrations of 0.5 and 2 mg/ml, which aligns with the findings of previous studies using this strategy (20).
Because chronic inflammatory conditions, including autoimmune diseases, often correlate with altered serum lipid profiles and an increased risk of cardiovascular disease (21), we hypothesized that anti–ApoA-I immunization of dyslipidemic mice would provide a reasonable model to observe anti–self-responses over time. Male mice lacking the low-density lipoprotein receptor (LDLr−/−) on a standard chow diet were immunized with liposomal ApoA-I and MPL or given a sham immunization and then followed until 48 wk of age. The immunization resulted in a robust antipeptide response (Fig. 1B), and both groups of mice maintained total serum cholesterol levels of ~250–300 mg/dl throughout the study (data not shown), which is considered normal for the strain (22). However, there was no detectable response against the full ApoA-I protein until 1 wk after a booster at 23 wk of age, and after that point, responses in both immunized and sham-immunized mice increased similarly until a peak at 43 wk of age (Fig. 1C). This suggested that either aging or dysregulated lipid homeo-stasis might be a trigger for development of anti–ApoA-I autoantibodies, which led us to explore the interactions of WD, sex, and aging and how each might contribute to anti–self-responses.
Chronic anti–ApoA-I responses develop with age in male and female wild-type mice
Many autoimmune conditions, including systemic lupus erythematosus, scleroderma, and Grave disease are strongly biased toward females (23). Levels of leptin, the satiety hormone, are also higher in females (24) and have been linked to development of autoimmune diabetes in female nonobese diabetic mice (25). To separately examine the contributions of diet and sex to anti–ApoA-I responses, male and female C57BL/6 mice were placed on WD or a CD with no added cholesterol, given a sham immunization, and monitored until 41 wk of age. Consumption of WD prompted a rapid increase in total serum cholesterol levels to more than 250 mg/dl in male mice; however, no significant change was observed in females (Fig. 2A). The difference was maintained or increased over the course of the study, with males ending at ~300 mg/dl. This observation agrees with previous studies performed over a shorter time span (26, 27).
Circulating levels of IL-6, a pleiotropic cytokine with roles in the acute phase response and development of autoimmunity (28), were also analyzed at 9 and 11 wk of age. IL-6 levels were not assayed at the outset of the study; however, females on both CD and WD displayed significantly higher levels of circulating IL-6 in comparison with males at 9 wk, averaging ~3–4 μg/ml, respectively (Fig. 2B). By 11 wk of age, serum IL-6 in female mice had begun to decrease and was not significantly different from the values found in male serum. IFN-γ levels were also measured at the same times; however, no measurable IFN-γ was found in any treatment group (data not shown).
Previous unpublished observations from our laboratory have indicated that a very small percentage of mice tend to have transient anti–ApoA-I responses regardless of immunization or age, but that these responses do not persist over time. When total IgG directed against full-length ApoA-I was assayed (Fig. 2C), we again observed some mice, particularly females, with high A450 readings at the outset of the study and an overall increase in responses as the mice aged. Males on CD tended to exhibit growing anti– ApoA-I responses over time, although the increase was quite variable, and females fed CD had no significant change in overall responses. Although female mice on WD showed a tendency toward increasing anti–ApoA-I responses over time, large variations made differences ultimately not significant. Male mice on WD exhibited a slow but significant increase in anti–ApoA-I IgG between 7 and 41 wk of age, with all values rising above the limit of detection. Together, these data indicated that a variable immune response toward ApoA-I started at a young age in both males and females, increased with time, and was not strictly dependent on diet.
WD suppresses age-dependent IgG2b and IgG2c increases in male mice
Sex and diet are known to influence immunity through diverse mechanisms such as microbiota metabolite production, TLR4 signaling, and inflammasome activation (29–34). Given that anti–ApoA-I responses increased with age and tended to be more pronounced in mice on WD, we hypothesized that consumption of WD would also cause an inflammatory shift in total IgG profiles and that the shift would become apparent as the mice aged. Therefore, total circulating IgG1, IgG2b, IgG2c, and IgG3 were monitored by ELISA in the same sham-immunized male and female mice until study termination at 41 wk of age. Of note, C57BL/6 mice do not possess the IgG2a allele, but instead produce IgG2c (35).
As shown in Fig. 3, IgG1 concentrations increased over time in both male and female mice by 2- to 3-fold regardless of diet. Females on either diet displayed a definite preference for IgG2b secretion, but male mice on WD had nearly 50% less IgG2b and 40% less IgG2c at 41 wk of age than their counterparts on CD. IgG3 increased by ~5-fold in males, regardless of diet, whereas in females the increases were more gradual and variable. These data suggest that although young wild-type male mice are Th2 inclined, consumption of WD might paradoxically prevent age-related increases in inflammatory IgG subclasses as anti–self-Abs simultaneously increase with age.
Robust responses against a single nonimmunization epitope are detectable regardless of diet or sex
The target of the anti–ApoA-I Abs that developed without immunization remained of interest. To assess the specificity of immune response, serum from sham-immunized mice with anti–full-length ApoA-I A450 values over 1.0 at study termination (old) was selected for analysis by peptide array (Fig. 4A). Where possible, corresponding samples that met the threshold from 11 wk of age were also included (young). As a comparison, samples from mice that had received anti–ApoA-I immunization were also included (Fig. 4B). Of note, there were more samples that met the A450 threshold in mice that had received immunization, but IgG RET against the full protein at study termination were generally low, regardless of treatment (Table I).
FIGURE 4. Robust responses to a single peptide are detectable regardless of treatment.

Eighteen serum samples from immunized and sham-immunized C57BL/6 mice (see Table I) with A450 values above 1.0 at 11 wk (young [Y]) or terminal bleed (old [O]) timepoints were analyzed on a peptide array. The array spanned the entire 243-aa sequence of murine ApoA-I with 58 peptides of 15 aa in length using a 4-aa offset. Portions of the heatmap and corresponding peptides where mouse sera were found to bind as determined using numerical values produced from scanning the fluorescent array at 635 nm are shown. A control (Ctl) lane is included in the microarray assay, which includes immobilized peptides and secondary Abs, but not mouse sera. (A) Values for sham-immunized mice are shown. (B) Values for ApoA-I immunized mice are shown.
TABLE I.
Anti–ApoA-I A450 values and RET for mouse sera evaluated by peptide array
| Heatmap Number | Young A450 (FL) | Old A450 (FL) | Old RET | |
|---|---|---|---|---|
| Sham immunization | ||||
| Male CD | 1 | 1.81 | 2174 | |
| 2 | 1.04 | 2.31 | 2472 | |
| Male WD | 3 | 1.44 | 1.43 | 1019 |
| Female WD | 4 | 1.10 | 1242 | |
| 5 | 1.32 | 1426 | ||
| Anti-ApoA-I141−184 immunization | ||||
| Male CD | 6 | 1.76 | 1.96 | 1000 |
| Male WD | 7 | 1.63 | 934 | |
| 8 | 1.30 | ND | ||
| Female CD | 9 | 1.20 | 2545 | |
| 10 | 2.08 | 2.87 | 7579 | |
| Female WD | 11 | 1.67 | 1.05 | 878 |
| 12 | 1.39 | 1.22 | 744 | |
Samples with A450 values >1.0 at study termination (41 wk) were chosen from each group and designated as Old. Where possible, matched samples from 11 wk of age were selected for mice with A450 >1.0, designated as Young.
FL, full-length ApoA-I.
The peptide array consisted of the entire 243-aa sequence divided into 58 15-mer peptides with a 4-aa offset. Remarkably, every sample analyzed showed a robust response to only one peptide: SQLQERLGPLTRDFW (ApoA-I57–71). Lower-intensity and more variable responses were detected in immunized mice to an area corresponding to the immunization peptide EEFRDRMRTHVDSLR (ApoA-I145–159) and the two overlapping peptides surrounding it. When recombinant full-length ApoA-I protein was assayed as a control, responses were as low as 10% of the values observed for the most reactive epitope; however, the conformation in which the full protein was affixed to the assay plate could have limited Ab binding in this case. Where samples were able to be analyzed from both young and old mice, there was little to no alteration in epitope specificity and no pattern observed in the intensity of the responses. Together, the data presented in this peptide array indicated that anti–ApoA-I responses were likely to begin early in life and that an aberrant lipid profile was not required for their initiation, although their role in the pathology of atherosclerosis and inflammation remains to be elucidated.
DISCUSSION
Once thought to be primarily a disease of overconsumption and imbalance, atherosclerosis is now beginning to be understood as a multifactorial process involving metabolic insult, chronic inflammation, modification of self-antigens, and Ab responses (36, 37). Previous studies have found that autoantibodies raised against oxLDL, HMGB-1, and HSP60 could be generated in mice fed a high-fat diet for as little as 2 mo (1, 3–5). Although some have suggested that the initial development of these autoantibodies facilitates the removal of waste products (4), it seems likely that over time, detrimental effects would develop. In this study, we modeled autoimmunity with anti–ApoA-I immunization and unexpectedly found that both male and female mice developed Abs against a separate, discrete 15-aa sequence contained within the ApoA-I protein irrespective of immunization. Furthermore, we showed WD suppressed age-dependent increases in IgG2b and IgG2c in male mice, but not female mice, suggesting links to Th1/Th2 balance and implications in immunity. These results emphasize the necessity of using both sexes in studies of immune contributions to chronic inflammatory conditions and indicate that further exploration of the immunogenicity of certain epitopes in ApoA-I is needed.
In general, female sex is somewhat protective against metabolic changes induced by WD. For example, Zhu et al. (27) recently reported that activation of estrogen-α receptors in hepatocytes protects female mice on WD against insulin resistance and lipid deposition in the artery wall, but not males. Additionally, premenopausal women have less atherogenic plasma lipid profiles compared with body-matched men, indicating roles for estrogen signaling in lipid clearance (38). Over the course of our study, female mice on WD gained weight as expected but had very little change in serum cholesterol levels in contrast to the male mice, which reached an average of 300 mg/dl. These data support previous studies suggesting estrogen-mediated mechanisms of cholesterol control (27, 38, 39).
In mice, IgG1 directs a Th2- like response, whereas IgG2a/2c, IgG2b, and IgG3 are associated with Th1-like responses and cellular immunity (40). Total serum Ig levels increase with age in both mice and humans; however, human studies have found decreased class switch recombination with aging (41, 42). In C57BL/6 mice, age- and sex-dependent differences in total IgG2b and IgG3 composition have been noted with and without immunization (43). The relationship of diet to changes in total, nonspecific IgG subclass composition and the physiological relevance of these changes, however, is still unclear. In this study, we observed significant increases in total serum cholesterol levels and a blunting of IgG2b and IgG2c increases in WT male mice fed WD, but not CD. Females, in contrast, were not significantly affected by diet. Jones et al. (43) and others have reported that estrogen can modulate Ig levels in both mice and humans (44, 45). This effect was also observed ex vivo using B cells stimulated with LPS and suggests a sex hormone–mediated modulation of the immune response to pathogens (43). In this context it may be reasonable to expect deficient complement fixation in aged male mice on WD, where one or more Th1-type subclasses are suppressed. To the best of our knowledge, no studies have found major sex-based differences in IgG2c; however, this may be because of the fact that more isotyping has been done for IgG2a. One study that did isotype IgG2c found that feeding male C57BL/6 mice a high-fat diet for 6–12 wk resulted in increased serum IgG2c and no change in IgG2b (15); however, that study differed from ours in length of feeding, absolute diet composition, and the delivery of a sham immunization. Our study also supports the work of Zhouet al. (46), who found that feeding male ApoE−/−C57BL/6 mice a high-cholesterol diet for only 5 wk resulted in a switch of anti-oxLDL Abs to IgG1, with little change in IgG2a/2c. They suggested that hypercholesterolemia created a Th2 bias in the Ab response, agreeing with our finding that only male mice exhibited both high cholesterol and aberrant total subclass profiles when placedon WD. Although studies have been limited, it is known that a high-fat, high-cholesterol diet increases circulating ApoA-I levels in male C57BL/6 mice but not females (47, 48). This suggests that in our mice, hypocholesterolemia-mediated increased availability of ApoA-I could have driven a T cell–mediated change in the Ab profile.
Despite being partially protected against WD-induced morbidity, females have been noted to have higher circulating levels of inflammatory mediators than study-matched males (49, 50). Sullivan et al. (50) recently showed that young women with coronary artery disease exhibited higher circulating levels of IL-6 after mental stress than did men. This study also suggested that IL-6 elevation following acute stress may play a causal role in early-onset coronary artery disease in young females. Because the mice all received a sham immunization, it is not possible to discern between immunization-related effects and simple stress; however, these data support the previous findings of Sullivan et al. and others. Interestingly, the IL-6 levels we report in this study are ~100-fold greater than what Schulte et al. (51) saw in a study of male ApoE−/− mice on a C57BL/6 background consuming an atherogenic diet. The authors of that study associated atherosclerotic lesion development with increased IL-6 levels but un-fortunately did not use female mice in their study.
The results of the peptide array showed that the most immunogenic sequence in mouse ApoA-I was found in aa 57–71. The specificity of the responses toward this very narrow sequence suggests molecular mimicry or a natural immune-mediated mechanism of ApoA-I regulation. Notably, although various epitopes have been described in human subjects, this is the first immunogenic epitope of ApoA-I identified in mice (18, 52). Although the function of the particular sequence identified in our studies has not been fully elucidated, data from humans and structural studies offer some insight. A mutant form of ApoA-I, known as ApoA-I Iowa, has been identified in the N-terminal segment and results in fibril formation (53). Although this mutation is outside of the identified epitope, modifications that alter the secondary structure of the protein may lead to increased immunogenicity in adjacent segments. Structural studies indicate that the N-terminal domain exhibits an open and closed state based on its interaction with the lipid content of HDL, which is influenced by cholesterol and ApoA-I content of the particle (54). However, the extent to which this conformational change occurs in vivo in a mouse is unclear. These data warrant continued evaluation of the immunogenicity of the identified epitope, which shares 66% sequence homology with human ApoA-I, and its significance in cardiovascular immunology.
Our work, along with that of others, demonstrates that autoantibodies develop naturally to ApoA-I and that dysregulation of Ab class composition occurs as a result of aging and various other environmental factors. It has also been noted that because several hundred 1000 Ig molecules are required to facilitate complement-mediated lysis of a single RBC, autoantibodies are nearly impotent unless their target is expressed very densely on self-cells, with a few notable exceptions (40). It remains to be seen, then, whether anti–ApoA-I autoantibodies are the exception or the rule, and how their development early in life is relevant to atherosclerotic plaque deposition. The potential ties between diet, sex, and Ig class switching should be further investigated in disease-prone mice for their biological relevance in the context of both atherosclerosis and diet-induced obesity, as well as overall immune system function, as these findings could help in the understanding of the immune components of atherosclerosis and may elucidate therapeutic targets to prevent or treat disease.
ACKNOWLEDGMENTS
We thank Nicholas Cheung, Robert Kline IV, Matthew Cox, and Michelle Marti for technical assistance and Beth Garvy for a critical reading of the manuscript.
This work was supported by the American Heart Association (17SDG32670001) and the National Institutes of Health (NIH) (P30GM127211, P20GM130456, R01HL152081). M.G.P. was supported by a training grant from the NIH (T32HL091812), D.N. was supported by a training grant from the National Center for Advancing Translational Sciences, NIH (TL1TR001997), and C.M.I. was supported by a Louis Stokes Alliance for Minority Participation scholarship, National Science Foundation (1826763).
Abbreviations used in this article:
- ApoA-I
apolipoprotein A-I
- CD
control diet
- CHEMS
cholesteryl hemisuccinate
- HDL
high-density lipoprotein
- LDLr−/−
low-density lipoprotein receptor knockout
- MPL
monophosphoryl lipid A
- oxLDL
oxidized low-density lipoprotein
- PBS-C
PBS containing 0.05% casein
- RET
reciprocal end point titer
- WD
Western diet
Footnotes
DISCLOSURES
The authors have no financial conflicts of interest.
REFERENCES
- 1.Palinski W, Tangirala RK, Miller E, Young SG, and Witztum JL. 1995. Increased autoantibody titers against epitopes of oxidized LDL in LDL receptor-deficient mice with increased atherosclerosis. Arterioscler. Thromb. Vasc. Biol 15: 1569–1576. [DOI] [PubMed] [Google Scholar]
- 2.Chistiakov DA, Orekhov AN, and Bobryshev YV. 2016. ApoA1 and ApoA1-specific self-antibodies in cardiovascular disease. Lab. Invest 96: 708–718. [DOI] [PubMed] [Google Scholar]
- 3.Şelli ME, Wick G, Wraith DC, and Newby AC. 2017. Autoimmunity to HSP60 during diet induced obesity in mice. Int. J. Obes 41: 348–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nilsson J, and Hansson GK. 2008. Autoimmunity in atherosclerosis: a protective response losing control? J. Intern. Med 263: 464–478. [DOI] [PubMed] [Google Scholar]
- 5.Pan Y, Ke H, Yan Z, Geng Y, Asner N, Palani S, Munirathinam G, Dasari S, Nitiss KC, Bliss S, et al. 2016. The western-type diet induces anti-HMGB1 autoimmunity in Apoe(−/−) mice. Atherosclerosis 251: 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lund-Katz S, and Phillips MC. 2010. High density lipoprotein structure-function and role in reverse cholesterol transport. Subcell. Biochem 51: 183–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao GJ, Yin K, Fu YC, and Tang CK. 2012. The interaction of ApoA-I and ABCA1 triggers signal transduction pathways to mediate efflux of cellular lipids. Mol. Med 18: 149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng L, Nukuna B, Brennan ML, Sun M, Goormastic M, Settle M, Schmitt D, Fu X, Thomson L, Fox PL, et al. 2004. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J. Clin. Invest 114: 529–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montecucco F, Vuilleumier N, Pagano S, Lenglet S, Bertolotto M, Braunersreuther V, Pelli G, Kovari E, Pane B, Spinella G, et al. 2011. Anti-Apolipoprotein A-1 auto-antibodies are active mediators of atherosclerotic plaque vulnerability. Eur. Heart J 32: 412–421. [DOI] [PubMed] [Google Scholar]
- 10.Batuca JR, Ames PR, Isenberg DA, and Alves JD. 2007. Antibodies toward high-density lipoprotein components inhibit paraoxonase activity in patients with systemic lupus erythematosus. Ann. N. Y. Acad. Sci 1108: 137–146. [DOI] [PubMed] [Google Scholar]
- 11.Srivastava R, Yu S, Parks BW, Black LL, and Kabarowski JH. 2011. Autoimmune-mediated reduction of high-density lipoprotein-cholesterol and paraoxonase 1 activity in systemic lupus erythematosus-prone gld mice. Arthritis Rheum. 63: 201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hahn BH, and McMahon M. 2008. Atherosclerosis and systemic lupus erythematosus: the role of altered lipids and of autoantibodies. Lupus 17: 368–370. [DOI] [PubMed] [Google Scholar]
- 13.Antiochos P, Marques-Vidal P, Virzi J, Pagano S, Satta N, Hartley O, Montecucco F, Mach F, Kutalik Z, Waeber G, et al. 2017. Anti-apolipoprotein A-1 IgG predict all-cause mortality and are associated with Fc receptor-like 3 polymorphisms. Front. Immunol 8: 437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teixeira PC, Cutler P, and Vuilleumier N. 2012. Autoantibodies to apolipoprotein A-1 in cardiovascular diseases: current perspectives. Clin. Dev. Immunol 2012: 868251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winer DA, Winer S, Shen L, Wadia PP, Yantha J, Paltser G, Tsui H, Wu P, Davidson MG, Alonso MN, et al. 2011. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat. Med 17: 610–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams JW, Elvington A, Kessler S, Wohltmann M, Wu GF, and Randolph GJ. 2019. B cell-mediated antigen presentation through MHC class II is dispensable for atherosclerosis progression. Immunohorizons 3: 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teixeira PC, Ducret A, Ferber P, Gaertner H, Hartley O, Pagano S, Butterfield M, Langen H, Vuilleumier N, and Cutler P. 2014. Definition of human apolipoprotein A-I epitopes recognized by autoantibodies present in patients with cardiovascular diseases. J. Biol. Chem 289: 28249–28259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vuilleumier N, Rossier MF, Pagano S, Python M, Charbonney E, Nkoulou R, James R, Reber G, Mach F, and Roux-Lombard P. 2010. Anti-apolipoprotein A-1 IgG as an independent cardiovascular prog-nostic marker affecting basal heart rate in myocardial infarction. Eur. Heart J 31: 815–823. [DOI] [PubMed] [Google Scholar]
- 19.Dinu AR, Merrill JT, Shen C, Antonov IV, Myones BL, and Lahita RG. 1998. Frequency of antibodies to the cholesterol transport protein apolipoprotein A1 in patients with SLE. Lupus 7: 355–360. [DOI] [PubMed] [Google Scholar]
- 20.Watson DS, and Szoka FC Jr. 2009. Role of lipid structure in the humoral immune response in mice to covalent lipid-peptides from the membrane proximal region of HIV-1 gp41. Vaccine 27: 4672–4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaplan MJ 2009. Management of cardiovascular disease risk in chronic inflammatory disorders. Nat. Rev. Rheumatol 5: 208–217. [DOI] [PubMed] [Google Scholar]
- 22.Ishibashi S, Brown MS, Goldstein JL, Gerard RD, Hammer RE, and Herz J. 1993. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J. Clin. Invest 92: 883–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubtsova K, Marrack P, and Rubtsov AV. 2015. Sexual dimorphism in autoimmunity. J. Clin. Invest 125: 2187–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saad MF, Damani S, Gingerich RL, Riad-Gabriel MG, Khan A, Boyadjian R, Jinagouda SD, el-Tawil K, Rude RK, and Kamdar V. 1997. Sexual dimorphism in plasma leptin concentration. J. Clin. Endocrinol. Metab 82: 579–584. [DOI] [PubMed] [Google Scholar]
- 25.Matarese G, Sanna V, Lechler RI, Sarvetnick N, Fontana S, Zappacosta S, and La Cava A. 2002. Leptin accelerates autoimmune diabetes in female NOD mice. Diabetes 51: 1356–1361. [DOI] [PubMed] [Google Scholar]
- 26.Jarrett KE, Lee C, De Giorgi M, Hurley A, Gillard BK, Doerfler AM, Li A, Pownall HJ, Bao G, and Lagor WR. 2018. Somatic editing of Ldlr with adeno-associated viral-CRISPR is an efficient tool for atherosclerosis research. Arterioscler. Thromb. Vasc. Biol 38: 1997–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu L, Shi J, Luu TN, Neuman JC, Trefts E, Yu S, Palmisano BT, Wasserman DH, Linton MF, and Stafford JM. 2018. Hepatocyte estrogen receptor alpha mediates estrogen action to promote reverse cholesterol transport during Western-type diet feeding. Mol. Metab 8: 106–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka T, Narazaki M, and Kishimoto T. 2014. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol 6: a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belkaid Y, and Hand TW. 2014. Role of the microbiota in immunity and inflammation. Cell 157: 121–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yurkovetskiy L, Burrows M, Khan AA, Graham L, Volchkov P, Becker L, Antonopoulos D, Umesaki Y, and Chervonsky AV. 2013. Gender bias in autoimmunity is influenced by microbiota. Immunity 39: 400–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hills RD Jr., Pontefract BA, Mishcon HR, Black CA, Sutton SC, and Theberge CR. 2019. Gut microbiome: profound implications for diet and disease. Nutrients 11: 1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christ A, Gunther P, Lauterbach MAR, Duewell P, Biswas D, Pelka K, Scholz CJ, Oosting M, Haendler K, Bassler K, et al. 2018. Western diet triggers NLRP3-dependent innate immune reprogramming. Cell 172: 162–175.e14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim K-A, Gu W, Lee I-A, Joh E-H, and Kim D-H. 2012. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS One 7: e47713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kramer B, França LM, Zhang Y, Paes AMA, Gerdes AM, and Carrillo-Sepulveda MA. 2018. Western diet triggers toll-like receptor 4 signaling-induced endothelial dysfunction in female Wistar rats. Am. J. Physiol. Heart Circ. Physiol 315: H1735–H1747. [DOI] [PubMed] [Google Scholar]
- 35.Morgado MG, Cam P, Gris-Liebe C, Cazenave PA, and Jouvin-Marche E. 1989. Further evidence that BALB/c and C57BL/6 gamma 2a genes originate from two distinct isotypes. EMBO J. 8: 3245–3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hansson GK, and Nilsson J. 2008. Introduction: atherosclerosis as inflammation: a controversial concept becomes accepted. J. Intern. Med 263: 462–463. [DOI] [PubMed] [Google Scholar]
- 37.Libby P, Lichtman AH, and Hansson GK. 2013. Immune effector mechanisms implicated in atherosclerosis: from mice to humans. [Published erratum appears in 2013 Immunity 39: 413.] Immunity 38: 1092–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Castelli WP 1984. Epidemiology of coronary heart disease: the Framingham study. Am. J. Med 76: 4–12. [DOI] [PubMed] [Google Scholar]
- 39.Fernández-Suárez ME, Escolà-Gil JC, Pastor O, Dávalos A, Blanco-Vaca F, Lasunción MA, Martínez-Botas J, and Gómez-Coronado D. 2016. Clinically used selective estrogen receptor modulators affect different steps of macrophage-specific reverse cholesterol transport. Sci. Rep 6: 32105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bretscher PA 2014. On the mechanism determining the TH1/TH2 phenotype of an immune response, and its pertinence to strategies for the prevention, and treatment, of certain infectious diseases. Scand. J. Immunol 79: 361–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frasca D, Landin AM, Lechner SC, Ryan JG, Schwartz R, Riley RL, and Blomberg BB. 2008. Aging down-regulates the transcription factor E2A, activation-induced cytidine deaminase, and Ig class switch in human B cells. J. Immunol 180: 5283–5290. [DOI] [PubMed] [Google Scholar]
- 42.Speziali E, Santiago AF, Fernandes RM, Vaz NM, Menezes JS, and Faria AM. 2009. Specific immune responses but not basal functions of B and T cells are impaired in aged mice. Cell. Immunol 256: 1–5. [DOI] [PubMed] [Google Scholar]
- 43.Jones BG, Sealy RE, Penkert RR, Surman SL, Maul RW, Neale G, Xu B, Gearhart PJ, and Hurwitz JL. 2019. Complex sex-biased antibody responses: estrogen receptors bind estrogen response elements centered within immunoglobulin heavy chain gene enhancers. Int. Immunol 31: 141–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Asaba J, Bandyopadhyay M, Kindy M, and Dasgupta S. 2015. Estrogen receptor signal in regulation of B cell activation during diverse immune responses. Int. J. Biochem. Cell Biol 68: 42–47. [DOI] [PubMed] [Google Scholar]
- 45.Kanda N, and Tamaki K. 1999. Estrogen enhances immunoglobulin production by human PBMCs. J. Allergy Clin. Immunol 103: 282–288. [DOI] [PubMed] [Google Scholar]
- 46.Zhou X, Paulsson G, Stemme S, and Hansson GK. 1998. Hypercholesterolemia is associated with a T helper (Th) 1/Th2 switch of the autoimmune response in atherosclerotic apo E-knockout mice. J. Clin. Invest 101: 1717–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Escolà-Gil JC, Llaverias G, Julve J, Jauhiainen M, Méndez-González J, and Blanco-Vaca F. 2011. The cholesterol content of Western diets plays a major role in the paradoxical increase in high-density lipoprotein cholesterol and upregulates the macrophage reverse cholesterol transport pathway. Arterioscler. Thromb. Vasc. Biol 31: 2493–2499. [DOI] [PubMed] [Google Scholar]
- 48.Srivastava RA, Tang J, Krul ES, Pfleger B, Kitchens RT, and Schonfeld G. 1992. Dietary fatty acids and dietary cholesterol differ in their effect on the in vivo regulation of apolipoprotein A-I and A-II gene expression in inbred strains of mice. Biochim. Biophys. Acta 1125: 251–261. [DOI] [PubMed] [Google Scholar]
- 49.Khera A, McGuire DK, Murphy SA, Stanek HG, Das SR, Vongpatanasin W, Wians FH Jr., Grundy SM, and de Lemos JA. 2005. Race and gender differences in C-reactive protein levels. J. Am. Coll. Cardiol 46: 464–469. [DOI] [PubMed] [Google Scholar]
- 50.Sullivan S, Hammadah M, Wilmot K, Ramadan R, Pearce BD, Shah A, Kaseer B, Gafeer MM, Lima BB, Kim JH, et al. 2018. Young women with coronary artery disease exhibit higher concentrations of interleukin-6 at baseline and in response to mental stress. J. Am. Heart Assoc 7: e010329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schulte S, Sukhova GK, and Libby P. 2008. Genetically programmed biases in Th1 and Th2 immune responses modulate atherogenesis. Am. J. Pathol 172: 1500–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pagano S, Gaertner H, Cerini F, Mannic T, Satta N, Teixeira PC, Cutler P, Mach F, Vuilleumier N, and Hartley O. 2015. The human autoantibody response to apolipoprotein A-I is focused on the C-terminal helix: a new rationale for diagnosis and treatment of cardiovascular disease? PLoS One 10: e0132780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kameyama H, Nakajima H, Nishitsuji K, Mikawa S, Uchimura K, Kobayashi N, Okuhira K, Saito H, and Sakashita N. 2016. Iowa mutant apolipoprotein A-I (ApoA-IIowa) fibrils target lysosomes. Sci. Rep 6: 30391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kono M, Okumura Y, Tanaka M, Nguyen D, Dhanasekaran P, Lund-Katz S, Phillips MC, and Saito H. 2008. Conformational flexibility of the N-terminal domain of apolipoprotein a-I bound to spherical lipid particles. Biochemistry 47: 11340–11347. [DOI] [PMC free article] [PubMed] [Google Scholar]


