Abstract
Importance
Electronic prescribing promises to improve the safety and clarity of prescriptions. However, it also can introduce miscommunication between prescribers and pharmacists. There are situations where information that is meant to be sent to pharmacists is not sent to them, which has the potential for dangerous errors.
Objective
To examine how frequently prescribers or administrative personnel put information intended for pharmacists in a field not sent to pharmacists, classify the type of information included, and assess the potential harm associated with these missed messages.
Design, Setting, Participants
Medication record data from our legacy electronic health record were requested for ambulatory care patients seen at an academic medical center from January 1, 2000, to May 31, 2015 (20 123 881 records). From this database, 6 060 272 medication orders met our inclusion criteria. We analyzed a random sample of 10 000 medication orders with internal comments.
Main Outcomes and Measures
Reviewers classified internal comments for intent. Comments intended for pharmacists were also sorted into descriptive categories and analyzed for the potential for patient harm.
Results
We found that 11.7% of the prescriptions in our sample contained comments that were intended to be sent to pharmacists. Many comments contained information about the dose, route, or duration of the prescription (38.0%). Approximately a third of the comments intended for pharmacists contained information that had the potential for significant or severe harm if not communicated.
Conclusion
We found undelivered comments that were clearly intended for pharmacists and contained important information for either pharmacists or patients. This poses a legitimate safety concern, as a portion of comments contained information that could have prevented severe or significant harm.
Keywords: electronic prescribing, safety, usability, pharmacy
INTRODUCTION
Team-based care delivery models are increasingly common and are known for being effective, especially with regard to management of chronic disease.1,2 Pharmacists are integral to the success of an integrated care team and complement the skills of other members of the team.3–6 They are trained in identifying and addressing problems relating to medications, such as adverse drug events, drug-drug or drug-disease interactions, and issues with patient adherence. Additionally, pharmacists provide medication therapy management services that help optimize patients’ pharmacotherapy and improve collaboration with clinicians.7 However, pharmacists are often underused in clinical settings.8,9
Poor communication is a key barrier to effective team-based care and is a major cause of medical errors.10–13 In an analysis of >23 000 medical malpractice cases, 30% of them included at least one breakdown in communication.13 Electronic prescribing has the potential to improve the safety and quality of care by reducing errors in legibility and transcription during the prescribing process.14–18 However, research has shown that electronic prescribing can also introduce new kinds of errors, be ambiguous, or allow missing information, which can lead to delays and adverse patient outcomes.19–23 A recent study of e-prescriptions using the SCRIPT standard and sent from prescribers to community pharmacies examined the content within the free text sent with electronic prescriptions, noting that free-text fields often contained content that was more appropriate for structured fields.24 Without structured data, clinical decision support rules are not able to activate and are therefore unable to potentially prevent patient harm.
In addition to information being sent to pharmacists in the incorrect form, there are situations where information that is meant to be sent to pharmacists is not sent to them. This presents the potential for dangerous errors, as providers could mistakenly include important information intended for patients or the pharmacy, thinking that they are communicating the information. In contrast to the SCRIPT study that examined whether free-text comments sent to pharmacists were appropriate or useful, we investigated the prevalence of providers inputting information meant for the pharmacy but was never received because it was entered in a free-text “Comments” field designed for internal use only.
METHODS
Setting
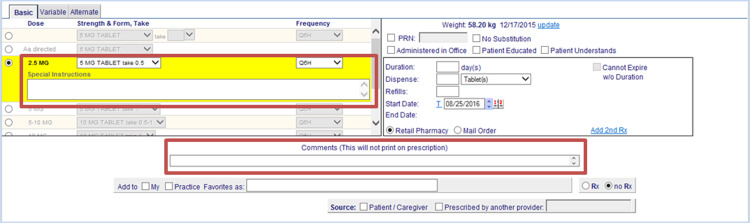
We conducted a retrospective analysis of medication records transmitted through the longitudinal medical record (LMR), the legacy electronic health record (EHR) system at Brigham and Women’s Hospital, a tertiary academic medical center that is part of the Partners HealthCare System. When prescribing medications in the Partners HealthCare LMR, practitioners have the option of writing additional information in 1 of 2 fields: Special Instructions or Comments (Figure 1). Text written in Special Instructions is electronically transmitted to the pharmacy. However, the Comments field is designed to contain internal notes to providers or administrative personnel and is not transmitted to the pharmacy or visible to the patient.
Figure 1.
Prescription entry window in the LMR. Boxes indicate “Special Instructions” and “Comments.”
Data extraction
LMR medication data were requested for Brigham and Women’s Hospital outpatient prescriptions written between January 1, 2000, and May 31, 2015. Data extracted included patient medical record number; medication information including medication name, route, and status (such as active, sent to the pharmacist, refill); date of prescription; start date and end date of prescription; prescribed quantity, strength, and frequency; special instructions; comments; prescriber name; and clinic. From this database, we extracted active orders that were sent to the pharmacy where the Comments field did not match the Special Instructions field. In some cases, the Comments field was autopopulated with information about the drug; we excluded these comments. An example of an autopopulated comment for isotretinoin is “Restricted Medication: Available only under a special restricted distribution program, iPLEDGE approved by the Food and Drug Administration.” From this database, a random sample of 10 000 medication records that included the Comments field were then evaluated for intent.
Determination of intent
The comments from each record of the random sample of 10 000 medication prescription records were categorized for intent by 2 independent reviewers, a clinical pharmacist (AW) and a research assistant (AA), both with previous experience in clinical informatics. The categories of comments in the Comments field were “intended for the pharmacist,” “not intended for the pharmacist,” and “uncertain intent.” When the 2 reviewers did not agree, a senior clinical pharmacist (MA) with >20 years of practice experience was consulted to reach consensus.
Categorization of free-text comments
A taxonomy for categorization of the free-text comments was developed iteratively using a grounded theory approach.25 The goal of the taxonomy was to group the comments by their intent and to permit analysis of the most frequent types of comments. Grounded theory is an inductive approach that starts with data collection: as data are collected, tags are applied and recurring themes (in this case, clinical intent) are identified. Study team members examined comments that had been coded as “intended for the pharmacist” in batches of 250. After discussion, preliminary categories were established, and each team member coded his or her batch of comments based on the preliminary categories. The research team reviewed all free-text comments and conducted a series of virtual card-sorting exercises to refine the taxonomy. As the categories emerged, we repeatedly reviewed and reclassified comments as needed and developed a code book with general rules for classification, as well as specific categories for comments intended for the pharmacist (Supplementary Appendix A). Additionally, we revisited comments that were initially marked as uncertain intent. If comments fell within the revised taxonomy categories, they were reclassified as intended for the pharmacist and coded.
Analysis of potential harm
Two independent pharmacist reviewers evaluated the messages determined to be intended for the pharmacist for the potential severity of harm if they were not relayed to the pharmacist. A third party was used in cases where consensus was not achieved between the 2 reviewers. Severity was judged on a scale of 0–3 (0: no harm, 1: potential significant harm, 2: potential severe harm, 3: potential life-threatening harm). The general rules stated that comments that asked for no substitutions for brand-name medications were scored as 1, because we were unsure of the reason why the brand name was needed. Additionally, in cases where the structured dose or directions did not match with the Comments field, they were scored as 1, except in the case of high-risk medications. We used the Institute for Safe Medical Practices List of High-Alert Medications in Community and Ambulatory Healthcare to define our list of high-risk medications.26 For these high-risk medications, such as benzodiazepines and opioids, harm was marked as 1 or 2 if there was the potential for overdose.
RESULTS
During our 15-year data period, 20 123 881 outpatient medication records were created in the LMR, our legacy EHR. Of those, 6 060 272 records were sent to the pharmacy and had text in the Comments field that was not autopopulated and did not match the Special Instructions field (30.1%).
Identification of notes intended for the pharmacist
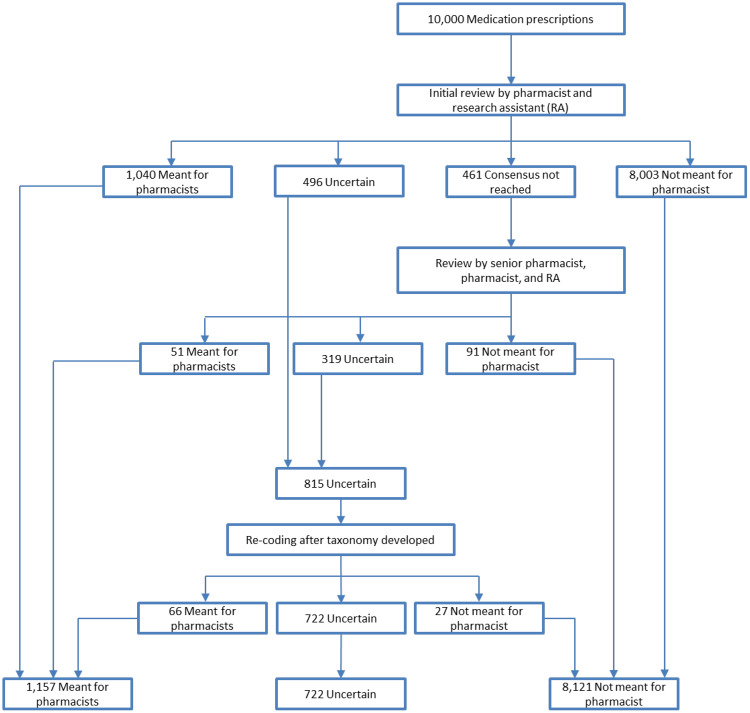
Initial review of the random sample of 10 000 comments indicated that 1040 (10.4%) free-text comments were intended by prescribers to be sent to the pharmacist, 8003 (80.0%) were not meant to be sent to the pharmacist, and for 496 the intent was uncertain (Figure 2). Interrater reliability was 95.4% (95% CI, 94.9%-95.8%). Consensus on 461 comments was not reached after multiple rounds of review by the 2 initial reviewers. A senior pharmacist adjudicator reviewed the 461 disputed comments and determined that 51 (11.1%) were intended for the pharmacist and 91 (19.7%) were not meant for the pharmacist, and was uncertain about 319 comments (69.2%). After the initial coding rounds were completed, 1091 comments (10.9%) were determined to be intended for the pharmacist, 8094 (80.9%) were not intended for the pharmacist, and 815 comments (8.2%) had uncertain intent. When the taxonomy categories were established, the entire research team reexamined the comments with uncertain intent. Of these 815 comments, 66 were reclassified as meant for the pharmacist and 27 as not meant for the pharmacist. In total, 1157 comments (11.6%) were determined to have been meant for the pharmacist and 722 comments (7.2%) were of uncertain intent.
Figure 2.
Analysis and classification electronic prescription comments for intent.
Classification of comments intended for the pharmacist
A total of 1236 codes were assigned to the 1157 comments (Table 1). The most frequent comment categories were related to dose, route, or duration (38.0%), transmission of the prescription (19.3%), or details on filling the prescription (16.6%). Of the comments relating to dose, route, or duration, 35.0% contained information that contradicted the information that would have been sent to the pharmacist, 33.6% contained additional information not sent to the pharmacist, and 31.4% contained only information that was already sent to the pharmacist in another field.
Table 1.
Categorization of themes in comments intended for the pharmacist
| Category | Number of comments, n (%) | Examples |
|---|---|---|
| Dose, route, or duration | 440 (38.0) | |
| Different from listed | 154 (35.0) | Structured sig: 1 tab Q6H; Comment: Take 1 to 2 tabs daily as needed |
| Structured sig: 150 mg QD of 150 mg tablet; Comment: Take ½ tab | ||
| Same as listed | 138 (31.4) | Structured sig: 500 mg tablet, take 2 BID; Comment: tab 2 po bid |
| Structured sig: BID; Comment: BID | ||
| Not listed information | 148 (33.6) | “Apply to wounds over fibrinous (yellow) tissue” |
| “do not exceed 6 tablets/day” | ||
| Transmission of prescription | 223 (19.3) | “refax” |
| “Please mail to patient” | ||
| Details on filling the prescription | 192 (16.6) | “Please disregard previous RX!! This is correct amount of pills” |
| “largest bottle” | ||
| Indication or diagnosis | 131 (11.3) | “Please take for kidney pain” |
| “Dx: ADHD.” | ||
| Substitution information | 120 (10.4) | |
| Other substitution | 81 (67.5) | “ok to substitute Ofloxacin if patient prefers for cost” |
| “PLEASE DISP GENERIC” | ||
| No substitution or Dispense as written (DAW) | 39 (32.5) | “PLEASE DISPENSE D.A.W. ONLY, NO SUBSTITUTIONS. THANK YOU!” |
| “please dispense as written” | ||
| Information for the pharmacist | 70 (6.1) | “Steroid side effects and correct use were discussed. Patient understands” |
| “this is the correct dosage and directions” | ||
| Other requests to the pharmacist | 60 (5.2) | “Please run medication compatibility with all meds” |
Examples are derived from real comments.
Harm potential analysis
A review of the comments for the potential for harm was conducted. There was initial agreement on 943 comments (81.5%). Interrater reliability, as measured by Cohen’s κ, was 0.611, which is interpreted as good strength of association. After discussion, consensus was reached on 1155 (99.8%) and a third party adjudicated on 2 records.
We found that almost two-thirds of comments contained information that had no potential for harm (65.7%) (Table 2). More than one-third of the prescriptions contained comments that had potential for harm if not communicated: 31.5% had the potential for significant harm and 2.8% had the potential for severe harm. No comments alluded to potentially life-threatening harm. We observed that there were several instances where the Special Instructions (transmitted) and Comments (not transmitted) were similar enough to what was not communicated to the pharmacist to likely not result in harm. These comments are included in those marked as no potential for harm. Additionally, there were instances where the comments did not make sense in context of the message, and instances where it was better for the message not to have been sent to the pharmacist. For example, the Comments field on a prescription for pravastatin 20 mg qHS stated “5 mg q 6 prn (120 tabs per month).” This is likely an error, as the comment text describes reasonable dosing for pravastatin (in this case, transmitting the comments may have led to confusion). Another comment in our database stated “sorry for prior rx.” It would be impossible to identify which prior prescription the comment is referencing.
Table 2.
Harm ratings for comments marked as intended for the pharmacist (n = 1157 comments)
| Potential harm | No harm, n (%) | Significant harm, n (%) | Severe harm, n (%) |
|---|---|---|---|
| Dose, route, and/or direction | 203 (26.7) | 210 (57.5) | 27 (84.4) |
| Different | 41 (20.2) | 98 (46.7) | 15 (55.6) |
| Same | 123 (60.6) | 14 (6.7) | 1 (3.7) |
| Not listed | 39 (19.2) | 98 (46.7) | 11 (40.7) |
| Transmission | 195 (25.6) | 24 (6.5) | 4 (12.5) |
| Fill details | 149 (19.6) | 42 (11.5) | 1 (3.1) |
| Indication/diagnosis | 120 (15.7) | 10 (2.7) | 1 (3.1) |
| Substitution | 49 (6.5) | 71 (19.5) | 0 (0) |
| Other substitution | 49 (100) | 32 (45.7) | 0 (0) |
| No substitution/DAW | 0 (0) | 39 (54.9) | 0 (0) |
| Information | 55 (7.2) | 14 (3.8) | 1 (3.1) |
| Other requests | 33 (4.3) | 26 (7.1) | 1 (3.1) |
| Total | 760a (65.7) | 365a (31.5) | 32a (2.8) |
aSome comments may have been placed into multiple categories.
DISCUSSION
In our analysis of a free-text field that was designed purely for office use, we found that 11.7% of comments were intended for the pharmacist and 7.2% contained content that could have been intended for the pharmacist. Many comments related to the dose, route, or duration of the prescription, with two-thirds of these comments containing information that contradicted or supplemented the information that would have been sent to the pharmacist (n = 302). Because these contradictory or supplementary comments were all written in the outpatient setting, it is very unlikely that they were seen by patients or anyone involved in administering the drug, rendering them (dangerously) moot. Additionally, many comments (16.6%) included instructions on how to fill the prescription. When we analyzed the potential harm if this field was not sent to the pharmacist, we found that 34.3% of comments that were intended for the pharmacist (4.0% of all analyzed comments) contained information that could have prevented significant or severe harm to the patient if not fully communicated to the pharmacist.
A proportion of comments that we deemed intended for the pharmacist included the indication for the prescription (10.6%). Over the past 20 years, there has been a push to add information about clinical indications to prescriptions to increase safety and awareness, and attention to this issue has increased.27 While there have been concerns about patient privacy and off-label use of medications when including indications, we believe that prescribers who include indications in the comments field intend for that information to be transmitted to the pharmacist, and that it is appropriate and useful information for the pharmacist. Supplying indications for therapy may help pharmacists identify potential medication errors and verify that correct medication, dose, and duration have been selected, as well as properly educate patients about the purpose and use of medications. Patients often do not know why they are taking particular medications, and many prefer to have information about the indications.28 We believe that clinicians who included this information in the Comments field would have intended for it to be sent to the pharmacist.
Additionally, the reviewed comments suggest that providers are not completely aware of regulations regarding substitution that pharmacists must follow. For example, in Massachusetts, pharmacists can only act on instructions written precisely as “dispense as written” or “no substitutions.”29 If comments such as those given as examples in Table 1, which are not appropriately written to conform with state laws, are supplied to pharmacists, this should result in some form of communication from the pharmacy to clarify the order. The lack of these comments for e-prescribed medications may result in potential patient harm from mandated exchange for generic medications unless properly specified, due to patient intolerance to generic medications or instances of narrow therapeutic index medications.
Communication between pharmacists and other care providers can be complicated. While electronic prescribing promises to reduce issues with legibility and transcription, it has created new issues. For example, previous studies have shown that prescription free-text fields frequently contain information that would be more suited as structured information.25 Another issue is the misdirection of communication, since information that clinicians intend for pharmacists might never be sent to them. For example, previous research has shown that free-text comments on computerized provider order entry (CPOE) alerts frequently contain relevant information on why the clinician overrode the alert and sometimes important information on potentially very important clinical safety issues.10 However, those comments were not sent to relevant personnel, and this misdirected communication could have led to patient care errors. Similarly, in our system, we found undelivered comments that were clearly intended for the pharmacist and contained important information for either the pharmacist or the patient. These fields pose a legitimate safety concern, as a portion of the comments contained information that could have prevented severe or significant harm. Fortunately, we identified that these situations are rare and are likely further mitigated by a pharmacist’s familiarity with patients through access to their past prescription claims, which would allow for identification of potential discrepancies.
Changes are needed to address this potential safety concern. One solution is to eliminate fields for internal use only and have all the information sent to the pharmacy. This has been done by some commercial systems. For example, all fields in our current CPOE system are sent to the pharmacist. Alternatively, the distinction between fields sent to the pharmacist and fields not sent to the pharmacist should be made clearer through interfaces designed with human factors in mind. Our new EHR no longer has an internal Comments field, and has replaced Special Instructions with a field labeled “Note to pharmacy.” Physicians are encouraged to place any relevant information in either the standard instructions field or this note field, and additional options about dispensing have also been added. The Safety Assurance Factors for EHR Resilience guide for clinician communication emphasizes that using health information technology safely requires accurate routing of clinician-to-clinician messages.30 When information is not sent to its expected recipient, patient safety is at risk.
Additional research is needed to identify communication failures that occur in other CPOE systems, and whether these failures lead to patient harm. Our study highlights the importance of clear system design and monitoring of how clinicians utilize free-text entry. This information can be shared and used to identify areas to focus on to implement strategies for effective communication in order to improve patient safety.
LIMITATIONS
Our study has several limitations. One is that our data come from outpatient care practices affiliated with a single large academic health care center using a locally developed legacy EHR system. We attempted to circumvent this by using data from a long study period and from a large sample. Another limitation is that it is difficult to gauge intent without directly contacting the person who wrote the comment, and we evaluated comments based on what we thought the comment meant based on a community practice setting. To address this, we only marked comments that we were confident were intended for the pharmacist and marked several comments as uncertain (see Supplementary Appendix A for details). Similarly, it is difficult to analyze the potential for harm. Theoretically, harm can occur with even a benign medication, and we did not have access to other communication the prescriber might have had with the pharmacist or patient to circumvent the harm, and did not attempt to assess whether harm actually occurred.
CONCLUSION
While the majority of comments in the LMR Comments field are meant for other providers or for office use, 11.7% of analyzed comments appear to have been meant for the pharmacist. This suggests that some providers may not realize that the Comments field is not transmitted to the pharmacy and include important information in this field. It is important to ensure that providers know where to put information that is meant to be seen by the pharmacy when sending electronic prescriptions.
FUNDING
The research was funded using departmental funds from Brigham and Women’s Hospital. There was no external public, commercial, or not-for-profit funding.
COMPETING INTERESTS
The authors have no competing interests to declare.
CONTRIBUTORS
AW had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis. All authors contributed to data acquisition and analysis. AA wrote the manuscript. All authors provided critical revisions of the manuscript for important intellectual content.
SUPPLEMENTARY MATERIAL
Supplementary material is available at Journal of the American Medical Informatics Association online.
Supplementary Material
REFERENCES
- 1. Proia KK, Thota AB, Njie GJ et al. , Team-based care and improved blood pressure control: a community guide systematic review. Am J Prev Med. 2014;471:86–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Misra-Hebert AD, Rabovsky A, Yan C, Hu B, Rothberg MB. A team-based model of primary care delivery and physician-patient interaction. Am J Med. 2015;1289:1025–28. [DOI] [PubMed] [Google Scholar]
- 3. Kaboli PJ, Hoth AB, McClimon BJ, Schnipper JL. Clinical pharmacists and inpatient medical care: a systematic review. Arch Intern Med. 2006;1669:955–64. [DOI] [PubMed] [Google Scholar]
- 4. Smith M, Bates DW, Bodenheimer TS. Pharmacists belong in accountable care organizations and integrated care teams. Health Aff (Millwood). 2013;3211:1963–70. [DOI] [PubMed] [Google Scholar]
- 5. Scott MA, Hitch B, Ray L, Colvin G. Integration of pharmacists into a patient-centered medical home. J Am Pharm Assoc (2003). 2011;512:161–66. [DOI] [PubMed] [Google Scholar]
- 6. Weber ZA, Skelley J, Sachdev G et al. , Integration of pharmacists into team-based ambulatory care practice models. Am J Health Syst Pharm. 2015;729:745–51. [DOI] [PubMed] [Google Scholar]
- 7. Giberson S, Yoder S, Lee M. Improving Patient and Health System Outcomes through Advanced Pharmacy Practice. A Report to the U.S. Surgeon General. Rockville, MD: US Public Health Service; 2011. [Google Scholar]
- 8. McBane SE, Dopp AL, Abe A et al. , Collaborative drug therapy management and comprehensive medication management ― 2015. Pharmacotherapy. 2015;354:e39–50. [DOI] [PubMed] [Google Scholar]
- 9. Viswanathan M, Kahwati LC, Golin CE et al. , Medication Therapy Management Interventions in Outpatient Settings. Rockville, MD: Agency for Healthcare Research and Quality; 2014. [PubMed] [Google Scholar]
- 10. Chused AE, Kuperman GJ, Stetson PD. Alert override reasons: a failure to communicate. AMIA Annu Symp Proc. 2008:Nov 6;111–15. [PMC free article] [PubMed] [Google Scholar]
- 11. Hess DR, Tokarczyk A, O'Malley M, Gavaghan S, Sullivan J, Schmidt U. The value of adding a verbal report to written handoffs on early readmission following prolonged respiratory failure. Chest. 2010;1386:1475–79. [DOI] [PubMed] [Google Scholar]
- 12. King BJ, Gilmore-Bykovskyi AL, Roiland RA, Polnaszek BE, Bowers BJ, Kind AJ. The consequences of poor communication during transitions from hospital to skilled nursing facility: a qualitative study. J Am Geriatr Soc. 2013;617:1095–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Malpractice Risks in Communication Failures: 2015 Annual Benchmarking Report: Risk Management Foundation of the Harvard Medical Institutions Incorporated. Rockville, MD: Agency for Healthcare Research and Quality; 2015. [Google Scholar]
- 14. Fischer MA, Vogeli C, Stedman M, Ferris T, Brookhart MA, Weissman JS. Effect of electronic prescribing with formulary decision support on medication use and cost. Arch Intern Med. 2008;16822:2433–39. [DOI] [PubMed] [Google Scholar]
- 15. Jani YH, Barber N, Wong IC. Paediatric dosing errors before and after electronic prescribing. Qual Saf Health Care. 2010;194:337–40. [DOI] [PubMed] [Google Scholar]
- 16. Schiff GD, Rucker TD. Computerized prescribing: building the electronic infrastructure for better medication usage. JAMA. 1998;27913:1024–29. [DOI] [PubMed] [Google Scholar]
- 17. Bates DW, Cohen M, Leape LL, Overhage JM, Shabot MM, Sheridan T. Reducing the frequency of errors in medicine using information technology. J Am Med Inform Assoc. 2001;84:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abramson EL, Barron Y, Quaresimo J, Kaushal R. Electronic prescribing within an electronic health record reduces ambulatory prescribing errors. Jt Comm J Qual Patient Saf. 2011;3710:470–78. [DOI] [PubMed] [Google Scholar]
- 19. Bates DW, Boyle DL, Teich JM. Impact of computerized physician order entry on physician time. Proc Annu Symp Comput Appl Med Care. 1994:996. [PMC free article] [PubMed] [Google Scholar]
- 20. Palchuk MB, Fang EA, Cygielnik JM et al. , An unintended consequence of electronic prescriptions: prevalence and impact of internal discrepancies. J Am Med Inform Assoc. 2010;174:472–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pevnick JM, Li N, Asch SM, Jackevicius CA, Bell DS. Effect of electronic prescribing with formulary decision support on medication tier, copayments, and adherence. BMC Med Inform Decis Mak. 2014;14:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ungar JP, Gandhi TK, Poon EG et al. , Impact of ambulatory computerized physician order entry on clinicians’ time. AMIA Annu Symp Proc. 2008:Nov 6;1158. [PubMed] [Google Scholar]
- 23. Donyai P, O’Grady K, Jacklin A, Barber N, Franklin BD. The effects of electronic prescribing on the quality of prescribing. Br J Clin Pharmacol. 2008;652:230–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dhavle AA, Yang Y, Rupp MT, Singh H, Ward-Charlerie S, Ruiz J. Analysis of prescribers’ notes in electronic prescriptions in ambulatory practice. JAMA Intern Med. 2016;1764:463–70. [DOI] [PubMed] [Google Scholar]
- 25. Glasier B, Strauss A. The Discovery of Grounded Theory: Strategies for Qualitative Research. New York: Routledge; 2017. [Google Scholar]
- 26. Institute for Safe Medication Practices. ISMP List of High-Alert Medications in Community/Ambulatory Healthcare. 2011. https://www.ismp.org/communityRx/tools/ambulatoryhighalert.asp. Accessed July 1, 2017. [Google Scholar]
- 27. Schiff GD, Seoane-Vazquez E, Wright A. Incorporating indications into medication ordering: time to enter the age of reason. N Engl J Med. 2016;3754:306–09. [DOI] [PubMed] [Google Scholar]
- 28. Zargarzadeh AH, Law AV. Design and test of preference for a new prescription medication label. Int J Clin Pharm. 2011;332:252–59. [DOI] [PubMed] [Google Scholar]
- 29. Commonwealth of Massachusetts. Policy on drug interchangeability and midstream interchange. Mass.gov 2002. https://www.mass.gov/service-details/policy-on-drug-interchangeability-and-midstream-interchange. Accessed February 7, 2018. [Google Scholar]
- 30. Sittig D, Ash J, Singh H. Clinician Communication SAFER Guide. SAFER Guides. 2016. https://www.healthit.gov/safer/guide/sg009. Accessed July 17, 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.