Abstract
Objective
To extract drug indications from a commercial drug knowledgebase and determine to what extent drug indications can discriminate between look-alike-sound-alike (LASA) drugs.
Methods
We extracted drug indications disease concepts from the MedKnowledge Indications module from First Databank Inc. (South San Francisco, CA) and associated them with drugs on the Institute for Safe Medication Practices (ISMP) list of commonly confused drug names. We used high-level concepts (rather than granular concepts) to represent the general indications for each drug. Two pharmacists reviewed each drug’s association with its high-level indications concepts for accuracy and clinical relevance. We compared the high-level indications for each commonly confused drug pair and categorized each pair as having a complete overlap, partial overlap or no overlap in high-level indications.
Results
Of 278 LASA drug pairs, 165 (59%) had no overlap and 58 (21%) had partial overlap in high-level indications. Fifty-five pairs (20%) had complete overlap in high-level indications; nearly half of these were comprised of drugs with the same active ingredient and route of administration (e.g., Adderall, Adderall XR).
Conclusions
Drug indications data from a drug knowledgebase can discriminate between many LASA drugs.
Keywords: indications, look-alike-sound-alike, LASA, medication safety
Introduction
Confusion involving look-alike, sound-alike (LASA) drug names is a known source of preventable medication error that can lead to adverse events, serious patient harm, and substantial healthcare costs.1–4 Various patient safety organizations, accrediting bodies, and regulatory agencies have advocated for strategies to identify and manage risks associated with LASA drugs.5–7 Examples include the use of bar code technology, Tall Man (mixed case) lettering, separate storage of LASA drugs, inclusion of both brand and generic medication names on orders and labels, specifying the purpose of the drug when it is prescribed, and checking that a patient’s active diagnosis matches the indication of a drug prior to dispensing or administering the drug.5–7 Regulatory agencies, including the Joint Commission, are encouraging organizations to proactively identify LASA pairs and take action to prevent medication errors with LASA drugs.8 The U.S. Food and Drug Administration (FDA) and Health Canada have also issued guides to identify potentially confusing drug names during the premarket phase of drug development.9–11 The expanding use of electronic health records has the potential to reduce LASA errors due to improved legibility and more efficient and accurate transmission of prescriptions. However, computerized systems can also introduce LASA errors. Pick-lists where LASA drugs appear next to each other and variation in the format of drug name displays on order screens, for example, can increase the risk of drug selection errors.12,13
As part of a larger project that advocates for incorporating indications in the prescription ordering phase, we conducted a study to determine whether drug indications knowledgebase content could be used to distinguish between LASA drugs.14 We used the MedKnowledge Indications module from First Databank, Inc. (South San Francisco, CA) as the source of drug indications data, and the nine-page listing of confused drug names maintained by the Institute for Safe Medication Practices (ISMP) as our source for LASA drugs.15,16 The purpose of this project was to determine whether drug indications could potentially be used to discriminate between LASA drug names.
Methods
Data Sources
ISMP’s list of confused drug names is a compilation of generic and brand drug name pairs reported to ISMP as having caused drug errors due to name confusion.16 Since the drug name pairs are a cumulative collection of drug name pairs over time, we used the drugs@fda database and FDA National Drug Code directory to identify and exclude drugs that were no longer marketed in the U.S. or branded products that had undergone a brand name change.17,18
We used the Indications module from First Databank as the source of drug indications.15 The Indications module is a proprietary knowledgebase that includes FDA-approved and “off-label” indications for prescription and non-prescription drugs and is used in many applications within health care, including clinical decision support. Module content is specific to the active ingredient, dose form, strength and route of administration for a given drug. Reference sources used to compile and update indications data include the manufacturer product labeling, medical reference texts, treatment guidelines, expert consensus statements and primary medical literature. FDA-approved indications may also be removed from a drug in the module if the drug is no longer the standard of care for the indication, e.g., penicillin G for treatment of Neisseria gonorrhea infection. The Indications module excludes most dietary supplements, herbal products, large volume parenteral products, diluents, veterinary drugs, medical supplies, medical devices, and bulk drug substances used in compounding.
The Indications module is used in conjunction with a proprietary concept-based medical vocabulary called the First Databank Medical Lexicon (FML).19 FML is comprised of disease concept codes and descriptions that represent medical diagnoses, disease states, and health-related conditions or procedures. Examples of disease concept descriptions include “mania associated with bipolar disorder” or “loss of bone mineral density due to aromatase inhibitor therapy.” Disease concept descriptions can be searched or displayed using either professional terminology (e.g., post herpetic neuralgia), medical abbreviations (e.g., PHN) or consumer-friendly terms (e.g., nerve pain after herpes). FML disease concepts are also mapped to interoperable terminologies (e.g., ICD, SNOMED) to allow for related concept searching.
We had considered using ICD or SNOMED terms to represent drug indications but found that the standardized code sets did not adequately address certain concepts such as disease prevention (e.g., prevention of gastrointestinal ulcer), diagnostic testing for disease, or treatment of symptoms related to disease (e.g., anemia due to bleeding uterine leiomyoma). We chose to use the FML medical vocabulary because we had access to the content, and it offered a more precise representation of drug indications than what we could find in the standardized terminologies.
Because disease concept descriptions exist at varying levels of granularity for different drugs, and drugs may be associated with many disease concepts, we used two data elements in the Indications module – the disease concept proxy and the disease concept grouping – to identify the broad indication(s) for a given drug.
The disease concept proxy represents a single general indication for a drug that is used to facilitate drug-disease interactions checking. For example, many antibiotics are assigned a proxy of “bacterial infection” that can be utilized as an inferred patient problem when screening a new prescription for drug-disease interactions. Similarly, antifungal and antiviral agents are assigned a proxy of “fungal infection” and “viral infection,” respectively. In addition, most antineoplastic drugs are assigned a proxy of “malignancy.” For this study, we used the disease concept proxy to represent the high-level indication for anti-infective (antibacterial, antiviral, and antifungal) agents and antineoplastic drugs. For all other drugs, we used the disease concept grouping.
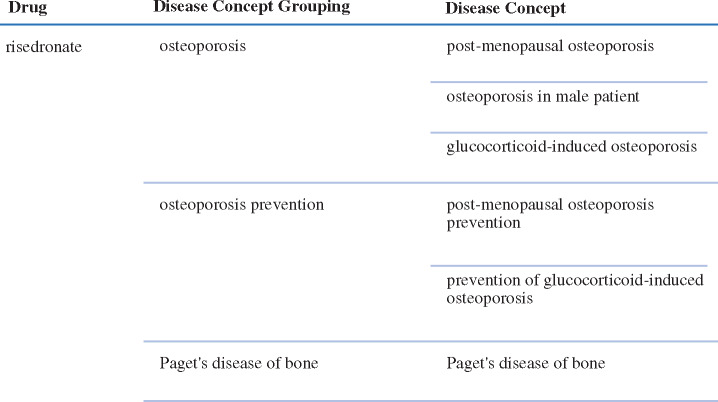
The disease concept grouping is a high-level disease concept (i.e., roll-up term) assigned to each drug-disease concept pair that can be used to shorten long tabular displays of granular indications lists. For example, insulin is associated with the granular disease concepts “gestational diabetes mellitus,” “type 1 diabetes mellitus” and “type 2 diabetes mellitus,” and each of these concepts is associated with the disease concept grouping “diabetes mellitus.” Similarly, for the drug risedronate, the granular disease concepts “post-menopausal osteoporosis” and “glucocorticoid-induced osteoporosis” are associated with the disease concept grouping “osteoporosis” (Figure 1). A given drug may be associated with one or more disease concept groupings in the Indications module.
Figure 1.
Example of disease concept and disease concept groupings for oral risedronate.
We presumed that using high-level concepts would be a conservative and more practical approach to evaluating overlaps in indications between drugs that were paired on the ISMP list.
Data Synthesis
In June 2017, one pharmacist (CC) extracted the high-level indications from the Indications module (either disease concept proxy or disease concept grouping) for each drug on the ISMP list. If only the active ingredient was listed on the ISMP list, then we extracted the high-level indications for every dose form, strength and route of administration available for the product. For example, cyclosporine is associated with distinct indications for its oral, intravenous, and ophthalmic formulations. Each drug-indication set was then compared to its corresponding confused drug-indication set. Two pharmacists (AS, MGA) reviewed the data for accuracy and clinical relevance. Any issues were resolved by consensus through discussion.
We recorded all instances where the drug and confused drug had the same high-level indication (i.e., indication overlap). We then categorized the degree of indication overlap within each drug pair. A “complete overlap” was defined as an instance where all high-level indications for a drug and its corresponding confused drug were the same. A “partial overlap” was defined as an instance where some of the high-level indications for a drug and its corresponding confused drug were the same. A “no overlap” was defined as an instance where none of the high-level indications for a drug and its corresponding confused drug were the same. All data were recorded on a Microsoft Excel (2016) worksheet.
Analysis
We used descriptive statistics to describe the number and proportion of drug pairs with complete, partial, and no overlap in high-level indications. For drug pairs with a partial overlap, we used the Dice Similarity Coefficient (DSC) to compare the similarity in indications between drugs within a pair.20 The DSC has been used to evaluate lexical similarity between text strings.21 We used DSC to measure the extent of overlap between indications within a drug pair. The DSC was calculated as follows:
DSC = (2 * number of shared indications between drug A and drug B)/(number of indications for drug A + number of indications for drug B), where drug A is one drug within a drug pair, and drug B is the corresponding confused drug of the pair.
The DSC value ranges from 0 (no overlap in high-level indications) to 1 (complete overlap in high-level indications). A DSC value between 0 and 1 can be interpreted as the probability that an overlap in high-level indications will occur within a given pair of drugs.
Results
There were 399 unique LASA drug pairs comprised of 646 unique drugs. A total of 123 drugs were excluded because they had been discontinued in the US (n = 89), were non-US products (n = 6), had had a name change (n = 4), represented a drug class instead of an individual drug (n = 2) or were out-of-scope for the Indications module (n = 22). The four drugs that had undergone a name change were Altocor (changed to Altoprev in 2004), Reminyl (changed to Razadyne in 2005), Kapidex (changed to Dexilant in 2010), and Brintellix (changed to Trintellix in 2016).18 The two names that represented a drug class instead of an individual drug were HMG Co-A reductase inhibitors (“statins”) and proton pump inhibitors. Drugs that were out-of-scope for the Indications module included dietary supplements (e.g., Floranex, Florastor, Glycotrol), medical devices (e.g., Healon and Arista AH), bulk fluids (e.g., acetic acid for irrigation), and antiseptic agents (e.g., Cidex, Betadine). A total of 121 drug pairs were excluded because one or both drugs within the pair met exclusion criteria.
The 278 included drug pairs were comprised of 452 unique drugs that were each associated with an average of 2.4 high-level indications (range 1 to 11). There were 197 drugs with a single high-level indication. There were 181 generic names and 271 brand names in our study sample.
There were 165 (59%) pairs with no overlap in high-level indications. There was an average of 5 distinct high-level indications within each drug pair (range 2 to 14) (Table 1).
Table 1.
ISMP confused drug name pairs with no overlap in high-level indications*
| Drug A |
Drug B |
Number of high-level indications within pair* | ||||||
|---|---|---|---|---|---|---|---|---|
| Name† | Name type | Active ingredient(s) | Route(s) | Name† | Name type | Active ingredient(s) | Route(s) | |
| Aggrastat | brand | tirofiban | IV | argatroban | generic | argatroban | IV | 2 |
| Anzemet | brand | dolasetron | oral, IV | Avandamet | brand | rosiglitazone /metformin | oral | 2 |
| BabyBIG | brand | botulism immune globulin | IV | HBIG (hepatitis B immune globulin) | brand | hepatitis b immune globulin | IM | 2 |
| Bidex | brand | guaifenesin | oral | Videx | brand | didanosine | oral | 2 |
| DACTINomycin | generic | dactinomycin | IV | DAPTOmycin | generic | daptomycin | IV | 2 |
| Denavir | brand | penciclovir | topical | indinavir | generic | indinavir | oral | 2 |
| Farxiga | brand | dapagliflozin | oral | Fetzima | brand | levomilnacipran | oral | 2 |
| flumazenil | generic | flumazenil | IV | influenza virus vaccine | generic | influenza virus vaccine | IM | 2 |
| influenza virus vaccine | generic | influenza virus vaccine | IM | perflutren lipid microspheres | generic | perflutren lipid microspheres | IV | 2 |
| influenza virus vaccine | generic | influenza virus vaccine | IM | tuberculin purified protein derivative (PPD) | generic | tuberculin, purified protein derivative | intradermal | 2 |
| Keflex | brand | cephalexin | oral | Keppra | brand | levetiracetam | oral, IV | 2 |
| methylene blue | generic | methylene blue | IV | VisionBlue | brand | trypan blue | intraocular | 2 |
| nalbuphine | generic | nalbuphine | SC, IM, IV | naloxone | generic | naloxone | injection, nasal | 2 |
| Oracea | brand | doxycycline | oral | Orencia | brand | abatacept | SC | 2 |
| Patanol | brand | olopatadine | ophthalmic | Platinol | brand | cisplatin | IV | 2 |
| sitaGLIPtin | generic | sitagliptin | oral | SUMAtriptan | generic | sumatriptan | SC, oral, nasal | 2 |
| tetanus diptheria toxoid (Td) | generic | tetanus and diphtheria toxoids, adult | IM | tuberculin purified protein derivative (PPD) | generic | tuberculin, purified protein derivative | intradermal | 2 |
| tiaGABine | generic | tiagabine | oral | tiZANidine | generic | tizanidine | oral | 2 |
| traMADol | generic | tramadol | oral | traZODone | generic | trazodone | oral | 2 |
| Vexol | brand | rimexolone | ophthalmic | Vosol | brand | acetic acid | otic | 2 |
| Xeloda | brand | capecitabine | oral | Xenical | brand | orlistat | oral | 2 |
| Antivert | brand | meclizine | oral | Axert | brand | almotriptan | oral | 3 |
| Apidra | brand | insulin glulisine | SC | Spiriva | brand | tiotropium | inhalation | 3 |
| Aricept | brand | donepezil | oral | Azilect | brand | rasagiline | oral | 3 |
| Avandia | brand | rosiglitazone | oral | Coumadin | brand | warfarin | oral | 3 |
| Clindesse | brand | clindamycin | vaginal | Clindets | brand | clindamycin | topical | 3 |
| dexmethylphenidate | generic | dexmethylphenidate | oral | methadone | generic | methadone | oral, injection | 3 |
| Diflucan | brand | fluconazole | oral, IV | Diprivan | brand | propofol | IV | 3 |
| Diprivan | brand | propofol | IV | Ditropan | brand | oxybutynin | oral | 3 |
| DOBUTamine | generic | dobutamine | IV | DOPamine | generic | dopamine | IV | 3 |
| Doribax | brand | doripenem | IV | Zovirax | brand | acyclovir | oral, topical | 3 |
| edetate calcium disodium | generic | edetate calcium disodium | injection | edetate disodium | generic | edetate disodium | IV | 3 |
| Enjuvia | brand | estrogens, conjugated, synthetic b | oral | Januvia | brand | sitagliptin | oral | 3 |
| Fanapt | brand | iloperidone | oral | Xanax | brand | alprazolam | oral | 3 |
| gentamicin | generic | gentamicin | injection, ophthalmic, topical | gentian violet | generic | gentian violet | topical | 3 |
| guaiFENesin | generic | guaifenesin | oral | guanFACINE | generic | guanfacine | oral | 3 |
| Jantoven | brand | warfarin | oral | Janumet | brand | sitagliptin /metformin | oral | 3 |
| Jantoven | brand | warfarin | oral | Januvia | brand | sitagliptin | oral | 3 |
| Janumet | brand | sitagliptin/metformin | oral | Sinemet | brand | carbidopa/levodopa | oral | 3 |
| Kaletra | brand | lopinavir/ritonavir | oral | Keppra | brand | levetiracetam | oral, IV | 3 |
| LaMICtal | brand | lamotrigine | oral | LamISIL | brand | terbinafine | topical, oral | 3 |
| Lanoxin | brand | digoxin | oral, injection | naloxone | generic | naloxone | injection, nasal | 3 |
| lanthanum carbonate | generic | lanthanum carbonate | oral | lithium carbonate | generic | lithium carbonate | oral | 3 |
| Lantus | brand | insulin glargine | SC | Latuda | brand | lurasidone | oral | 3 |
| levETIRAcetam | generic | levetiracetam | oral, IV | levofloxacin | generic | levofloxacin | oral, IV, ophthalmic | 3 |
| lithium | generic | lithium | oral | Ultram | brand | tramadol | oral | 3 |
| Lunesta | brand | eszopiclone | oral | Neulasta | brand | pegfilgrastim | SC | 3 |
| Matulane | brand | procarbazine | oral | Materna | brand | prenatal vitamins with calcium/ferrous fumarate/folic acid | oral | 3 |
| methadone | generic | methadone | oral, injection | methylphenidate | generic | methylphenidate | oral | 3 |
| methimazole | generic | methimazole | oral | metolazone | generic | metolazone | oral | 3 |
| Mucinex | brand | guaifenesin | oral | Mucinex Allergy | brand | fexofenadine | oral | 3 |
| Neulasta | brand | pegfilgrastim | SC | Neumega | brand | oprelvekin | SC | 3 |
| Neulasta | brand | pegfilgrastim | SC | Nuedexta | brand | dextromethorphan /quinidine | oral | 3 |
| Prenexa | brand | prenatal vitamin,calcium/iron/folic acid/docusate/docosahexaenoic acid | oral | Ranexa | brand | ranolazine | oral | 3 |
| Razadyne | brand | galantamine | oral | Rozerem | brand | ramelteon | oral | 3 |
| Salagen | brand | pilocarpine | oral | selegiline | generic | selegiline | oral, transdermal | 3 |
| sotalol | generic | sotalol | oral, IV | Sudafed | brand | pseudoephedrine | oral | 3 |
| sulfADIAZINE | generic | sulfadiazine | oral | sulfaSALAzine | generic | sulfasalazine | oral | 3 |
| Tenex | brand | guanfacine | oral | Xanax | brand | alprazolam | oral | 3 |
| Tracleer | brand | bosentan | oral | Tricor | brand | fenofibrate,micronized | oral | 3 |
| zolpidem | generic | zolpidem | oral, sublingual | Zyloprim | brand | allopurinol | oral | 3 |
| Zostrix | brand | capsaicin | topical | Zovirax | brand | acyclovir | oral, topical | 3 |
| Zovirax | brand | acyclovir | oral, topical | Zyvox | brand | linezolid | oral, IV | 3 |
| Metadate | brand | methylphenidate | oral | methadone | generic | methadone | oral, injection | 3 |
| Actonel | brand | risedronate | oral | Actos | brand | pioglitazone | oral | 4 |
| atomoxetine | generic | atomoxetine | oral | atorvastatin | generic | atorvastatin | oral | 4 |
| Brevibloc | brand | esmolol | IV | Brevital | brand | methohexital | injection | 4 |
| Clozaril | brand | clozapine | oral | Colazal | brand | balsalazide | oral | 4 |
| Colace | brand | docusate | oral, rectal | Cozaar | brand | losartan | oral | 4 |
| Foradil | brand | formoterol | inhalation | Fortical | brand | calcitonin,salmon | nasal | 4 |
| Levemir | brand | insulin detemir | SC | Lovenox | brand | enoxaparin | SC | 4 |
| metFORMIN | generic | metformin | oral | metroNIDAZOLE | generic | metronidazole | oral, topical, vaginal, IV | 4 |
| Miralax | brand | polyethylene glycol 3350 | oral | Mirapex | brand | pramipexole | oral | 4 |
| Neumega | brand | oprelvekin | SC | Neupogen | brand | filgrastim | injection | 4 |
| penicillamine | generic | penicillamine | oral | penicillin | generic | penicillin v potassium; penicillin g sodium; penicillin g procaine | oral, injection | 4 |
| Sonata | brand | zaleplon | oral | Soriatane | brand | acitretin | oral | 4 |
| Tiazac | brand | diltiazem | oral | Ziac | brand | bisoprolol /hydrochlorothiazide | oral | 4 |
| Allegra | brand | fexofenadine | oral | Viagra | brand | sildenafil | oral | 4 |
| Allegra (fexofenadine) | brand | fexofenadine | oral | Allegra Anti-Itch (diphenhydrAMINE/allantoin) | brand | diphenhydramine/allantoin | topical | 4 |
| amantadine | generic | amantadine | oral | amiodarone | generic | amiodarone | oral, IV | 5 |
| Amicar | brand | aminocaproic acid | oral, IV | Omacor | brand | omega-3 acid ethyl esters | oral | 4 |
| Asacol | brand | mesalamine | oral | Os-Cal | brand | calcium carbonate | oral | 5 |
| azaCITIDine | generic | azacitidine | injection | azaTHIOprine | generic | azathioprine | oral, injection | 5 |
| buPROPion | generic | bupropion | oral | busPIRone | generic | buspirone | oral | 5 |
| Femara | brand | letrozole | oral | Femhrt | brand | norethindrone/ethinyl estradiol | oral | 4 |
| Flonase | brand | fluticasone propionate | nasal | Flovent | brand | fluticasone | inhalation | 4 |
| Inspra | brand | eplerenone | oral | Spiriva | brand | tiotropium | inhalation | 4 |
| lamoTRIgine | generic | lamotrigine | oral | levothyroxine | generic | levothyroxine | oral, IV | 4 |
| Lanoxin | brand | digoxin | oral, injection | levothyroxine | generic | levothyroxine | oral, IV | 4 |
| Mephyton | brand | phytonadione (vit k1) | oral | methadone | generic | methadone | oral, injection | 4 |
| Metadate ER | brand | methylphenidate | oral | methadone | generic | methadone | oral, injection | 4 |
| methadone | generic | methadone | oral, injection | metolazone | generic | metolazone | oral | 4 |
| oxaprozin | generic | oxaprozin | oral | OXcarbazepine | generic | oxcarbazepine | oral | 4 |
| Rapaflo | brand | silodosin | oral | Rapamune | brand | sirolimus | oral | 4 |
| Reprexain | brand | hydrocodone/ibuprofen | oral | ZyPREXA | brand | olanzapine | oral, IM | 5 |
| rifampin | generic | rifampin | oral, IV | rifaximin | generic | rifaximin | oral | 5 |
| silodosin | generic | silodosin | oral | sirolimus | generic | sirolimus | oral | 4 |
| Zelapar (Zydis formulation) | brand | selegiline | oral | ZyPREXA Zydis | brand | olanzapine | oral | 5 |
| desipramine | generic | desipramine | oral | disopyramide | generic | disopyramide | oral | 6 |
| Effexor XR | brand | venlafaxine | oral | Enablex | brand | darifenacin | oral | 6 |
| flavoxATE | generic | flavoxate | oral | fluvoxaMINE | generic | fluvoxamine | oral | 6 |
| Intuniv | brand | guanfacine | oral | Invega | brand | paliperidone | oral | 6 |
| NexAVAR | brand | sorafenib | oral | NexIUM | brand | esomeprazole | oral, IV | 6 |
| Restoril | brand | temazepam | oral | RisperDAL | brand | risperidone | oral | 6 |
| Zestril | brand | lisinopril | oral | Zetia | brand | ezetimibe | oral | 6 |
| Cardura | brand | doxazosin | oral | Coumadin | brand | warfarin | oral | 5 |
| colchicine | generic | colchicine | oral | Cortrosyn | brand | cosyntropin | injection | 5 |
| Doxil | brand | doxorubicin pegylated liposomal | IV | Paxil | brand | paroxetine | oral | 7 |
| leucovorin calcium | generic | leucovorin calcium | oral, injection | Leukeran | brand | chlorambucil | oral | 5 |
| Lotronex | brand | alosetron | oral | Protonix | brand | pantoprazole | oral, IV | 7 |
| NIFEdipine | generic | nifedipine | oral | niMODipine | generic | nimodipine | oral | 5 |
| Paxil | brand | paroxetine | oral | Taxol | brand | paclitaxel | IV | 7 |
| Proscar | brand | finasteride | oral | Provera | brand | medroxyprogesterone | oral | 5 |
| protamine | generic | protamine | IV | Protonix | brand | pantoprazole | oral | 7 |
| Zerit | brand | stavudine | oral | ZyrTEC | brand | cetirizine | oral | 5 |
| cycloSERINE | generic | cycloserine | oral | cycloSPORINE | generic | cyclosporine | oral, IV, ophthalmic | 8 |
| Thalomid | brand | thalidomide | oral | Thiamine | brand | thiamine | oral, injection | 8 |
| Advair | brand | fluticasone /salmeterol | inhalation | Advicor | brand | niacin/lovastatin | oral | 6 |
| CeleBREX | brand | celecoxib | oral | Cerebyx | brand | fosphenytoin | injection | 6 |
| cetirizine | generic | cetirizine | oral | stavudine | generic | stavudine | oral | 6 |
| clomiPHENE | generic | clomiphene | oral | clomiPRAMINE | generic | clomipramine | oral | 6 |
| Diovan | brand | valsartan | oral | Zyban | brand | bupropion | oral | 6 |
| Enbrel | brand | etanercept | SC | Levbid | brand | hyoscyamine | oral | 6 |
| heparin | generic | heparin | injection | Hespan | brand | hetastarch in 0.9% sodium chloride | IV | 6 |
| lamiVUDine | generic | lamivudine | oral | lamoTRIgine | generic | lamotrigine | oral | 6 |
| Prograf | brand | tacrolimus | oral, IV | PROzac | brand | fluoxetine | oral | 9 |
| Pyridium | brand | phenazopyridine | oral | pyridoxine | generic | pyridoxine | oral, injection | 6 |
| Aldara | brand | imiquimod | topical | Alora | brand | estradiol | transdermal | 6 |
| antacid | generic | magnesium /aluminum/sodium bicarbonate | oral | Atacand | brand | candesartan | oral | 6 |
| Cozaar | brand | losartan | oral | Zocor | brand | simvastatin | oral | 6 |
| Zocor | brand | simvastatin | oral | ZyrTEC | brand | cetirizine | oral | 6 |
| Aciphex | brand | rabeprazole | oral | Aricept | brand | donepezil | oral | 7 |
| fomepizole | generic | fomepizole | IV | omeprazole | generic | omeprazole | oral | 7 |
| Lasix | brand | furosemide | oral, injection | Luvox | brand | fluvoxamine | oral | 7 |
| risperiDONE | generic | risperidone | oral | rOPINIRole | generic | ropinirole | oral | 7 |
| Xanax | brand | alprazolam | oral | Zantac | brand | ranitidine | oral, injection | 7 |
| CeleXA | brand | citalopram | oral | Cerebyx | brand | fosphenytoin | injection | 8 |
| hydrALAZINE | generic | hydralazine | oral, injection | hydrOXYzine | generic | hydroxyzine | oral, IM | 8 |
| Lexiva | brand | fosamprenavir | oral | Pexeva | brand | paroxetine | oral | 8 |
| Lipitor | brand | atorvastatin | oral | ZyrTEC | brand | cetirizine | oral | 7 |
| PARoxetine | generic | paroxetine | oral | piroxicam | generic | piroxicam | oral | 7 |
| chlordiazePOXIDE | generic | chlordiazepoxide | oral | chlorproMAZINE | generic | chlorpromazine | oral, injection | 9 |
| chlorproMAZINE | generic | chlorpromazine | oral, injection | chlorproPAMIDE | generic | chlorpropamide | oral | 9 |
| Hydrea | brand | hydroxyurea | oral | Lyrica | brand | pregabalin | oral | 9 |
| Accupril | brand | quinapril | oral | Aciphex | brand | rabeprazole | oral | 8 |
| PriLOSEC | brand | omeprazole | oral | Pristiq | brand | desvenlafaxine | oral | 11 |
| Zantac | brand | ranitidine | oral, injection | ZyrTEC | brand | cetirizine | oral | 8 |
| Zegerid | brand | omeprazole/sodium bicarbonate | oral | Zestril | brand | lisinopril | oral | 8 |
| Diabinese | brand | chlorpropamide | oral | Diamox | brand | acetazolamide | oral | 10 |
| ZyPREXA | brand | olanzapine | oral, IM | ZyrTEC | brand | cetirizine | oral | 8 |
| Paxil | brand | paroxetine | oral | Plavix | brand | clopidogrel | oral | 9 |
| sertraline | generic | sertraline | oral | Soriatane | brand | acitretin | oral | 9 |
| Adderall | brand | dextroamphetamine /amphetamine | oral | Inderal | brand | propranolol | oral | 12 |
| Zestril | brand | lisinopril | oral | ZyPREXA | brand | olanzapine | oral, IM | 9 |
| cyclophosphamide | generic | cyclophosphamide | oral, IV | cycloSPORINE | generic | cyclosporine | oral, IV, ophthalmic | 10 |
| LORazepam | generic | lorazepam | oral, injection | Lovaza | brand | omega-3 acid ethyl esters | oral | 17 |
| CeleBREX | brand | celecoxib | oral | CeleXA | brand | citalopram | oral | 10 |
| cetirizine | generic | cetirizine | oral | sertraline | generic | sertraline | oral | 14 |
| ARIPiprazole | generic | aripiprazole | oral, IM | RABEprazole | generic | rabeprazole | oral | 10 |
| Benadryl | brand | diphenhydramine | topical, oral, injection | benazepril | generic | benazepril | oral | 11 |
| clonazePAM | generic | clonazepam | oral | cloNIDine | generic | clonidine | oral, transdermal, epidural | 13 |
| cloNIDine | generic | clonidine | transdermal, oral, epidural | KlonoPIN | brand | clonazepam | oral | 12 |
| SandIMMUNE | brand | cyclosporine | oral, IV | SandoSTATIN | brand | octreotide | injection | 12 |
| PriLOSEC | brand | omeprazole | oral | PROzac | brand | fluoxetine | oral | 13 |
| Lopressor | brand | metoprolol tartrate | oral | Lyrica | brand | pregabalin | oral | 14 |
disease concept grouping or disease concept proxy
Drug A and Drug B names are as represented on ISMP’s list of confused drug names
IM = intramuscular, IV= intravenous, SC = subcutaneous
There were 58 drug pairs (21%) with a partial overlap of high-level indications. Each pair had an average of 5 distinct high-level indications (range 2 to 14). The mean DSC was 0.53 (range 0.17 to 0.93) (Table 2).
Table 2.
ISMP confused drug name pairs with partial overlap in high-level indications*
| Drug A |
Drug B |
Dice similarity coefficient | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Name† | Name type | Active ingredient(s) | Route(s) | Number of high-level indications* | Name† | Name type | Active ingredient(s) | Route(s) | Number of high-level indications* | |
| Activase | brand | alteplase | IV | 4 | TNKase | brand | tenecteplase | IV | 1 | 0.40 |
| Activase | brand | alteplase | IV | 5 | Cathflo Activase | brand | alteplase | injection | 2 | 0.57 |
| Alkeran | brand | melphalan | oral, IV | 1 | Leukeran | brand | chlorambucil | oral | 2 | 0.67 |
| Alkeran | brand | melphalan | oral, IV | 1 | Myleran | brand | busulfan | oral | 2 | 0.67 |
| ALPRAZolam | generic | alprazolam | oral | 1 | LORazepam | generic | lorazepam | oral, injection | 11 | 0.17 |
| aMILoride | generic | amiloride | oral | 3 | amLODIPine | generic | amlodipine | oral | 2 | 0.40 |
| captopril | generic | captopril | oral | 4 | carvedilol | generic | carvedilol | oral | 3 | 0.57 |
| carBAMazepine | generic | carbamazepine | oral | 4 | OXcarbazepine | generic | oxcarbazepine | oral | 2 | 0.67 |
| Cardene | brand | nicardipine | 1 | Cardizem | brand | diltiazem | oral | 3 | 0.50 | |
| CeleXA | brand | citalopram | oral | 2 | ZyPREXA | brand | olanzapine | oral, IM | 5 | 0.29 |
| clobazam | generic | clobazam | oral | 2 | clonazePAM | generic | clonazepam | oral | 5 | 0.57 |
| clonazePAM | generic | clonazepam | oral | 5 | LORazepam | generic | lorazepam | oral, injection | 12 | 0.35 |
| Cymbalta | brand | duloxetine | oral | 5 | Symbyax | brand | olanzapine/fluoxetine | oral | 1 | 0.33 |
| Depo-Medrol | brand | methylprednisolone | injection | 7 | Solu-MEDROL | brand | methylprednisolone | injection | 8 | 0.93 |
| Depo-Provera | brand | medroxyprogesterone | IM | 2 | Depo-subQ Provera 104 | brand | medroxyprogesterone | SC | 2 | 0.50 |
| dimenhyDRINATE | generic | dimenhydrinate | oral, injection | 2 | diphenhydrAMINE | generic | diphenhydramine | oral, injection, topical | 11 | 0.46 |
| DULoxetine | generic | duloxetine | oral | 5 | FLUoxetine | generic | fluoxetine | oral | 8 | 0.46 |
| Effexor | brand | venlafaxine | oral | 5 | Effexor XR | brand | venlafaxine | oral | 4 | 0.89 |
| ePHEDrine | generic | ephedrine | oral, injection | 2 | EPINEPHrine | generic | epinephrine | injection | 11 | 0.31 |
| fentaNYL | generic | fentanyl | transdermal, nasal, sublingual, transmucosal, buccal, epidural | 1 | SUFentanil | generic | sufentanil | IV | 2 | 0.67 |
| FLUoxetine | generic | fluoxetine | oral | 8 | PARoxetine | generic | paroxetine | oral | 6 | 0.86 |
| folic acid | generic | folic acid | oral, injection | 2 | folinic acid (leucovorin calcium) | generic | leucovorin calcium | oral, injection | 3 | 0.40 |
| HumaLOG | brand | insulin lispro | SC | 1 | HumuLIN | brand | insulin regular | SC | 4 | 0.40 |
| HumuLIN R U-100 | brand | insulin regular | SC | 4 | HumuLIN R U-500 | brand | insulin regular | SC | 1 | 0.40 |
| HYDROmorphone | generic | hydromorphone | oral, injection, rectal | 1 | morphine | generic | morphine | oral, injection, rectal | 3 | 0.50 |
| inFLIXimab | generic | infliximab | IV | 3 | riTUXimab | generic | rituximab | IV | 5 | 0.25 |
| Isordil | brand | isosorbide dinitrate | oral | 2 | Plendil | brand | felodipine | oral | 3 | 0.40 |
| ISOtretinoin | generic | isotretinoin | oral | 3 | tretinoin | generic | tretinoin | oral, topical | 4 | 0.57 |
| Ketalar | brand | ketamine | injection | 3 | ketorolac | generic | ketorolac | oral, ophthalmic, injection, nasal | 3 | 0.33 |
| ketorolac | generic | ketorolac | oral, ophthalmic, injection, nasal | 3 | methadone | generic | methadone | oral, injection | 2 | 0.40 |
| lamoTRIgine | generic | lamotrigine | oral | 2 | levETIRAcetam | generic | levetiracetam | oral, injection | 1 | 0.67 |
| leucovorin calcium | generic | leucovorin calcium | oral, injection | 3 | levoleucovorin | generic | levoleucovorin | IV | 2 | 0.80 |
| Leukeran | brand | chlorambucil | oral | 2 | Myleran | brand | busulfan | oral | 2 | 0.50 |
| levothyroxine | generic | levothyroxine | oral, IV | 2 | liothyronine | generic | liothyronine | oral | 3 | 0.80 |
| Maxzide | brand | triamterene/hydrochlorothiazide | oral | 2 | Microzide | brand | hydrochlorothiazide | oral | 4 | 0.67 |
| Metadate CD | brand | methylphenidate | oral | 1 | Metadate ER | brand | methylphenidate | oral | 2 | 0.67 |
| metoprolol succinate | generic | metoprolol succinate | oral | 7 | metoprolol tartrate | generic | metoprolol tartrate | oral, IV | 7 | 0.86 |
| mifepristone | generic | mifepristone | oral | 3 | misoprostol | generic | misoprostol | oral | 0.67 | |
| mitoMYcin | generic | mitomycin | IV | 1 | mitoXANTRONE | generic | mitoxantrone | IV | 2 | 0.67 |
| Motrin | brand | ibuprofen | oral | 4 | Neurontin | brand | gabapentin | oral | 5 | 0.22 |
| Mucinex D | brand | guaifenesin/pseudoephedrine | oral | 2 | Mucinex DM | brand | guaifenesin/dextromethorphan | oral | 1 | 0.67 |
| niCARdipine | generic | nicardipine | oral, IV | 3 | NIFEdipine | generic | nifedipine | oral | 3 | 0.67 |
| NovoLIN | brand | insulin regular | SC | 3 | NovoLOG | brand | insulin aspart | SC | 1 | 0.50 |
| OLANzapine | generic | olanzapine | oral, IM | 4 | QUEtiapine | generic | quetiapine | oral | 4 | 0.75 |
| opium tincture | generic | opium tincture | oral | 2 | paregoric (camphorated tincture of opium) | generic | paregoric | oral | 1 | 0.67 |
| PENTobarbital | generic | pentobarbital | oral, injection | 3 | PHENobarbital | generic | phenobarbital | oral, injection | 2 | 0.40 |
| Plavix | brand | clopidogrel | oral | 3 | Pradaxa | brand | dabigatran | oral | 2 | 0.40 |
| prednisoLONE | generic | prednisolone | oral, ophthalmic, injection | 9 | predniSONE | generic | prednisone | oral | 7 | 0.88 |
| Provera | brand | medroxyprogesterone | oral | 3 | PROzac | brand | fluoxetine | oral | 8 | 0.18 |
| quiNIDine | generic | quinidine | oral, injection | 2 | quiNINE | generic | quinine | oral | 2 | 0.50 |
| Ritalin LA | brand | methylphenidate | oral | 1 | Ritalin SR | brand | methylphenidate | oral | 2 | 0.67 |
| Sudafed 12 Hour | brand | pseudoephedrine | oral | 1 | Sudafed 12 Hour Pressure + Pain | brand | naproxen /pseudoephedrine | oral | 3 | 0.50 |
| TNKase | brand | tenecteplase | IV | 1 | t-PA | generic | alteplase | injection | 5 | 0.33 |
| Tobradex | brand | tobramycin/dexamethasone | ophthalmic | 2 | Tobrex | brand | tobramycin | ophthalmic | 1 | 0.67 |
| Topamax | brand | topiramate | oral | 5 | Toprol-XL | brand | metoprolol succinate | oral | 7 | 0.17 |
| Tylenol | brand | acetaminophen | oral | 2 | Tylenol PM | brand | acetaminophen/diphenhydramine | oral | 4 | 0.33 |
| Wellbutrin SR | brand | bupropion | oral | 4 | Wellbutrin XL | brand | bupropion | oral | 3 | 0.57 |
| ZyrTEC | brand | cetirizine | oral | 4 | ZyrTEC-D | brand | cetirizine /pseudoephedrine | oral | 2 | 0.67 |
disease concept grouping or disease concept proxy
Drug A and Drug B names are as represented on ISMP’s list of confused drug names
IM = intramuscular, IV= intravenous, SC = subcutaneous
There were 55 drug pairs (20%) with complete overlap in high-level indications. Each of these pairs shared a single, common high-level indication (Table 3). Most comparisons were between antineoplastic drugs (n = 13, 24%), anti-infective agents (n = 10, 18%), antidiabetic drugs (n = 9, 16%), or opioid analgesics (n = 5, 9%). Nearly half of the drug pairs (n = 24, 44%) were comprised of drugs with the same active ingredient and route of administration.
Table 3.
ISMP confused drug name pairs with complete overlap in high-level indications*
| Drug A |
Drug B |
Number of high-level indications within pair* | ||||||
|---|---|---|---|---|---|---|---|---|
| Name† | Name type | Active ingredient(s) | Route(s) | Name† | Name type | Active ingredient(s) | Route(s) | |
| Abelcet | brand | amphotericin b lipid complex | IV | amphotericin B | generic | amphotericin b | IV | 1 |
| Adacel (Tdap) | brand | diphtheria,pertussis(acellular),tetanus vaccine | IM | Daptacel (DTaP) | brand | diphtheria, pertussis (acell), tetanus pediatric vaccine | IM | 1 |
| ado-trastuzumab emtansine | generic | ado-trastuzumab emtansine | IV | trastuzumab | generic | trastuzumab | IV | 1 |
| Ambisome | brand | amphotericin b liposome | IV | amphotericin B | generic | amphotericin b | IV | 1 |
| Avandia | brand | rosiglitazone | oral | Prandin | brand | repaglinide | oral | 1 |
| Bicillin C-R | brand | penicillin g benzathine/penicillin g procaine | IM | Bicillin L-A | brand | penicillin g benzathine | IM | 1 |
| CARBOplatin | generic | carboplatin | IV | CISplatin | generic | cisplatin | IV | 1 |
| ceFAZolin | generic | cefazolin | injection | cefTRIAXone | generic | ceftriaxone | injection | 1 |
| coagulation factor IX (recombinant) | generic | factor IX human recombinant | IV | factor IX complex, vapor heated | generic | factor IX complex, prothrombin complex concentrate, 3-factor | IV | 1 |
| DAUNOrubicin | generic | daunorubicin | IV | DAUNOrubicin citrate liposomal | generic | daunorubicin citrate liposomal | IV | 1 |
| DAUNOrubicin | generic | daunorubicin | IV | DOXOrubicin | generic | doxorubicin | IV | 1 |
| DAUNOrubicin | generic | daunorubicin | IV | IDArubicin | generic | idarubicin | IV | 1 |
| Dilaudid | brand | hydromorphone | oral, injection | Dilaudid-5 | brand | hydromorphone | oral | 1 |
| DOXOrubicin | generic | doxorubicin | IV | DOXOrubicin liposomal | generic | doxorubicin pegylated liposomal | IV | 1 |
| DOXOrubicin | generic | doxorubicin | IV | IDArubicin | generic | idarubicin | IV | 1 |
| Dulcolax (bisacodyl) | brand | bisacodyl | oral, rectal | Dulcolax (docusate sodium) | brand | docusate sodium | oral | 1 |
| Engerix-B adult | brand | hepatitis b virus vaccine recombinant | IM | Engerix-B pediatric/adolescent | brand | hepatitis b virus vaccine recombinant | IM | 1 |
| epirubicin | generic | epirubicin | IV | eribulin | generic | eribulin | IV | 1 |
| Fioricet | brand | butalbital/acetaminophen/caffeine | oral | Fiorinal | brand | butalbital/aspirin/caffeine | oral | 1 |
| glipiZIDE | generic | glipizide | oral | glyBURIDE | generic | glyburide | oral | 1 |
| HumaLOG | brand | insulin lispro | SC | NovoLOG | brand | insulin aspart | SC | 1 |
| HumaLOG Mix 75/25 | brand | insulin lispro protamine and insulin lispro | SC | HumuLIN 70/30 | brand | insulin NPH /insulin regular | SC | 1 |
| HYDROcodone | generic | hydrocodone | oral | oxyCODONE | generic | oxycodone | oral | 1 |
| Janumet | brand | sitagliptin /metformin | oral | Januvia | brand | sitagliptin | oral | 1 |
| Menactra | brand | meningococcal vaccine a,c,y,w-135,diphtheria toxoid conjugate | IM | Menomune | brand | meningococcal vaccine a,c,y,w-135 | SC | 1 |
| morphine – non-concentrated oral liquid | generic | morphine | oral | morphine – oral liquid concentrate | generic | morphine | oral | 1 |
| MS Contin | brand | morphine | oral | OxyCONTIN | brand | oxycodone | oral | 1 |
| NovoLIN 70/30 | brand | insulin NPH /insulin regular | SC | NovoLOG Mix 70/30 | brand | insulin aspart protamine /insulin aspart | SC | 1 |
| NovoLOG FLEXPEN | brand | insulin aspart | SC | NovoLOG Mix 70/30 FLEXPEN | brand | insulin aspart protamine /insulin aspart | SC | 1 |
| oxyCODONE | generic | oxycodone | oral | OxyCONTIN | brand | oxycodone | oral | 1 |
| PACLitaxel | generic | paclitaxel | IV | PACLitaxel protein-bound particles | generic | paclitaxel protein-bound | IV | 1 |
| pazopanib | generic | pazopanib | oral | ponatinib | generic | ponatinib | oral | 1 |
| PEMEtrexed | generic | pemetrexed | IV | PRALAtrexate | generic | pralatrexate | IV | 1 |
| Renagel | brand | sevelamer | oral | Renvela | brand | sevelamer | oral | 1 |
| Rifadin | brand | rifampin | oral, IV | Rifater | brand | rifampin/isoniazid/pyrazinamide | oral | 1 |
| Rifamate | brand | rifampin/isoniazid | oral | rifampin | generic | rifampin | oral, IV | 1 |
| Sudafed | brand | pseudoephedrine | oral | Sudafed PE | brand | phenylephrine | oral | 1 |
| SUMAtriptan | generic | sumatriptan | oral, nasal, SC, transdermal | ZOLMitriptan | generic | zolmitriptan | oral, nasal | 1 |
| Taxol | brand | paclitaxel | IV | Taxotere | brand | docetaxel | IV | 1 |
| TOLAZamide | generic | tolazamide | oral | TOLBUTamide | generic | tolbutamide | oral | 1 |
| valACYclovir | generic | valacyclovir | oral | valGANciclovir | generic | valganciclovir | oral | 1 |
| Valcyte | brand | valganciclovir | oral | Valtrex | brand | valacyclovir | oral | 1 |
| vinBLAStine | generic | vinblastine | IV | vinCRIStine | generic | vincristine | IV | 1 |
| Viracept | brand | nelfinavir | oral | Viramune | brand | nevirapine | oral | 1 |
| Adderall | brand | dextroamphetamine /amphetamine | oral | Adderall XR | brand | dextroamphetamine /amphetamine | oral | 2 |
| Claritin-D | brand | loratadine/pseudoephedrine | oral | Claritin-D 24 | brand | loratadine/pseudoephedrine | oral | 2 |
| Retrovir | brand | zidovudine | oral, IV | ritonavir | generic | ritonavir | oral | 2 |
| Depakote | brand | divalproex | oral | Depakote ER | brand | divalproex | oral | 3 |
| HumuLIN | brand | insulin regular | SC | NovoLIN | brand | insulin regular | SC | 4 |
| Lupron Depot-3 Month | brand | leuprolide | IM | Lupron Depot-Ped | brand | leuprolide | IM | 4 |
| Ortho Tri-Cyclen | brand | norgestimate-ethinyl estradiol | oral | Ortho Tri-Cyclen LO | brand | norgestimate-ethinyl estradiol | oral | 4 |
| SEROquel | brand | quetiapine | oral | SEROquel XR | brand | quetiapine | oral | 4 |
| TEGretol | brand | carbamazepine | oral | TEGretol XR | brand | carbamazepine | oral | 4 |
| Yasmin | brand | ethinyl estradiol/drospirenone | oral | Yaz | brand | ethinyl estradiol/drospirenone | oral | 4 |
| Solu-CORTEF | brand | hydrocortisone | injection | Solu-MEDROL | brand | methylprednisolone | injection | 8 |
disease concept grouping or disease concept proxy
Drug A and Drug B names are as represented on the ISMP’s list of confused drug names
IM = intramuscular, IV= intravenous, SC = subcutaneous
Discussion
This study shows that nearly 60% of the ISMP confused drug pairs included in our dataset had no overlap in indications, and another 21% of the drug pairs had just a partial overlap in indications. Associating indications with these drugs may help to differentiate these pairs and eliminate confusion between them. The remaining 20% of the drug pairs contained drugs with the same high-level indication. Many of these drug pairs were comprised of drugs with the same active ingredient and route of administration and differed only in dosage form (e.g., Seroquel vs Seroquel XR), strength (e.g., Ortho Tri-Cyclen vs Ortho Tri-Cyclen LO) or formulation (e.g., paclitaxel vs paclitaxel protein-bound). Our results are consistent with a recent study that used only FDA-approved indications to differentiate a smaller set (33 drug pairs) of LASA drug names.22 To our knowledge, this is the first study to systematically evaluate the degree of overlapping indications (FDA approved and unapproved) between LASA drugs reported to ISMP.
For decades, leading authorities have recommended adding indications to medication orders to prevent errors and confusion between LASA drugs.5,16 However, there is limited quantitative data on how many LASA drugs mix-ups could be prevented using indications. Our study shows that associating indications to LASA medications is a potentially powerful lever since more than half of these medication pairs could be distinguished even with high-level indications concepts.
Current strategies for avoiding LASA errors include use of bar code technology, storage of LASA drugs apart from each other, use of Tall Man lettering, adding extra security labels to the outer packaging of LASA drugs, and performing medication reconciliation at every clinical encounter.5–7 Adding indications to LASA pairs during prescription ordering is a newer, potentially less labor-intensive way to prevent name confusion electronically and make computerized medication ordering safer.23–28 Drug indications content that is integrated within the electronic health record can allow users to associate indications with drugs being prescribed, dispensed or administered. This may help prevent errors due to choosing the wrong medication because of drug name confusion, as well as facilitate the incorporation of indications in the electronic medical record.14,22,24–25
In 2010, the Joint Commission added a LASA requirement to the Medication Management Standards for organizations to identify confused drug name pairs such as the list provided by ISMP.8 Our study demonstrates that providing information about drug indications could provide organizations and clinicians with another practical tool to prevent drug name confusion errors.
Incorporating indications onto prescription orders and labels might also allow for better medication counseling and potentially empower patients to point out wrong-patient-wrong-medication errors if the reason for use on the medication label is not consistent with their diagnosis.
Limitations
We intentionally used high-level indications rather than granular indications associated with each drug to facilitate indication review and comparison and model an approach to displaying drug indications to prescribers when ordering medications. Thus, our evaluation of indication overlaps is conservative, particularly for anti-infective agents and antineoplastic drugs, where the proxy disease concept was used to represent a single, broad indication for the drug.
Had we reviewed the more granular indications for these drugs, we likely would have had fewer indication overlaps within the drug pairs in our dataset. For example, pazopanib (Votrient), is used to treat renal cell carcinoma and advanced soft tissue sarcoma. Ponatinib (Iclusig), treats chronic myeloid leukemia or Philadelphia chromosome positive acute lymphoblastic leukemia. However, both drugs were associated with “malignancy” in our data and were categorized as having overlapping indications. Thus, our results are likely an underestimate of the number of LASA drugs that could be distinguished by indications.
Similarly, valacyclovir and valganciclovir were both associated with “viral infection” even though the former treats herpes virus infections and the latter treats infections caused by cytomegalovirus. Use of the proxy disease concept may have been too broad for drug pairs where both constituents were anti-infectives or both were antineoplastic drugs. The level of granularity required to distinguish between indications for these drugs would be a valuable area of further study. Most oncology agents, in particular, have a high likelihood of causing significant patient harm if used in error.29,30
Another limitation is that we used a single source of drug indication information rather than cross-referencing multiple sources. The advantage of using the Medknowledge Indications Module was that the content included both FDA-approved and unapproved indications and, once mapped to the products on the ISMP list, could be extracted to a tabular format and reviewed. We used clinician review to evaluate the data for appropriateness and thus felt that the data was valid and appropriate for our study.
We would have liked to have weighted the indications according to the likelihood that a drug would be used for one indication versus another. For example, the most likely indication for regular insulin is diabetes. However, it may also be used with glucose to treat hyperkalemia or as part of a diagnostic procedure to assess cortisol and growth hormone response to hypoglycemic stress. For drug pairs with a partial overlap, defining how common the indication is for a drug could be helpful for determining the likelihood that having an indication associated with the drug would help reduce confusion with a similarly named drug.
We also did not account for how frequently the drug is used. The addition of indications to a drug order or prescription may have more impact in terms of reducing the incidence of inadvertent interchange for commonly prescribed LASA drugs, if associating an indication can differentiate between them. While we are aware that the frequency of prescribing (and how common or uncommon a drug is used) varies among practices and practice settings, we noted that several drug pairs with no overlap in high-level indications were comprised of drugs that were among the top 200 U.S. drugs.31 Examples include sitagliptin/sumatriptan, tramadol/trazodone, atomoxetine/atorvastatin, metformin/metronidazole, bupropion/buspirone, lamotrigine/levothyroxine, and clonazepam/clonidine. Future studies using real-world drug utilization data in different practice settings would better inform the patient safety and electronic medical record design communities on the impact of associating indications with drugs. Nevertheless, we feel that our results give a good general sense of the potential for indications to reduce medication error.
Additionally, we did not evaluate the relative seriousness of adverse outcomes from confusing two similarly named drugs since some drug mix-ups may not be as dangerous as others; confusing drugs with the same pharmacologic action (e.g., antidiabetic drugs glyburide and glipizide) may be less concerning than confusing two drugs with very different uses (e.g., Keflex for bacterial infection versus Keppra for seizures). These are potential areas for future studies.
The ISMP list is also based on reported errors. Since many errors are unreported, the list is likely an underestimate of LASA drugs. Nevertheless, this study shows that associating indications with prescriptions, even at a general and non-granular level, can provide an additional piece of information that clinicians can use to distinguish between many otherwise confusing drug names. Our findings add to the growing body of literature on the use of indications information to prevent medication errors, guide therapy selection, support medication counseling and education, and facilitate deprescribing of medications.23–28 Further study is needed on optimal ways of representing drug indications within drug knowledge bases to support different applications of the data (e.g., indications-based prescribing, patient problem list maintenance, drug regimen review, claims adjudication).32 More research is also needed on practical ways to integrate indications content within the electronic health record such that clinicians can associate a drug’s indication easily and accurately at any point in the medication use process.
Conclusion
Indications can help differentiate the majority of drugs with look-alike-sound-alike names in the current version of the ISMP list, and thus may potentially be used to reduce harm from errors that occur from drug name confusion. Further studies are needed to assess the optimal structuring of indications content, the impact of adding indications to drug prescriptions, and optimal ways to integrate indications content into electronic medication ordering systems and other areas of the clinical workflow.
Funding
This work received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests
Dr Cheng is employed by First Databank Inc. All other authors have no competing interests to declare.
Contributors
Dr Cheng was involved in the study design, data acquisition, analysis, interpretation, and drafting of the manuscript. Drs Salazar and Amato were involved with the design, data validation, data analysis, and critical review of the manuscript. Dr Lambert was involved with the study design, and critical review of the manuscript. Ms. Volk was involved with the study design, data analysis and critical review of the manuscript. Dr Schiff was involved with the study design, data analysis, and critical review of the manuscript. All authors approved of the final manuscript version.
Acknowledgments
The authors acknowledge Jeff Bubp, PharmD and Jim Breen, PharmD for supporting the study and manuscript preparation.
References
- 1. Hoffman JM, Proulx SM.. Medication errors caused by confusion of drug names. Drug Saf 2003; 267: 445–52. [DOI] [PubMed] [Google Scholar]
- 2. Hicks RW, Becker SC, Cousins DD, eds. MEDMARX Data Report: A Report on the Relationship of Drug Names and Medication Errors in Response to the Institute of Medicine’s Call for Action. Rockville, MD: U.S. Pharmacopeia; 2008. [Google Scholar]
- 3. Basco WT, Garner SS, Ebeling M et al. , . Evaluating the potential severity of look-alike, sound-alike drug substitution errors in children. Acad Pediatr 2016; 162: 183–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bryan R, Aronson JK, ten Hacken P et al. , . Patient safety in medication nomenclature: orthographic and semantic properties of international nonproprietary names. PLoS One 2015; 1012: e0145431.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Look-Alike, Sound-Alike Medication Names WHO Collaborating Centre for Patient Safety Solutions. Patient Safety Solutions, May 2007; volume 1, solution 1. http://www.who.int/patientsafety/solutions/patientsafety/PS-Solution1.pdf. Accessed April 24, 2018.
- 6. U.S. Food & Drug Administration Strategies to Reduce Medication Errors: Working to Improve Medication Safety https://www.fda.gov/Drugs/ResourcesForYou/Consumers/ucm143553.htm. Accessed October 1, 2017.
- 7. Joint Commission for Accreditation of Healthcare Organizations. Sound-alike drug names product risk of medication interchange errors. J Pain Palliat Care Pharmacother 2005; 193: 47–53. [PubMed] [Google Scholar]
- 8. The Joint Commission Look-alike/Sound-alike Drug List https://www.jointcommission.org/lasa/. Accessed November 21, 2017.
- 9. U.S. Department of Health and Human Services. Draft Guidance for Industry: Best Practices in Developing Proprietary Names for Drugs May 2014. https://fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM398997.pdf. Accessed October 24, 2017.
- 10. Health Canada Guidance Document for Industry—Review of Drug Brand Names July 2014. https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/dhp-mps/alt_formats/pdf/pubs/medeff/guide/2014-review-examen_drug-medicament_names-marques/2014-review-examen_drug-medicament_names-marques-eng.pdf. Accessed October 24, 2017.
- 11. Trbovich PL, Hyland S.. Responding to the challenge of look-alike, sound-alike drug names. BMJ Qual Saf 2017; 265: 357–9. [DOI] [PubMed] [Google Scholar]
- 12. Quist AJL, Hickman T-TT, Amato MG et al. , . Analysis of variations in the display of drug names in computerized prescriber-order entry systems. Am J Health Syst Pharm 2017; 747: 499–509. [DOI] [PubMed] [Google Scholar]
- 13. Shamliyan TA, Duval S, Du J et al. , . Just what the doctor ordered. Review of the evidence of the impact of computerized physician order entry system on medication errors. Health Serv Res 2007; 43 (1p1): 32–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schiff G, Seoane-Vazquez E, Write A.. Incorporating indications into medication ordering: time to enter the age of reason. N Engl J Med 2016; 3754: 306–9. [DOI] [PubMed] [Google Scholar]
- 15. First Databank MedKnowledge Indications module (INDM) South San Francisco, CA: First Databank Inc.; 2017.
- 16.Institute of Safe Medication Practice (ISMP). List of Confused Drug Names, updated February 2015. https://www.ismp.org/Tools/confuseddrugnames.pdf. Accessed January 10, 2017.
- 17. US Food & Drug Administration Drugs@FDA: FDA Approved Drug Products Database https://www.accessdata.fda.gov/scripts/cder/daf/. Accessed January 10, 2017.
- 18. US Food & Drug Administration National Drug Code Directory Database https://www.accessdata.fda.gov/scripts/cder/ndc/default.cfm. Accessed March 8, 2017.
- 19. First Databank Medical Lexicon. South San Francisco, CA: First Databank Inc; 2017 [Google Scholar]
- 20. Dice LR. Measures of the amount of ecologic association between species. Ecology 1945; 263: 297–302. [Google Scholar]
- 21. Griffon N, Kerdelhue G, Soualmia LF et al. , . Evaluating alignment quality between iconic language and reference terminologies using similarity metrics. BMC Med Inform Decis Mak 2014; 141: 17.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Seoane-Vazquez E, Rodriguez-Monguio R, Alqahtani S et al. , . Exploring the potential for using drug indications to prevent look-alike and sound-alike drug errors. Expert Opin Drug Saf 2017; 1610: 1103. 1103-1009. [DOI] [PubMed] [Google Scholar]
- 23. Rash-Foanio C, Galanter W, Bryson M et al. , . Automated detection of look-alike/sound-alike medication errors. Am J Health Syst Pharm 2017; 747: 521–7. [DOI] [PubMed] [Google Scholar]
- 24. Galanter W, Falck S, Burns M et al. , . Indication-based prescribing prevents wrong-patient medication errors in computerized provider order entry (CPOE). J Am Med Inform Assoc 2013; 203: 477–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Galanter WL, Bryson ML, Falck S et al. , . Indication alerts intercept drug name confusion errors during computerized entry of medication orders. PLoS One 2014; 97: e101977.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Falck S, Adimadhyam S, Meltzer DO et al. , . A trial of indication based prescribing of antihypertensive medications during computerized order entry to improve problem list documentation. Int J Med Inform 2013; 8210: 996–1003. [DOI] [PubMed] [Google Scholar]
- 27. Linsky A, Meterko M, Stolzmann K et al. , . Supporting medication discontinuation: provider preferences for interventions to facilitate deprescribing. BMC Health Serv Res 2017; 171: 447.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wong J, Motulsky A, Abrahamowicz M et al. , . Off-label indications for antidepressants in primary care: descriptive study of prescriptions from an indication based electronic prescribing system. BMJ 2017; 365: j603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Institute for Safe Medication Practices (ISMP). List of High-Alert Medications in Acute Care Settings http://www.ismp.org/Tools/highalertmedications.pdf. Accessed October 24, 2017.
- 30. Kovacic L, Chambers C.. Look-alike, sound-alike drugs in oncology. J Oncol Pharm Pract 2011; 172: 104–18. [DOI] [PubMed] [Google Scholar]
- 31. Kane SP. About the ClinCalc DrugStats Database, Version 18.0 ClinCalc. http://clincalc.com/DrugStats/About.aspx. Updated February 3, 2018. Accessed March 25, 2018.
- 32. Nelson SJ, Oprea TI, Ursu O et al. , . Formalizing drug indications on the road to therapeutic intent. J Am Med Inform Assoc 2017; 246: 1169–72. [DOI] [PMC free article] [PubMed] [Google Scholar]