Abstract
Antigen-specific memory B cell (MBC) populations mediate the rapid, strong, and high affinity secondary antibody responses that play a key role in combating infection and generating protective responses to vaccination. Recently, cell staining with fluorochrome-labeled antigens together with sequencing methods such as Drop-seq and CITE-seq have provided information on the specificity, phenotype, and transcriptome of single MBCs. However, characterization of MBCs at the level of antigen-reactive populations remains an important tool for assessing an individual’s B cell immunity and responses to antigen exposure. This is readily performed using a long-established method based on in vitro polyclonal stimulation of MBCs to induce division and differentiation into antibody-secreting cells (ASCs). Post-stimulation antigen-specific measurement of the MBC-derived ASCs (or the secreted antibodies) indicates the size of precursor MBC populations. Additional information about the character of antigen-reactive MBC populations is provided by analysis of MBC-derived antibodies of particular specificities for binding avidity and functionality. This article outlines a simple and reliable strategy for efficient in vitro MBC stimulation and use of the ELISpot assay as a post-stimulation readout to determine the size of antigen-specific MBC populations. Other applications of the in vitro stimulation technique for MBC analysis are discussed. The following protocols are included.
Basic Protocol 1: Polyclonal stimulation of memory B cells using unfractionated PBMCs
Alternate Protocol 1: Stimulation of small PBMC numbers using 96-well plates with U-bottomed wells
Basic Protocol 2: ELISpot essay for enumeration of memory B cell-derived antibody-secreting cells
Keywords: Human memory B cells, in vitro stimulation, R848, polyclonal antibodies, ELISpot assay
INTRODUCTION
B cell memory generated by antigen exposure consists of antibodies (Abs) and memory B cells (MBCs). Typically, MBCs are the isotype-switched products of germinal centers, where they have undergone somatic hypermutation and selection for high affinity B cell receptor recognition of the activating antigen. Populations of antigen-reactive MBCs are the basis for the rapid, vigorous, and high affinity secondary Ab response on antigen re-exposure. The nature of the secondary Ab response reflects not only the number of antigen-reactive MBCs, but also cell-intrinsic characteristics of MBCs that reduce their dependence on T cell-associated signals for activation. As a result, MBCs are more readily activated to proliferate and differentiate into Ab-secreting cells (ASCs) than are naïve B cells. Features of MBCs that facilitate generation of activating signals include expression of the IgG B cell receptor and constitutive, high level expression of toll-like receptors (TLRs) (Cyster & Allen, 2019; Good-Jacobson, 2018; Inoue et al., 2018; Sangster et al., 2019).
Enumeration of antigen-specific MBCs provides important information on an individual’s preexisting B cell memory and capacity to generate strong Ab responses to particular antigens, and also on the effectiveness of immunization strategies in generating and expanding B cell memory (Topham et al., 2018). In addition, MBC measurements provide insights into mechanisms underlying suboptimal or pathological patterns of Ab production (Cobey & Hensley, 2017; Karrar & Cunninghame Graham, 2018; Saunders et al., 2019). Importantly, antigen-specific MBC analysis can reveal MBCs capable of generating protective Ab responses, even in cases where circulating protective Abs cannot be detected. For example, when the pandemic H1N1 (pH1N1) influenza virus emerged in 2009, the H1 hemagglutinin (HA) was highly novel to young adults, and most lacked Abs that bound the H1 head domain and neutralized virus infectivity. However, almost all young adults carried MBCs reactive to the pH1 head, presumably generated by seasonal H1N1 viruses, that were the basis for strong virus-neutralizing Ab production after pH1N1 infection or a single dose of the pandemic vaccine (Andrews et al., 2015; Li et al., 2012; Sangster et al., 2013; Wrammert et al., 2011).
A number of approaches have been used to enumerate antigen-specific MBCs and to investigate their characteristics and potential to generate protective Ab responses. Fluorochrome-labeled antigens have been used in flow cytometry panels to identify and sort antigen-specific MBCs for cloning and characterization of expressed immunoglobulins (Andrews et al., 2019). More recently, fluorochrome-labeled antigens (potentially carrying barcoding oligonucleotide tags) have been combined with methods such as Drop-seq and CITE-seq for characterizing the specificity, phenotype, and transcriptome of single MBCs (Macosko et al., 2015; Stoeckius et al., 2017). Characterization of MBCs at the level of antigen-reactive polyclonal populations is performed simply and effectively by in vitro stimulation of mixed cell populations, typically peripheral blood mononuclear (PBMCs) for human MBC analysis, to induce polyclonal MBC activation, proliferation, and differentiation into ASCs. After stimulation, total and antigen-specific MBC-derived ASCs or secreted Abs are quantified by ELISpot assay or ELISA, respectively, to provide a measure of the size of antigen-specific precursor MBC populations (Amanna & Slifka, 2006; Crotty et al., 2004; Halliley et al., 2015; Muir et al., 2018; Pinna et al., 2009; Tesini et al., 2019). Importantly, this technique also provides the basis for additional assays for MBC analysis. For example, polyclonal Abs derived from in vitro-stimulated MBCs can be analyzed by multiplexed binding assays to comprehensively probe the breadth of MBC reactivity, by virus neutralization assays to assess Ab functionality, and by techniques that measure Ab avidity to monitor affinity maturation of MBCs (Tesini et al., 2019).
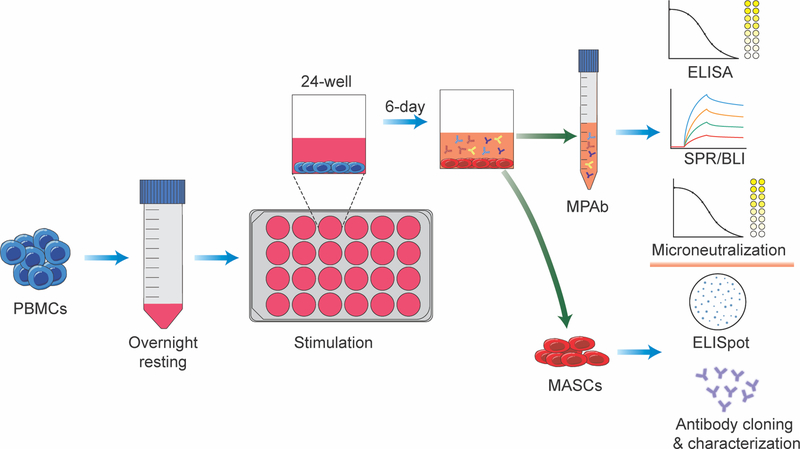
In this article, we describe a method for quantifying antigen-reactive MBC populations in humans based on in vitro polyclonal MBC stimulation with a simple and efficient activating cocktail. Basic Protocol 1 describes polyclonal stimulation of MBCs in unfractionated human PBMCs and Basic Protocol 2 describes application of an ELISpot essay to enumerate ASCs derived from stimulated MBCs. Steps covered include the thawing of cryopreserved PBMCs to maximize recovery of viable cells, choice of plate type and cell seeding strategy to optimize yields of ASCs and secreted Abs from stimulated MBCs, selection of an appropriate stimulation cocktail, application of assays for measurement of MBC-derived ASCs and polyclonal Abs, and use of results to quantify antigen-reactive MBC populations. We also describe how the technique serves as a foundation for additional MBC analyses at the individual and population level. An overview is shown in Figure 1.
Figure 1.
Overview of in vitro stimulation of MBCs in a sample of unfractionated PBMCs. MBC-derived Ab-secreting cells (MASCs) and MBC-derived polyclonal Abs (MPAbs) collected post-stimulation can be used in the indicated ways to characterize antigen-specific MBC populations.
BASIC PROTOCOL 1
POLYCLONAL STIMULATION OF MEMORY B CELLS USING UNFRACTIONATED PBMCs
A large range of culture conditions have been evaluated for effectiveness in polyclonally activating human MBCs and supporting their division and differentiation into ASCs. In 2004, Crotty et al. (Crotty et al., 2004) described a highly effective activation cocktail containing pokeweed mitogen (PWM), Staphylococcus aureus Cowan strain (SAC), and CpG oligodeoxynucleotide (a TLR9 agonist) that is still widely used. However, standardization of stimulation cocktails is an important consideration. For instance, different PWM preparations can vary in effectiveness as B cell stimulants (Bekeredjian-Ding et al., 2012; Yang et al., 2006). Over about the last 10 years, many additional studies have identified simple, standard cocktails for efficient and reliable human MBC activation. In this protocol, we describe use of a combination of the TLR7/8 agonist R848 (Resiquimod) and cytokines IL-2 and IL-10 for highly efficient polyclonal stimulation of human MBCs, but not naïve B cells, to form IgG ASCs. Stored PBMCs are stimulated as unfractionated populations containing approximately 5–20% B cells. Stimulation cultures are incubated to allow peak numbers of MBC-derived IgG ASCs to form. Post-stimulation IgG ASCs are then enumerated by ELISpot assay (see Basic Protocol 2) to provide a measure of the size of precursor antigen-specific IgG MBC populations.
CAUTION: Human samples can be a source of pathogens and appropriate biosafety practices must be followed.
NOTE: Proper sterile techniques and sterile solutions must be used for cell culture procedures.
Materials
PBMCs, gradient-purified from heparinized human blood and cryopreserved. Typically, 1 vial containing approximately 107 cryopreserved PBMCs is used for a stimulation assay (see Anticipated Results). Blood samples are collected through approved study protocols and after informed donor consent.
R10 medium (see recipe)
15-ml conical centrifuge tubes (e.g. BD Falcon, cat. no. 1495953A)
50-ml conical centrifuge tubes (e.g. BD Falcon, cat. no. 14–432-22)
24-well tissue culture plates (e.g. Corning, cat. no. 3526)
Beckman benchtop centrifuge with SX4750 rotor (or equivalent)
Trypan Blue solution, cell counter, and microscope for counting viable cells
2× R848/IL-2/IL-10 stimulation cocktail (see reagents and solutions)
PBMC thawing and resting (day −1 relative to plating cells for in vitro stimulation)
-
1Quickly thaw frozen vials of PBMCs in a 37°C water bath for 90–120 seconds until only a small fragment of ice remains in each vial. Transfer the contents of vials to 15-ml tubes, with a maximum of 3 vials of a sample in one 15-ml tube.PBMCs are routinely cryopreserved in 1 ml aliquots at 5–15×106 PBMC/ml for storage in liquid nitrogen. Vials of PBMCs should be thawed as soon as possible after removal from liquid nitrogen; hold vials on dry ice until they can be thawed.Use of a cryovial floating rack during thawing and carefully spraying down cryovials with 70% EtOH before transfer to the BSL2 cabinet for cell collection helps to minimize contamination.Cell recovery after centrifugation is generally reduced when 50-ml tubes are used instead of 15-ml tubes.
-
2
Rinse cryovials with 1 ml of R10 (pre-warmed to 37°C in a water bath) to collect remaining cells and add to other cells in the sample.
-
3
Slowly run R10 down the wall of the tube to bring the volume to 14 ml. Cap the tube and invert 2–3 times to mix. Centrifuge for 10 min at 300 × g, 23–25°C.
-
4Quickly pour out the supernatant. Resuspend the pellet by pipetting up and down with 1 ml of R10.The cell pellet will loosen over time and it recommended that the supernatant be removed within 2 min following centrifugation. If tubes sit for more than 2 min, remove the supernatant by aspiration instead of pouring, taking care to avoid disturbing cell pellets.
-
5Add an appropriate volume of additional R10 and mix for counting.Cells should be resuspended in an appropriate R10 volume for convenient and accurate counting after diluting 1:1 with trypan blue (i.e., resuspend at an estimated cell concentration of 1–3×106 PBMC/ml).
-
6
Count viable cells by trypan blue exclusion (see Current Protocols article: Phelan & May, 2015).
-
7Centrifuge for 10 min at 300 × g, 23–25°C.The centrifugation steps are important to reduce the concentration of DMSO that was present in the freezing medium.
-
8Resuspend cell pellets in R10 and transfer to 50-ml tubes (up to 15×106 PBMC/tube) for resting overnight. Rinse the tubes with R10 medium to collect residual cells and transfer to the 50-ml tubes.It is important that PBMCs are rested in R10 medium. Overnight resting in serum-free RPMI could increase cell loss.For overnight resting, 50-ml tubes should optimally contain 5–7 ml of PBMCs at 2–3×106 cells/ml.
-
9
Incubate at 37°C and 5% CO2 overnight in 50-ml tubes with caps loosened.
PBMC stimulation (day 0)
-
10Resuspend rested cells and transfer to 15-ml tubes.PBMC samples that were rested in more than one 50-ml tube because of high cell numbers can be pooled in a single 15-ml tube at this stage.
-
11
Rinse the 50-ml tubes with 2–5 ml of R10 to collect residual cells and add to cells in 15-ml tubes.
-
12
Centrifuge for 10 min at 300 × g, 23–25°C.
-
13
Repeat steps 4–6 to count viable PBMCs.
-
14
During this time, freshly prepare a sufficient volume (~500 μl per 1 million cells) of the 2× R848/IL-2/IL-10 stimulation cocktail (with 2-mercaptoethanol) in R10 (see recipe).
-
15Add R10 (at 37°C) to adjust concentrations of counted PBMCs to 2×106 cells/ml.If necessary, transfer PBMCs to a 50-ml tube to accommodate a larger volume of cell suspension.
-
16Add equal volumes of the 2× stimulation cocktail to the PBMC suspensions (the final concentration is 1×106 cells/ml) and add 1 ml per well to 24-well plates.Unstimulated control PBMC samples are prepared with R10 supplemented with 2-mercaptoethanol instead of with the complete stimulation cocktail.
-
17Incubate at 37°C and 5% CO2 for 6 days.Isolated small clusters of cells are apparent from day 3 onwards. These become a little larger and more numerous over the incubation period, but do not coalesce. The composition of the clusters has not been characterized, but they are likely to include proliferating and differentiating MBCs. Outside of the cell clusters, individual cells maintain a loose monolayer that changes little during incubation. The cell number at the end of the incubation period ranges from 1.0–1.5× the input cell number and reflects cell death and proliferation.
ALTERNATE PROTOCOL 1
STIMULATION OF SMALL PBMC NUMBERS USING 96-WELL PLATES WITH U-BOTTOMED WELLS
Occasionally, the number of PBMCs available for analysis is small (<106 cells), especially when working with samples from infants and children. In this situation, efficient MBC stimulation is achieved by a modification of Basic Protocol 1 in which a smaller number of PBMCs is cultured in 96-well plates with U-bottomed wells. Complete recovery of all cells post-stimulation for analysis by ELISpot assay is facilitated by the shape of the wells. Basic Protocol 1 and Alternate Protocol 1 produce similar post-stimulation yields of IgG ASCs and secreted IgG.
Materials
PBMCs, gradient-purified from heparinized human blood and cryopreserved
R10 medium (see recipe)
15-ml conical centrifuge tubes (e.g. BD Falcon, cat. no. 1495953A)
50-ml conical centrifuge tubes (e.g. BD Falcon, cat. no. 14–432-22)
96-well plate with U-bottomed wells (Corning, cat no. 3799)
Beckman benchtop centrifuge with SX4750 rotor (or equivalent)
Trypan Blue solution, cell counter, and microscope for counting viable cells
2× R848/IL-2/IL-10 stimulation cocktail (see reagents and solutions)
PBS (Corning, cat. no. 21040CV)
Perform Basic Protocol 1, steps 1–14.
Add R10 to adjust PBMC concentrations to 1×106 cells/ml.
- Add equal volumes of 2× R848/IL-2/IL-10 stimulation cocktail to the PBMC suspensions (such that the final concentration is 0.5×106 cells/ml) and plate 0.2 ml per well.Do not use wells around the edge of the plate because of the loss of well volume through evaporation. To reduce this “edge effect”, add 0.2 ml PBS to wells surrounding those seeded with cells.
Incubate at 37°C and 5% CO2 for 6 days.
BASIC PROTOCOL 2
ELISPOT ASSAY FOR ENUMERATION OF MEMORY B CELL-DERIVED ANTIBODY-SECRETING CELLS
After in vitro MBC stimulation, analysis of MBC-derived IgG ASC formation provides a measure of the number of precursor IgG MBCs. This protocol describes analysis of the post-stimulation cell population by ELISpot essay to determine the number of total IgG ASCs and the number of IgG ASCs specific for antigens of interest. Enumeration of total as well as antigen-specific ASCs enables antigen-specific IgG MBCs to be represented as a percentage of total IgG MBCs, an approach that assumes equivalent proliferation and differentiation of all IgG MBCs. The count of MBC-derived ASCs (MASCs) of particular specificities can be simply shown per 106 PBMCs as a measure of the size of precursor MBC populations. Designation of these cells as “MASCs” rather than “ASCs” can help avoid confusion, since they are not preexisting circulating ASCs and, because of MBC proliferation in the stimulation cultures, do not relate one-to-one with MBCs. Antigen-specific concentrations of MBC-derived secreted IgG in stimulation culture supernatants correlate directly with IgG ASC counts and can also be used as a measure of the size of precursor MBC populations.
Materials
Coating antigens, for example, H1 hemagglutinin from influenza A virus A/California/07/2009 (H1N1)pdm09 (BEI Resources, NIAID, NIH, cat. no. NR-51668)
(Optional): use other coating antigens as needed
Goat anti-human IgG (Invitrogen, cat. no. 62–8400)
PBS (Corning, cat. no. 21040CV)
R10 medium (see recipe)
PBS with 0.1% Tween-20 (PBS-T)
ELISpot buffer (see recipe)
Alkaline phosphatase AffiniPure goat anti-human IgG, Fcγ fragment specific, (Jackson ImmunoResearch Laboratories, cat. no. 109–055-008)
Vector® Blue Alkaline Phosphatase (AP) Substrate Kit (Vector Laboratories, cat. no. SK-5300)
100 mM Tris HCl buffer, pH 8.2
15-ml conical centrifuge tubes (e.g. BD Falcon, cat. no. 1495953A)
MultiScreenHTS 96-well filter plate, 0.45 μm pore size, PVDF membrane (Millipore, cat. no. MSIPN4W50)
10-ml syringe (BD, cat. no. 302995)
Syringe filter, cellulose acetate, 0.45 μm (VWR International, cat. no. 28145–481)
Plastic/glass bucket of sufficient capacity for submerging ELISpot plates
(Optional): Biotek ELISA plate washer
(Optional): Cellular Technologies CTL immunospot reader
Coating ELISpot plates (days 3–5)
-
1Dilute each coating reagent/antigen in sterile PBS to the empirically determined optimal coating concentration.The optimal coating concentration of reagents/antigens should be determined by titration. Generally, optimal spot formation requires coating with 5–20 μg/ml (0.25–1.0 μg/well). As an example, a frequently used antigen is the H1 hemagglutinin of the H1N1 influenza A virus A/California/07/2009. Most individuals have circulating MBCs reactive to this H1 because of exposure through seasonal influenza infection and vaccination. Based on titration of coating concentrations in an ELISpot assay, dilution of the H1 in PBS to 10 μg/ml (0.5 μg/well in 50 μl) optimizes the sensitivity of spot detection while conserving antigen.Note: The approach described above for coating with the influenza virus H1, a viral glycoprotein, is appropriate for proteins and glycoproteins. However, for detection of anti-polysaccharide Abs, a modified procedure is required to improve the efficiency of attachment of negatively charged polysaccharides to plates. An approach for coating ELISpot plates of the type used in Basic Protocol 2 with polysaccharide antigen is to add 100 μl/well of a mixture of the polysaccharide and methylated human serum albumen (positively charged), each at 5 μg/ml in PBS.
-
2
Wet plates with 150–200 μl of sterile PBS per well.
-
3Aspirate PBS and add 50 μl of each coating reagent to the appropriate wells. An example of a plate layout is shown in Figure 2.The assay should include at least one negative control well that receives PBS or an irrelevant antigen. BSA is frequently used as a negative control protein. A well that contained only PBS during the coating incubation is essentially coated with BSA during the blocking step (step 6) when R10 (which contains 10% fetal bovine serum) is incubated in wells.Take care to avoid damaging well membranes with the pipette tips.
-
4
Gently tap the plates to ensure that the bases of wells are completely covered by the coating solutions.
-
5Wrap plates in dampened paper towels covered by plastic wrap and store at 4°C for 1–3 days before use.Plates can be coated by incubating for 2 h at 37°C if this is necessary for a “same-day” ELISpot assay. However, coating overnight at 4°C generally reduces a diffuse blue background in ELISpot plates after color development.An appropriate plate sealer is an alternative to wrapping plates during the coating incubation.
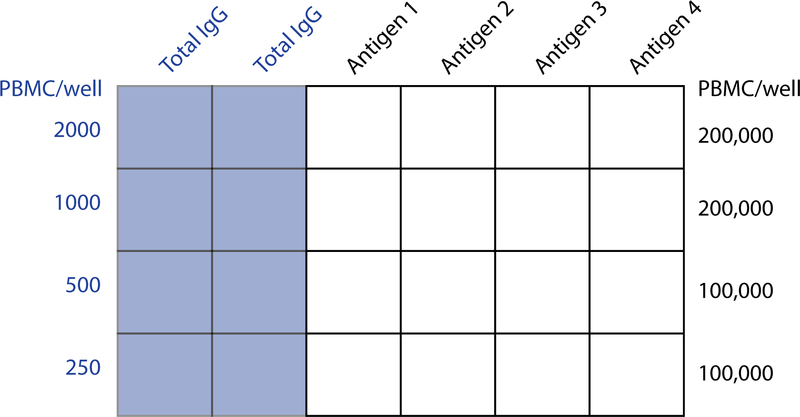
Figure 2.
Example of ELISpot plate layout for measurement of antigen-specific MBCs following in vitro polyclonal stimulation of human PBMCs. The layout can be modified according to the availability of cells and the number of antigens tested. The illustrated example allows 4 PBMC samples to be analyzed against 4 antigens on the same 96-well plate. The numbers of antigen-specific IgG ASCs generated by MBC stimulation is generally small and detection requires seeding wells with large numbers of cells. Much smaller numbers of cells need to be seeded for measurement of total IgG ASCs derived from MBCs. Antigen-specific MASCs as a percentage of total IgG MASCs can be calculated from the results. This value represents the size of the antigen-specific MBC population as a percentage of total IgG MBCs. Measurement of total IgG MASCs is also a valuable control for effectiveness of the in vitro MBC stimulation procedure. As a rough guide, total IgG MASCs ranging from 50,000 to >250,000 per million cells should be expected after stimulation of PBMC samples from adults. Note that the numbers of seeded cells in the ELISpot assay represent PBMC numbers prior to stimulation.
Collection of stimulated cells and transfer to ELISpot plates (day 6)
-
6
Aspirate coating solutions from ELISpot plates and block wells with 150 μl/well of R10. Incubate at 37°C and 5% CO2 for at least 2 h. Remove blocking solution immediately prior to addition of cells as subsequently described in step 13.
-
7Resuspend the stimulated PBMCs and pool the cells for each sample in one or more 15-ml tubes. Cells in this “first set” of 15-ml tubes will be pelleted as described below. In addition to collection of these cells for analysis by ELISpot assay, supernatants should also be collected and stored as samples of MBC-derived polyclonal Abs or MPAbs.It is recommended that multiple 15-ml tubes are used for large volume samples, since cell recovery after centrifugation is generally reduced when 50-ml tubes are used.To maximize recovery of stimulated cells, wells can be rinsed with 1 ml of R10 (at 37°C) and the rinses collected in a second set of 15-ml tubes (“rinse” tubes). However, it is important that these rinses are not added to the first set of 15-ml tubes before MPAb collection, since that would dilute the MPAb samples. Cells pelleted from the rinse tubes can be combined with cells in the first set of 15-ml tubes after MPAbs have been collected.After collection of stimulated cells, it is prudent to examine wells under a microscope to establish that cell collection was sufficiently complete.
-
8
Centrifuge the first set of 15-ml tubes for 10 min at 350 x g, 23–25°C, to pellet cells. Collect the supernatants (MPAb samples) and store in cryotubes at −80°C.
-
9Resuspend the pellet in each tube with 1 ml of R10 at 37°C.Cells from the same sample that were processed in multiple tubes because of a large culture volume can be combined in a single 15-ml tube at this stage.Resuspended cells pelleted from rinse tubes can be combined with other cells from the same sample at this stage.
-
10
Bring the volume in tubes to 14 ml with R10. Centrifuge for 10 min at 350 x g, 23–25 °C, to pellet cells.
-
11Pour off supernatants and repeat the wash (steps 9 and 10). Pour off supernatants in preparation for cell resuspension.Multiple washes are important to minimize background in the ELISpot assay.
-
12(Optional) A cell counting step can be included to quantify post-stimulation cell recovery. Resuspend pellets with 1 ml of R10, and add R10 to an appropriate volume for counting viable cells by trypan blue exclusion.Cell yields on d6 post-stimulation with the R848/IL-2/IL-10 cocktail are approximately 100–150% of the input cell number.
-
13Dilute cell suspensions to the appropriate concentration (calculated based on the pre-stimulation input number of PBMCs) and add cells to ELISpot plates according to the assay plan. An example of an ELISpot plate with recommended seeding cell numbers for measurement of total and antigen-specific IgG MASCs is shown in Figure 2.Note: Cell seeding concentrations should be optimized empirically depending on expected spot numbers. As a guide, 2-fold dilutions starting with 2000 PBMC/well (in 100 μl) is recommended for measurement of total IgG MASCs. Antigen-specific MASCs are much less frequent and seeding with 100,000–200,000 PBMC/well is recommended. Seeding wells with more than 200,000 PBMCs “overcrowds” wells and reduces spot quality and sensitivity of spot detection.
-
14
Incubate plates overnight at 37°C with 5% CO2.
Completion of ELISpot assay
-
15
Wash the plates 6 times with PBS-T using a microtiter plate washer or manually with a multichannel pipette (150 μl of PBS-T per well). Flick out remaining liquid from wells before adding secondary Ab.
-
16Dilute alkaline phosphatase AffiniPure goat anti-human IgG to 1 μg/ml in ELISpot buffer and filter through a 0.2 μm membrane into a reagent reservoir.Filtering the detection Ab preparation can reduce small, false-positive spots, especially when precipitates have formed during storage of the reagent at 4°C.
-
17
Add 50 μl of filtered detection Ab to appropriate wells. Incubate plates at RT for 2 h.
-
18
Wash plates as in step 15.
-
19Remove the plastic base of plates and submerge plates in a tub of PBS-T for 0.5–1 h.Our experience is that this step substantially reduces a diffuse blue background after color development.
-
20Flick plates to remove PBS-T from wells and replace plastic bases.It is important that the plastic base fits tightly when replaced to prevent leakage during color development.
-
21
Prepare the required volume of AP substrate using the Vector® Blue AP Substrate Kit and following the manufacturer’s instructions: add 4 drops of each of the provided reagents to 10 ml of 100 mM Tris HCl (pH 8.2), shake well, and add 100 μl of substrate per well.
-
22Incubate plates at RT until development of blue spots is optimal. Wells for measurement of total IgG spots (coated with goat anti-human IgG) serve as positive controls for spot development.Blue spots typically appear within 5–30 min. Optimal incubation time is typically about 20 min and represents a balance between the time required for development of easily visualized spots and blue background development.
-
23
Remove plastic bases and wash both sides of plates under cold tap water.
-
24Flick water from plates and air-dry for 1–2 h. After drying is complete, replace plastic bases and store plates in the dark.Air flow in a running BSL2 hood facilitates drying, but lengthy exposure can crack well membranes.Clarity of spots is improved when plates have completely dried.
-
25Scan plates and count spots using a CTL immunospot reader.Antigen-specific spots differ in size and intensity and are frequently best counted manually using a dissecting microscope.Typical variation in spot appearance is shown in Figure 3.
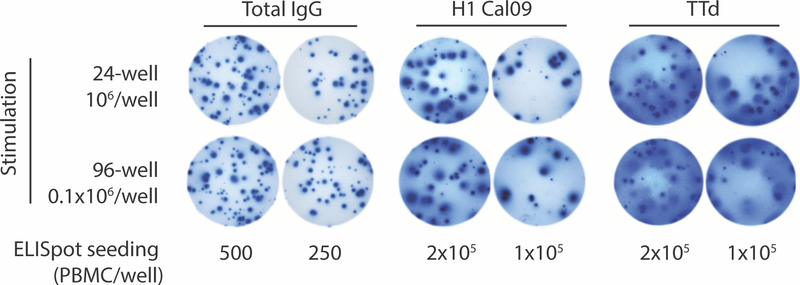
Figure 3.
A portion of a developed ELISpot plate used to enumerate total and antigen-specific IgG MASCs after in vitro MBC stimulation. PBMCs from a healthy donor were stimulated and analyzed by ELISpot assay as described in Basic Protocols 1 and 2. Wells in the ELISpot plate were coated with goat anti-human IgG for measurement of total IgG MASCs and with influenza virus HA (H1 Cal09) or tetanus toxoid (TTd) for measurement of MASCs specific for those antigens. Note the differences in spot size and appearance. This can reflect the amount of IgG secreted by individual MASCs and the affinity of the interaction between secreted IgG and the anti-IgG capture Ab or the target antigens. The quality of well coating is also an important determinant of spot appearance. True spots are typically round with a darker center and a faint edge, reflecting less Ab with distance from the MASC.
REAGENTS AND SOLUTIONS
ELISpot buffer
PBS (Corning, cat. no. 21–040-CV)
0.1% (v/v) Tween-20 (VWR, cat. no. 0777–1L)
2% (w/v) bovine serum albumin, heat shock fraction (Sigma, cat. no. A9647)
Filter (Nalgene rapid-flow filter unit, cat. no. 156–4020) and store at 4°C
Heat-inactivated fetal bovine serum
Completely thaw fetal bovine serum (Hyclone, cat. no. SH30071.03) at 4°C (takes 2–4 days)
Warm the fetal bovine serum in a 37°C water bath for 60 min
Heat-inactivate in a 56°C water bath for 30 min
Filter (Nalgene rapid-flow filter unit, cat. no. 156–4020) and store in aliquots at −80°C
R10 medium
500 ml RPMI 1640 (ThermoFisher Scientific, cat. no. MT10040CV)
50 ml heat-inactivated fetal bovine serum (Hyclone, cat. no. SH30071.03)
5 ml 100× penicillin/streptomycin (Gibco, cat. no. 15140–122)
Store up to 6 months at 4°C
2× R848/IL-2/IL10 stimulation cocktail
10 ml R10 medium
4 μl 5 mg/ml Resiquimod/R848 (Sigma, cat. no. SML0196–10MG, dissolved in DMSO, aliquots stored at −20°C)
2 μl 100 μg/ml IL-2 (ThermoFisher Scientific, cat. no. PHC0021, dissolved in 100 mM acetic acid, aliquots stored at −20°C)
5 μl 100 μg/ml IL-10 (STEMCELL Technologies, cat. no. 78024, dissolved in water, aliquots stored at −20°C)
18.2 μl 2-mercaptoethanol (Gibco, cat. no. 21985023, stored at 4°C)
Freshly prepare on day of use
2-mercaptoethanol is a reducing agent that is essential for lymphocyte culture. It is conveniently added when preparing the stimulation cocktail.
COMMENTARY
BACKGROUND INFORMATION:
In vitro polyclonal stimulation of MBCs, together with antigen-specific readout assays that measure MBC-derived IgG ASC formation and IgG secretion, is a long-established strategy for determining the size of antigen-reactive MBC populations in humans. A major goal over many years of assay development has been identification of a stimulation cocktail that (i) is simple and contains standard components, (ii) activates MBCs, but not naïve B cells, and (iii) optimizes MBC “burst size”, i.e. the number of IgG ASCs generated from a single MBC. An important advance was identification of R848 plus IL-2 as a highly effective cocktail for in vitro stimulation of human MBCs that did not induce proliferation of naïve B cells (Pinna et al., 2009). Weiss et al. (Weiss et al., 2012) demonstrated that addition of IL-10 to a PWM/SAC/CpG stimulation cocktail resulted in a 10-fold increase in the number of responding MBCs, but naïve B cells were not stimulated to form IgG ASCs. Notably, the addition of IL-10 to the cocktail increased the sensitivity of detection of antigen-specific MBCs to that achieved by flow cytometry with a labeled antigen. Our experience is that the addition of IL-10 to the R848/IL-2 cocktail results in a small but consistent increase in the yield of IgG ASCs and secreted IgG from stimulated human MBCs.
The R848/IL-2/IL-10 stimulation cocktail is a highly effective strategy for driving human IgG MBCs to form IgG ASCs, but it is less clear whether it is superior to other cocktails for stimulating IgA MBCs to form IgA ASCs. PBMC culture with the PWM/SAC/CpG or PWM/SAC/R848 stimulation cocktails has generated IgA ASCs for IgA MBC analysis (He et al., 2015; Ramani et al., 2015). Our experiments comparing the R848/IL-2/IL-10 and PWM/SAC/CpG/IL-10 cocktails for generating IgA ASCs from stimulated PBMCs were inconclusive, but the trend was towards a greater effectiveness of PWM/SAC/CpG/IL-10. In addition, Tengvall et al. (Tengvall et al., 2010) suggested that a combination of CpG, IL-15, and B cell-activating factor belonging to the TNF family (BAFF) is also an effective alternative to PWM/SAC/CpG for IgA MBC stimulation.
In vitro stimulation strategies for human MBC analysis have been developed almost exclusively using easy-to-obtain PBMC samples. However, in the steady-state, circulating MBCs sampled as PBMCs reflect the low-level recirculation of much larger MBC reservoirs in lymphoid tissues such as spleen, lymph nodes, and tonsils. Only the most abundant of the MBC specificities present in lymphoid tissues are likely to be consistently detected in PBMC samples. The in vitro stimulation strategy is well suited for analysis of lymphoid tissue-resident MBC populations, but limited work has been done to establish protocols that are optimal for unfractionated lymphoid tissue cells (Cao et al., 2010). Tonsillar cell suspensions have been stimulated with a PWM/SAC/CpG cocktail for IgG MBC analysis (Sharma et al., 2019) and our preliminary experiments suggest that R848/IL-2/IL-10 is effective for stimulating IgG MBCs in splenocyte preparations.
Protocols described in this article emphasize in vitro polyclonal MBC stimulation (Basic Protocol 1) as the first step in a strategy to measure the size of antigen-reactive MBC populations. Importantly, in vitro MBC stimulation also serves as a foundation for other techniques that extend the antigen-specific analysis and provide additional information on the character of antigen-specific MBC populations (Figure 1). The ELISpot assay provides a simple and effective post-stimulation readout of ASC formation (Basic Protocol 2), but the number of antigens that can be tested is limited by the number of cells used to seed stimulation cultures.. MBC-derived polyclonal Abs or MPAbs in the stimulation culture supernatants can be collected and stored for later analysis of reactivity to an almost unlimited number of antigens, with the concentrations of binding Abs reflecting the size of precursor antigen-reactive MBC populations. Analysis of MPAbs by multiplexed binding assays greatly facilitates screening for reactivity to a broad range of antigens (Tesini et al., 2019; Wang et al., 2015). MPAbs can also be analyzed by surface plasmon resonance or biolayer interferometry to provide a measure of the net strength of binding of MBC populations reactive to particular antigens. This is most accurately done using IgG purified from MPAb samples (Tesini et al., 2019). Often, an important question is whether Abs expressed by IgG MBC populations are functionally active without further affinity maturation. This can be addressed using MPAb-purified IgG, for example, in assays that measure the functionality of antiviral Abs (Tesini et al., 2019). In vitro MBC stimulation also has value as a step to facilitate cloning of MBC-expressed Ab genes through enrichment of MBCs and increasing transcript levels (Rajan et al., 2018; Waltari et al., 2019).
CRITICAL PARAMETERS
PBMC quality
The quality of PBMCs is a critical determinant of the effectiveness of in vitro MBC stimulation. On occasions, poor outcomes can reflect problems at steps in the process of cryopreservation of cells from collected blood samples. This could be indicated by an unexpectedly low recovery of viable cells from stored vials. Problems with PBMC quality are more likely to be encountered when they are collected during the acute response to infectious agents. For example, viral infections in humans are frequently associated with a period of lymphopenia and potentially decreased lymphocyte function that can persist for at least 2 wk after infection.
A commonly held view is that cryopreservation reduces the number and functionality of MBCs. However, a number of studies of in vitro MBC stimulation with a range of stimulation cocktails have reported similar results for fresh and frozen PBMCs (Buisman et al., 2009; Crotty et al., 2004; Fecher et al., 2018; Pinna et al., 2009; Weiss et al., 2012).
Stimulation cocktails
Special attention must be paid to preparation of the stimulation cocktail as a critical determinant of the effectiveness of in vitro stimulation of human MBCs. Over many years, a large range of stimulation strategies have been developed and shown to work well for driving human MBC activation and differentiation in vitro. The R848/IL-2/IL-10 cocktail used in our protocol is highly effective for IgG MBC activation, as well as being simple and without non-standard components such as PWM. It is easy to prepare and requires no more than standard consideration of the quality of individual components. The combination of only R848 and IL-2 is also a frequently used and highly effective stimulation cocktail (Fecher et al., 2018; Jahnmatz et al., 2013; Pinna et al., 2009). We have found that the addition of IL-10 results in a small but consistent 3–4-fold increase in the yield of IgG MASCs and MPAbs from PBMCs stimulated as described in Basic Protocol 2. Interestingly, Weiss et al. (Weiss et al., 2012) described a 10-fold increase in the efficiency of IgG MBC activation when IL-10 was added to a PWM/SAC/CpG cocktail, but this was done using PBMCs cultured in a limiting dilution format.
Incubation duration
ELISpot assay analysis of MASCs generated in cultures of stimulated PBMCs is timed to coincide with the MASC peak. A pattern of peak MASC numbers after 5–6 days incubation and a decrease on day 7 is well established for PBMCs stimulated with a range of cocktails. However, other sample types, such as lymphoid tissues, have received much less attention and optimization of the sampling time after stimulation is recommended. Although the MASC peak is reached by day 6 for PBMCs, the decline is gradual and MPAb concentrations continue to increase. Collection of MPAbs after 10 days stimulation is recommended for antigen-specific MBC analysis based on MPAb measurements (Pinna et al., 2009; Weiss et al., 2012).
Troubleshooting
In vitro stimulation of PBMCs to activate IgG MBCs is generally a reliable procedure. A rough indicator of a poor outcome is <50,000 total IgG MASC/106 stimulated PBMCs in the ELISpot assay readout. Typically, this is the result of poor sample quality, a deficiency in the stimulation cocktail, or a problem during the culture of stimulated cells. Otherwise, the most common challenges relate to evaluating spots in antigen-coated wells of the ELISpot assay.
MBCs of particular specificities are only consistently detected in the circulation when their numbers have been expanded by strong B cell responses. In many studies of PBMCs, the MBCs of interest will be at or below the limit of detection and there will be few, if any, spots in antigen-coated wells in the ELISpot assay. Even one spot in an antigen-coated well can be an important indicator of a precursor MBC population. Such spots are not the result of preexisting IgG ASCs in the PBMC sample, primarily because these cells are unlikely to survive the 6-day period of in vitro stimulation. Furthermore, circulating IgG ASCs are generally scarce; those formed in an immune response circulate for only a few days or weeks while that response is ongoing and are not present in blood during steady-state sampling.
Because of the potential importance of a small number of antigen-specific spots, it is essential that the ELISpot assay is optimized to facilitate identification of true spots. Key determinants of the appearance of antigen-specific spots are the quality of the coating antigen and the amount used for coating. Correct handling and storage of the antigen is essential and molecular characterization if informative is recommended. Antigens should be titrated to identify the coating amount required for good spot clarity. This can be done by stimulating PBMC samples known to contain MBCs of the required specificity. Alternatively, mice can be immunized to generate ASCs for analysis by ELISpot assay (remembering to use an anti-mouse detection Ab in the ELISpot assay). Generally, 0.5 μg of coating antigen per well (sometimes more) is required to generate clear spots, but this needs to be determined for each antigen. Large, faint spots can reflect insufficient coating antigen or Abs with low affinity for the coating antigen. Increasing the incubation time for color development can improve the clarity of spots, but this needs to be balanced against development of background color. Beware of spot-like artifacts that occasionally form. Generally, these are uniformly dark and irregular, in contrast to true spots, which are round with a dark center and a faint periphery.
Anticipated Results
One vial (1 ml) of cryopreserved PBMCs at 5–15×106 cells/ml and of good viability provides, on average, approximately 107 PBMCs for in vitro stimulation. Assuming that the number of cells recovered post-stimulation is 100% of the input cell number (usually the number is a little higher), 107 input PBMCs is comfortably sufficient to measure the number of MASCs specific for 10–15 different antigens by ELISpot assay (see Figure 2 for plate layout). As discussed above, collection of MPAbs enables MBC reactivity to a much larger number of antigens to be measured.
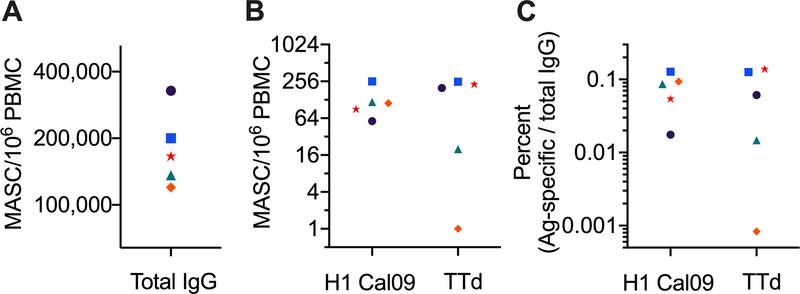
An example of a developed ELISpot assay plate used as readout after stimulation of one PBMC sample is shown in Figure 3. Total IgG MASCs were measured, as well as IgG MASCs specific for an influenza virus H1 HA and tetanus toxoid (TTd). Data from that plate are used to provide measures of the size of H1-reactive and TTd-reactive IgG MBC populations (Figure 4). One approach is to simply represent the size of an MBC population specific for a particular antigen as the number of antigen-specific MASCs generated per 106 PBMCs (Figure 4B). The number of IgG MASCs correlates directly with the number of precursor IgG MBCs. However, the number of MASCs is higher because of proliferation following MBC activation. It is estimated that a single MBC generates approximately 10–20 MASCs (Weiss et al., 2012), but the extent to which this estimate might differ between MBCs because of factors such as age or proliferation history is not known.
Figure 4.
Expression of the size of antigen-specific MBC populations based on ELISpot assay data. PBMCs from five healthy donors were stimulated and analyzed by ELISpot assay as shown in Figure 3. Total IgG spots (A) were counted using a CTL analyzer; spots against the H1 Cal09 and TTd antigens were counted manually. The size of antigen-specific IgG MBC populations can be simply represented as the antigen-specific MASC count per 106 PBMCs (B). Note that this is per pre-stimulation PBMCs, not per cells collected after stimulation. Alternatively, antigen-specific IgG MBC populations can be shown as a percentage of total IgG MBCs in a PBMC sample (C).
The size of an antigen-specific IgG MBC population can also be represented as the percentage of total IgG MBCs, calculated as antigen-specific IgG MASCs as a percentage of total IgG MASCs (Crotty, 2004) (Figure 4C). An advantage of this approach is that it normalizes for variation in the effectiveness of in vitro MBC stimulation. However, an assumption is that in vitro stimulation results in equivalent proliferation and differentiation of all IgG MBCs; this is not well established. The calculation is also affected by the proportion of PBMCs that are IgG MBCs, which is likely to differ between individuals and fluctuate within an individual.
An important question relates to the efficiency of activation of IgG MBCs stimulated with particular cocktails. Studies have used a limiting dilution approach to estimate that approximately one-third of IgG MBCs classified by flow cytometry form ASCs when stimulated with R848/IL-2 and other cocktails (Amanna & Slifka, 2006; Pinna et al., 2009). It is unclear whether the non-responding IgG MBCs include those that generate high affinity secondary Ab responses. Importantly, Weiss et al. (Weiss et al., 2012) demonstrated that addition of IL-10 to the PWM/SAC/CpG cocktail increased the efficiency of MBC activation in limiting dilution cultures and produced a measure of TTd-specific MBC frequency similar to that identified by flow cytometry with fluorochrome-labeled TTd.
It is important to appreciate that, in the steady-state, IgG MBCs specific for particular antigens circulate at low frequency. Counts of ≥100 MASC/106 PBMC are generally only measured against immunogenic proteins that individuals are likely to have “seen” multiple times, such as the influenza virus HA and TTd. Assuming a yield of 20 ASCs from a single stimulated MBC, 100 antigen-specific MASC/106 PBMC would indicate only 50 MBCs of that specificity in a starting vial of 107 PBMCs. MBCs of many specificities are likely to be much less frequent. Thus, one or two clear antigen-specific spots per 600,000 PBMCs plated post-stimulation (as in the Figure 3 assay layout) are likely to be meaningful.
Time Considerations
Thawing up to 6 vials of stored PBMCs and washing and preparing the cells for the overnight rest takes approximately 1.5 h. Processing more than 6 vials at a time by a single investigator is not recommended because of the need to minimize exposure of thawed cells to DMSO in the freezing medium. The collection, counting, and plating of up to 10 PBMC samples in stimulation cultures takes 2–3 h. Each additional sample adds 5–10 min to the total time. A team of two investigators for this step is optimal.
Coating of ELISpot plates can take 30 min to 1.5 h depending on the size of the antigen panel. Collection, washing, and plating stimulated cells in the ELISpot assay takes approximately 5 h (longer for additional samples). A team of two investigators for this step is preferable for large experiments.
Development of ELISpot plates takes approximately 4.5 h: blocking (1 h), addition of secondary Abs (2 h), soaking (30 min to 1 h), and color development (30 min). Image reading by the CTL analyzer takes 20–30 min for the first plate and 2–5 min for additional plates. Spot counting can be performed using the CTL counter; 2–5 min per plate for total IgG spots. Antigen-specific spots are best counted manually (5–15 minutes per plate).
SIGNIFICANCE STATEMENT:
The size and character of an individual’s antigen-specific memory B cell populations, which are important determinants of protective immunity, can be measured by analysis of antibodies derived from in vitro-stimulated memory B cells.
Acknowledgments
This work was supported with Federal funds from the National Institute of Allergy and Infectious Diseases, NIH, Department of Health and Human Services through awards to the New York Influenza Center of Excellence (NYICE, Contract No. HHSN272201400005C), a member of the NIAID Centers of Excellence for Influenza Research and Surveillance (CEIRS), and to the Respiratory Pathogens Research Center (RPRC; contract HHSN272201200005C).
LITERATURE CITED:
- Amanna IJ, & Slifka MK (2006). Quantitation of rare memory B cell populations by two independent and complementary approaches. Journal of Immunological Methods, 317(1–2), 175–185. doi: 10.1016/j.jim.2006.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews SF, Chambers MJ, Schramm CA, Plyler J, Raab JE, Kanekiyo M, … McDermott AB (2019). Activation Dynamics and Immunoglobulin Evolution of Preexisting and Newly Generated Human Memory B cell Responses to Influenza Hemagglutinin. Immunity, 51(2), 398–410.e395. doi: 10.1016/j.immuni.2019.06.024 [DOI] [PubMed] [Google Scholar]
- Andrews SF, Huang Y, Kaur K, Popova LI, Ho IY, Pauli NT, … Wilson PC (2015). Immune history profoundly affects broadly protective B cell responses to influenza. Science Translational Medicine, 7(316), 316ra192–316ra192. doi: 10.1126/scitranslmed.aad0522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekeredjian-Ding I, Foermer S, Kirschning CJ, Parcina M, & Heeg K (2012). Poke Weed Mitogen Requires Toll-Like Receptor Ligands for Proliferative Activity in Human and Murine B Lymphocytes. PLOS ONE, 7(1), e29806. doi: 10.1371/journal.pone.0029806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisman AM, de Rond CGH, Öztürk K, ten Hulscher HI, & van Binnendijk RS (2009). Long-term presence of memory B-cells specific for different vaccine components. Vaccine, 28(1), 179–186. doi: 10.1016/j.vaccine.2009.09.102 [DOI] [PubMed] [Google Scholar]
- Cao Y, Gordic M, Kobold S, Lajmi N, Meyer S, Bartels K, … Atanackovic D (2010). An optimized assay for the enumeration of antigen-specific memory B cells in different compartments of the human body. Journal of Immunological Methods, 358(1–2), 56–65. doi: 10.1016/j.jim.2010.03.009 [DOI] [PubMed] [Google Scholar]
- Cobey S, & Hensley SE (2017). Immune history and influenza virus susceptibility. Current Opinion in Virology, 22, 105–111. doi: 10.1016/j.coviro.2016.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S, Aubert RD, Glidewell J, & Ahmed R (2004). Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. Journal of Immunological Methods, 286(1–2), 111–122. doi: 10.1016/j.jim.2003.12.015 [DOI] [PubMed] [Google Scholar]
- Cyster JG, & Allen CDC (2019). B Cell Responses: Cell Interaction Dynamics and Decisions. Cell, 177(3), 524–540. doi: 10.1016/j.cell.2019.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecher P, Caspell R, Naeem V, Karulin A, Kuerten S, & Lehmann P (2018). B Cells and B Cell Blasts Withstand Cryopreservation While Retaining Their Functionality for Producing Antibody. Cells, 7(6), 50. doi: 10.3390/cells7060050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good-Jacobson KL (2018). Strength in diversity: Phenotypic, functional, and molecular heterogeneity within the memory B cell repertoire. Immunological Reviews, 284(1), 67–78. doi: 10.1111/imr.12663 [DOI] [PubMed] [Google Scholar]
- Halliley JL, Khurana S, Krammer F, Fitzgerald T, Coyle EM, Chung KY, … Sangster, M. Y. (2015). High-Affinity H7 Head and Stalk Domain–Specific Antibody Responses to an Inactivated Influenza H7N7 Vaccine After Priming With Live Attenuated Influenza Vaccine. Journal of Infectious Diseases, 212(8), 1270–1278. doi: 10.1093/infdis/jiv210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W, Mullarkey CE, Duty JA, Moran TM, Palese P, & Miller MS (2015). Broadly Neutralizing Anti-Influenza Virus Antibodies: Enhancement of Neutralizing Potency in Polyclonal Mixtures and IgA Backbones. Journal of Virology, 89(7), 3610–3618. doi: 10.1128/JVI.03099-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Moran I, Shinnakasu R, Phan TG, & Kurosaki T (2018). Generation of memory B cells and their reactivation. Immunological Reviews, 283(1), 138–149. doi: 10.1111/imr.12640 [DOI] [PubMed] [Google Scholar]
- Jahnmatz M, Kesa G, Netterlid E, Buisman A-M, Thorstensson R, & Ahlborg N (2013). Optimization of a human IgG B-cell ELISpot assay for the analysis of vaccine-induced B-cell responses. Journal of Immunological Methods, 391(1–2), 50–59. doi: 10.1016/j.jim.2013.02.009 [DOI] [PubMed] [Google Scholar]
- Karrar S, & Cunninghame Graham DS (2018). Review: Abnormal B Cell Development in Systemic Lupus Erythematosus: What the Genetics Tell Us. Arthritis & Rheumatology, 70(4), 496–507. doi: 10.1002/art.40396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GM, Chiu C, Wrammert J, McCausland M, Andrews SF, Zheng NY, … Ahmed R (2012). Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proceedings of the National Academy of Sciences, 109(23), 9047–9052. doi: 10.1073/pnas.1118979109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macosko Evan Z., Basu A, Satija R, Nemesh J, Shekhar K, Goldman M, … McCarroll Steven A. (2015). Highly Parallel Genome-wide Expression Profiling of Individual Cells Using Nanoliter Droplets. Cell, 161(5), 1202–1214. doi: 10.1016/j.cell.2015.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir L, McKay PF, Petrova VN, Klymenko OV, Kratochvil S, Pinder CL, … Shattock RJ (2018). Optimisation of ex vivo memory B cell expansion/differentiation for interrogation of rare peripheral memory B cell subset responses. Wellcome Open Research, 2, 97. doi: 10.12688/wellcomeopenres.11386.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan K, & May KM (2015). Basic techniques in mammalian cell tissue culture. Curr Protoc Cell Biol, 66, 1 1 1–1 1 22. doi: 10.1002/0471143030.cb0101s66 [DOI] [PubMed] [Google Scholar]
- Pinna D, Corti D, Jarrossay D, Sallusto F, & Lanzavecchia A (2009). Clonal dissection of the human memory B-cell repertoire following infection and vaccination. European Journal of Immunology, 39(5), 1260–1270. doi: 10.1002/eji.200839129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan S, Kierny MR, Mercer A, Wu J, Tovchigrechko A, Wu H, … Chowdhury PS (2018). Recombinant human B cell repertoires enable screening for rare, specific, and natively paired antibodies. Communications Biology, 1(1), 5. doi: 10.1038/s42003-017-0006-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramani S, Neill FH, Opekun AR, Gilger MA, Graham DY, Estes MK, & Atmar RL (2015). Mucosal and Cellular Immune Responses to Norwalk Virus. Journal of Infectious Diseases, 212(3), 397–405. doi: 10.1093/infdis/jiv053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangster MY, Baer J, Santiago FW, Fitzgerald T, Ilyushina NA, Sundararajan A, … Subbarao K (2013). B cell response and hemagglutinin stalk-reactive antibody production in different age cohorts following 2009 H1N1 influenza virus vaccination. Clinical and vaccine immunology: CVI, 20(6), 867–876. doi: 10.1128/CVI.00735-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangster MY, Nguyen PQT, & Topham DJ (2019). Role of Memory B Cells in Hemagglutinin-Specific Antibody Production Following Human Influenza A Virus Infection. Pathogens, 8(4), 167. doi: 10.3390/pathogens8040167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders SP, Ma EGM, Aranda CJ, & Curotto de Lafaille MA (2019). Non-classical B Cell Memory of Allergic IgE Responses. Frontiers in Immunology, 10, 715. doi: 10.3389/fimmu.2019.00715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Hagbom M, Nordgren J, Frodlund J, Hinkula J, Ledin T, & Svensson L (2019). Detection of rotavirus‐ and norovirus‐specific IgG memory B cells in tonsils. Journal of Medical Virology, 91(2), 326–329. doi: 10.1002/jmv.25247 [DOI] [PubMed] [Google Scholar]
- Stoeckius M, Hafemeister C, Stephenson W, Houck-Loomis B, Chattopadhyay PK, Swerdlow H, … Smibert P (2017). Simultaneous epitope and transcriptome measurement in single cells. Nature Methods, 14(9), 865–868. doi: 10.1038/nmeth.4380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tengvall S, Lundgren A, Quiding-Järbrink M, & Svennerholm A-M (2010). BAFF, stimulatory DNA and IL-15 stimulates IgA+ memory B cells and provides a novel approach for analysis of memory responses to mucosal vaccines. Vaccine, 28(33), 5445–5450. doi: 10.1016/j.vaccine.2010.06.001 [DOI] [PubMed] [Google Scholar]
- Tesini BL, Kanagaiah P, Wang J, Hahn M, Halliley JL, Chaves FA, … Sangster MY (2019). Broad Hemagglutinin-Specific Memory B Cell Expansion by Seasonal Influenza Virus Infection Reflects Early-Life Imprinting and Adaptation to the Infecting Virus. Journal of Virology, 93(8), e00169–00119, /jvi/00193/00168/JVI.00169–00119.atom. doi: 10.1128/JVI.00169-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topham DJ, Nguyen P, & Sangster MY (2018). Pandemic influenza vaccines: what they have taught us about B cell immunology. Current Opinion in Immunology, 53, 203–208. doi: 10.1016/j.coi.2018.06.004 [DOI] [PubMed] [Google Scholar]
- Waltari E, McGeever A, Friedland N, Kim PS, & McCutcheon KM (2019). Functional Enrichment and Analysis of Antigen-Specific Memory B Cell Antibody Repertoires in PBMCs. Frontiers in Immunology, 10, 1452. doi: 10.3389/fimmu.2019.01452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Hilchey SP, Hyrien O, Huertas N, Perry S, Ramanunninair M, … Zand MS (2015). Multi-dimensional measurement of antibody-mediated heterosubtypic immunity to influenza. PLoS ONE, 10(6), e0129858. doi: 10.1371/journal.pone.0129858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss GE, Ndungu FM, McKittrick N, Li S, Kimani D, Crompton PD, … Pierce SK (2012). High efficiency human memory B cell assay and its application to studying Plasmodium falciparum-specific memory B cells in natural infections. Journal of Immunological Methods, 375(1–2), 68–74. doi: 10.1016/j.jim.2011.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrammert J, Koutsonanos D, Li G-M, Edupuganti S, Sui J, Morrissey M, … Wilson PC (2011). Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. The Journal of Experimental Medicine, 208(1), 181–193. doi: 10.1084/jem.20101352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JS, Kim HJ, Ryu YH, Yun C-H, Chung DK, & Han SH (2006). Endotoxin contamination in commercially available pokeweed mitogen contributes to the activation of murine macrophages and human dendritic cell maturation. Clinical and vaccine immunology: CVI, 13(3), 309–313. doi: 10.1128/CVI.13.3.309-313.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]