Abstract
Biologically functional cationic phospholipid-gold nanoplasmonic carriers have been designed to simultaneously exhibit carrier capabilities, demonstrate improved colloidal stability, and show no cytotoxicity under physiological conditions. These carriers are able to retain their unique nano-scale optical properties under physiological conditions, making them particularly useful in a wide range of imaging, therapeutic, and gene delivery applications that utilize selective nanoplasmonic properties.
Introduction
Gold nanoparticles (GNPs) in the near infrared (NIR) spectral region, due to their size and core material, display unique optical properties that make them attractive candidates for drug delivery (1–4), gene delivery (5–7), biomedical and molecular imaging (8–11), and therapeutics (12–20). When GNPs are specifically used to convert light into heat, otherwise known as photothermal conversion (21–23), the NIR wavelength regime is well suited for biomedical applications since tissues and cells are essentially transparent at 800–1300 nm (24, 25). It is possible to obtain very efficient photothermal conversion of energy when the NIR light is matched to the plasmon resonance wavelength of the GNP. Additionally, heat transfer from the surface of GNPs to the surrounding cellular environment is highly localized, decaying exponentially within a few nanometers (7, 21, 26, 27) and therefore is thought to have minimal adverse effects on cells. Among the GNPs, rod-shaped GNPs, known as nanorods, are of particular interest due to their large absorption cross-section, a narrow spectral width of the longitudinal plasmon resonance band, and tunability of the longitudinal plasmon resonance wavelength based on aspect ratio. The unique optical properties of gold nanorods arise due to their nanoscale asymmetric geometry and gold core material.
Despite these unique optical properties, the combination of three key factors – carrier functionality, colloidal stability, and cytotoxicity - have hindered the widespread use of gold nanorods as carriers in biological and biomedical applications. Therefore, in order to harness the full potential of nanomaterials in these applications, the surface coating material as well as the gold core material deserves attention. With respect to cytotoxicity, while the gold core is widely accepted as being biocompatible (16), bare gold nanoparticles are known to interact with proteins and induce mis-folding at physiological conditions (28). Specifically, in the case of gold nanorods, the asymmetric geometry is obtained by synthesizing the nanorods in the presence of a high concentration (> 0.1 M) of cetyltrimethylammonium bromide (CTAB), a cationic micellar surfactant that associates preferentially with the {110} crystallographic facet of gold (29). However CTAB is known to degrade biomembranes and peptides (30), raising significant concern about the cytotoxicity of CTAB-coated nanorods in vitro and in vivo (30–35).
The cytotoxic effects of CTAB-coated nanorods can be minimized by reducing the CTAB concentration below the critical micellar concentration (37), but at the expense of the nanorod suspension stability (Fig. 1b), consequently compromising their unique optical properties in biological environments. Notably, under physiological conditions, aggregation of nanoparticles has been shown to significantly red-shift and decrease the amplitude of the plasmon resonance band due to closely interacting nanoparticles (28, 38).
Fig. 1. Concept of biologically functional cationic phospholipid-gold nanoplasmonic carriers.
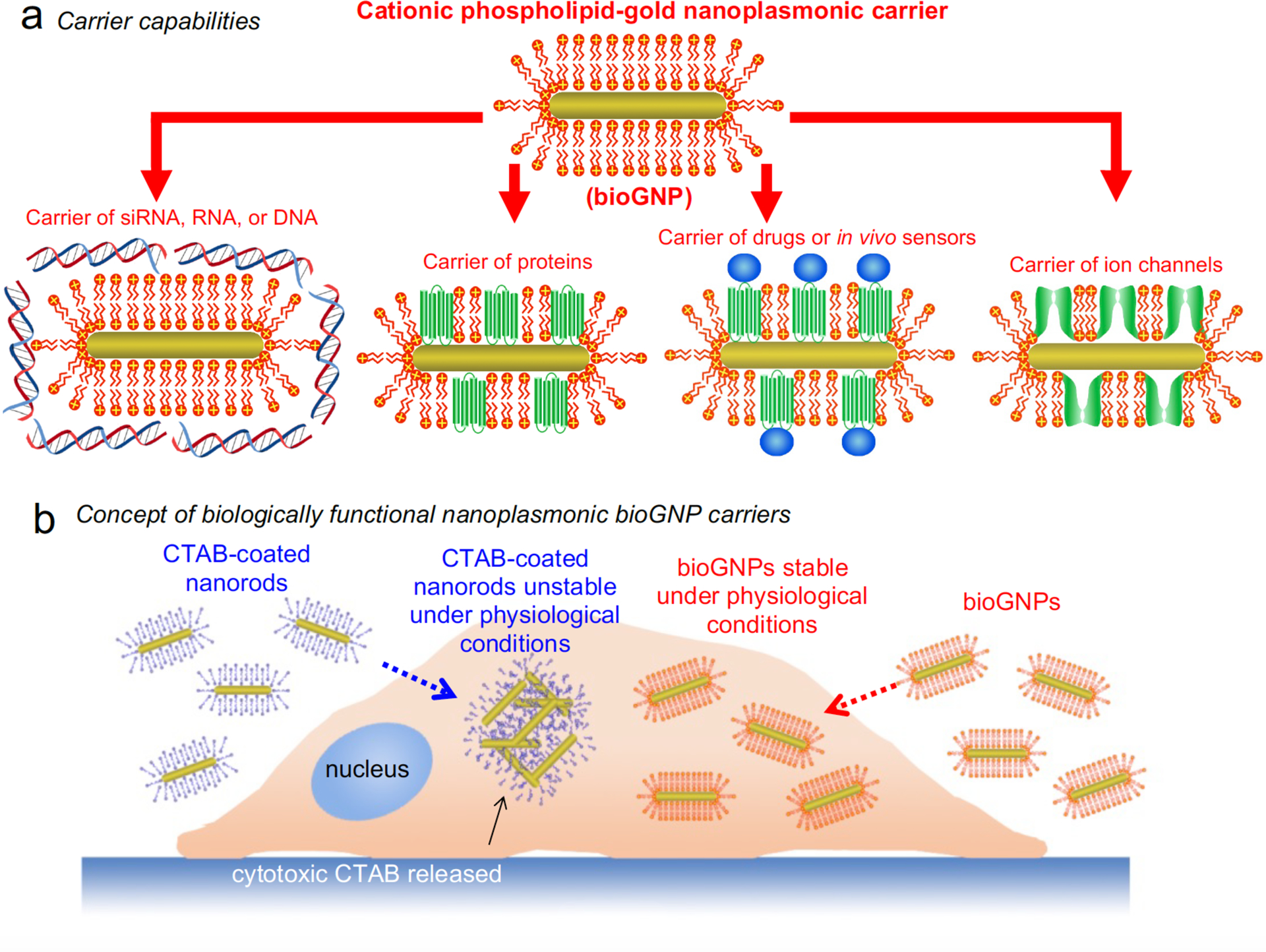
(a) The cationic phospholipid bilayer membrane is formed around the gold nanoplasmonic carriers for carrier capabilities. These carriers (bioGNPs) can then be used to carry a variety of biomolecules such as RNA oligonucleotides, DNA oligonucleotides, siRNA, proteins, and drugs by binding to the positively-charged surface or by incorporation into the membrane. (b) When CTAB concentration is reduced below the critical micellar concentration, CTAB-coated gold nanorods aggregate under physiological conditions, thereby compromising their unique optical properties. Since CTAB is also known to degrade biomembranes and peptides, CTAB dissociated from the nanorods’ surface raises concern regarding cytotoxicity. In contrast, nontoxic bioGNPs are highly stable under physiological conditions, thereby able to retain their unique optical properties for plasmonic-based applications.
Finally, in order for plasmon-resonant nanorods to function as biological carriers, biomolecules such as DNA oligonucleotides, RNA oligonucleotides, or small interfering RNA (siRNA) must be able to adsorb on their surface. Poly(ethylene glycol) (PEG) has previously been used to modify gold nanorods for in vivo applications (39–41); however, their ability to maximally carry subsequent biomolecules is limited due to the absence of surface charge. Organothiols and polystyrenesulfonate,have been used to coat gold nanorods; however, attachment of nucleic acids have not been demonstrated (42 43). Synthetic lipids have also been used to modify gold nanorods (34, 35, 44); however carrier capabilities and/or biocompatibility have not yet been addressed. Polyelectrolyte coating schemes have also been to coat gold nanorods (45, 46); however, plasmonic properties under physiological conditions have not yet been discussed. Alternatively, since cationic lipid formulations have already been optimized for both in vitro and in vivo gene transfer over the past decade (47, 48) and many cationic lipid-based gene delivery approaches are currently being tested at the clinical level (49, 50), validated cationic lipids make ideal candidates for modifying ordinary nanorods into biologically compatible nanorods that simultaneously satisfy carrier functionality, colloidal stability, and non-cytotoxicity. The positively charged surface can be used to adsorb negatively-charged biomolecules such as RNA oligonucleotides, siRNA, or DNA oligonucleotides (Fig. 1b). In addition to carrying siRNA, RNA oligonucleotides, and DNA oligonucleotides, these carriers can also be used to carry a variety of other compounds such as proteins and drugs by incorporation into or binding to the cationic phospholipid membrane (Fig. 1a).
In this article, we present biologically functional cationic phospholipid-gold plasmonic carriers (bioGNPs) that exhibit carrier capabilities, demonstrate improved colloidal stability, and show no cytotoxicity under physiological conditions. Using an adaptation of vesicle-to-nanoparticle fusion (44), we exchanged the cytotoxic CTAB surfactant at the nanorod surface with commercially available cationic phospholipids (Fig. 1b) successfully used for in vivo studies (51, 52). We first demonstrate that bioGNPs are stable under physiological conditions thus retaining their unique plasmonic properties. We then show that the positively charged surface of nanorods can adsorb cargo such as DNA oligonucleotides, RNA oligonucleotides, or siRNA. We finally demonstrate the biocompatibility of bioGNPs via viability/cytotoxicity and cell proliferation studies.
Experimental Procedures
Cell preparation.
The human breast carcinoma line MCF-7 was purchased from the American Type Culture Collection (ATCC). Dulbecco’s modified eagle’s media (DMEM) was purchased from Invitrogen and was supplemented with 10% heat-inactivated fetal bovine serum, 0.1% nonessential amino acids, and 1% sodium pyruvate. Cells were cultured in the supplemented media and maintained in a 37˚C incubator with 5% CO2 humidified air.
Synthesis of DNase/RNase-free rod-shaped GNPs.
Gold nanorods of aspect ratio 3.0 were synthesized by adapting a previously reported seed-mediated growth method (29, 53) to a DNase/RNase-free environment. Hexadecyltrimethylammonium bromide (CTAB) was purchased from Alfa Aeser. Silver nitrate (AgNO3), L-ascorbic acid, sodium tetrahydroborate (NaBH4), and hydrogentetrachloroaurate (HAuCl4) were purchased from Alfa Aeser. All solutions were prepared using 0.2 μm filtered DEPC-treated water. All glassware and metalware were baked at 240°C for 24 hours to remove exogenous RNase. All pipetting devices and counter space was treated with 70% ethanol. All disposable plastic pipette tips and centrifuge tubes were certified to be free of RNase.
To prepare the seed solution, 5 mL of 0.2 M CTAB solution was mixed with 5 mL of 0.0005 M HAuCl4. Ice-cold 0.010 M NaBH4 (0.60 mL) was then added and the solution was continuously stirred for 2 minutes at room temperature. To prepare the growth solution, 9.5 mL of 0.1 M CTAB was mixed with 60 μL of 0.10 M AgNO3, 0.5 mL of 0.01 M HAuCl4, 55 μL of 0.10 M ascorbic acid, and 12 μL of seed solution with continuous stirring. The gold nanorods were aged overnight at room temperature. At this point, the CTAB concentration in the gold nanorod solution was approximately 0.1 M. The nanorod concentration (approximately 50 μg/mL) was confirmed by adjusting to an absorbance of 1 at the longitudinal plasmon resonance wavelength using UV-VIS spectroscopy.
Synthesis of bioGNPs.
Commercially available cationic phospholipids Oligofectamine, Lipofectamine 2000 and sc29528 were purchased from Invitrogen and Santa Cruz Biotechnology. Non-ionic surfactant Brij56 was purchased from Fluka and prepared in DEPC-treated water.
To remove excess CTAB surfactant, 500 μL unmodified CTAB-coated nanorods in 0.1 mM CTAB were centrifuged at 5000 rpm for 10 minutes. A 10 μL pellet was transferred to a new microcentrifuge tube, redispersed in 500 μL of DEPC-treated water such that the final concentration was approximately 0.1 mM, briefly vortexed, and sonicated for 1 minute.
To replace CTAB with non-ionic Brij56 surfactant at the nanorod surface, nanorods were then centrifuged again at 5000 rpm for 10 minutes. A 10 μL pellet was transferred to a new microcentrifuge tube, resuspended in 500 μL of 0.01 mM Brij56, briefly vortexed, and sonicated for 1 minute.
To replace Brij56 surfactant with a phospholipid bilayer membrane at the nanorod surface, the aforementioned procedure was repeated again to resuspend particles in 50 μL of either Oligofectamine, sc29528, or Lipofectamine 2000. Finally, to remove excess cationic phospholipids in the solution, bioGNPs were washed twice with DEPC-treated water by centrifugation at 5000 rpm for 10 minutes.
Preparation of bioGNP carriers.
Phosphorothioate 21-mer RNA oligonucleotides conjugated to fluorescein (FAM) dye (488 nm excitation, 532 nm emission) were purchased from Integrated DNA Technologies. The sequence was: 5’ GUAGAUUACCACUGGAGUCUU - FAM −3’. Rnase-free 20X Tris-EDTA (TE) buffer was purchased from Invitrogen and was used to prepare 1X TE buffer solution using DEPC-treated water.
Stock solutions of RNA oligonucleotides were prepared in RNase-free 1X TE buffer. To 500 μL of bioGNPs, 0.25 μL of 100 μM RNA oligonucleotides was added. The solution was vortexed and allowed to incubate for 1 hour. To remove excess oligonucleotides from solution, bioGNPs were washed twice with DEPC-treated water by centrifugation at 5000 rpm for 10 minutes. BioGNP carriers of fluorescently-labeled RNA oligonucleotides were finally visualized using fluorescent microscopy.
Dynamic light scattering of bioGNP carriers.
DLS data were collected for all samples using a DynaPro-99-E-15 DLS apparatus from Wyatt Technologies. For each sample, at least 20 scans were performed, each with a 10 s acquisition time, using a scattering angle of 90°. Optimem medium was purchased from Invitrogen.
CTAB-coated nanorods, Brij56-coated nanorods, are bioGNPs were prepared as described above. DLS measurements were taken of each sample (diluted 1:60 in a quartz cuvette) as-prepared, after washing twice in DEPC-treated water, and after resuspending in Optimem medium.
Internalization assay of bioGNP carriers.
BioGNP carriers of FAM-labeled RNA oligonucleotides (500 μL) were concentrated into a 5 μL pellet by centrifugation at 5000 rpm for 10 minutes. MCF-7 cells were harvested and resuspended in DMEM media to a concentration of 240,000 cells/mL. To each well of a 6-well plate, 0.5 mL of cell suspension and 5 μL of concentrated FAM-RNA functionalized bioGNPs were added (120,000 cells/well). The cells were allowed to incubate for 10 hours. After 10 hours, cells were carefully washed with 1X phosphate buffered solution (PBS), detached using 0.25% trypsin for 2 minutes, mixed with media 4:1 to deactivate trypsin, collected using centrifugation (1800 rpm, 4 minutes), and resuspended in 2% paraformaldehyde. Trypan blue was finally added to the cell suspension at 50% concentration to quench uninternalized bioGNP carriers.
As a control, surface receptor ERBB2 on ERBB2-positive BT474 cells was labeled with antibodies conjugated to FITC dye (340553, BD Biosciences). Cells were harvested using 1 mM EDTA for 10 minutes. Cells were resuspended in surface-staining buffer containing 0.5% bovine serum albumin (BSA) and 0.1% sodium azide in 1X PBS. 5 μL of normal mouse IgG1 (I8765, Sigma Aldrich) was added and cells were incubated for 15 minutes to prevent non-specific binding of antibodies to Fc receptors. 15 μL of anti-ERBB2-FITC was added and cells were incubated for 45 minutes at room temperature. Cells were then washed in surface-staining buffer. Cells were resuspended in 1X PBS containing 2% paraformaldehyde. Trypan blue was finally added to the cell suspension at 50% concentration to quench surface receptors labeled with fluorescent antibodies. LSRII flow cytometer (BD Biosciences) and FlowJo software (Tree Star, Ashland, Oregon) was used to analyze samples.
Visualization of internalized bioGNP carriers by darkfield light microscopy.
500 μL of bioGNPs were concentrated into a 5 μL pellet by centrifugation at 5000 rpm for 10 minutes. Sterile and RNase-free glass coverslips were placed into each well of a 6-well plate. MCF-7 cells were harvested and resuspended in cell culture media to a concentration of 240,000 cells/mL. To each well, 0.5 mL of cell suspension and 5 μL of concentrated bioGNPs were added on top of the coverslips (120,000 cells/well). The cells were allowed to incubate for 10 hours. After 10 hours, 2% paraformaldehyde was added to each well to fix cells onto the glass coverslips. For visualization purposes, cell nuclei were stained with DAPI, purchased from Invitrogen, by incubating cells in 300 nM DAPI to each well for 5 minutes. 1X PBS was then added to each well to wash the cells. A coverslip containing fix, adhered cells was then placed facedown on a microscope slide and broadband white light was shined onto the adhered cells from an oblique angle using a darkfield condenser lens. The scattered light alone was collected using a microscope objective lens with a numerical aperture (NA) of 0.65 that was smaller than the NA (1.2–1.4) of the illumination condenser lens.
Viability/cytotoxicity and proliferation analysis.
As a control, unmodified CTAB-coated nanorods were initially resuspended in 6 mM CTAB. Then, 500 μL (50 μg/mL) of bioGNPs or unmodified CTAB-coated nanorods were washed once with DEPC-treated water to remove excess lipids/CTAB and were then concentrated into a 5 μL pellets by centrifugation at 5000 rpm for 10 minutes.
MCF-7 cells were harvested and resuspended in cell culture media to a concentration of 240,000 cells/mL. To each well of a 6-well plate, 0.5 mL of cell suspension and 5 μL of concentrated bioGNPs or unmodified CTAB-coated nanorods were added (120,000 cells/well). The cells were allowed to incubate for 24 hours. After 24 hours, wells containing cells which were to be analyzed 72 hours and 120 hours later are exchanged with fresh media. Cells which were to be analyzed after 24 hours were washed with 1X PBS, detached using 0.25% trypsin for 2 minutes, mixed with media 4:1 to deactivate trypsin, collected using centrifugation (1800 rpm, 4 minutes), and resuspended in 0.5 mL of 0.125 μM Calcein AM from Invitrogen. Immediately prior to flow cytometric analysis, 5 μL of PI from BD Pharmingen was added to stain dead cells for cytotoxicity analysis. Cells are simultaneously analyzed for viability and cytotoxicity using the LSRII flow cytometer within 30 minutes. This same viability/cytotoxicity staining procedure was repeated after 72 hours and 120 hours of incubation. Cell proliferation was assessed by cell count analysis using a hemocytometer after 120 hours of incubation.
Results and Discussion
Gold nanorod carriers were synthesized by modifying a seed-mediated growth approach (29, 53) to be free of DNase/RNase contamination. Since the resultant gold nanorod carriers were coated with CTAB that yielded a net positive surface charge from the quaternary ammonium surfactant head group (29), negatively-charged, fluorescently-conjugated 21-mer RNA oligonucleotides readily attached to the CTAB-coated nanorod carriers, enabling visualization by fluorescent microscopy (Fig. 2b). Using dynamic light scattering (DLS), in Fig. 2a, the resultant CTAB-coated nanorod carriers show a size distribution with an average radius of ~15 nm (30 nm diameter). Since the nanorod’s length was short and the rotational diffusion was therefore rapid, the nanorod was approximated as a translationally diffusing sphere whose diameter equaled the length of the nanorod (30 nm). Lengths based on DLS measurements were in agreement with lengths seen in SEM images (Fig. 4a).
Fig. 2. Synthesis of bioGNP carriers.

(a) CTAB-coated nanorod carriers are prepared using seed-mediated growth process. Using dynamic light scattering (DLS), CTAB-coated nanorod carriers show a size distribution with an average radius of ~15 nm (30 nm diameter). (b) Fluorescent image showing CTAB-coated nanorods carry negatively-charged, fluorescent FAM-conjugated 21-mer RNA oligonucleotides. (c) The CTAB surfactant is then place-exchanged with a non-ionic surfactant Brij56 surfactant. Using DLS, brij56-coated nanorods show a size distribution with an average radius ~50 nm (100 nm length). (d) Fluorescent image showing that brij56-coated nanorods are resistant to coupling with fluorescently-conjugated RNA oligonucleotides. (e) The brij56 coating is then place-exchanged with cationic phospholipids to form bioGNPs. Using DLS, bioGNPs show an average radius of ~15 nm (30 nm length) with a narrow size distribution. (f) Fluorescent image showing bioGNPs carry negatively-charged, fluorescent FAM-conjugated RNA oligonucleotides.
Fig. 4. Experimental characterization and internalization of bioGNP carriers.
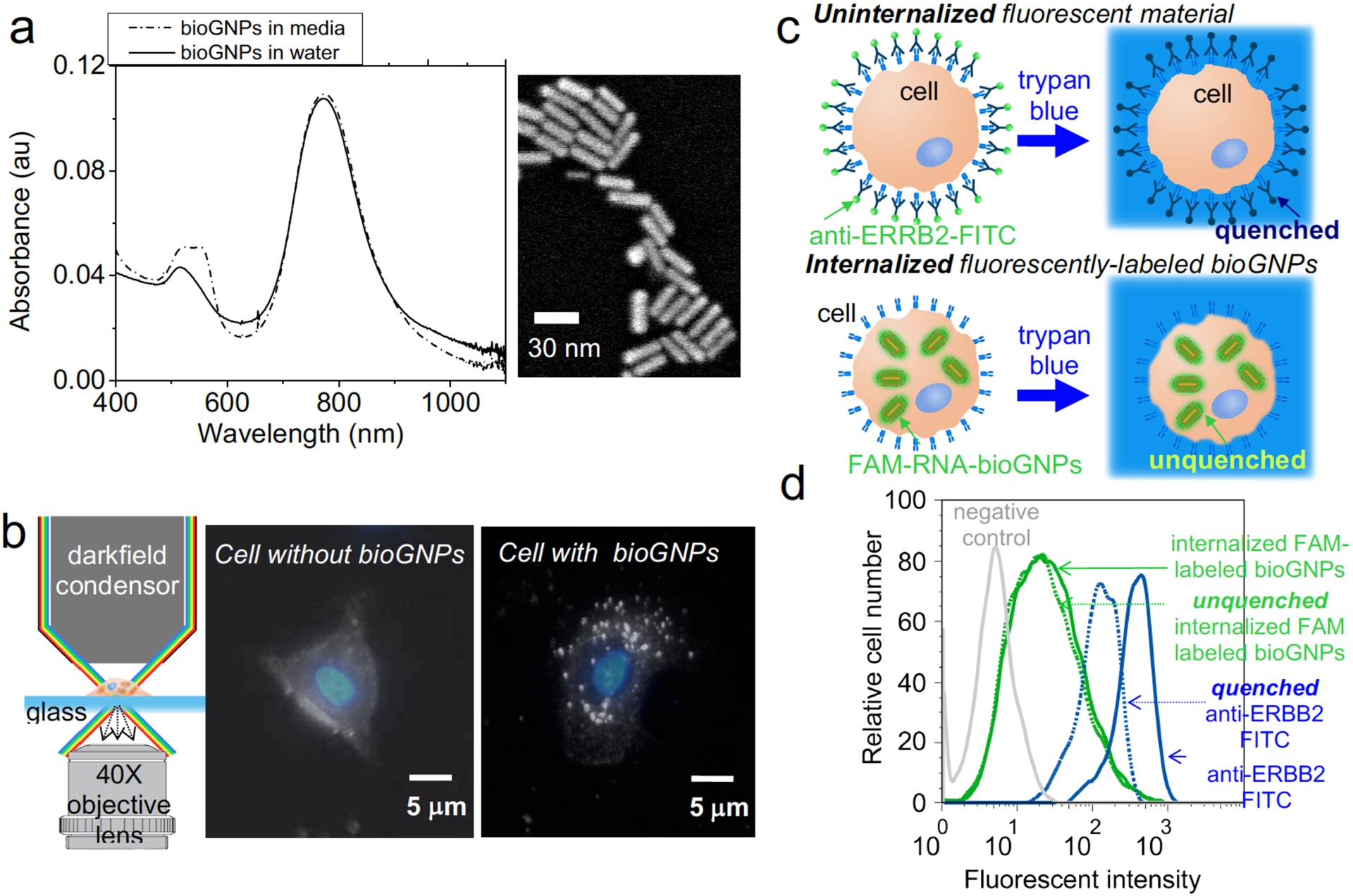
(a) UV-VIS absorbance spectra of bioGNPs suspended in water (solid line), UV-VIS absorbance spectra of bioGNPs suspended in media (dash line), and scanning electron microscopy image of bioGNPs, (b) Schematic of microscope setup, darkfield scattering image of a cell without bioGNPs overlaid with DAPI-stained nuclei image, and darkfield scattering image of a cell containing single bioGNPs overlaid with DAPI-stained nuclei image. (c) concept of internalization assay where uninternalized fluorescent material (ie. FITC conjugated ERBB2 antibody) is quenched by trypan blue and internalized fluorescent material (ie. bioGNP carriers of FAM conjugated RNA oligonucleotides) remains unquenched by trypan blue. (d) flow cytometric comparison of MCF-7 cells containing unquenched bioGNP carriers of fluorescently-labeled RNA oligonucleotides and quenched surface-labeled antibodies (FITC conjugated) recognizing ERBB2 surface receptor on ERBB2-positive BT474 cells. Green solid line represents cells containing bioGNP carriers of fluorescently-labeled RNA oligonucleotides in the absence of trypan blue quencher. Green dotted line represents cells containing unquenched bioGNP carriers of fluorescently-labeled RNA oligonucleotides in the presence of trypan blue quencher. Blue solid line represents cells that are surface-labeled with fluorescent antibodies in the absence of trypan blue quencher. Blue dotted line represents cells that are quenched surface-labeled with fluorescent antibodies in the presence of trypan blue quencher. Grey solid line represents negative control cells.
The first stage in demonstrating stable bioGNPs was place-exchanging CTAB with cationic phospholipids vesicles at the nanorod surface by using a vesicle-to-nanorod fusion approach. Excess CTAB surfactant was first removed from the CTAB-coated nanorod solution to yield a final CTAB concentration was approximately 0.1 mM. These CTAB-coated nanorods were then dispersed in various commercially available liposome formulations in approximately <50 fold excess. The phospholipid bilayer coating at the nanorods’ surface readily replaced the CTAB surfactant (Fig.1b).
An alternative route to phospholipid-coated nanorods was to first place-exchanged the CTAB surfactant with the non-ionic surfactant Brij56, followed by place-exchange with cationic phospholipid vesicles. Since CTAB removal from the nanorods is essential to minimize cytotoxicity and to eliminate any electrostatic contribution, the latter procedure was developed since it is known that CTAB is capable of inserting itself into lipid bilayers (54). Therefore, all the results henceforth describe the Brij56-to-phospholipid exchange process for coating gold nanorods with a phospholipid bilayer. In Fig. 2c, Brij56-coated nanorods show a size distribution with an average radius ~50 nm (100 nm length). The narrow width of the size distribution suggests that Brij56-coated nanorods were stable and with no observed aggregation over a period of weeks. As seen in the fluorescent image of Fig. 2d, the Brij56-coated nanorods were resistant to coupling with fluorescently-conjugated RNA oligonucleotides. The Brij56 coating was then place-exchanged with various commercially-available cationic phospholipids to form bioGNPs by simply exposing surfactant-coated gold nanorods with cationic phospholipid vesicles. BioGNPs show an average radius of ~15 nm (30 nm length) with a narrow size distribution (Fig. 2e and Supporting Information Fig. S1). The smaller average lengths suggest that the phospholipid bilayer did not layer on top of the Brij56 coating, but instead successfully place-exchanged with the Brij56 at the bioGNPs’ surface. Furthermore, negatively-charged, fluorescently-conjugated RNA oligonucleotides readily attach to the bioGNP carriers, strongly suggesting that the non-ionic surfactant was successfully place-exchanged by a positively-charged phopholipid bilayer (Fig. 2f and Supporting information Fig. S2). The formation of phospholipid bilayer coatings on gold nanorods and simultaneous loss of surfactant coatings been previously determined by NMR and FTIR spectroscopy and zeta potential measurements (44).
Having established that the CTAB coating can be place-exchanged with a cationic phospholipid membrane, the stability of bioGNPs was then studied and compared against the stability of the CTAB-coated nanorods under biological conditions. CTAB-coated nanorods, which were washed twice and resuspended in DEPC-treated water, showed an average radius of ~15 nm (30 nm length) with a broad distribution in Fig. 3c. The twice-washed CTAB-coated nanorods showed a slightly wider size distribution compared to as-prepared CTAB-coated nanorods in 0.1 M CTAB solution (Fig. 3b), suggesting the onset of aggregation. After resuspending the twice-washed CTAB-coated particles in cell culture media, further broadening was seen of the size distribution centered at radius of 60 nm, suggesting that particles were highly unstable in culture media (Fig. 3d). In comparison, bioGNPs which were washed twice and resuspended in DEPC-treated water were highly stable (Fig. 2g). When resuspended in cell culture media, bioGNPs continue to exhibit a narrow size distribution (Fig. 2h and Fig. S1), thus affirming their excellent stability under biological conditions. Furthermore, optical properties were confirmed using UV-VIS spectroscopy. BioGNPs were suspended in water and were compared to bioGNPs suspended in cell culture media by UV-VIS spectroscopy in Fig. 4a. BioGNPs suspended in cell culture media continue to display a large absorption cross-section and a narrow spectral width of the longitudinal plasmon resonance band, thus confirming bioGNPs’ excellent stability under biological conditions.
Fig. 3. Experimental characterization of bioGNP stability.
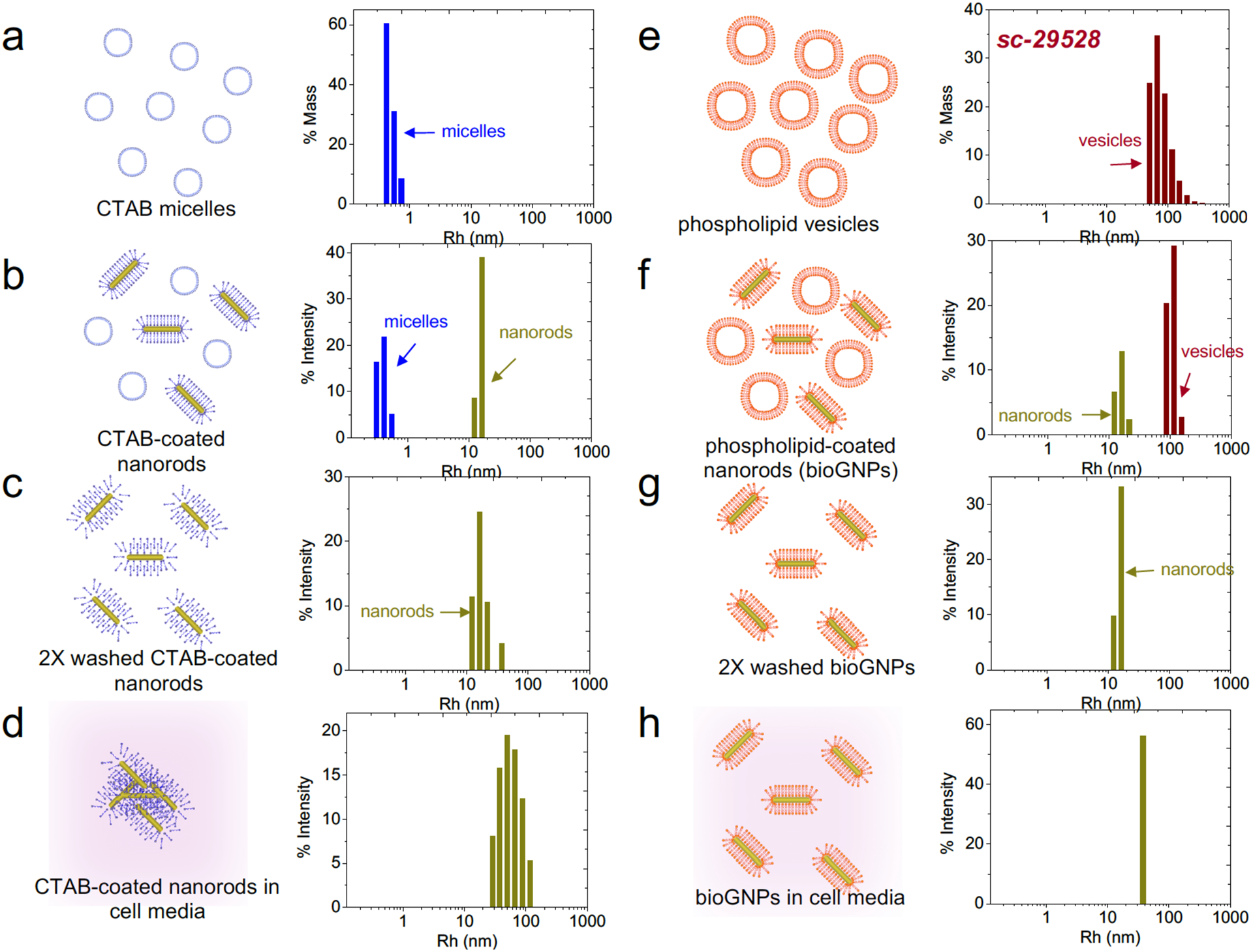
(a) DLS size distribution of CTAB micelles, (b) DLS size distribution of CTAB-coated nanorods, (c) DLS size distribution of CTAB-coated nanorods that have been washed 2X and resuspended in water, (d) DLS size distribution of CTAB-coated nanorods that have been resuspended cell culture media, (e) DLS size distribution of cationic phospholipid vesicles (sc-29528), (f) DLS size distribution of bioGNPs, (g) DLS size distribution of bioGNPs that have been washed 2X and resuspended in water, (h) DLS size distribution of bioGNPs that have been resuspended cell culture media.
Having confirmed bioGNPs’ stability, we then investigated the internalization of bioGNPs into human cells. Net-positively charged bioGNPs were endocytosed by MCF-7 human breast carcinoma cells by incubation for 10 hours. To visualize internalized bioGNPs, the cells were then illuminated with unpolarized white light from an oblique angle using a darkfield condenser lens, and scattered light was collected using a transmission-mode darkfield microscope (Fig. 4b). To locate cells’ nuclei, darkfield scattering images were overlaid with DAPI-stained images. It is clearly evident from Fig. 4b that scattered light from cells containing bioGNPs are easily differentiated from cells lacking bioGNPs.
To further confirm that bioGNPs were in fact internalized within cells and not externally adsorbed onto the cells’ surface, MCF-7 cells were first exposed for 10 hours to bioGNP carriers of fluorescently-labeled RNA oligonucleotides. Uninternalized carriers were then rendered non-fluorescent by using trypan blue as a quencher. Since trypan blue absorbs light between 475 and 675 nm (55, 56), the emission of fluorescent dyes within this wavelength range are quenched in the presence of trypan blue. If bioGNP carriers of FAM-labeled RNA oligonucleotides (excitation 495 nm, emission 520 nm) are indeed internalized within cells, it is expected that their fluorescence should remain unquenched when cells are resuspended in trypan blue. Flow cytometry results displayed in Fig. 4 confirm that the majority of the bioGNP carriers of FAM-labeled RNA oligonucleotides remained fluorescent within the protective confines of intact cell membranes. In contrast, as a control, surface receptor ERBB2 (also known as HER-2, neu, and EGFR-2) on ERBB2-positive BT474 breast carcinoma cells were labeled with antibodies conjugated to FITC dye (excitation 488 nm, emission 532 nm). To inhibit internalization by receptor-mediated endocytosis, FITC-conjugated antibodies were bound in the presence of sodium azide. Flow cytometry results in Fig. 4 show that surface-bound FITC on control cells was efficiently quenched in the presence of trypan blue (Fig. 4d).
Finally, the biocompatibility of bioGNPs was investigated and compared against unmodified CTAB-coated nanorods by conducting a long term viability/cytoxicity and proliferation studies in MCF-7 cells. Viability/cytotoxicity was conducted using a two-color fluorescence assay to simultaneously determine numbers of live and dead cells. Calcein AM - a dye which converts from nonfluorescent cell-permeant calcein AM into fluorescent cell-impermeant calcein by intracellular esterase enzymes in living cells - was used as a measure of cell viability. Propidium iodide (PI) - a dye that enters through permeabilized membranes of compromised cells - was used as a measure of cell cytotoxicity. The concentration of commercial cationic phospholipids was estimated to be approximately 6 mM based on DLS measurements. Unmodified CTAB-coated nanorods were therefore initially resuspended in 6 mM CTAB to obtain comparable results. Unmodified CTAB-coated nanorods and bioGNPs were then washed with DEPC-treated water to remove excess lipids/CTAB and resuspended in cell culture media. Based on DLS measurements, bioGNPs were highly stable while unmodified CTAB-coated nanorods were only moderately stable in cell culture media. In order to retain unique optical properties of stable nanoparticles, further washing was avoided since twice-washed CTAB-coated nanorods tend to strongly aggregate in cell culture media as seen in DLS measurements in Fig. 3d. After 72 hours and 120 hours of incubation, based on flow cytometric data, viability (Fig. 5a) and cytotoxicity (Fig. 5b) of cells containing bioGNPs were statistically significant from cells containing unmodified CTAB-coated nanorods (also see supporting information Fig. S3). It is apparent that cells containing bioGNPs are viable whereas cells containing CTAB-coated nanorods are compromised. Clearly, bioGNP carriers are a superior alternative to unmodified CTAB-coated nanorods for biological studies.
Fig. 5. Biocompatibility analysis of bioGNPs.

(a) Viability analysis - flow cytometric results of viability using Calcein AM after 24 hrs, 72hrs, and 120 hrs incubation with nothing, CTAB-coated nanorods, Oligofectamine-coated bioGNPs, sc29528-coated bioGNPs, and Lipofectamine2000-coated bioGNPs, (b) Cytotoxicity analysis – flow cytometric results of cytotoxcity using PI after 24 hrs, 72hrs, and 120 hrs incubation with nothing, CTAB-coated nanorods, Oligofectamine-coated bioGNPs, sc29528-coated bioGNPs, and Lipofectamine2000-coated bioGNPs, (c) Proliferation analysis - table summarizing initial cell count (Ninitial), final cell count after 120 hrs (Nfinal), and calculated doubling times for cells containing nothing, Oligofectamine-coated bioGNPs, sc29528-coated bioGNPs, and Lipofectamine2000-coated bioGNPs
Cell proliferation was also assessed by cell count analysis after 120 hours of incubation with and without bioGNPs. Based on cell count analysis, doubling times were then determined for bioGNP-containing samples and compared to an untreated control sample. Doubling time of 55 hours for the untreated control sample was in agreement with doubling times previously reported in literature for MCF-7 cells (57). Cells containing bioGNPs exhibited approximately similar doubling times as the untreated control sample (Fig. 5c), verifying that incubation with bioGNPs did not adversely affect proliferation of MCF-7 cells.
While in vitro experiments were successfully demonstrated here, we anticipate developing bioGNP carriers for use in vivo mammalian and non-mammalian model systems. BioGNP carriers can be coated with cationic phospholipid formulations specifically optimized for distribution in vivo. In addition to carrying siRNA, RNA oligonucleotides, and DNA oligonucleotides for gene delivery applications, bioGNPs can also be used to carry a variety of other compounds such as proteins and drugs for in vivo applications.
Conclusions
In closing, we have designed biologically functional phospholipid-gold plasmonic bioGNP carriers that exhibit carrier capabilities, demonstrate improved nanoparticle stability, and show no cytotoxicity under physiological conditions. In addition to these advantages, since bioGNPs are able to retain their unique optical properties under physiological conditions, bioGNPs will be particularly useful in a wide range of applications that utilize selective nanoplasmonic properties. We therefore expect bioGNPs to have important implications in advancing drug delivery, gene delivery, biomedical and molecular imaging, and therapeutics.
Supplementary Material
Acknowledgements
The authors acknowledge the National Physical Science Consortium (NPSC) graduate fellowship for support of S.E.Lee. The authors thank Terry Johnson and Dave Robinson for technical insight. The authors thank the UC Berkeley undergraduate students Dana Donnenwirth, Daniel Rosen, Gabe Sudario, and Alan Wilk for assistance with viability/cytotoxicity experiments. The authors also thank Ann Fischer and Michelle Yasukawa of the UC Berkeley Tissue Culture Facility for long-term maintenance of MCF-7 and BT474 cell lines. The authors acknowledge the Division of Materials Science and Engineering in the Department of Energy Office of Basic Energy Sciences for financial support of D.Y.Sasaki at Sandia. Sandia is a multiprogram laboratory operated by Sandia Corporation, a Lockheed Martin Company, for the United States Department of Energy under Contract DE-AC04-94AL85000.
Footnotes
Supplementary Data
Supporting figures available in the supplementary data section.
References
- (1).Sershen SR, Westcott SL, Halas NJ, West JL Temperature-sensitive polymer–nanoshell composites for photothermally modulated drug delivery. J. Biomed. Mater. Res, 2000; 51: 293–298 [DOI] [PubMed] [Google Scholar]
- (2).Ren L, Chow GM Materials Science and Engineering. Synthesis of nir-sensitive Au–Au2S nanocolloids for drug delivery, 2003; C23: 113–116 [Google Scholar]
- (3).Skirtach AG, Javier AM, Kreft O; Kohler K, Alberola AP, Mohwald H, Parak WJ, Sukhorukov GB Laser induced release of encapsulated material inside living cells, Angew. Chem. Int. Ed, 2006; 45: 4612–4617 [DOI] [PubMed] [Google Scholar]
- (4).Wijaya A, Schaffer SB, Pallares IG, Hamad-Schifferli K Selective release of multiple DNA oligonucleotides from gold nanorods, ACS Nano, 2009; 3: 80–86 [DOI] [PubMed] [Google Scholar]
- (5).Chen C; Lin Y, Wang C, Tzeng H, Wu C, Chen Y, Chen C, Chen L, Wu Y DNA gold conjugates for remote control localized gene expression by near infrared irradiation, J. Am. Chem. Soc, 2006; 128: 3709–3715 [DOI] [PubMed] [Google Scholar]
- (6).Horiguchi Y, Niidome T, Yamada S, Nakashima N, Niidome Y Expression of plasmid DNA released from DNA conjugates of gold nanorods, Chemistry Letters., 2007; 36: 952–953 [Google Scholar]
- (7).Lee SE, Liu GL, Kim F, Lee LP Remote optical switch for localized and selective control of gene interference, Nanoletters, 2009; 9: 562–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Copland JA, Eghtedari M, Popov VL, Kotov N, Mamedova N, Motamedi M, Oraevsky AA Bioconjugated gold nanoparticles as a molecular based contrast agent: implications for imaging of deep tumors using optoacoustic tomography, Molecular Imaging & Biology, 2004; 6: 341–349 [DOI] [PubMed] [Google Scholar]
- (9).Javier DJ, Nitin N, Roblyer DM, Richards-Kortum R Metal-based nanorods as molecule-specific contrast agents for reflectance imaging in 3D tissues, Journal of Nanophotonics, 2008; 2: 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Choi Y, Kang T, Lee LP Plasmon Resonance Energy Transfer (PRET)-based Molecular Imaging of Cytochrome c in Living Cells, Nanoletters, 2009; 9: 85–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Nallathamby PD; Lee KJ; Xu XN Design of stable and uniform single nanoparticle photonics for in vivo dynamics imaging of nanoenvironments of zebrafish embryonic fluids, ACS Nano, 2008, 2, 1371–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Huang X, El-Sayed IH, Qian W, El-Sayed MA Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods, J. Am.Chem.Soc, 2006; 128: 2115–2120 [DOI] [PubMed] [Google Scholar]
- (13).Huang YF, Sefah K, Bamrungsap S, Chang H; Tan W Selective photothermal therapy for mixed cancer cells using aptamer-conjugated nanorods, Langmuir, 2008, [DOI] [PubMed] [Google Scholar]
- (14).Pissuwan D, Valenzuela SM, Killingsworth MC, Xu X Targeted destruction of murine macrophage cells with bioconjugated gold nanorods. Journal of Nanoparticle Research, 2007; 9: 1109–1124 [Google Scholar]
- (15).Choi M, Stanton-Maxey KJ, Stanley JK, Levin CS, Bardhan R, Akin D, Badve S, Sturgis J, Robinson JR, Bashir R, Halas NJ; Clare SE A cellular trojan horse for delivery of therapeutic nanoparticles to tumors, Nanoletters, 2007; 7: 3759–3765 [DOI] [PubMed] [Google Scholar]
- (16).Loo C, Hirsch L, Lee M, Chang E, West J, Halas N, Drezek R Gold nanoshell bioconjugates for molecular imaging in living cells, Optics Letters, 2005; 30: 1012–1014 [DOI] [PubMed] [Google Scholar]
- (17).Skabalak SE, Chen J, Au L, Lu X, Li X, Xia Y Gold nanocages for biomedical applications, Advanced Materials, 2007; 19: 3177–3184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Gobin AM, O’Neal DP, Watkins DM, Halas NJ, Drezek RA, West JL Near infrared laser-tissue welding using nanoshells as an exogenous absorber, Laser in Surgery and Medicine, 2005; 37: 123–129 [DOI] [PubMed] [Google Scholar]
- (19).Hauck TS, Jennings TL, Yatsenko T, Kumaradas JC, Chan WC Advanced Materials, 2008; 1–7 [Google Scholar]
- (20).Norman RS, Stone JW, Gole A, Murphy CJ, Sabo-Attwood TL Targeted photothermal lysis of the pathogenic bacteria, pseudomonas aeruginosa, with gold nanorods, Nanoletters, 2008:8: 302–306 [DOI] [PubMed] [Google Scholar]
- (21).Cortie M, Xu X, Chowdhury H, Zareie H, Smith G Plasmonic heating of gold nanoparticles and its exploitation, Proceedings SPIE, 2005; 5649: 565–573 [Google Scholar]
- (22).Khlebtsov B, Zharov V, Melnikov A, Tuchin V, Khlebtsov N Optical amplification of photothermal therapy with gold nanoparticles and nanoclusters, Nanotechnology, 2006; 17: 5167–5179 [Google Scholar]
- (23).Liu GL, Kim J, Lu Y, Lee LP Optofluidic control via photothermal nanoparticles, Nature Methods, 2006; 5: 27–32 [DOI] [PubMed] [Google Scholar]
- (24).Svoboda K, Block SM Biological applications of optical forces, Annu.Rev. Biomol. Struct, 1994; 23: 247–285 [DOI] [PubMed] [Google Scholar]
- (25).Hirsch L, Stafford R, Bankson J, Sershen S, Price R, Hazle J, Halas N, West J Targeted photothermal tumor therapy using metal nanoshells, Proceedings Second Joint EMBS BMES Conference, 2002; 1: 530 [Google Scholar]
- (26).Skirtach A, Dejugnat C, Braun D, Susha A, Rogach A, Parak W, Mohwald H, Sukhorukov G The role of metal nanoparticles in remote release of encapsulated material, Nano Letters, 2005; 5: 1371–1377. [DOI] [PubMed] [Google Scholar]
- (27).Lu Y, Liu GL, Kim J, Mejia YX, Lee LP Nanophotonic crescent moon structures with sharp edge for ultrasensitive biomolecular detections by local electromagnetic field enhancement effect, Nano Letters, 2005; 5: 119–124. [DOI] [PubMed] [Google Scholar]
- (28).Zhang D, Neumann O, Wang H, Yuwono VM, Barhoumi A, Perham M, Hartgerink JD, Wittung-Stafshede P, Halas NJ Gold nanoparticles can induce the formation of protein-based aggregates at physiological pH, Nanoletters, 2009, [DOI] [PubMed] [Google Scholar]
- (29).Nikoobakht B, El-Sayed MA Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method, Chem. Mater, 2003; 15: 1957–1962 [Google Scholar]
- (30).Broderick G, Craig M J. Dairy Sci, Metabolism of peptides and amino acids during in vitro protein degradation by mixed rumen organisms, 1989; 72: 2540–2548 [DOI] [PubMed] [Google Scholar]
- (31).Cortesi R, Esponito E, Menegatti E, Gambari R, Nastruzzi C Effect of cationic liposome composition on in vitro cytotoxicity and protective effect on carried DNA, International Journal of Pharmaceutics, 1996; 139: 69–78 [Google Scholar]
- (32).Ruissen F, Le M, Carroll JM, van der Walk PGM, Schalkwijk J Differential effects of detergents on keratinocyte gene expression, Journal of Investigative Dermatology, 1998; 110: 359–363 [DOI] [PubMed] [Google Scholar]
- (33).Huff TB, Hansen MN, Cheng J, Wei A Controlling the cellular uptake of gold nanorods, Langmuir, 2007; 23: 1596–1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Niidome Y, Honda K, Higashimoto K, Kawazumi H, Yamada S, Nakashima N, Sasaki Y, Ishida Y, Kikuchi J Surface modification of gold nanorods with synthetic cationic lipids, Chem. Commun, 2007; 3777–3779 [DOI] [PubMed] [Google Scholar]
- (35).Takahashi H, Niidome T, Kawano T, Yamada S, Niidome Y Surface modification of gold nanorods using layer-by-layer technique for cellular uptake, J. Nanopart Res, 2008; 10: 221–228 [Google Scholar]
- (36).Didychuk CL, Ephrat P, Belton M, Carson JJL Synthesis and in vitro cytotoxicity of mPEG-SH modified gold nanorods, Proc. SPIE, 2008; 6856: 68560M1–8 [Google Scholar]
- (37).Connor E, Mwamuka J, Gole A, Murphy CJ, Wyatt MD Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity, Small, 2005; 1: 325–327 [DOI] [PubMed] [Google Scholar]
- (38).Wang S, Lu W, Tovmachenko O, Rai U, Yu H, Ray PC Challenge in understanding size and shape dependent toxicity of gold nanomaterials in human skin keratinocytes, Chemical Physics Letters, 2008; 463: 145–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Niidome T, Yamagata M, Okamoto Y, Akiyama Y, Takahashi H, Kawano T, Katayama Y, Niidome Y Peg-modified gold nanorods with a stealth character for in vivo applications, Journal of Controlled Release, 2006; 114: 343–347 [DOI] [PubMed] [Google Scholar]
- (40).Eghtedari M, Liopo AV, Copland JA, Oraevsky AA, Motamedi M Engineering of hetero-functional gold nanorods for the in vivo molecular targeting of breast cancer cells. Nanoletters, 2009; 9: 287–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Liao H, Hafner JH Gold Nanorod Bioconjugates. Chem. Mat 2005; 17; 4636–4641 [Google Scholar]
- (42).Yu C, Varghese L, Irudayaraj. Surface Modification of Cetyltrimethylammonium Bromide-Capped Gold Nanorods to Make Molecular Probes. Langmuir. 2007; 23: 9114–9119 [DOI] [PubMed] [Google Scholar]
- (43).Leonov AP, Zheng J, Clogston JD, Stern ST, Patri AK, Wei A Detoxification of Gold Nanorods by Treatment with Polystyrenesulfonate. ACS Nano. 2008; 12:2481–2488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Orendorff CJ, Alam TM, Sasaki DY, Bunker BC, Voigt JA Phospholipid-gold nanorod composites, ACS Nano., 2009; 3: 971–983 [DOI] [PubMed] [Google Scholar]
- (45).Hauck TS; Ghazani AA; Chan WCW Assessing the effect of surface chemistry on gold nanorod uptake, toxicity, and gene expression in mammalian cells, Small, 2008; 4: 153–159 [DOI] [PubMed] [Google Scholar]
- (46).Gole A, Murphy CJ Polyelectrolyte-Coated Gold Nanorods: Synthesis, Characterization and Immobilization. Chem. Mat 2005; 17: 1325–1330 [Google Scholar]
- (47).Felgner PL, Tsai YJ, Sukhu L, Wheeler CJ, Manthorpe M, Marshall J, Cheng SH Improved cationic lipid formulations for in vivo gene therapy, Ann N Y Acad Sci, 1995; 772: 126–139 [DOI] [PubMed] [Google Scholar]
- (48).Bergan D, Galbraith T, Sloane DL Gene transfer in vitro and in vivo by cationic lipids is not significantly affected by levels of supercoiling of a reporter plasmid, Pharmaceutical Research, 2000; 17: 967–973 [DOI] [PubMed] [Google Scholar]
- (49).Rubin J; Galanis ME; Pitot HC; Richardson RL; Burch PA; Charboneau JW; Reading CC; Lewis BD; Stahl S; Akporiaye ET; Harris DT Phase I study of immunotherapy of hepatic metastases of colorectal carcinoma by direct gene transfer of an allogeneic histocompatibility antigen, HLA-B7, Gene Therapy, 1997: 4; 419–425 [DOI] [PubMed] [Google Scholar]
- (50).Madhusudan S, Tamir A, Bates N, Flanagan E, Gore ME, Barton DPJ, Harper P, Seckl M, Thomas H, Lemoine NR, Charnock M, Habib NA, Lechler R, Nicholls J, Pignatelli M, Ganesan TS A Multicenter Phase I Gene Therapy Clinical Trial Involving Intraperitoneal Administration of E1A-Lipid Complex in Patients with Recurrent Epithelial Ovarian Cancer Overexpressing HER-2/neu Oncogene, Clinical Cancer Research, 2004; 10: 2986–2996 [DOI] [PubMed] [Google Scholar]
- (51).Magin-Lachmann C, Kotzamanis G, D’Aiuto L, Cooke H, Huxley C, Wagner E In vitro and in vivo delivery of intact BAC DNA – comparison of different methods, J. Gene Med, 2004; 6: 195–209 [DOI] [PubMed] [Google Scholar]
- (52).Tompkins SM, Lo C, Tumpey TM, Epstein SL Protection against lethal influenza virus challenge by RNA interference in vivo, PNAS, 2004; 101: 8682–8686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Gou L, Murphy CJ Fine-tuning the shape of gold nanorods, Chem. Mater, 2005; 17: 3668–3672 [Google Scholar]
- (54).Thomas CF, Luisi PL RNA selectively interacts with vesicles depending on their size, J. Phys. Chem, 2005; 109: 14544–14550 [DOI] [PubMed] [Google Scholar]
- (55).Wang R, Kovalchin JT, Muhelnkamp P, Chandawarkar RY Exogenous heat shock protein 70 binds macrophage lipid raft microdomain and stimulates phagocytosis, processing, and MHC-II presentation of antigens, Blood, 2006; 107: 1636–1642. [DOI] [PubMed] [Google Scholar]
- (56).Perroud TD, Kaiser JN, Sy. JC, Lane TW, Branda CS, Singh AK, Patel KD Microfluidic-Based Cell Sorting of Francisella tularensis Infected Macrophages Using Optical Forces, Anal. Chem, 2008; 80: 6365–6372 [DOI] [PubMed] [Google Scholar]
- (57).Barnes JA, Dix DJ, Collins BW, Luft C, Allen JW Expression of inducible hsp70 enhances the proliferation of mcf-7 breast cancer cells and protects against cytotoxic effects of hyperthermia, Cell Stress & Chaperones, 2001; 6: 316–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


