Abstract
Objective
Translating clinical evidence to daily practice remains a challenge and may improve with clinical pathways. We assessed interest in and usability of clinical pathways by primary care professionals.
Methods
An online survey was created. Interest in pathways for patient care and learning was assessed at start and finish. Participants completed baseline questions then pathway-associated question sets related to management of 2 chronic diseases. Perceived pathway usability was assessed using the system usability scale. Accuracy and confidence of answers was compared for baseline and pathway-assisted questions.
Results
Of 115 participants, 17.4% had used clinical pathways, the lowest of decision support tool types surveyed. Accuracy and confidence in answers significantly improved for all pathways. Interest in using pathways daily or weekly was above 75% for the respondents.
Conclusion
There is low utilization of, but high interest in, clinical pathways by primary care clinicians. Pathways improve accuracy and confidence in answering written clinical questions.
Keywords: Clinical pathways, clinical decision support, primary health care, usability
Introduction
The rapid increase in the volume and scope of medical literature, in the setting of time and resource constraints in primary care, has increased the difficulty for primary care professionals to stay current on and adhere to the latest medical evidence. Regional- and provider-level variability in clinical care persists, often associated with increased costs and worse clinical outcomes.1,2 To address variability in medical practice, bridging the evidence-to-practice gap has become the focus of type 2 translational research – translating medical knowledge from clinical trials to everyday clinical practice for the benefit of patients.3
Clinical decision support tools are one intervention in addressing the evidence-to-practice gap. One such tool is the clinical pathway. A clinical pathway is a structured plan of care, used to translate current medical evidence into a framework of recommendations, at times tailored to the local healthcare setting. It details the steps in management and aims to standardize care and reduce variation for a specific clinical context.4 Clinical pathways are often presented in visual or algorithmic format. In emergency department and inpatient settings, implementation of clinical pathways, as compared to usual care, has been shown to reduce in-hospital complications and improve documentation with no negative effects on cost or length of stay.5
Given success in acute care settings, clinical pathways have been studied in ambulatory care, with mixed results with respect to their effect on clinical outcomes and resource utilization.6 These variable results may be in part dependent on the willingness of clinicians to use such a decision support tool and on the perceived usability. Research on the design of decision support tools other than clinical pathways and on decision aids have shown that design has a significant impact on usability and ultimately effectiveness of such tools.7–9 The current use of, interest in, and perceived usability of clinical pathways by primary care professionals has not yet been studied.
Through a survey-based study amongst healthcare professionals working in primary care, we sought to assess current use of and interest in clinical pathways as a tool for learning and for patient care. We also sought to assess the perceived usability of clinical pathways for 2 frequently encountered conditions in primary care: chronic gout and chronic obstructive pulmonary disease (COPD).
Methods
Study Design and Participants
We designed an online survey using Google Forms (Google Inc., Mountain View, CA, USA). Physicians, advanced registered nurse practitioners, clinical pharmacists, and physician assistants who currently worked in primary care were eligible to participate. Physicians still in a primary care residency were eligible. Invitations to participate were sent by email, through which participants could directly access the survey. Participants were recruited by emails sent to primary care teaching organizations, community-based primary care organizations, primary care-related email lists, and primary care thought-leaders, with requests that individuals receiving invites pass along the invitation to other primary care professionals in their networks. Through this snowball sampling, primary care professionals could choose to participate. Participation was anonymous and voluntary.
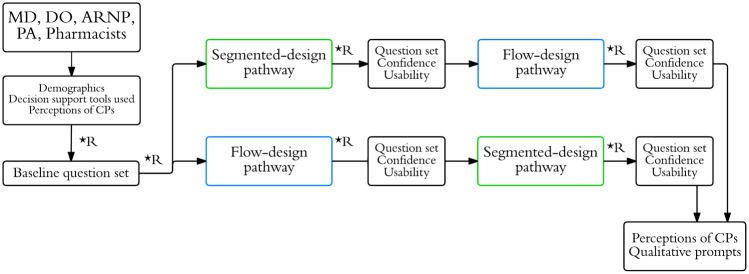
The survey design is outlined in Figure 1. The survey first assessed which kinds of clinical decision support tools participants currently used. Participants were then shown an example of a clinical pathway and asked how frequently (daily, weekly, monthly, rarely, never, or not sure) they would anticipate using such a tool, if provided for common conditions, as a learning tool and for real-time patient care. Participants were then randomized to a baseline question set related to management of the chronic condition (gout or COPD), followed by 2 pathway-associated question sets. The 2 pathway designs were a traditional flow-chart design and a segmented design (described below). The purpose of the clinical questions was to assess change in participants’ accuracy and confidence when using pathways and to require that participants use the pathway in order to more fully assess usability. Randomization, the purpose of which was to better allow for comparison of accuracy when answering baseline questions as compared to pathway-associated questions, occurred by participants selecting one of several equally appearing, nondescript radio buttons throughout the survey. For every clinical question answered, participants noted the confidence of their answer, on a scale of 1–5. Accuracy was measured as the percentage of questions correctly answered (Supplementary Appendix S1). Perceived usability of the clinical pathways was assessed using the system usability scale (SUS). The SUS is a validated 10-item questionnaire that can effectively differentiate between usable and unusable tools.10 Participants had the option to complete only one or both surveys for gout and COPD management. Lastly, participants re-answered questions on their interest in using clinical pathways, to assess for changes in the intensity of their interest, followed by several qualitative questions on their experience.
Figure 1.
Survey study design.
Abbreviations: MD: medical doctor; DO: doctor of osteopathy; ARNP: advance registered nurse practitioner; PA: physician assistant; CP: clinical pathway; *R: randomization point.
Pathway Design
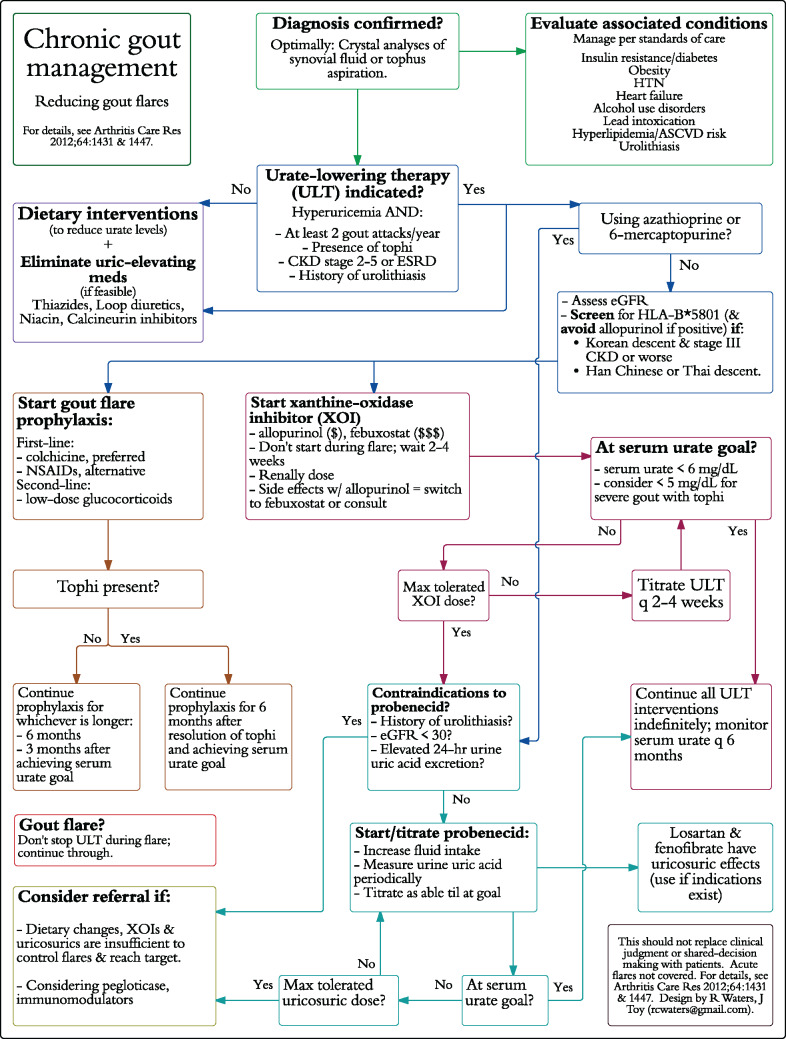
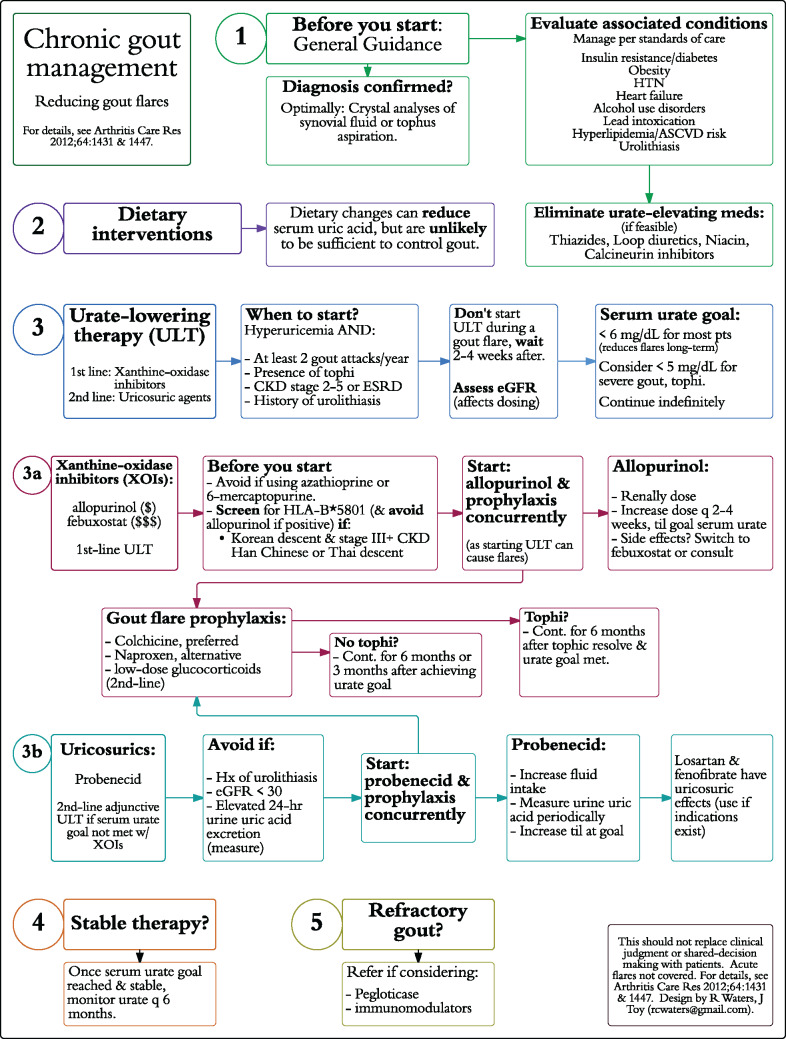
Pathways for chronic gout and COPD management focused only on chronic management, not acute exacerbations. The gout pathways (Figures 2 and 3) were developed based on the 2012 American College of Rheumatology guidelines.11,12 The COPD pathways (Supplementary Figures S1 and S2) were based on guidelines from the American College of Physicians and American Thoracic Society.13 The flow-chart pathways were designed such that every subsequent step in management stemmed directly from a prior step. The segmented pathways grouped similar types of interventions in the algorithm, based on groupings in the practice guidelines. Two designs were used to assess perceived usability in 2 different formats
Figure 2.
Chronic gout flow-chart design pathway.
Abbreviations: HTN: hypertension; ASCVD: atherosclerotic cardiovascular disease; CKD: chronic kidney disease; ESRD: end-stage renal disease; eGFR: estimated glomerular filtration rate; NSAID: non-steroidal anti-inflammatory drug.
Figure 3.
Chronic gout segmented design pathway.
Abbreviations: HTN: hypertension; ASCVD: atherosclerotic cardiovascular disease; CKD: chronic kidney disease; ESRD: end-stage renal disease; eGFR: estimated glomerular filtration rate; NSAID: non-steroidal anti-inflammatory drug.
Statistical Analysis
Statistical analyses were performed using Stata statistical software release 11 (StataCorps LP, College Station, TX, USA). The number of correct responses, the average confidence of the responses and SUS scores were compared by t-tests and multiple linear regression.
Results
There were 115 participants who completed the survey (Table 1). Participants included physicians, nurse practitioners, and primary care clinical pharmacists. Most participants had up to 5 years of primary care practice (53%), with others in practice from 6 to 10 years, 11 to 20 years, and >20 years. Primary care clinic types were mostly university-based and residency-affiliated, nonuniversity.
Table 1.
Characteristics of Survey Participants
| Characteristic | Number of participants | Percentage of total |
|---|---|---|
| Profession | ||
| Physician | 48 | 41.7 |
| Nurse practitioner | 5 | 4.4 |
| Primary care clinical pharmacist | 62 | 53.9 |
| Years of practice | ||
| 0–2 | 36 | 31.3 |
| 3–5 | 25 | 21.7 |
| 6–10 | 19 | 16.5 |
| 11–20 | 16 | 13.9 |
| 21+ | 19 | 16.5 |
| Practice type | ||
| University-based | 50 | 43.8 |
| Residency-affiliated, nonuniversity | 37 | 32.5 |
| Nonteaching site | 27 | 23.7 |
| Practice characteristics | ||
| Solo practice (one clinician only) | 0 | 0 |
| 2–4 primary care clinicians | 24 | 20.9 |
| 5 or more primary care clinicians | 91 | 79.1 |
| Presence of interprofessional primary care teams | 77 | 70.0 |
| Care management teams, led by nonclinicians | 38 | 33.0 |
| Ongoing quality improvement projects | 69 | 60.0 |
Participants reported having used various decision support tools or resources. The decision support tools used by participants included UpToDate (n = 96, 83.5%), clinical practice guidelines (81, 70.4%), smart phone applications of any kind (77, 67.0%), medical reference books of any kind (46, 40.0%), electronic health record-based support tools (38, 33.0%), and Dynamed (21, 18.3%). Only 20 participants (17.4%) reported having used clinical pathways.
Seventy-one participants completed a survey utilizing the 2 pathways for chronic gout management, while 72 participants did so for chronic management of COPD. The accuracy and confidence rates for questions answered when assisted by any pathway significantly increased compared to those for the baseline question sets. The average SUS score ranged from 49 to 66 (Table 2). Multiple linear regression showed that neither profession, years in practice or practice type significantly affected the SUS of pathways nor accuracy of questions answered when using a pathway.
Table 2.
System Usability Score, Accuracy Rate, and Answering Confidence by Pathway Type
| Outcome measures | Baseline question set | Flow-chart design | Segmented design |
|---|---|---|---|
| Gout pathways | |||
| System usability score (confidence interval) | 49.7 (45.7-53.7) | 59.4 (55.9-63.0) | |
| Accuracy rate (%, 0–100) | 39.7 | 83.4* | 82.8* |
| Confidence in answers (%, 0–100) | 50.3 | 78.1* | 79.0* |
| COPD pathways | |||
| System usability score | 66.2 (62.3-70.1) | 63.5 (59.9-67.2) | |
| Accuracy rate (%, 0–100) | 41.7 | 77.2* | 73.9* |
| Confidence in answers (%, 0–100) | 59.2 | 81.4* | 77.2* |
*Indicates P < 0.0001 when compared to corresponding baseline question set value.
At the start of the survey, after being shown an examples of clinical pathways – but prior to starting the question set exercises for gout and COPD management – 84.2% of respondents anticipated that they would use clinical pathways, if provided, for real-time patient care on a daily or weekly basis, decreasing to 77.4% at the conclusion of the survey. The percentage of respondents who would use clinical pathways, if provided, as a learning tool on a daily or weekly basis was 82.5% at the start of the survey, and 82.7% after the survey. Neither of these changes reached statistical significance.
Discussion
Our survey of 115 professionals in current primary care practice has several notable findings. We found that clinical pathways were one of the least utilized types of clinical decision support tools used by our surveyed population, with only 17% reporting use. Lack of availability of clinical pathways and ease of use of other nonpathway clinical decision support tools may be explanatory factors. Interest in using clinical pathways, however, for both patient care and learning was high at the start and finish of the survey, with over 75% reporting anticipated daily or weekly use. Exposure to clinical pathways, by means of engaging with pathways during the survey, did not significantly change this interest. Using a proposed scale for evaluating the SUS score, the perceived usability for the different pathways ranged from the “okay” range (SUS score range 39–52) to the “good” range (52–73).14,15 Thus, despite less-than-excellent ratings for perceived usability, interested in pathways remained high. This suggests that if made readily available, there may be high uptake of clinical pathways within primary care.
All pathways significantly improved accuracy and confidence of answering clinical questions, as anticipated. Such improvements, combined with the high interest in and anticipated use of clinical pathways, support further study of pathways as decision support tools. It remains unclear, though, if our findings would translate into improved clinical outcomes for patients, and studies to date have had mixed outcomes.16
Qualitative responses suggested that participants’ interest was related to a desire for clear recommendations and the potential for reduced variability in care. These goals, however, are also the goals of clinical practice guidelines (CPGs). CPGs are “statements that include recommendations intended to optimize patient care that are informed by a systematic review of evidence and an assessment of the benefits and harms of alternative care options.”17 CPGs have the same goal as clinical pathways: the standardization, when able, of medical care to reduce variability and maximize effectiveness, based on best evidence. While CPGs have undoubtedly assisted in the synthesis of and dissemination of medical knowledge, their implementation has faced challenges and barriers to implementation.18–22
We support the notion of clinical pathways as “translators” of guidelines – adjuncts to, rather than replacing, guidelines – as means to assist in their implementation. Pathways may be easier to use in real-time or as a brief learning tool, which may explain the high interest in both these areas. Requiring further study, we hypothesize that if every CPG released had a well-designed accompanying clinical pathway for the elements relevant to primary care practice, there may be a higher likelihood of implementation of those guidelines by primary care teams.
There are several limitations to our study. These include small sample size, only 2 types of chronic conditions tested, overrepresentation of clinical pharmacists in the sample, and no measurement of the direct effect on clinical practice or resulting quality of patient care. Also, there is a risk of selection bias, as participants most interested in clinical pathways are most likely to complete a voluntary survey on the topic. The pathway designs were based on a few published guidelines from the United States, which may be different from other published guidelines and may not have contained the most updated medical literature.
Conclusion
This study suggests that there is a significant interest in clinical pathways amongst primary care professionals, but that despite this interest, clinical pathways are among the least utilized of the types of clinical decision support tools available.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Competing interests
None.
Contributors
JMT, AD, and RCW each contributed to the conception and design of this investigation, to the analysis and interpretation of data, and to the drafting and finalization of the manuscript.
SUPPLEMENTARY MATERIAL
Supplementary material is available at Journal of the American Medical Informatics Association online.
Supplementary Material
References
- 1. Fisher ES, Bynum JP, Skinner JS. Slowing the growth of health care costs–lessons from regional variation. N Engl J Med 2009; 3609:849–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Korn L, Corrigan J, Donaldson M. To Err is Human: Building a Safer Health System. Washington: Institute of Medicine; 1999. [Google Scholar]
- 3. Woolf SH. The meaning of translational research and why it matters. JAMA 2008; 2992:211–213. [DOI] [PubMed] [Google Scholar]
- 4. Lawal AK, Rotter T, Kinsman L et al. What is a clinical pathway? Refinement of an operational definition to identify clinical pathway studies for a Cochrane systematic review. BMC Med 2016; 14:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rotter T, Kinsman L, James E et al. Clinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev 2010; 3:CD006632. [DOI] [PubMed] [Google Scholar]
- 6. Rotter T, Kinsman L, Machotta A et al. Clinical Pathways for Primary Care: Effects on Professional Practice, Patient Outcomes, and Costs. The Cochrane Library [Internet]; 2013. http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD010706/pdf/abstract [Google Scholar]
- 7. Tsopra R, Jais J-P, Venot A, Duclos C. Comparison of two kinds of interface, based on guided navigation or usability principles, for improving the adoption of computerized decision support systems: application to the prescription of antibiotics. J Am Med Inform Assoc 2014; 21(e1):e107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Horsky J, Schiff GD, Johnston D, Mercincavage L, Bell D, Middleton B. Interface design principles for usable decision support: a targeted review of best practices for clinical prescribing interventions. J Biomed Inform 2012; 456:1202–1216. [DOI] [PubMed] [Google Scholar]
- 9. Elwyn G, O’Connor A, Stacey D et al. Developing a quality criteria framework for patient decision aids: online international Delphi consensus process. BMJ 2006; 3337565:417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brooke J. SUS-A quick and dirty usability scale. Usability Evaluation in Industry 1996; 189194:4–7. [Google Scholar]
- 11. Khanna D, Fitzgerald JD, Khanna PP et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res 2012; 6410:1431–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Khanna D, Khanna PP, Fitzgerald JD et al. American College of Rheumatology. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res 2012; 6410:1447–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Qaseem A, Wilt TJ, Weinberger SE et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med 2011; 1553:179–191. [DOI] [PubMed] [Google Scholar]
- 14. Bangor A, Kortum PT, Miller JT. An empirical evaluation of the system usability scale. Int J Human–Comp Interact 2008; 246:574–594. [Google Scholar]
- 15. Bangor A, Kortum P, Miller J. Determining what individual SUS scores mean: adding an adjective rating scale. J Usability Stud 2009; 43:114–123. [Google Scholar]
- 16. Garg AX, Adhikari NKJ, McDonald H et al. Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: a systematic review. JAMA 2005; 29310:1223–1238. [DOI] [PubMed] [Google Scholar]
- 17. Graham R, Mancher M, Miller Wolman D. Committee on Standards for Developing Trustworthy Clinical Practice Guidelines; Institute of Medicine. Clinical Practice Guidelines We Can Trust. 2011. Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- 18. Hysong SJ, Best RG, Pugh JA. Clinical practice guideline implementation strategy patterns in Veterans Affairs primary care clinics. Health Serv Res 2007; 42(1 Pt 1):84–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cabana MD, Rand CS, Powe NR et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA 1999; 28215:1458–1465. [DOI] [PubMed] [Google Scholar]
- 20. Flores G, Lee M, Bauchner H, Kastner B. Pediatricians’ attitudes, beliefs, and practices regarding clinical practice guidelines: a national survey. Pediatrics 2000; 105(3 Pt 1):496–501. [DOI] [PubMed] [Google Scholar]
- 21. Carlsen B, Glenton C, Pope C. Thou shalt versus thou shalt not: a meta-synthesis of GPs’ attitudes to clinical practice guidelines. Br J Gen Pract 2007; 57545:971–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grol R, Grimshaw J. From best evidence to best practice: effective implementation of change in patients’ care. Lancet 2003; 3629391:1225–1230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.