Abstract
Introduction:
As South Africa, especially the Western Cape Province, faces an epidemic of methamphetamine use disorder (MUD), therapeutic approaches suited to the South African context are needed.
Aims:
This secondary analysis assessed retention and methamphetamine abstinence outcomes in response to an 8-week pilot contingency management (CM) intervention trial of neural correlates of methamphetamine abstinence, exploring sociodemographic and clinical differences between responders and nonresponders.
Design and Methods:
Research participants provided thrice-weekly, monitored urine samples, which were analyzed by qualitative radioimmunoassay. The primary outcome for this analysis was therapeutic response, defined as abstinence from methamphetamine (≥23 of 24 possible methamphetamine-negative urine samples).
Results:
Data from 30 adults living in Cape Town, South Africa (34 ± 6.1 years of age, mean age ± SD, 21 men) were included. Sixty-three percent (12 men) were responders. In bivariate comparisons, baseline measurements showed fewer responders reported monthly household income ≥25,000+ South African Rand (ZAR; ~ USD $1,880; vs. ZAR <25,000) than nonresponders (15.8% vs. 63.6%; p=0.007). Furthermore, responders had higher median years of education (12 vs. 10; Kruskal-Wallis χ2 = 4.25, DF=1, p=0.039) and lower median body mass index (BMI) than nonresponders (19 vs. 24; Kruskal-Wallis χ2 = 6.84, p=0.008).
Discussions and Conclusions:
Therapeutic response in this study were comparable to those obtained with CM for MUD in North America and Europe. Our findings suggest that CM may be a useful component of treatment strategies to boost retention and continuous abstinence from methamphetamine in Cape Town, South Africa. Larger efficacy studies are needed in this setting.
Keywords: Contingency Management, Behavioral Treatment, Methamphetamine Use Disorder, Stimulants, South Africa
1. Introduction
South Africa, particularly the Western Cape Province, is facing an ongoing epidemic of methamphetamine use disorder (MUD), as the prevalence of methamphetamine has increased steadily in the past decade (1). Data from the South African Community Epidemiological Network on Drug Use, which examines data from specialist substance use treatment centers in the nine provinces of South Africa, show that in 2016, of the 5,784 patients presenting for drug abuse treatment, 31% (n=1,766) reported methamphetamine as the primary substance of use (1). As in other countries, MUD in South Africa is associated with a broad range of negative health issues, including HIV sexual transmission behaviors (2,3), HIV seroconversion, (4) and mental disorders (e.g., mood and psychotic disorders) (5).
Treatment for MUD in South Africa has predominantly focused on cognitive behavioral therapy, motivational interviewing, and problem-solving skills (6–8). In response to the methamphetamine epidemic in 2007, the City of Cape Town introduced Matrix treatment centers, which use an evidence-based treatment developed in the U.S. in the 1980s, at primary health care clinics (9). The treatment approach includes psychoeducation, individual sessions, early recovery groups, substance-use monitoring, peer support, family support and relapse prevention (10). In one recent study of Matrix Model, conducted within a community health center outside of Cape Town, only 129 of the 986 clients (majority whom presented with methamphetamine or heroin as the primary drug of choice) that initiated treatment (13%) completed the 16-week program. Most of these program enrollees presented with methamphetamine or heroin as the primary drug of choice, underscoring the need for alternative or complementary therapies to those already in place. Contingency management (CM), a behavioral therapy that provides positive reinforcers (such as financial incentives) in exchange for objective evidence of abstinence from substance use, has demonstrated efficacy in promoting continued abstinence from methamphetamine use and other stimulants with treatment effect sizes (Cohen’s d) ranging from .32 to .42 (12–14). Furthermore, CM produces better retention rates than other psychosocial treatments, with 63% of individuals completing a 16 weeks CM program for stimulant use compared to 40% in cognitive behavioral therapy arm (15). Thus, CM may provide a better approach to improving treatment retention by reducing the previously documented financial and transportation access barriers to treatment utilization and engagement experienced in South Africa (16). The aim of this article was to assess retention, methamphetamine abstinence outcomes in response to an 8-week pilot CM program and potential sociodemographic and clinical differences in response to the CM program among treatment-seeking adults who meet criteria for MUD in Cape Town, South Africa.
2. Methods
2.1. Study setting and participants
This was a secondary analysis of a pilot trial that assessed the neurobiological correlates underpinning outcomes for CM as a treatment for MUD (Clinical Trial Identifier: NCT02907853). Participants were treatment-seeking persons who use methamphetamine enrolled between August 2016 and January 2017 in an 8-week clinical trial of CM in Cape Town, South Africa. Participants were recruited via referrals from drug rehabilitation centers as well as using flyers distributed in homeless shelters, community centers, and shopping centers. They were eligible to participate in the study if they met DSM-5 criteria for MUD, provided a urine sample that tested positive for methamphetamine during screening, were aged 18–45 years, and had a good understanding of English. Participants were also included in the study if they reported secondary use of methaqualone, marijuana or tobacco. The exclusion criteria were: currently in treatment for addiction to a substance other than stimulants; meeting DSM-5 criteria for a substance-related diagnosis other than Methamphetamine, Tobacco, Marijuana or Methaqualone Use Disorder; current use of a prescribed psychoactive medication; inability to attend ≥4 visits during a 2-week screening period or to complete screening measures; physical or mental illness that would (a) require intervention (inpatient treatment) or (b) alter brain imaging findings, or (c) interfere with safe study participation; pregnancy, claustrophobia, or presence of metal prostheses, cardiac pacemakers, or metal clips that are incompatible with the MRI environment; HIV seropositive status; and previous head injury.
All participants provided written informed consent. A total of 269 individuals were screened for the study. Of these 148 (55%) were excluded as they did not meet inclusion/exclusion criteria. An additional 88 (33%) did not come for the enrollment visit, yielding a sample of 33 participants (12%). The Institutional Review Boards of the University of Cape Town, South Africa and the University of California, Los Angeles oversaw all aspects of the study, ensuring that it complied with ethical standards laid out by the Declaration of Helsinki (citation).
2.2. Contingency management (CM) procedure
Urine Testing Procedures.
Throughout, participants attended clinic thrice weekly to provide a monitored urine sample at each visit and to complete all other study activities. Eligibility for the CM program was determined during a 2-week baseline screening period. Participants who tested positive for methamphetamine at least once during screening and who met all inclusion and no exclusion criteria were allowed to begin the 8-week CM program. Urine sample integrity was verified using a urine collection cup with an affixed temperature strip and personal escort to the toilet. Participants voided into the cup, placed a lid on the cup and handed the specimen to the research assistant. Valid samples required a reading of 92°-96° F on the temperature strip. If any of these procedures were not met exactly, participants were required to provide a different urine sample.
The CM Program.
The CM program provided vouchers that increased in value in exchange for consecutive urine samples documenting abstinence from MA. Over the course of the 8-week program, including three weekly visits, participants could provide up to 24 methamphetamine-negative samples. Using a high-value (in the setting of South Africa), escalating schedule, the first urine sample negative for methamphetamine was worth 25 South African Rand (ZAR; ~ USD $1.88; 2017 exchange rates used). Consecutive negative samples increased in value by ZAR 12.50 (~USD $0.94). For every three consecutive negative samples, a ZAR 100 (~USD $7.53) bonus was provided. Thus, the total possible earnings for providing 24 methamphetamine-negative urine samples was ZAR 4,850 (~ USD $366). To maintain motivation, a ‘rapid reset’ rule was employed, such that participants with a methamphetamine-positive or missing urine sample (on the third consecutive methamphetamine-negative urine test) returned to their prior place in the escalating schedule.
2.3. Measures
The primary outcomes for this analysis were retention rates and methamphetamine abstinence. Retention was defined as the percentage of participants enrolled that completed the 8-week CM program. Methamphetamine abstinence: urine samples were analyzed qualitatively for methamphetamine or amphetamine (500 ng/mL threshold indicating use of methamphetamine or amphetamine in the 72 hours before testing) via immunoassay-based dip cards (CLIAwaived, Inc., San Diego, CA, USA). For this study, responders to the CM-program were defined as those providing 23 of a possible 24 methamphetamine-negative urine samples across the 8-week CM program all others were defined as nonresponders. Furthermore, at the baseline screening visit, participants completed questionnaires providing sociodemographic information including age, income, years of schooling, race/ethnicity, alcohol, and other drug use history. Height and weight collected during the clinical appointment at baseline were used to calculate Body Mass Index (BMI). Drug and alcohol use in the prior 30 days was collected using the Addiction Severity Index (ASI). In addition, a skilled practitioner conducted the Structured Clinical Interview for DSM-5 (SCID) interview (to confirm diagnosis of MUD), the Revised Hamilton Rating Scale for Depression (RHRSD) (17), the Fagerström Test for Nicotine Dependence (FTND) (18) and the Wechsler Abbreviated Scale of Intelligence (WASI) (19).
Data analysis
Results were summarized using descriptive statistics, including means, medians, and percentages. For this study, responders to the CM-program were defined as those providing 23 of a possible 24 methamphetamine-negative urine samples across the 8-week CM program. We compared characteristics of responders to the CM-program with nonresponders using chi-square or Fisher’s exact test (for categorical variables) and t-tests/Kruskal-Wallis χ2 test (for continuous variables).
3. Results
Of the 33 individuals who completed an enrollment visit, 3 were excluded, for the following reasons: meningitis or brain abnormality (n = 2), previous history of cocaine dependence (n = 1). Of the 30 remaining individuals, who were included in the analysis, 2 dropped out of the study — a 93% treatment-completion rate. At baseline, median age was 34 years [standard deviation (SD) = 6.1], median number of days of methamphetamine use in the month prior to enrollment was 21, median years of schooling was 11, and median WASI-IQ score was 86 (Table 1). Seventy percent (n = 21) were males, 60% (n = 18) identified English as their primary language, 67% (n = 20) had a monthly household income less than ZAR 25,000 (~ USD $1,880), and 37% (n = 11) were classified as having moderate to high nicotine dependence (score ≥5 on the Fagerström Test for Nicotine Dependence).
Table 1.
Participant characteristics stratified by response to CM for methamphetamine addiction
| Overall | Responders | Non-responders | p-value | ||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Total | 30 | 100 | 19 | 67.9 | 11 | 32.1 | |
| Sex | |||||||
| Male | 21 | 70.0 | 12 | 63.2 | 9 | 81.8 | 0.2825 |
| Female | 9 | 30.0 | 7 | 36.8 | 2 | 18.2 | |
| Monthly household income | |||||||
| ZAR5,001–R25,000 | 20 | 66.7 | 16 | 84.2 | 4 | 36.4 | 0.0074 |
| ZAR25,000+ | 10 | 33.3 | 3 | 15.8 | 7 | 63.6 | |
| Primary language | |||||||
| NOT-English | 12 | 40.0 | 9 | 47.4 | 3 | 27.3 | 0.2789 |
| English | 18 | 60.0 | 10 | 52.6 | 8 | 72.7 | |
| Antisocial Personality Disorder | |||||||
| No | 25 | 83.3 | 16 | 84.2 | 9 | 81.8 | 0.8655 |
| Yes | 5 | 16.7 | 3 | 15.8 | 2 | 18.2 | |
| Other substance use | |||||||
| No | 13 | 43.3 | 9 | 47.4 | 4 | 36.4 | 0.5578 |
| Yes | 17 | 56.7 | 10 | 52.6 | 7 | 63.6 | |
| Fagerström Test for Nicotine Dependence | |||||||
| < 5 | 19 | 63.3 | 11 | 57.9 | 8 | 72.7 | 0.4166 |
| ≥ 5 | 11 | 36.7 | 8 | 42.1 | 3 | 27.3 | |
| Median | Q1, Q3 | Median | Q1, Q3 | Median | Q1, Q3 | ||
| Age | 34 | 30, 40 | 33 | 28, 40 | 38 | 31, 41 | 0.3315 |
| No of years of MA use | 12 | 10, 15 | 12 | 8, 14 | 12 | 10, 15 | 0.5017 |
| Days used MA in previous 30 days | 21 | 15, 26 | 21 | 20, 25 | 20 | 7, 28 | 0.5157 |
| Cigarettes smoked in previous 30 days | 5 | 4, 10 | 6 | 4, 10 | 5 | 3, 10 | 0.7945 |
| Education | 11 | 9, 12 | 12 | 10, 14 | 10 | 8, 11 | 0.0390 |
| BMI | 22 | 19, 26 | 20 | 19, 22 | 24 | 22, 28 | 0.0070 |
| WASI-IQ | 86 | 73, 94 | 87 | 73, 101 | 85 | 70, 89 | 0.3544 |
| RHRSD | 22 | 7, 42 | 20 | 10, 42 | 24 | 7, 47 | 0.9485 |
| Number of psychotic symptoms | 3 | 2, 6 | 3 | 1, 6 | 4 | 2, 8 | 0.4227 |
Note - BMI =Body Mass Index; WASI-IQ= Wechsler Abbreviated Scale of Intelligence – Intelligence Quotient; RHRSD= Revised Hamilton Rating Scale for Depression
Across the 8-week CM program, 30 participants provided 704 urine samples; of these 579 (82%) were methamphetamine-negative (range across visits: 76% to 88%; Figure 1). Participants earned vouchers worth an average ZAR 3,375 (~USD 253), representing 70% of the total possible earnings in the program. Sixty-eight percent of the sample (n = 19) provided ≥23 methamphetamine-negative urine samples over the 8-week CM program and were classified as responders. Furthermore, 61% of the sample (n = 17), remained abstinent from methamphetamine throughout the 8 weeks. Median number of methamphetamine-negative urine samples for non-responders was 10 [interquartile range (IQR) = 6, 15]. Of the 248 urine samples provided by the nonresponders, 50% (n = 125) were methamphetamine-negative, and the percent of methamphetamine-negative samples across the visit ranged from 29% to 72% (Figure 1). Nonresponders earned vouchers worth a median of ZAR 987.50 (~USD 73) per month (range: ZAR 175 to ZAR 2, 425; ~USD 13.16 to ~USD 182.46) across the 8-week CM program compared to ZAR 4, 850 among responders. In bivariable analysis, income, body mass index (BMI) and education differed significantly between nonresponders and responders. Specifically, significantly fewer responders reported ZAR 25, 000+ of monthly household baseline income compared to nonresponders (15.8% vs. 66.7%; p = 0.007), median BMI was significantly lower in responders than in nonresponders (19 vs 24; Kruskal-Wallis χ2 = 6.84, DF=1, p=0.008), and median number of years of education was significantly higher in responders compared to nonresponders (12 vs 10; Kruskal-Wallis χ2 = 4.25, DF=1, p=0.039).
Figure 1:
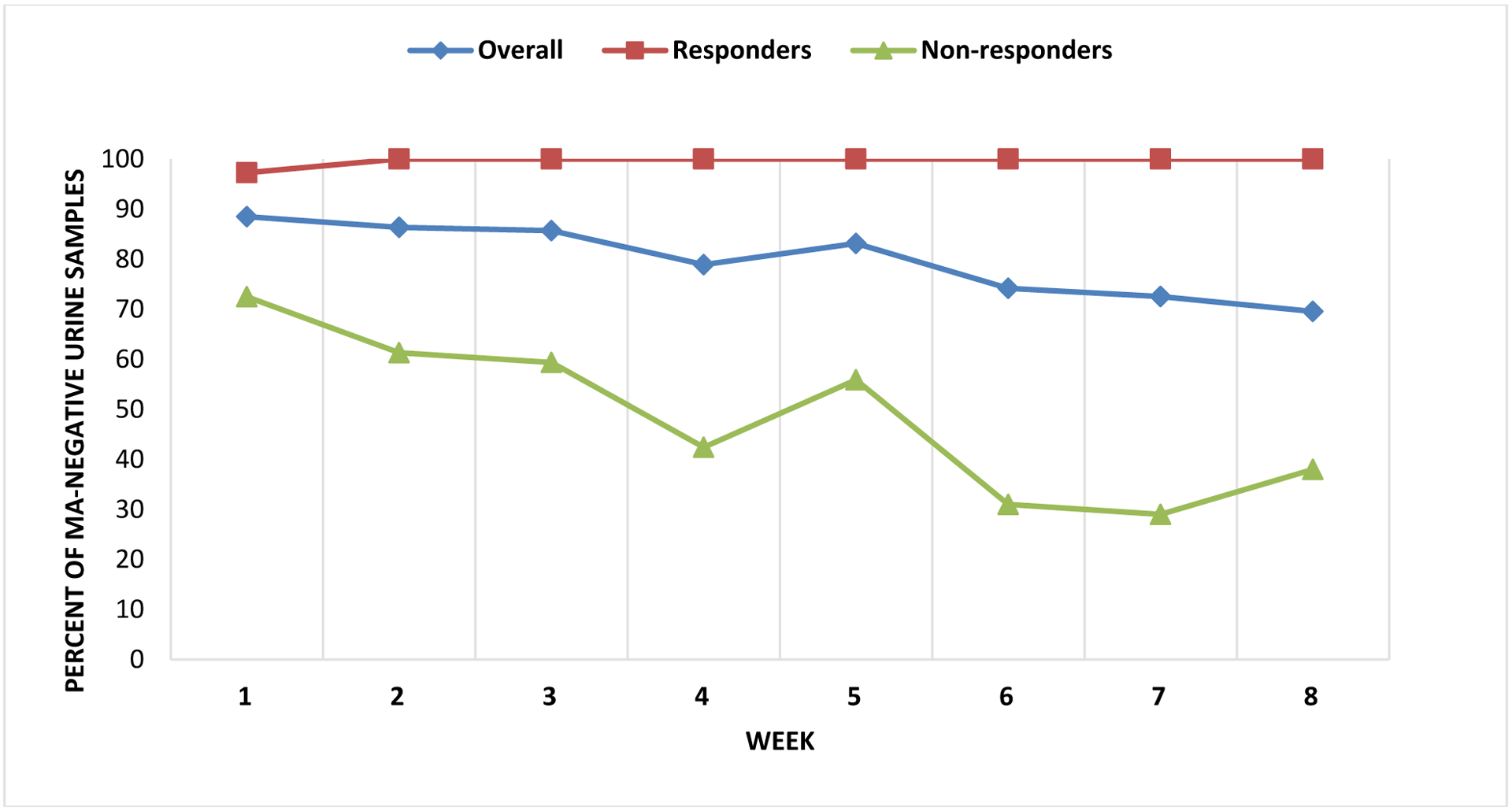
Methamphetamine-negative urine samples over the 8-week CM program
4. Discussion
This study shows that in an 8-week CM program for MA dependence, 93% of participants completed the 8-week CM program and over two-thirds of the sample were responders, maintaining continuous abstinence from MA throughout the program. Studies of CM for methamphetamine in North America have found between 18% to 60% continuous methamphetamine abstinence following CM programs of varied duration (20–22). As such, the response rates observed were higher than expected, and only 2 participants dropped out of the study without completing the full 24 sessions (i.e., 93% treatment retention rate). The Matrix Model, a 16-week program, consisting of 8 sessions of early recovery, 32 sessions of relapse prevention and other optional weekly individual or conjoint sessions was recently implemented in South Africa. In that study, of 986 who initiated treatment, only 45% attended at least 4 sessions, with 13% completing the full 16-week program. Furthermore, of the 57% of those who had a urine toxicology screen at treatment exit, just over half had a negative result for all substances. These findings suggest that a voucher-based reinforcement therapy CM program may be a useful component of treatment approaches to increase treatment retention and abstinence rates and addressing the methamphetamine problem in South Africa.
That individuals with lower baseline monthly household income were more likely to respond to the CM program compared to those with higher income is inconsistent with observations in the majority of studies in North America and Europe, where income did not affect the efficacy of CM for substance use disorders (23–25). We speculate that for some participants, the high-value CM program in this study provided some stability in their socioeconomic status that promoted abstinence from methamphetamine. The CM schedule was targeted at a total possible payout of approximately 10% of monthly household income in Cape Town, a guide used in our high-value CM schedules in the U.S (19,23). Findings showed that the Cape Town and U.S. schedules approximated each other, though both retention and maximum earned were substantially greater in the application of CM in Cape Town. It is encouraging that this level of success was observed and is consistent with findings from conditional incentive programs in low- to moderate-income countries, showing that earnings from the programs enhance healthy behaviors for the index participants (and their families) over and above improvement for the specific target behavior – in this case, methamphetamine addiction (26)
Responders had significantly lower median BMI than nonresponders, and BMI was significantly positively correlated with income, such that individuals with lower baseline monthly household income (i.e., ZAR <25,000) had lower BMI than those with higher income (ZAR ≥25,000). As associations of CM success with income and BMI are not separable in this study, it is possible that both reflect socioeconomic links in response to the high-value CM program in this study. We caution that although these findings suggest potential differences in CM for MUD in this setting, the small sample size limits the interpretability of these results.
Our finding that responders had significantly more years of education compared to nonresponders merits discussion. This finding is inconsistent with the current finding of a positive association between lower-income and responding to the CM program. It is possible that the positive association between level of education and income, observed in many North American and European studies (27,28), is not applicable to the current setting in South Africa. Indeed, in additional analysis (results not shown), we did not find a significant association between education and income in this sample. Moreover, additional investigations on the association between education and response to CM is warranted, given that prior studies have found that persons who use substances including smoking (24) and cocaine addiction (29) respond equally well to CM regardless of educational level.
Our study had some limitations. Importantly, the absence of a control group that did not receive CM limits our ability to claim efficacy of CM for MUD. The sample size was small, which precluded additional analysis on differences between the responders and nonresponders to CM. This was a secondary analysis of data from a pilot trial assessing the neural correlates of methamphetamine-abstinence in adults with MUD. Accordingly, some participants were excluded as a result of ineligibility based on the parent study inclusion/exclusion criteria, limiting the generalizability of the findings reported here. Particularly, individuals with a use disorder involving a substance other than methamphetamine were excluded, which may have bearing on the high response rates, as individuals with single substance use disorders may be easier to treat than those with polysubstance use disorders. Additionally, because this sample included treatment-seeking participants, it is possible that some participants sought out and engaged in unmeasured behavioral support at local agencies. Finally, our study relied on self-reported data on some of the demographic data including income – recorded as monthly household income – and years of education which may be prone to reporting biases.
5. Conclusion
As one of the first investigations of CM for methamphetamine use disorder in a treatment-seeking sample of adults in Cape Town, South Africa, our findings provide initial evidence that CM is feasible, may increase treatment retention and potentially promote continuous methamphetamine abstinence in this setting. These findings suggest the need for further research, testing the efficacy of CM alone or in combination with other psychosocial or pharmacological treatment. Additionally, further research will benefit from involving larger samples drawn from a range of socio-economic backgrounds, longer treatment durations, and different reinforcement schedules, to address the problem of methamphetamine addiction in Low- and Middle-Income Countries, such as South Africa.
Acknowledgments
We thank the individuals who participated in this study. Research reported in this publication was supported by the National Institute on Drug Abuse (R21DA040492, PI: Shoptaw). SS is supported by NIMH P30 058107 - CHIPTS UCLA CFAR grant AI028697. CNO is supported by the UCLA Postdoctoral Fellowship Training Program in Global HIV Prevention Research (T32MH080634, PIs: Currier and Gorbach). TK is supported by an Institutional K-award at the University of Pittsburgh (NIH KL2 TR001856.) DJS is supported by the SAMRC. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interests
None of the authors have any conflict of interest to report
References
- 1.Dada S, Burnhams BH, Erasmus J, Parry C, Bhana A, Timol F, et al. South African Community Epidemiology Network on Drug Use (SACENDU): Monitoring alcohol, tobacco and other drug abuse treatment admissions in South Africa, March 2018 (Phase 42). 2018. [cited 2018 Nov 26]; Available from: http://www.samrc.ac.za/sites/default/files/attachments/2018-05-09/SACENDUFullReportPhase42.pdf
- 2.Wechsberg WM, Jones HE, Zule WA, Myers BJ, Browne FA, Kaufman MR, et al. Methamphetamine (“tik”) Use and Its Association with Condom Use among Out-of-School Females in Cape Town, South Africa. Am J Drug Alcohol Abuse. 2010. July 1;36(4):208–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meade CS, Watt MH, Sikkema KJ, Deng LX, Ranby KW, Skinner D, et al. Methamphetamine use is associated with childhood sexual abuse and HIV sexual risk behaviors among patrons of alcohol-serving venues in Cape Town, South Africa. Drug Alcohol Depend. 2012. November 1;126(1–2):232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gouse H, Joska JA, Lion RR, Watt MH, Burnhams W, Carrico AW, et al. HIV testing and sero-prevalence among methamphetamine users seeking substance abuse treatment in Cape Town. Drug Alcohol Rev. 2016;35(5):580–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akindipe T, Wilson D, Stein DJ. Psychiatric disorders in individuals with methamphetamine dependence: prevalence and risk factors. Metab Brain Dis. 2014. June;29(2):351–7. [DOI] [PubMed] [Google Scholar]
- 6.Jones HE, Myers B, O’Grady KE, Gebhardt S, Theron GB, Wechsberg WM. Initial feasibility and acceptability of a comprehensive intervention for methamphetamine-using pregnant women in South Africa. Psychiatry J. 2014;2014:929767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sorsdahl K, Stein DJ, Corrigall J, Cuijpers P, Smits N, Naledi T, et al. The efficacy of a blended motivational interviewing and problem solving therapy intervention to reduce substance use among patients presenting for emergency services in South Africa: A randomized controlled trial. Subst Abuse Treat Prev Policy [Internet]. 2015. November 14 [cited 2018 May 24];10 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4650345/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sorsdahl K, Myers B, Ward CL, Matzopoulos R, Mtukushe B, Nicol A, et al. Adapting a blended motivational interviewing and problem-solving intervention to address risky substance use amongst South Africans. Psychother Res J Soc Psychother Res. 2015;25(4):435–44. [DOI] [PubMed] [Google Scholar]
- 9.Magidson JF, Gouse H, Burnhams W, Wu CYY, Myers B, Joska JA, et al. Beyond methamphetamine: Documenting the implementation of the Matrix model of substance use treatment for opioid users in a South African setting. Addict Behav. 2017. March 1;66:132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rawson RA, Shoptaw SJ, Obert JL, McCann MJ, Hasson AL, Marinelli-Casey PJ, et al. An intensive outpatient approach for cocaine abuse treatment. The Matrix model. J Subst Abuse Treat 1995. April;12(2):117–27. [DOI] [PubMed] [Google Scholar]
- 11.Gouse H, Magidson JF, Burnhams W, Remmert JE, Myers B, Joska JA, et al. Implementation of Cognitive-Behavioral Substance Abuse Treatment in Sub-Saharan Africa: Treatment Engagement and Abstinence at Treatment Exit. PLOS ONE. 2016. January 27;11(1):e0147900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: a meta-analysis. Addict Abingdon Engl. 2006. November;101(11):1546–60. [DOI] [PubMed] [Google Scholar]
- 13.Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addict Abingdon Engl. 2006. February;101(2):192–203. [DOI] [PubMed] [Google Scholar]
- 14.Benishek LA, Dugosh KL, Kirby KC, Matejkowski J, Clements NT, Seymour BL, et al. Prize-based Contingency Management for the Treatment of Substance Abusers: A Meta-analysis. Addict Abingdon Engl. 2014. September;109(9):1426–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rawson RA, McCann MJ, Flammino F, Shoptaw S, Miotto K, Reiber C, et al. A comparison of contingency management and cognitive-behavioral approaches for stimulant-dependent individuals. Addiction. 2006;101(2):267–74. [DOI] [PubMed] [Google Scholar]
- 16.Myers BJ, Louw J, Pasche SC. Inequitable access to substance abuse treatment services in Cape Town, South Africa. Subst Abuse Treat Prev Policy. 2010. November 15;5:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riskind JH, Beck AT, Brown G, Steer RA. Taking the measure of anxiety and depression. Validity of the reconstructed Hamilton scales. J Nerv Ment Dis. 1987. August;175(8):474–9. [DOI] [PubMed] [Google Scholar]
- 18.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991. September;86(9):1119–27. [DOI] [PubMed] [Google Scholar]
- 19.Wechsler Adult Intelligence Scale® - Third Edition [Internet]. [cited 2016 Jun 10]. Available from: http://www.pearsonclinical.com/psychology/products/100000243/wechsler-adult-intelligence-scale--third-edition-wais-iii.html [Google Scholar]
- 20.Chudzynski J, Roll JM, McPherson S, Cameron JM, Howell DN. Reinforcement Schedule Effects on Long-Term Behavior Change. Psychol Rec. 2015. June 1;65(2):347–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roll JM, Petry NM, Stitzer ML, Brecht ML, Peirce JM, McCann MJ, et al. Contingency management for the treatment of methamphetamine use disorders. Am J Psychiatry. 2006. November;163(11):1993–9. [DOI] [PubMed] [Google Scholar]
- 22.Shoptaw S, Huber A, Peck J, Yang X, Liu J, Jeff Dang, et al. Randomized, placebo-controlled trial of sertraline and contingency management for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2006. October 15;85(1):12–8. [DOI] [PubMed] [Google Scholar]
- 23.Rash CJ, Olmstead TA, Petry NM. Income Does Not Affect Response to Contingency Management Treatments Among Community Substance Abuse Treatment-seekers. Drug Alcohol Depend. 2009. October 1;104(3):249–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.López-Núñez C, Secades-Villa R, Peña-Suárez E, Fernández-Artamendi S, Weidberg S. Income Levels and Response to Contingency Management for Smoking Cessation. Subst Use Misuse. 2017. June 7;52(7):875–83. [DOI] [PubMed] [Google Scholar]
- 25.Secades-Villa R, García-Fernández G, Peña-Suárez E, García-Rodríguez O, Sánchez-Hervás E, Fernández-Hermida JR. Contingency management is effective across cocaine-dependent outpatients with different socioeconomic status. J Subst Abuse Treat. 2013. March 1;44(3):349–54. [DOI] [PubMed] [Google Scholar]
- 26.Baird SJ, Garfein RS, McIntosh CT, Özler B. Effect of a cash transfer programme for schooling on prevalence of HIV and herpes simplex type 2 in Malawi: a cluster randomised trial. The Lancet. 2012. April 7;379(9823):1320–9. [DOI] [PubMed] [Google Scholar]
- 27.Blanden J, Gregg P. Family Income and Educational Attainment: A Review of Approaches and Evidence for Britain. Oxf Rev Econ Policy. 2004. June 1;20(2):245–63. [Google Scholar]
- 28.Lynch SM. Explaining Life Course and Cohort Variation in the Relationship between Education and Health: The Role of Income. J Health Soc Behav. 2006. December 1;47(4):324–38. [DOI] [PubMed] [Google Scholar]
- 29.García-Fernández G, Secades-Villa R, García-Rodríguez O, Alvarez-López H, Sánchez-Hervás E, Fernández-Hermida JR, et al. Individual characteristics and response to Contingency Management treatment for cocaine addiction. Psicothema. 2011. February;23(1):114–8. [PubMed] [Google Scholar]


