Abstract
The altered expression of glycan antigens has been reported during cervix transformation, demonstrating increased mRNA levels of certain glycogenes. Human papillomavirus (HPV) is the aetiological agent of cervical cancer. High risk HPV E5 is considered an oncogene and has been implicated in cell transformation. E6 and E7 HPV oncoproteins modify the expression of certain glycogenes. The role of the E5 HPV protein in glycogene expression changes has not yet been reported. The aim of the present study was to determine the effects of HPV16 E5 oncoprotein on glycogene expression. For these, a microarray assay was performed using the HaCaT cell line and altered glycogenes were identified. The mRNA levels of certain glycogenes were determined via reverse transcription-quantitative PCR (RT-qPCR). Using in silico analysis, the present study identified that glycosylation pathways were altered by E5. Microarray analysis revealed alterations in certain glycogenes, including the upregulation of ST6GAL1, ST3GAL3, CHST2 and MANBA, and the downregulation of UGT2B15, GALNT11, NDST2 and UGT1A10. Increased mRNA levels were confirmed via RT-qPCR for sialyltransferases genes. Additionally, in silico analysis was performed to identify glycosylation networks altered in the presence of the E5 oncoprotein. The analysis revealed that E5 could modify glycan sialylation, the N-glycosylation pathway, keratan sulfate and glycosaminoglycan synthesis. To the best of our knowledge, the current study was the first to determine the role of the HPV16 E5 oncoprotein in glycogene expression changes. The results indicated that increased sialyltransferase mRNA levels reported in pre-malignant and malignant cervical tissues could be the result of E5 oncoprotein expression. The results provide a possible role of HPV infection on glycosylation changes reported during cervix transformation.
Keywords: human papillomavirus 16, E5 oncoprotein, human papillomavirus, glycogene, glycosylation, microarray expression
Introduction
Human papillomavirus (HPV) is the aetiological agent of cervical cancer (CC) (1). HPV16 is the most prevalent genotype and is responsible for greater than 50% of CC cases worldwide (2,3). The oncogenic potential of HPV is attributed to the following three viral proteins: E5, E6 and E7. E6 and E7, the best characterized viral proteins, promote cell transformation by several mechanisms; for example, they destabilize and induce the degradation of the tumour suppressor proteins p53 and pRB, respectively (4). Meanwhile, E5, a transmembrane protein present in the Golgi apparatus, endoplasmic reticulum and nuclear envelope (5), has several roles in cell transformation. For example, E5 can force cells through the cell cycle and promote the evasion of the immune response (6). Additionally, E5 can modify gene expression; HPV16 E5 induces the expression of prostaglandin E2 receptor by stimulating the binding of the cAMP-response element binding protein (CREB) to its promoter, which activates mitogenic activated protein kinase (MAPK) and increases C-FOS and C-JUN transcription (7,8). Moreover, E5 increases the expression of genes related to cell adhesion, cell motility, and mitogenic signalling, suggesting that the protein plays an important role in the events associated with cellular transformation (9).
Altered glycosylation is another characteristic of cancer cells (10), and it can be caused by the altered expression of different glycosyltransferases that can lead to changes in glycan structures. Increased mRNA levels of some sialyltransferases have been reported in different cancer types (11). Specifically, CC and premalignant lesions display increased mRNA levels of the sialyltransferases ST3GAL3 and ST6GAL1 (12,13), which are related to increased expression of sialic acid (14,15) and of sialylated antigens such as sTn and sLe(x) (16–18). Studies in the HeLa cell line show that E6/E7 HPV18 oncogene knockdown modified glycogene expression, some of which participate in the synthesis of O-glycans such as sTn (19). These results suggest that viral infection could modify glycogene expression and the glycosylation of the cervical epithelium.
The objective of this work was to identify glycogenes that displayed modified expression patterns in the presence of the HPV16 E5 oncoprotein. The results showed that the HPV16 E5 oncoprotein could increase the expression of the sialyltransferases ST3GAL3 and ST6GAL1, which have been reported to be altered in premalignant and malignant cervical tissues. The network interaction constructed with the altered glycogenes in the presence of E5 showed that not only the glycan sialylation but also some glycan structures, such keratan sulphate and glycosaminoglycans, could also be altered.
Materials and methods
Cell culture
The HaCaT cell line from human skin keratinocytes stably transfected with the HPV16 E5 oncogene (HaCaT-E5) (20) or the vector pMSG (HaCaT-pMSG) (kindly donated by Dr. A. Alonso from German Cancer Research Centre, University of Heidelberg, Germany) was cultured and maintained in Dulbecco's modified Eagle's medium (DMEM) containing Earle's salts and L-glutamine (DMEM; Sigma-Aldrich; Merck KGaA) and supplemented with 10% foetal bovine serum (Biowest), 100 µg/ml streptomycin (Sigma-Aldrich; Merck KGaA). The CasKi cell line from a squamous CC (kindly donated by Dr A. Aguilar-Lemarroy from Centro de Investigación Biomédica de Occidente, IMSS) was cultured and maintained in RPMI-1640 medium (Sigma-Aldrich; Merck KGaA) containing L-glutamine and supplemented with 10% foetal bovine serum, and 100 µg/ml streptomycin (Sigma-Aldrich; Merck KGaA). Cells were maintained at 37°C with an atmosphere of 5% CO2. The culture medium was replaced every two days. Sub-confluent cells were harvested using a mixture of trypsin (0.025%) and EDTA (0.02%; Sigma-Aldrich; Merck KGaA) and were washed with phosphate-buffered saline.
Microarray expression assay
The microarray expression assay was performed at the Cellular Physiology Institute of UNAM. The microarray contained 10,000 gene-specific oligonucleotide probes representing the best-annotated genes from human. For the probe preparation, total RNA from HaCaT/E5 and HaCaT/pGSM monocultures was obtained with the ReliaPrep™ RNA Cell Miniprep System (Promega Corporation) and 10 µg of each RNA was used for cDNA synthesis incorporating dUTP-Alexa555 or dUTP-Alexa647 and employing the First-Strand cDNA labelling kit (Invitrogen; Thermo Fisher Scientific, Inc.). Acquisition and quantification of the array images were performed in GenePix 4100A with its accompanying software GenePix from Molecular Devices. Microarray data analysis was performed with the free software GenArise developed at the Computing Unit of Cellular Physiology Institute of UNAM (http://www.ifc.unam.mx/genarise/). The software identifies differentially expressed genes by calculating an intensity-dependent z-score. The elements with a z-score >2 standard deviations would be the significantly differentially expressed genes. The analysed data were submitted to the NCBI Gene Expression Omnibus (access no. GSE118776).
Expression analysis of E5 and glycogenes
Total RNA from CasKi, HaCaT-E5 and HaCaT-pMSG monocultures was obtained with the NucleoSpin II RNA kit (Macherey-Nagel).
To determine the amplification efficiencies of E5, ST3GAL3 and ST6GAL1, standard curves were performed with the following RNA concentrations: 10, 1, 0.1, 0.01, and 0.001 ng/µl. HPRT was used as an endogenous gene. Based on these curves we determined the better concentration to perform the relative quantification assays, considering that these genes have different expression levels. cDNA was synthesized using random primers and the RevertAid First-Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.). qPCR reactions were performed in a final volume of 10 µl with the following components: 5 µl of 2X Maxima SYBR-Green/Rox qPCR Master Mix (Thermo Fisher Scientific, Inc.) and 0.5 µl of 10 mM forward and reverse primers (Table I). The reactions were performed with a StepOne Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.), and the cycling conditions were as follows: 95°C for 10 min, followed by 40 cycles of 95°C for 30 sec, an annealing temperature for 30 sec, and 70°C for 30 sec. For HPV16 E5, the Tms for HPV16 E5, ST3GAL3 and ST6GAL1 were 55°C, 57°C and 60°C, respectively.
Table I.
Sequences of the oligonucleotides used in the reverse transcription-quantitative PCR assays.
| Primer | Forward (5–3) | Reverse (5–3) | Length (bp) Product |
|---|---|---|---|
| HPRT | CCTGGCGTCGTGATTAGTGATGAT | CGAGCAAGACGTTCAGTCCTGTC | 150 |
| HPV16 E5 | CGCTGCTTTTGTCTGTGTCT | GCGTGCATGTGTATGTATTAAAAA | 146 |
| ST3GAL3 | CATGTGAAGATGGGACTCTTGG | CCTCCCACTGGAGTAAGTGTAG | 118 |
| ST6GAL1 | TATCGTAAGCTGCACCCCAATC | TTAGCAGTGAATGGTCCGGAAG | 372 |
HPRT, hypoxanthine-guanine phosphoribosyltransferase; HPV, human papilloma virus; ST3GAL3, ST3 β-galactoside α-2,3-sialyltransferase 3; ST6GAL1, β-galactoside α-2,6-sialyltransferase 1; bp, base pair.
Relative quantification was performed using the comparative CT method as follows: 2−ΔΔCT. The qPCR reaction was performed on a StepOne Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.). The final 10 µl reaction volume included 1 µl of cDNA template (E5 VPH16-0.5 ng/µl; ST3GAL3−10 ng/µl, or ST6GAL1−3 ng/µl), 5 µl of 2X Maxima SYBR-Green/Rox qPCR Master Mix (Thermo Fisher Scientific, Inc.), 0.5 µl of forward and reverse primers (0.5 µM final concentration) and 3 µl of RNase free water. qPCR was performed under with following conditions: 95°C 10 min, followed 40 cycles of 95°C for 30 sec, the annealing temperature for 30 sec (60°C for ST6GAL1 and 57°C for ST3GAL3) and 70°C for 30 sec. The gene transcript levels were analysed and normalised to HPRT expression.
Identification of glycogenes
For the analysis of the glycogenes in the microarray displaying altered expression, we considered 336 glycogenes reported to date using the GlycoGene database (http://riodb.ibase.aist.go.jp/rcmg/ggdb/), the Consortium for Functional Glycomics-CAZy database (http://www.cazy.org/CAZY/) and the published reports on glycogenes not included in the databases, including DPY19L1 (21) and MANBAL (22). We identified glycogenes displaying altered expression in the microarray as those with a z-score >2.
Protein-protein interaction network
The downregulated or upregulated set of glycogenes in the HaCaT-E5 cells were submitted separately, to the STRING database (http://string-db.org/). The following parameter were applied for the analysis: text mining, experiments, databases, co-expression, neighbourhood, gene fusion and co-occurrence as interaction sources, no more than 5 interactor, minimum interaction score of 0.9 as confidence level, and a protein-protein enrichment P-value at least ≤0.05 and FDR at least <0.05.
Statistical analysis
Statistical analysis of the qPCR results was performed using the GraphPad program. The Students t-test was performed. A P-value <0.05 was considered statistically significant.
Results
E5 oncogene expression by RT-qPCR
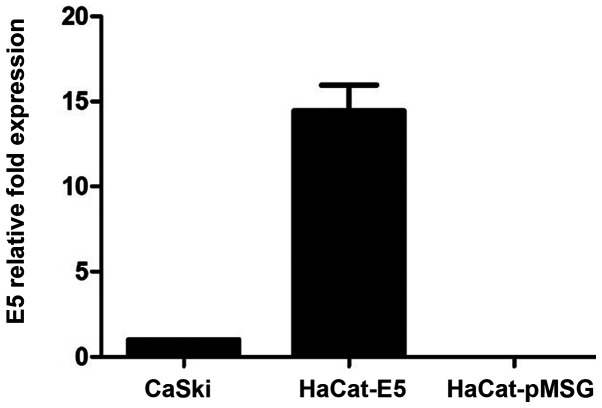
RT-qPCR was performed to quantify E5 mRNA expression levels from HaCaT-E5 cells, as a positive control it was used mRNA of CasKi cells (a cell line that contains HPV16-integrated genomes and expresses E5) and as a negative control was used mRNA of HaCaT-pMSG. Fig. 1 shows the relative expression of E5, showing that E5 expression is greater in HaCaT-E5 than in CasKi cells.
Figure 1.
Human papillomavirus-16 E5 mRNA expression. E5 mRNA expression was 14.4 times higher in HaCaT-E5 cells compared with CasKi cells. HaCaT-pMSG was used as a negative control. Data are presented as the mean ± SEM (n=3).
Glycogene expression altered by the E5 oncoprotein
From a total of 336 glycogenes reported to date, we searched those altered in the HaCaT-E5 microarray with respect to HaCaT-pGSM. We identified four upregulated glycogenes, including ST3GAL3, CHST2 and MANBA with a z-score >2 and ST6GAL1 with a z-score of 1.8. The latter was included because of its importance in CC. We also identified four downregulated glycogenes, including GALNT11, NDST2, UGT2B15 and UGT1A10 with a z-score <2. The microarray data analysed herein are included in the NCBI Gene Expression Omnibus Database (accession no. GSE118776) and in the article text.
E5 increased the expression of the sialyltransferases ST3GAL3 and ST6GAL1
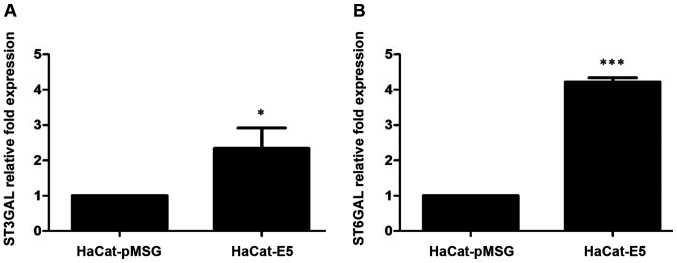
Because the microarray results showed that the presence of E5 can lead to an increase in sialyltransferases expression, we evaluated the mRNA levels of ST3GAL3 and ST6GAL1 by RT-qPCR in HaCaT-E5 and HaCaT-pMSG cells. ST3GAL3 and ST6GAL1 mRNA levels were increased in HaCaT-E5 cells (Fig. 2).
Figure 2.
Expression levels of sialyltransferases genes. (A) mRNA levels of ST3GAL3 in HaCaT-pMSG and HaCaT-E5 cells determined by RT-qPCR. (B) mRNA levels of ST6GAL1 in HaCaT-pMSG and HaCaT-E5 cells determined by RT-qPCR. The mRNA levels of sialyltransferases genes were increased in presence of E5. Data are presented as the mean ± SD of three independent experiments performed in triplicate assays. *P<0.05 and ***P<0.001. RT-qPCR, reverse transcription-quantitative PCR.
We next analysed literature data for E5-modified glycogenes that also have been previously reported as altered in cancerous tissues [Tables II and III; (11,12,22–45)]. First, we analysed the upregulated genes. As previously described, ST3GAL3 and ST6GAL1 were increased in CC and premalignant lesions; in contrast, CHST2 and MANBA have not been reported in CC, but display altered expression in other cancer types. The results showed that the four glycogenes are aberrantly expressed in several types of cancer and have clinical relevance (Table II).
Table II.
Upregulated glycogenes in HaCaT-E5 cells.
| Alteration of mRNA/enzyme in cancer | ||||
|---|---|---|---|---|
| Gene/enzyme | Enzyme function | mRNA | Enzyme | Clinical relevance |
| ST3GAL3/ST3 β-galactoside α2,3-sialyltransferase 3 | Transfers sialic acid from CMP-sialic acid to the structure Galβ1→3/4GlcNAc- and is involved in the synthesis of sialyl Lewis(a) (NeuAcα2→3Galb1→3 (Fucα1→4) GlcNAc-). | Upregulation in cervical cancer (12), breast cancer (23), extrahepatic bile duct carcinoma (24), colorectal carcinoma (25), glioblastoma, renal clear cell carcinoma and lung squamous carcinoma (26). Downregulation in breast invasive carcinoma, colon adenocarcinoma (26) and ovarian cancers (11). | High levels of enzyme activity in the tumor tissue correlated with secondary local tumor recurrence in gastric cancer (27). | In cervical carcinoma the overexpression of mRNA is associated with lymph node metastases (12). In breast cancer, high expression of mRNA is associated with poor prognosis (23). |
| ST6GAL1/ST6 β-galactoside α-2,6-sialyltransferase 1 | Transfers sialic acid from CMP-sialic acid to the Galβ1→4GlcNAc structure on glycoproteins. | Upregulation in squamous cell carcinoma (12), breast cancer (23), extrahepatic bile duct carcinoma (24), ovarian cancer (11), and colorectal carcinoma and non-metastasic colorectal tumors (28). | Increased enzyme expression in colon tumors (28), ovarian and pancreatic carcinomas (29,30). Increased enzyme activity in gastric and colorectal cancer (27,31). | In breast cancer, high expression of mRNA is associated with poor prognosis (23). In pancreatic ductal adenocarcinoma promotes chemoresistance to gemcitabine (32). |
| CHST2/carbohydrate sulfotransferase 2 | Sulfotransferase that utilizes 3-phospho-5-adenylyl sulfate as donor to catalyze the transfer of sulfate to position 6 of non-reducing GlcNAc within keratan-like structures on N-glycans and mucin-associated glycans. | Upregulation in breast cancer (33), osteosarcoma (34) and esophageal cancer (35). Increased enzyme in uterine cervical and corpus cancer (36). | Increased enzyme expression in metastasic osteosarcoma (34). | In osteosarcoma, weak protein expression is associated with improved survival (34). |
| MANBA/β-mannosidase | Exoglycosidase that cleaves the | Upregulation in dysplastic esophageal | No reports | Candidate for molecular |
| single β-linked mannose residue | tissues and esophageal | target for early detection | ||
| from the non-reducing end of N-glycoproteins oligosaccharides. | squamous cell carcinoma (37). | of esophageal cancer (37). | ||
ST3GAL3, ST3 β-galactoside α-2,3-sialyltransferase 3; ST6GAL1, β-galactoside α-2,6-sialyltransferase 1; bp, base pair.
Table III.
Downregulated glycogenes in HaCaT-E5 cells.
| Alteration of mRNA/enzyme in cancer | ||||
|---|---|---|---|---|
| Gene/enzyme | Enzyme function | mRNA | Enzyme | Clinical relevance |
| UGT2B15/UDP | An enzyme of the glucuronidation | Upregulation in castration | Upregulation of expression | In gastric cancer, patients |
| glucuronosyltransferase | pathway, involved in the metabolism | resistant prostate cancer (38). | in gastric cancer (39). | with higher UGT2B15 mRNA |
| family 2 member B15 | and elimination of toxic compounds. | Upregulation in gastric | Low level of expression in aggressive | expression have poor |
| Serves a role in the regulation of estrogens and androgens. | cancer (39). | prostate tumors and undetectable expression in prostate cancer with lymph node metastasis (40). | prognosis (39). | |
| GALNT11/polypeptide | Catalyzes the initiation of protein | Upregulation in chronic | No reports. | GALNT11 expression is |
| N-acetylgalactosaminyltransferase 11 | O-linked glycosylation and | lymphocytic leukemia (41). | associated with prognosis | |
| is involved in left/right asymmetry by mediating O-glycosylation of NOTCH1. | of chronic lymphocytic leukemia (41). | |||
| NDST2/N-deacetylase | Enzyme with dual functions: | Moderately upregulated | No reports. | No reports. |
| and N-sulfotransferase 2 | Participates in processing glucosamine | in hepatocellular cancer (42). | ||
| and heparin polymers, including | Decreased levels in II, III | |||
| N-deacetylation and N-sulfation. | and IV stages of glioma (43). | |||
| UGT1A10/UDP | An enzyme of the glucuronidation | Upregulated in stomach cancer (44). | Downregulated in breast | No reports. |
| glucuronosyltransferase | pathway that transforms small | Downregulated in breast cancer (45). | cancer (45). | |
| family 1 member A10 | lipophilic molecules, such as steroids, bilirubin, hormones and drugs, into water-soluble, excretable metabolites. | |||
ST3GAL3, ST3 β-galactoside α-2,3-sialyltransferase 3; ST6GAL1, β-galactoside α-2,6-sialyltransferase 1; bp, base pair.
We next compared the downregulated glycogenes in HaCaT-E5 cells with those reported in cancerous tissues. The four glycogenes have been reported in cancer. GALNT11 is overexpressed in chronic lymphocytic leukaemia (CLL), and UGT2B15 is downregulated in prostate cancer, but none of the downregulated glycogenes have been previously reported to be altered in CC (Table III).
E5 and glycosylation pathways
To identify possible functional associations among the enzymes identified as altered glycogenes under E5 regulation, we analysed the data with STRING software to generate predicted protein-protein interactions with a higher confidence level (0.9). For the analysis, we considered the altered glycogenes and five additional proteins with the goal of identifying possible glycosylation pathways.
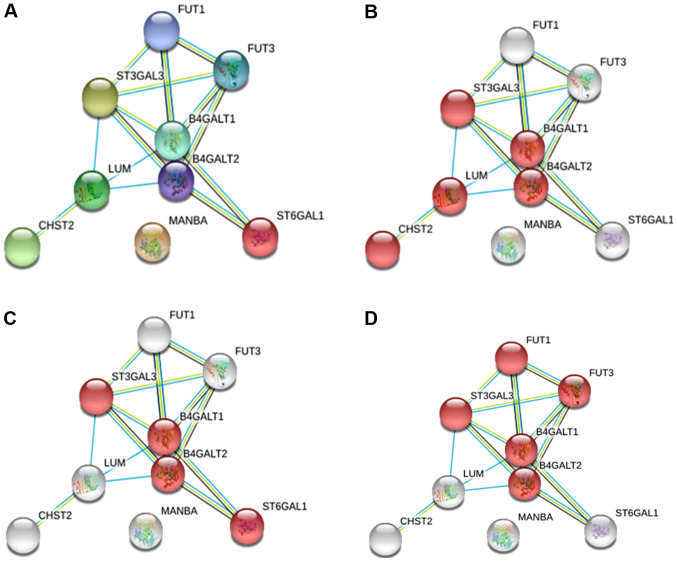
For the upregulated glycogenes (ST3GAL3, CHST2, MANBA and ST6GAL1), the results displayed an interacting network with eight proteins where MANBA did not interact with any protein but participates in N-glycosylation (Fig. 3A). Specifically, we identified the keratan sulfate biosynthesis pathway, which includes CHST2 and ST3GAL3 (Fig. 3B); the N-glycosylation pathway, in which both the sialyltransferase genes are involved in the sialylation of N-glycans (Fig. 3C); and the glycosphingolipid biosynthesis pathway, where ST3GAL3 participates with fucosyltransferases and galactosyltransferases (Fig. 3D).
Figure 3.
Protein-protein interaction network of the upregulated glycogenes. (A) Network of upregulated glycogenes with five additional proteins that interacted with the altered glycogenes. (B) The red network indicates proteins that participate in the keratan sulfate biosynthetic process. (C) The red network indicates the proteins that participate in N-glycosylation. (D) The red network indicates the proteins that participate in glycosphingolipid biosynthesis.
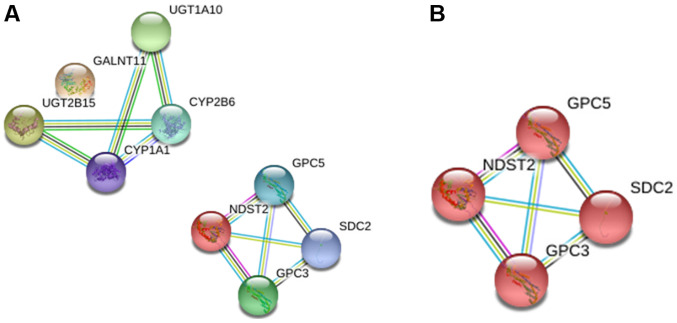
For the downregulated glycogenes (UGT2B15, GALNT11, NDST2 and UGT1A10), the interaction analysis showed two different networks, among which GALNT11 was excluded from the first network and NDST2 establishes a separate network (Fig. 4A). The analysis showed that the latter is involved in glycosaminoglycan biosynthesis (Fig. 4B).
Figure 4.
Protein-protein interaction network of the downregulated glycogenes. (A) Network of downregulated glycogenes including five additional proteins that interacted with the altered glycogenes. (B) The red network indicates proteins that participate in glycosaminoglycan biosynthesis.
Discussion
Aberrant glycosylation is a characteristic of tumour cells. Changes in glycan structures in cancer have been related with altered glycogene expression (46). Increased sialylation is one of the most frequent alterations in cancer (47). Increased activity of sialyltransferases, enzymes that transfer sialic acid to glycoconjugates, has been reported during tumour transformation (11,48). Increased α2,3 and α2,6 sialic acid levels have been reported in premalignant lesions from the cervical epithelium (14). Increased expression of the sialylated antigens sLe(a) and sLe(x) have also been reported in CC and premalignant lesions (17,18). These findings could be the result of enhanced ST3GAL3 and ST6GAL1 (sialyltransferase genes) mRNA levels, which have been previously reported during cervical transformation (12,13). HPV is the aetiological agent of CC, and the HPV genome encodes three oncoproteins, E5, E6 and E7 (4); however, their roles in altering glycogene expression have been poorly investigated. Our research group is interested in the role of HPV infection and its relationship with glycogene expression changes in the cervical epithelium. Our group recently reported that the oncoproteins E6 and E7 from HPV18 modify the expression of some glycogenes, some of which participate in the glycosylation of the Notch receptor and O-glycosylation type mucin (19). However, similar reports focused on the E5 oncoprotein do not exist. E5 is a protein expressed during the early stages of viral infection, and this protein has different targets in the cell that promote cellular transformation (6,49).
With the aim of determining whether E5 modifies the expression of some glycogenes, we performed an expression microarray on the HaCaT cell line stably transfected with the HPV16 E5 oncoprotein. We identified four upregulated glycogenes (CHST2, MANBA, ST3GAL3 and ST6GAL1) and four downregulated glycogenes (UGT2B15, GALNT11, NDST2 and UGT1A10). All these genes have been reported to be altered in cancer either at the transcript or protein level (Tables II and III).
Increased CHST2 mRNA levels have been reported in osteosarcoma and breast and oesophageal cancer (33–35), while increased protein levels have been reported in ovarian cancer and CC (36). With regards to the glycogene MANBA, increased mRNA levels have only been reported in oesophageal cancer (37). Interestingly, of the four upregulated glycogenes, two correspond to the sialyltransferases genes ST3GAL3 and ST6GAL1. ST3GAL3 expression has been reported as altered in different cancer types, such as gastric, bile, colon, kidney, lung, breast, and ovarian cancers and glioblastoma (11,23–27). Increased ST6GAL1 mRNA and protein expression has been reported in gastric and biliary cancers (24,27), colon and colorectal cancers (25,29,31), and ovarian and pancreatic cancers (11,30). Here, we showed that the mRNA levels of the ST6GAL1 and ST3GAL3 genes can be increased by the presence of E5; interestingly, both mRNA levels are increased in premalignant and malignant tissues in the cervical epithelium (12,13). However, whether these phenotypes could be a consequence of HPV infection remains unclear. Additionally, the upregulation of these genes agrees with the increased levels of sialic acid reported for CC and with the increased levels of α2,3 and α2,6 sialic acid in premalignant lesions in the cervix (14). These results suggest that the sialyltransferase expression changes that occur in the early stages of cervical cell transformation could be related to HPV infection and due to E5, but not E6 or E7, activity (19). Nevertheless, this hypothesis requires more investigation. With respect to E5 and sialylation, a previous study found no important changes in the sialylation status of keratinocytes in the presence of HPV16 E5 (50), however, in this study the authors analysed the expression of different monosaccharaides and disaccharides, using a panel of seven lectins, but they did not perfom glycogene expression analysis. Additionally, as they use an inducible vector, they incubated the transfected cells with dexamethasone to increase the expression of E5, so the glycosylation pattern could be influenced by the effect of this molecule, as has been reported in different studies where the dexamethasone increase the sialyltransferases expression (51,52).
Regarding the downregulated genes in the presence of HPV16 E5, GALNT11 has been reported as diminished in breast cancer (33) and increased in chronic lymphocytic leukaemia, and it was proposed to be implicated in O-glycosylation changes in chronic lymphocytic leukaemia (CLL) cells (41). NDST2 has demonstrated increased mRNA levels in hepatocellular cancer (42). It was also interesting to find expression changes in two UGT genes (UGT2B15 and UGT1A10) that encode uridine 5′-diphospho-glucuronosyltransferases as these enzymes play important roles in the biotransformation of drugs, xenobiotics, and toxic compounds (44). Expression changes in UGT genes could modify the response to some cancer drugs. UGT2B15 has been reported as diminished in prostate cancer (40), and UGT1A10 has been reported as downregulated in breast cancer (45) but upregulated in gastric cancer (44). These UGT genes have not been previously reported as altered in CC; thus, it could be interesting to analyse their expression status and the response of patients to treatment when their expression is altered.
Additionally, we also identified possible glycosylation pathways altered in the presence of E5 by analysing protein-protein interactions. We identified keratan sulfate and glycosphingolipid synthesis as including the involvement of the glycogenes ST3GAL3, ST6GAL1 and CHST2. Keratan sulfate belongs to the family of glycosaminoglycans, which participates in the regulation of cellular functions in epithelial and mesenchymal tissues (53).
The glycogenes ST3GAL3 and ST6GAL1 are involved in sialylation of glycosphingolipid. These glycolipids can be located in the cellular membrane, participate in cell-cell interactions and modulate transduction pathways, cell growth, apoptosis, cell proliferation, endocytosis, cell migration, senescence and embryogenesis (54,55). The aberrant expression of glycosphingolipids and the enzymes that participate in their biosynthesis has been reported in different cancer types (56). The expression of the ganglioside GM1 has been reported as being increased in ectocervix cells expressing VPH16 E5; gangliosides are expressed at high levels on tumour cells and inhibit cytotoxic T lymphocytes (56). Our results support these findings, demonstrating that E5 could be modifying ganglioside synthesis.
The glycogenes present in the N-glycosylation pathway are ST3GAL3 and ST6GAL1. Changes in sialic acid expression have been reported for different cancer types including CC, as was mention previously (11). Increased expression of sialylated antigens such as sLe(x) and sLe(a) has been reported in premalignant lesions and CC, and the enzyme ST3Gal III participates in the synthesis of both of these antigens (17,18,57). sLe(x) antigens can be modified by the enzyme sulfotransferase CHST2 to produce 6-sulfosialil Lewis-x (6-sulfo sLex), which can be a ligand of L-, P- and E-selectin (58). Moreover, ST6Gal I catalyses the α2,6 sialylation of N-glycans, and its expression is increased in different types of cancer (59). ST6Gal I had been implicated in the hypersialylation of the cell membrane protein β1 integrin in tumour cells, which leads to increased migration capacity (60). ST6Gal I also participates in the sialylation of epidermal growth factor receptor (EGFR) (61), which showed increased expression due to HPV16 E5 (62).
For the downregulated genes, the network interactions implicated in a glycosylation pathway involved only one glycogene. Fig. 4 shows a network of four proteins implicated in glycosaminoglycan biosynthesis, but only one glycogene, NDST2, was present in this network. The upregulation of NDST2 has been related to an increase in heparan sulfate (63).
For the glycogenes downregulated in the presence of E5, there are no reports focused on CC; thus, it would be important to evaluate their expression and roles in cervical transformation.
The results of this study provide important information related to glycogenes that modify their expression in the presence of the HPV-16 E5 protein. The expression microarray generated large amounts of information on the genes that were altered, but the results must be validated. The study present some limitations, it is important to confirm that the expression of the gene is related with the expression level of its corresponding protein, additionally, the study was performed in a cell line, so the role of the HPV infection and the expression of the other viral proteins could be participating in the glycosylation changes, so the effect of E5 could be affected by other factors. Therefore, the results must be confirmed with more in vitro experiments, followed by the evaluation of glycogene expression in different stages in cervical neoplasias positive for HPV16, as some of the altered glycogenes have not been previously reported in premalignant and malignant tissues. Also, it could be of interest to evaluate if the protein E5 from different viral genotypes, plays a different role in the glycosylation changes. Studying the effects of E5 of high and low risks HPV, will certainly provide relevant additional information of the effects of this viral infection.
The expression of the HPV16 E5 protein in HaCaT cells increased ST3GAL3 and ST6GAL1 mRNA levels, suggesting that the E5 protein could participate in the glycosylation changes found during cervical transformation in the cervical epithelium infected with HPV, especially those related to increased α2,3 and α2,6 sialylation, which have been reported as enhanced in premalignant lesions and CC. Additionally, E5 could participate in UGT gene expression changes implicated in treatment response. Changes in UGT gene expression have not been previously reported; thus, it would be interesting to analyse their levels in CC samples as well as to examine the correlation of these changes with responses to treatment.
Acknowledgements
The authors would like to thank Dr Lorena Chávez González, Dr Simón Guzmán León, Dr José Luis Santillán Torres and Dr Jorge Ramírez for their technical assistance with microarray determinations. The authors would also like to thank Mr. Gerardo Coello, Mr. Gustavo Corral and Ms. Ana Patricia Gómez for their assistance with the genArise software. All the previously mentioned individuals are affiliated to the Cellular Physiology Institute of National Autonomous University of Mexico.
Funding
The current study was supported by the Consejo Nacional de Ciencia y Tecnología (grant no. SALUD-2017-01-290068). JRL has a fellowship from the Fundación IMSS (grant no. 8066205). DCR was supported by a Ph.D. fellowship from CONACYT (grant no. 242745) and IMSS (grant no. 98227564). Funding was also provided by the Programa Cátedras CONACYT 2016 (grant no. 485) and Fondo Redes Temáticas de Investigación CONACYT (grant no. 253596).
Availability of data and materials
The datasets generated and analysed during the current study are available in the repository NCBI Gene Expression Omnibus database, (accession no. GSE118776; http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE118776).
Authors' contributions
DCR performed molecular biology experiments, in silico analysis, data analysis and revised the manuscript. YML participated in data analysis and critically revised the manuscript. PMM participated in the in silico and data analyses, and critically revised the manuscript. AAL analysed the data and critically revised the manuscript. LFJS participated in the in silico analysis and critically revised the manuscript. GSL participated in the molecular biology experiments, analysed the data and revised the manuscript. JRL designed the current study, analysed the data and critically revised the manuscript. VVR conceived and designed the current study, coordinated the study and draft the manuscript. All the authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.zur Hausen H. Papillomaviruses in the causation of human cancers - a brief historical account. Virology. 2009;384:260–265. doi: 10.1016/j.virol.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 2.Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, Clifford GM. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: A meta-analysis update. Int J Cancer. 2007;121:621–632. doi: 10.1002/ijc.22527. [DOI] [PubMed] [Google Scholar]
- 3.Aguilar-Lemarroy A, Vallejo-Ruiz V, Cortés-Gutiérrez EI, Salgado-Bernabé ME, Ramos-González NP, Ortega-Cervantes L, Arias-Flores R, Medina-Díaz IM, Hernández-Garza F, Santos-López G, et al. IMSS Research Network on HPV Human papillomavirus infections in Mexican women with normal cytology, precancerous lesions, and cervical cancer: Type-specific prevalence and HPV coinfections. J Med Virol. 2015;87:871–884. doi: 10.1002/jmv.24099. [DOI] [PubMed] [Google Scholar]
- 4.Münger K, Howley PM. Human papillomavirus immortalization and transformation functions. Virus Res. 2002;89:213–228. doi: 10.1016/S0168-1702(02)00190-9. [DOI] [PubMed] [Google Scholar]
- 5.Conrad M, Bubb VJ, Schlegel R. The human papillomavirus type 6 and 16 E5 proteins are membrane-associated proteins which associate with the 16-kilodalton pore-forming protein. J Virol. 1993;67:6170–6178. doi: 10.1128/JVI.67.10.6170-6178.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Venuti A, Paolini F, Nasir L, Corteggio A, Roperto S, Campo MS, Borzacchiello G. Papillomavirus E5: The smallest oncoprotein with many functions. Mol Cancer. 2011;10:140. doi: 10.1186/1476-4598-10-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen SL, Huang CH, Tsai TC, Lu KY, Tsao YP. The regulation mechanism of c-jun and junB by human papillomavirus type 16 E5 oncoprotein. Arch Virol. 1996;141:791–800. doi: 10.1007/BF01718155. [DOI] [PubMed] [Google Scholar]
- 8.Chen SL, Lin YK, Li LY, Tsao YP, Lo HY, Wang WB, Tsai TC. E5 proteins of human papillomavirus types 11 and 16 transactivate the c-fos promoter through the NF1 binding element. J Virol. 1996;70:8558–8563. doi: 10.1128/JVI.70.12.8558-8563.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kivi N, Greco D, Auvinen P, Auvinen E. Genes involved in cell adhesion, cell motility and mitogenic signaling are altered due to HPV 16 E5 protein expression. Oncogene. 2008;27:2532–2541. doi: 10.1038/sj.onc.1210916. [DOI] [PubMed] [Google Scholar]
- 10.Varki A, Kannagi R, Toole B, Stanley P. Glycosylation Changes in Cancer. In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH, Prestegard JH, et al., editors. Essentials of Glycobiology [Internet] 3rd edition. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2017. [Google Scholar]
- 11.Wang PH. Altered Glycosylation in Cancer: Sialic Acids and Sialyltransferases. J Cancer Mol. 2005;1:73–81. [Google Scholar]
- 12.Wang PH, Li YF, Juang CM, Lee YR, Chao HT, Ng HT, Tsai YC, Yuan CC. Expression of sialyltransferase family members in cervix squamous cell carcinoma correlates with lymph node metastasis. Gynecol Oncol. 2002;86:45–52. doi: 10.1006/gyno.2002.6714. [DOI] [PubMed] [Google Scholar]
- 13.López-Morales D, Velázquez-Márquez N, Valenzuela O, Santos-López G, Reyes-Leyva J, Vallejo-Ruiz V. Enhanced sialyltransferases transcription in cervical intraepithelial neoplasia. Invest Clin. 2009;50:45–53. [PubMed] [Google Scholar]
- 14.López-Morales D, Reyes-Leyva J, Santos-López G, Zenteno E, Vallejo-Ruiz V. Increased expression of sialic acid in cervical biopsies with squamous intraepithelial lesions. Diagn Pathol. 2010;5:74. doi: 10.1186/1746-1596-5-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roy A, Chakraborty S. Detection of cancer cervix by estimation of sialic acid. J Indian Med Assoc. 2005;103:589–590. [PubMed] [Google Scholar]
- 16.Terasawa K, Furumoto H, Kamada M, Aono T. Expression of Tn and sialyl-Tn antigens in the neoplastic transformation of uterine cervical epithelial cells. Cancer Res. 1996;56:2229–2232. [PubMed] [Google Scholar]
- 17.Engelstaedter V, Fluegel B, Kunze S, Mayr D, Friese K, Jeschke U, Bergauer F. Expression of the carbohydrate tumour marker Sialyl Lewis A, Sialyl Lewis X, Lewis Y and Thomsen-Friedenreich antigen in normal squamous epithelium of the uterine cervix, cervical dysplasia and cervical cancer. Histol Histopathol. 2012;27:507–514. doi: 10.14670/HH-27.507. [DOI] [PubMed] [Google Scholar]
- 18.Velázquez-Márquez N, Santos-López G, Jiménez-Aranda L, Reyes-Leyva J, Vallejo-Ruiz V. Sialyl Lewis × expression in cervical scrapes of premalignant lesions. J Biosci. 2012;37:999–1004. doi: 10.1007/s12038-012-9261-z. [DOI] [PubMed] [Google Scholar]
- 19.Aco-Tlachi M, Carreño-López R, Martínez-Morales PL, Maycotte P, Aguilar-Lemarroy A, Jave-Suárez LF, Santos-López G, Reyes-Leyva J, Vallejo-Ruiz V. Glycogene expression profiles based on microarray data from cervical carcinoma HeLa cells with partially silenced E6 and E7 HPV oncogenes. Infect Agent Cancer. 2018;13:25. doi: 10.1186/s13027-018-0197-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kabsch K, Alonso A. The human papillomavirus type 16 E5 protein impairs TRAIL- and FasL-mediated apoptosis in HaCaT cells by different mechanisms. J Virol. 2002;76:12162–12172. doi: 10.1128/JVI.76.23.12162-12172.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buettner FF, Ashikov A, Tiemann B, Lehle L, Bakker H. C. elegans DPY-19 is a C-mannosyltransferase glycosylating thrombospondin repeats. Mol Cell. 2013;50:295–302. doi: 10.1016/j.molcel.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Milde-Langosch K, Karn T, Schmidt M, zu Eulenburg C, Oliveira-Ferrer L, Wirtz RM, Schumacher U, Witzel I, Schütze D, Müller V. Prognostic relevance of glycosylation-associated genes in breast cancer. Breast Cancer Res Treat. 2014;145:295–305. doi: 10.1007/s10549-014-2949-z. [DOI] [PubMed] [Google Scholar]
- 23.Recchi MA, Hebbar M, Hornez L, Harduin-Lepers A, Peyrat JP, Delannoy P. Multiplex reverse transcription polymerase chain reaction assessment of sialyltransferase expression in human breast cancer. Cancer Res. 1998;58:4066–4070. [PubMed] [Google Scholar]
- 24.Jin XL, Zheng SS, Wang BS, Chen HL. Correlation of glycosyltransferases mRNA expression in extrahepatic bile duct carcinoma with clinical pathological characteristics. Hepatobiliary Pancreat Dis Int. 2004;3:292–295. [PubMed] [Google Scholar]
- 25.Petretti T, Kemmner W, Schulze B, Schlag PM. Altered mRNA expression of glycosyltransferases in human colorectal carcinomas and liver metastases. Gut. 2000;46:359–366. doi: 10.1136/gut.46.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ashkani J, Naidoo KJ. Glycosyltransferase gene expression profiles classify cancer types and propose prognostic subtypes. Sci Rep. 2016;6:26451. doi: 10.1038/srep26451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gretschel S, Haensch W, Schlag PM, Kemmner W. Clinical relevance of sialyltransferases ST6GAL-I and ST3GAL-III in gastric cancer. Oncology. 2003;65:139–145. doi: 10.1159/000072339. [DOI] [PubMed] [Google Scholar]
- 28.Zhang S, Lu J, Xu Z, Zou X, Sun X, Xu Y, Shan A, Lu J, Yan X, Cui Y, et al. Differential expression of ST6GAL1 in the tumor progression of colorectal cancer. Biochem Biophys Res Commun. 2017;486:1090–1096. doi: 10.1016/j.bbrc.2017.03.167. [DOI] [PubMed] [Google Scholar]
- 29.Swindall AF, Londoño-Joshi AI, Schultz MJ, Fineberg N, Buchsbaum DJ, Bellis SL. ST6Gal-I protein expression is upregulated in human epithelial tumors and correlates with stem cell markers in normal tissues and colon cancer cell lines. Cancer Res. 2013;73:2368–2378. doi: 10.1158/0008-5472.CAN-12-3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schultz MJ, Holdbrooks AT, Chakraborty A, Grizzle WE, Landen CN, Buchsbaum DJ, Conner MG, Arend RC, Yoon KJ, Klug CA, et al. The tumor-associated glycosyltransferase ST6Gal-I regulates stem cell transcription factors and confers a cancer stem cell phenotype. Cancer Res. 2016;76:3978–3988. doi: 10.1158/0008-5472.CAN-15-2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vázquez-Martín C, Gil-Martín E, Fernández-Briera A. Elevation of ST6Gal I activity in malignant and transitional tissue in human colorectal cancer. Oncology. 2005;69:436–444. doi: 10.1159/000089999. [DOI] [PubMed] [Google Scholar]
- 32.Chakraborty A, Dorsett KA, Trummell HQ, Yang ES, Oliver PG, Bonner JA, Buchsbaum DJ, Bellis SL. ST6Gal-I sialyltransferase promotes chemoresistance in pancreatic ductal adenocarcinoma by abrogating gemcitabine-mediated DNA damage. J Biol Chem. 2018;293:984–994. doi: 10.1074/jbc.M117.808584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Potapenko IO, Haakensen VD, Lüders T, Helland A, Bukholm I, Sørlie T, Kristensen VN, Lingjaerde OC, Børresen-Dale AL. Glycan gene expression signatures in normal and malignant breast tissue; possible role in diagnosis and progression. Mol Oncol. 2010;4:98–118. doi: 10.1016/j.molonc.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen X, Yang TT, Qiu XC, Ji ZG, Li CX, Long H, Zhou Y, Ma BA, Ma Q, Zhang X, et al. Gene expression profiles of human osteosarcoma cell sublines with different pulmonary metastatic potentials. Cancer Biol Ther. 2011;11:287–292. doi: 10.4161/cbt.11.2.13966. [DOI] [PubMed] [Google Scholar]
- 35.Warnecke-Eberz U, Metzger R, Hölscher AH, Drebber U, Bollschweiler E. Diagnostic marker signature for esophageal cancer from transcriptome analysis. Tumour Biol. 2016;37:6349–6358. doi: 10.1007/s13277-015-4400-4. [DOI] [PubMed] [Google Scholar]
- 36.Seko A, Kataoka F, Aoki D, Sakamoto M, Nakamura T, Hatae M, Yonezawa S, Yamashita K. N-Acetylglucosamine 6-O-sulfotransferase-2 as a tumor marker for uterine cervical and corpus cancer. Glycoconj J. 2009;26:1065–1073. doi: 10.1007/s10719-008-9227-4. [DOI] [PubMed] [Google Scholar]
- 37.Sud N, Sharma R, Ray R, Chattopadhyay T, Ralhan R. Differential expression of beta mannosidase in human esophageal cancer. Int J Cancer. 2004;112:905–907. doi: 10.1002/ijc.20469. [DOI] [PubMed] [Google Scholar]
- 38.Pfeiffer MJ, Smit FP, Sedelaar JP, Schalken JA. Steroidogenic enzymes and stem cell markers are upregulated during androgen deprivation in prostate cancer. Mol Med. 2011;17:657–664. doi: 10.2119/molmed.2010.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen X, Li D, Wang N, Yang M, Liao A, Wang S, Hu G, Zeng B, Yao Y, Liu D, et al. Bioinformatic analysis suggests that UGT2B15 activates the Hippo YAP signaling pathway leading to the pathogenesis of gastric cancer. Oncol Rep. 2018;40:1855–1862. doi: 10.3892/or.2018.6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pâquet S, Fazli L, Grosse L, Verreault M, Têtu B, Rennie PS, Bélanger A, Barbier O. Differential expression of the androgen-conjugating UGT2B15 and UGT2B17 enzymes in prostate tumor cells during cancer progression. J Clin Endocrinol Metab. 2012;97:E428–E432. doi: 10.1210/jc.2011-2064. [DOI] [PubMed] [Google Scholar]
- 41.Libisch MG, Casás M, Chiribao M, Moreno P, Cayota A, Osinaga E, Oppezzo P, Robello C. GALNT11 as a new molecular marker in chronic lymphocytic leukemia. Gene. 2014;533:270–279. doi: 10.1016/j.gene.2013.09.052. [DOI] [PubMed] [Google Scholar]
- 42.Tátrai P, Egedi K, Somorácz A, van Kuppevelt TH, Ten Dam G, Lyon M, Deakin JA, Kiss A, Schaff Z, Kovalszky I. Quantitative and qualitative alterations of heparan sulfate in fibrogenic liver diseases and hepatocellular cancer. J Histochem Cytochem. 2010;58:429–441. doi: 10.1369/jhc.2010.955161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ushakov VS, Tsidulko AY, de La Bourdonnaye G, Kazanskaya GM, Volkov AM, Kiselev RS, Kobozev VV, Kostromskaya DV, Gaytan AS, Krivoshapkin AL, et al. Heparan sulfate biosynthetic system is inhibited in human glioma due to EXT1/2 and HS6ST1/2 D}down-regulation. Int J Mol Sci. 2017;18:E2301. doi: 10.3390/ijms18112301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cengiz B, Yumrutas O, Bozgeyik E, Borazan E, Igci YZ, Bozgeyik I, Oztuzcu S. Differential expression of the UGT1A family of genes in stomach cancer tissues. Tumour Biol. 2015;36:5831–5837. doi: 10.1007/s13277-015-3253-1. [DOI] [PubMed] [Google Scholar]
- 45.Starlard-Davenport A, Lyn-Cook B, Radominska-Pandya A. Identification of UDP-glucuronosyltransferase 1A10 in non-malignant and malignant human breast tissues. Steroids. 2008;73:611–620. doi: 10.1016/j.steroids.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pinho SS, Reis CA. Glycosylation in cancer: Mechanisms and clinical implications. Nat Rev Cancer. 2015;15:540–555. doi: 10.1038/nrc3982. [DOI] [PubMed] [Google Scholar]
- 47.Magalhães A, Duarte HO, Reis CA. Aberrant glycosylation in cancer: A novel molecular mechanism controlling metastasis. Cancer Cell. 2017;31:733–735. doi: 10.1016/j.ccell.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 48.Harduin-Lepers A, Krzewinski-Recchi MA, Colomb F, Foulquier F, Groux-Degroote S, Delannoy P. Sialyltransferases functions in cancers. Front Biosci (Elite Ed) 2012;4:499–515. doi: 10.2741/e396. [DOI] [PubMed] [Google Scholar]
- 49.Estêvão D, Costa NR, Gil da Costa RM, Medeiros R. Hallmarks of HPV carcinogenesis: The role of E6, E7 and E5 oncoproteins in cellular malignancy. Biochim Biophys Acta Gene Regul Mech. 2019;1862:153–162. doi: 10.1016/j.bbagrm.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 50.Oetke C, Auvinen E, Pawlita M, Alonso A. Human papillomavirus type 16 E5 protein localizes to the Golgi apparatus but does not grossly affect cellular glycosylation. Arch Virol. 2000;145:2183–2191. doi: 10.1007/s007050070048. [DOI] [PubMed] [Google Scholar]
- 51.Vandamme V, Pierce A, Verbert A, Delannoy P. Transcriptional induction of beta-galactoside alpha-2,6-sialyltransferase in rat fibroblast by dexamethasone. Eur J Biochem. 1993;211:135–140. doi: 10.1111/j.1432-1033.1993.tb19879.x. [DOI] [PubMed] [Google Scholar]
- 52.Coughlan CM, Burger PG, Berger EG, Breen KC. The biochemical consequences of alpha2,6(N) sialyltransferase induction by dexamethasone on sialoglycoprotein expression in the rat H411e hepatoma cell line. FEBS Lett. 1997;413:389–393. doi: 10.1016/S0014-5793(97)00923-X. [DOI] [PubMed] [Google Scholar]
- 53.Caterson B, Melrose J. Keratan sulfate, a complex glycosaminoglycan with unique functional capability. Glycobiology. 2018;28:182–206. doi: 10.1093/glycob/cwy003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.D'Angelo G, Capasso S, Sticco L, Russo D. Glycosphingolipids: Synthesis and functions. FEBS J. 2013;280:6338–6353. doi: 10.1111/febs.12559. [DOI] [PubMed] [Google Scholar]
- 55.Zhuo D, Li X, Guan F. Biological roles of aberrantly expressed glycosphingolipids and related enzymes in human cancer development and progression. Front Physiol. 2018;9:466. doi: 10.3389/fphys.2018.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suprynowicz FA, Disbrow GL, Krawczyk E, Simic V, Lantzky K, Schlegel R. HPV-16 E5 oncoprotein upregulates lipid raft components caveolin-1 and ganglioside GM1 at the plasma membrane of cervical cells. Oncogene. 2008;27:1071–1078. doi: 10.1038/onc.2008.357. [DOI] [PubMed] [Google Scholar]
- 57.Harduin-Lepers A, Vallejo-Ruiz V, Krzewinski-Recchi MA, Samyn-Petit B, Julien S, Delannoy P. The human sialyltransferase family. Biochimie. 2001;83:727–737. doi: 10.1016/S0300-9084(01)01301-3. [DOI] [PubMed] [Google Scholar]
- 58.Ohmori K, Kanda K, Mitsuoka C, Kanamori A, Kurata-Miura K, Sasaki K, Nishi T, Tamatani T, Kannagi R. P- and E-selectins recognize sialyl 6-sulfo Lewis X, the recently identified L-selectin ligand. Biochem Biophys Res Commun. 2000;278:90–96. doi: 10.1006/bbrc.2000.3768. [DOI] [PubMed] [Google Scholar]
- 59.Lu J, Gu J. Significance of β-galactoside α2,6 sialyltranferase 1 in cancers. Molecules. 2015;20:7509–7527. doi: 10.3390/molecules20057509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seales EC, Jurado GA, Brunson BA, Wakefield JK, Frost AR, Bellis SL. Hypersialylation of beta1 integrins, observed in colon adenocarcinoma, may contribute to cancer progression by up-regulating cell motility. Cancer Res. 2005;65:4645–4652. doi: 10.1158/0008-5472.CAN-04-3117. [DOI] [PubMed] [Google Scholar]
- 61.Park JJ, Yi JY, Jin YB, Lee YJ, Lee JS, Lee YS, Ko YG, Lee M. Sialylation of epidermal growth factor receptor regulates receptor activity and chemosensitivity to gefitinib in colon cancer cells. Biochem Pharmacol. 2012;83:849–857. doi: 10.1016/j.bcp.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 62.Tomakidi P, Cheng H, Kohl A, Komposch G, Alonso A. Modulation of the epidermal growth factor receptor by the human papillomavirus type 16 E5 protein in raft cultures of human keratinocytes. Eur J Cell Biol. 2000;79:407–412. doi: 10.1078/0171-9335-00060. [DOI] [PubMed] [Google Scholar]
- 63.Deligny A, Dierker T, Dagälv A, Lundequist A, Eriksson I, Nairn AV, Moremen KW, Merry CLR, Kjellén L. NDST2 (N-Deacetylase/N-Sulfotransferase-2) enzyme regulates heparan sulfate chain length. J Biol Chem. 2016;291:18600–18607. doi: 10.1074/jbc.M116.744433. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analysed during the current study are available in the repository NCBI Gene Expression Omnibus database, (accession no. GSE118776; http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE118776).