Abstract
Cancer cell identity and plasticity are required in transition states, such as epithelial-mesenchymal transition (EMT) and mesenchymal-epithelial transition (MET), in primary tumor initiation, progression and metastasis. The functional roles of EMT, MET and partial state (referred to as p-EMT) may vary based upon the type of tumor, state of dissemination and degree of metastatic colonization. Herein, we review EMT, MET, pEMT and plasticity in the context of tumor metastasis.
Keywords: EMT, MET, pEMT, cellular plasticity, colonization, metastasis
Plasticity in cancer
Cellular identity reflects developmental pathways as well as cell non-autonomous and cell autonomous factors during the lifespan of a cell. Cellular plasticity implies the ability of a cell to adapt to changing circumstances, and this adaptation is either reversible or irreversible. There is increasing application of cellular plasticity in stem cell biology, regenerative medicine and cancer biology, although the term plasticity itself remains in evolution [1,2].
A number of genomic, genetic, epigenomic, transcriptomic and proteomic factors conspire for the conversion of a normal cell to a malignant cell. These factors have a complex interplay with diverse cell types in the tumor microenvironment. Once tumor cells invade through the tumor stroma, tumor cells have the capacity to disseminate in circulation and colonize distant organs, the latter influenced by the pre-metastatic niche and organotropism. It is becoming increasingly clear that epithelial-mesenchymal transition (EMT, Glossary), where cells lose their epithelial cell identity and acquire features of mesenchymal cells, is not only critical in development and wound healing, but also represents a salient property of primary tumor formation and metastasis. In turn, mesenchymal-epithelial transition (MET) is the reversion of EMT and is apparent during development, induced pluripotent stem cell reprogramming and tumor metastasis [3]. In this article, we review in vivo evidence of EMT; summarize the current evidence of whether EMT is necessary for tumor progression; discuss the evidence of MET in metastatic outgrowth and potential clinical associations; evaluate potential therapeutic implications; and, conclude with outstanding questions in the field, building upon recent review articles [4–9].
Tumor cell plasticity
Through investigation of EMT in breast, pancreatic and ovarian cancers, it has been found that the conversion from epithelial-like to mesenchymal-like cells is less akin to a light-switch analogy and more like a dimmer switch. In many malignant cases, the phenotypic and functional changes appear to shift across a continuum [10]. These different cases have given rise to the definitions of complete EMT (cEMT) and partial, mixed or hybrid EMT (Box 1). For consistency, we will refer to incomplete transition to a mesenchymal state as “partial EMT” or pEMT. Recent work by Panchy et al. used transcriptomic analysis to demonstrate the high degree to which cells can be along the epithelial and mesenchymal spectrum [11]. Several review articles have highlighted this phenomenon [12–14].
Box 1: Partial, hybrid and mixed EMT.
The field of cellular plasticity has generated several terms to describe cells residing between complete epithelial or mesenchymal phenotypes: partial EMT (pEMT), hybrid EMT and mixed EMT. While the terms are often used interchangeably, it is important to recognize the origins of these terms and nuances.
The term ‘mixed EMT’, or mixed tumors, might have arisen from Chase et al. [72] in which they describe keratin+/Vimentin+ sarcoma cells with epithelioid morphology. Stromal cells are thought to response to growth of malignant epithelial cells, but we now know that this may be the result of cellular plasticity [73].
The existence of a spontaneous ‘hybrid EMT’ state has been demonstrated. Pastushenko et al. discovered that primary skin and mammary tumors undergo EMT. Within the tumors, subpopulations of cells were found displaying different degrees of epithelial and mesenchymal characteristics, including cells with intermediate or hybrid characteristics. The cells along this epithelial and mesenchymal spectrum displayed differences in cellular plasticity, invasiveness and metastatic potential. Importantly, subpopulations of cells with hybrid phenotypes were more effective at forming metastases [74]. The importance of cellular plasticity in metastasis is highlighted by Kröger et al. demonstrated that hybrid EMT cells cannot be phenocopied by mixing cells in a stable epithelial state with cells in a stable mesenchymal state. It is the dynamic characteristic of cells in the hybrid state that is important for endowing increased metastatic potential [10].
Lastly, the term pEMT has been used to describe cells stably or dynamically in an epithelial-mesenchymal intermediate state [75]. This ‘partial’ phenotype can be manifest by the simultaneous expression of epithelial and mesenchymal markers, or by the loss of epithelial markers without the gain of mesenchymal markers. Recently, pEMT has gained traction in the field as an alternative to the observation of complete EMT (cEMT), which is characterized by the repression or inactivation of epithelial markers and the simultaneous gain of mesenchymal markers [23].
cEMT and pEMT in tumor models
A central challenge to the hypothesis that EMT occurs during tumorigenesis is that much of the initial evidence was predicated upon manipulation of EMT transcription factors (herein EMT-TFs), thereby leading to controversy over whether EMT was a physiologically relevant process [15,16]. The development of spontaneously metastatic genetically engineered mouse models (GEMMs) with fluorescent lineage labeling became a powerful tool to study in vivo EMT driven by endogenous EMT-TF expression. Here, we define characteristics of complete cEMT and pEMT and review evidence for de novo EMT under physiological conditions across multiple tumor types (Figures 1 and 2).
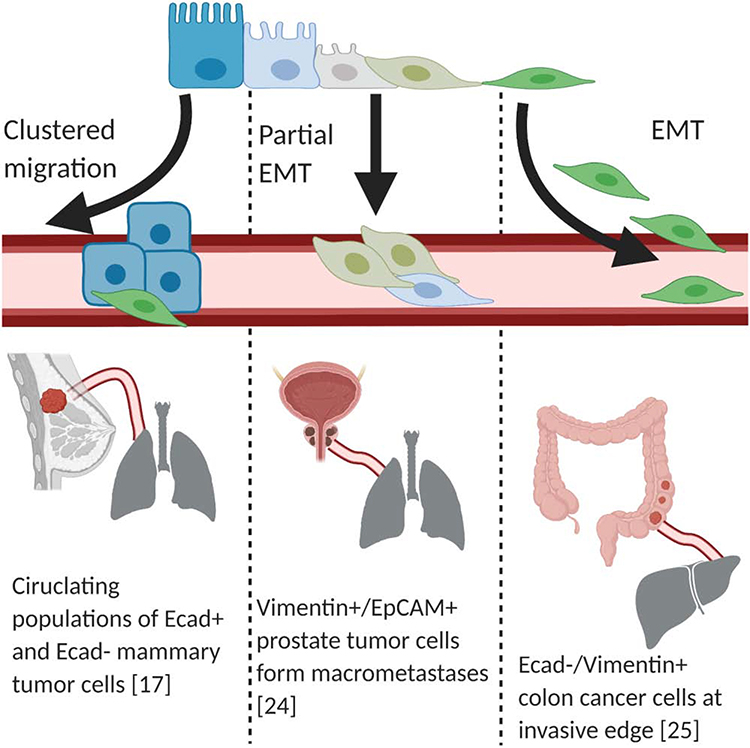
Figure 1: Primary tumor cells undergo intravasation into the lymphatic-vascular systems.
There are multiple mechanisms by which tumor cells (blue rectangles) are thought to detach from the primary site and intravasate into the lymphatic-vascular systems. One proposed mechanism is complete EMT or cEMT (green spheres), whereby cells lose epithelial markers and gain mesenchymal characteristics. During partial EMT or pEMT, cells retain some of their epithelial characteristics. Another possibility is that cells undergoing EMT represent only a small portion of the total population that ultimately metastasize.
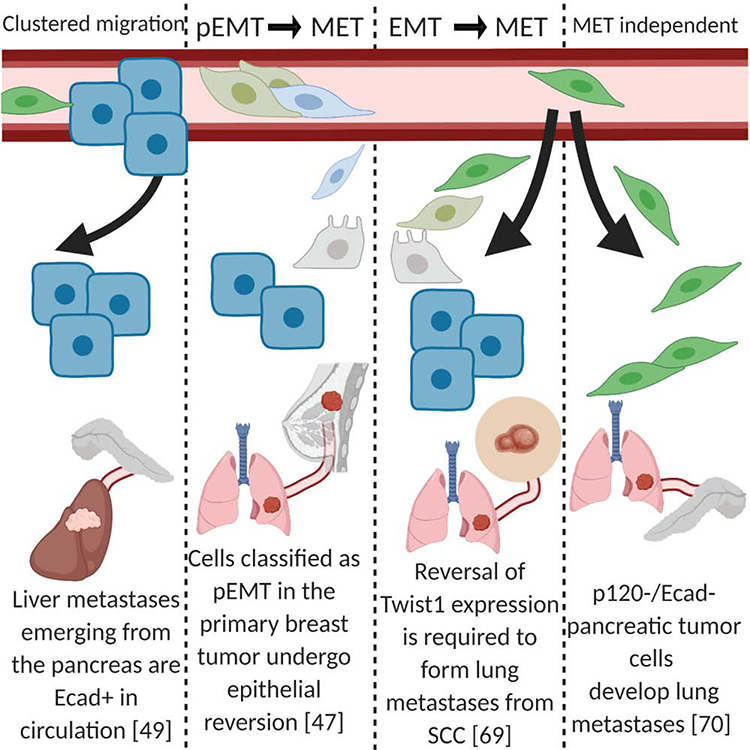
Figure 2: Primary tumor cells undergo extravasation and metastatic colonization.
Evidence in multiple model systems demonstrates that macrometastases have strong expression of epithelial markers (blue), which are likely a requirement for expansion within the metastatic site. One model suggests cells that previously underwent cEMT or pEMT regain epithelial markers and lose mesenchymal characteristics (green). Lastly, in mixed populations of metastatic cells, it is thought that the clusters of epithelial cells are the exclusive or dominant population capable of expanding and forming macrometastases.
Beerling et al. introduced a tumor-cell specific yellow fluorescent protein (YFP) lineage label to a mouse breast cancer model, dependent upon the expression of the polyoma virus middle T antigen (pyVT) and under the control of the mammary mouse tumor virus promoter, (referred to as the MMTV-PyMT mouse (Table 1)). This model allowed a glimpse into the role that classic EMT-TFs play as a cell undergoes EMT [17]. To that end, subpopulations of E-CADHERIN negative (Ecad-) and Ecad+ tumor cells were identified with upregulation of classic EMT-TFs (such as Snail, Slug, Twist, and Zeb1/2) in the Ecad- population (Figure 1) [17]. Importantly, orthotopic transplantation and intravital microscopy revealed that these Ecad- tumor cells, undergoing EMT, were precisely the cells that migrated and presumably initiated the metastatic cascade. While this does not prove necessity, the existence of EMT is demonstrated. In a related model, mouse MMTV-PyMT cells with an R(red)FP-to-G(green)FP switch, under the control of a Fsp1-cre promoter, were transplanted into NOD-scid Il2rynullB2mnull (NSG-β2 m−/−) mice (Table 1). Under these conditions, any cell that undergoes EMT, as defined by Fsp1 expression, would change fluorescence from red to green [18]. A population of GFP+ cells that had undergone EMT was observed with simultaneous Ecad loss, indicating that the mesenchymal conversion did in fact occur in this GEMM. The observation of a subset of RFP+/Ecad- cells suggests that additional cells underwent EMT, yet through an alternative, Fsp1-independent, pathway [18]. However, another group using a MMTV-Her2 model demonstrated that early disseminating cells downregulated Ecad, although these were defined as ECAD “low”, arguing for potential pEMT [19]. These results were largely in agreement with a previous study in a HER2-driven GEMM, which revealed spontaneous upregulation of Snail accompanied by EMT [20]. Thus, it is not surprising that a PyMT model with Snail-driven YFP resulted in YFP+ cells lacking Cdh1 and Zeb1 expression [21].
Table 1:
Relevant mouse models.
| Model | Primary organ site | Lineage label | Definition of EMT | Reference |
|---|---|---|---|---|
| MMTV-PyMT MMTV-Cre |
Breast |
RosaLSL-YFP Ecad-mCFP |
YFP+/mCFPlow EMT cells | [17] |
| MMTV-PyMT | Breast |
Fsp-Cre RosaloxP-RFP-STOP-loxP-GFP |
RFP−/GFP+ EMT cells after Fsp-cre mediated recombination | [18] |
| MMTV-Her2/c-neu/Erbb2 | Breast | Snail-YFP | YFP+ EMT cells after induction of Snail during EMT | [21] |
| “KPCY” KrasLSL-G12D p53LSL-R172H or loxP Pdx1-Cre |
Pancreas | RosaLSL-YFP | YFP+/ECAD− EMT cells | [22] |
|
Pb-Cre PtenloxP/loxP KrasLSL-G12D |
Prostate | Vimentin-GFP | GFP+/EpCAM− EMT cells after induction of Vimentin during EMT | [24] |
| AT3 tumor cell injection | Prostate | CMV-loxP-DsRed-Stop-loxP-eGFP | RFP+ cells that have undergone EMT and GFP+ cells that remain epithelial | [71] |
In pancreatic ductal adenocarcinoma (PDAC), spontaneously metastatic GEMMs with fluorescent lineage labeling of tumor cells have provided definitive evidence for the existence of EMT in vivo. The first model used a pan-epithelial lineage Pdx1-Cre driver to express mutant KrasG12D with concomitant p53 loss and a YFP label (herein KPCY, Table 1) [22]. This study demonstrated the existence of a YFP+, ZEB1+, Ecad- population of EMT cells at the preneoplastic stage. Using the same KPCY model, Aiello et al. demonstrated the existence of a pEMT population with co-expression of epithelial and mesenchymal markers that disseminated through collective migration, and a cEMT population that preferentially disseminated as single cells [23]. These studies demonstrated that EMT occurs early during primary tumor progression, and that different EMT states exist along the metastatic cascade.
Although breast and pancreatic cancer GEMMs have been the most well-defined models to demonstrate EMT in vivo, other models have provided corroborating evidence. Ruscetti et al. used a Pten and KrasG12D driven model with a VimentinGFP lineage label (Table 1), which marks the mesenchymal state, to demonstrate that EMT occurs in prostate cancer (Figure 1) [24]. This group co-stained for another epithelial cell marker, EpCAM, and found both a pEMT population that was EpCAM+/GFP+ and a cEMT population that was EpCAM-GFP+. In colon cancer, a model emerging from Notch activation and p53 loss, found a distinct population of invasive cells with mesenchymal morphology that was VIMENTIN+/GFP+, but lacked ECAD, suggesting that EMT was observed (Figure 1) [25].
Thus, these models have demonstrated that EMT occurs de novo without forced EMT-TF expression, and is a likely driver in the invasive and metastatic phenotypes. Yet, it is conceivable these phenotypes could be incidental, and EMT-TFs might have their own oncogenic EMT-independent effects. We next turn to the question of whether EMT is necessary or dispensable in tumor initiation and progression.
EMT: necessary vs. dispensable?
To evaluate the potential dispensability of EMT in tumor metastasis, work in breast, pancreas, skin, and lung cancers has used a similar paradigm: delete known EMT-TFs in transplantation or genetic mouse models and evaluate whether metastatic burden is affected.
In a pancreas cancer model (Pdx1-cre; KRASG12D; p53R172H), when Snail or Twist1 is independently knocked out, notably primary tumor histology, tumor progression, and metastasis are unaffected [26]. Each model showed a decrease in Zeb1, Zeb2, Sox4, and Slug, suggesting that at least these EMT-TFs are not compensating for Snail or Twist loss, although compensation by other EMT-TFs cannot be ruled out. Interestingly, the EMT-TF knockout correlated with chemosensitivity attributed to increased nucleoside transporter expression. These data suggest a possible explanation for the clinical observation that patients with more mesenchymal tumors have worse outcomes [8].
While Snail or Twist is dispensable individually, Zeb1 knockout in the same genetic background impairs multiple stages of tumorigenesis, ranging from premalignant lesions to metastatic tumors [27]. Corroborating the importance of Zeb1 in pancreatic cancer, short hairpin knockdown of ZEB1 in two human pancreatic cancer lines decreases progression in an orthotopic transplantation mouse model [28]. This suggests that potential EMT dispensability is dependent upon which EMT-TF is driving the process and implies a possible hierarchy amongst EMT-TFs. By contrast, in the Snail and Twist knockout mouse models, amongst other EMT-TFs, Zeb1 was decreased as well. This argues for testing whether there is a temporal dependent requirement for EMT-TFs mediated actions – for example, whether an inducible Zeb1 knockout would reveal different effects of metastatic potential depending upon whether Zeb1 was knocked out in the preneoplastic or neoplastic stages.
The question of potential EMT dispensability extends to other cancers. This was interrogated in breast cancer using a triple transgenic breast cancer mouse model MMTV-PyMT [29]. Using this system, the authors demonstrated that spontaneous lung metastatic lesions at 12 weeks of age remain Ecad+/RFP+. Any cell that underwent EMT would have turned on Cre recombinase and irreversibly lost RFP expression (Table 1). This demonstrates that these metastatic cancer cells never expressed Fsp1, and also opening the premise for a potential Fsp1-independent EMT program.
In another study, the positive predictive value of high miR-200, an inhibitor of Zeb1 and Zeb2, for successful breast cancer metastasis was established in both mouse models and human tissues [30]. Metastasis-free survival was associated significantly with higher miR-200 family member expression, thereby indicating lower Zeb1/Zeb2 correlated with reduced metastasis. Intriguingly, Ecad was not prognostic for survival, suggesting that Ecad expression did not differentiate between those patients with or without relapse. This reveals the following considerations in this specific context: (1) Zeb1/Zeb2-driven EMT is dispensable; (2) suppression of EMT promotes metastasis through Ecad independent mechanisms. The authors were able to show further that miR-200 ectopic overexpression enhances metastasis in a mammary fat pad injection model. Additionally, they demonstrated that ectopic Ecad expression enhances metastasis but not as effectively as miR-200. This reveals that Ecad restoration is only part of the MET program and that suppression of EMT-TFs promotes metastasis through other mechanisms as well, including modulation of secreted factors.
The dispensability of Zeb1 here stands in contrast to the requirement for Zeb1 in pancreatic cancer [27], although there are two differences: the pancreatic cancer model was a direct genetic knockout while the breast cancer data depends upon miR-200 inhibition of Zeb1 in a transplantation model. It does, however, serve as a reminder that different EMT-TFs have divergent effects, and that different cancers might have different hierarchies of EMT-TF functionality.
Reminiscent of the Snail/-Twist1 knockout model in the pancreas, this breast cancer model demonstrates that chemoresistance is associated with EMT. In the latter case, treatment with cyclophosphamide (a commonly used chemotherapeutic agent for breast cancer) revealed only RFP+ cells (i.e., the ones that at no point underwent EMT) had greater apoptosis, whereas GFP+ EMT cells were unaffected. Remarkably, chemotherapy also altered the EMT profiles of the metastatic lesions. Whereas control mice exhibited entirely RFP-+/non-EMT lung metastasis, chemotherapy-treated mice showed significant GFP+/EMT lung metastasis, suggesting that EMT is “turned on” under certain conditions.
The dispensability of EMT-TFs has been challenged vigorously with responses to uphold the role of EMT in both pancreatic [31] and breast [32] cancer metastasis. It was reasoned that deletion of Snail or Twist did not completely eliminate EMT programs and that pEMT programs likely persisted in metastatic pancreatic cancer [26]. Furthermore, they concluded that EMT is achieved through a complex interplay between multiple EMT-TFs and that a system that deletes one factor is unlikely to capture the entire complexity [26]. In particular, Zeb1, Sox4, and Slug persisted after deletion of Snail or Twist, likely indicating a residual persistence of EMT. In a similar manner, the perspective on breast cancer suggested that the Fsp1-Cre and Vim-CreER transgenes to drive the RFP-to-GFP switch (and thus mark cells that have undergone EMT) did not fully capture the entire EMT cell population32. The experimental evidence to show that Fsp1 is required for EMT was performed in renal cells [33], which are likely to activate an EMT program distinct from that in breast cells. Furthermore, the authors introduced studies to show that Fsp1 is not integral to all EMT programs [34] and that whole body Fsp1 knockout mice are still able to undergo EMT during development [35]. Only a small fraction of cancer cells undergoing EMT, as defined by Snail or Zeb1 expression, co-expresses Fsp1. These results bring into question the utility of the Fsp1-Cre transgene as a driver to mark cells that have undergone EMT in breast cancer.
It is possible that the desire to suppress EMT by forced expression of miR-200 (known to directly inhibit Zeb1) might also activate EMT-independent phenotypes that are important for metastasis. Fischer and colleagues explored the potential dispensability of EMT in breast cancer by presenting additional data to show that Zeb1 was expressed exclusively in GFP+ mesenchymal, but not in RFP+ epithelial cells [36]. They indicated that in the Vim-CreER model GFP+ EMT cells constituted ~5% of the primary tumor, but were not found in metastases. Finally, miR-200 overexpression recapitulates EMT suppression with increased Ecad and occludin levels and concomitant decreased Vimentin and Fsp1 levels [36].
In summary, the necessity or dispensability of EMT seems to be context dependent with more evidence underscoring the necessity of EMT. In addition, discrepancies may be the result of pEMT and differences in sensitivities of the techniques used, or low enrichment of the cells that underwent pEMT. In order to demonstrate that metastasis occurs in an EMT-independent fashion, it might require the ability to track Ecad expression and cellular localization throughout intravasation, circulation and extravasation of tumor cells. Evaluating Ecad expression as “positive or negative” will not be able to account for pEMT, where a subtle loss of Ecad expression may be required and sufficient for metastasis. Therefore, tracking tumor cells throughout the metastatic cascade in vivo, as well as more effective methods to isolate single cells at discrete time-points, will improve our understanding of EMT in various malignancies.
MET and tumor metastasis
Ecad expression at a metastatic site is employed as possible evidence for MET. This relies upon the assumption that EMT occurred earlier in the metastatic cascade, and there is re-expression of Ecad at the metastatic site. Alternatively, it is possible that tumor cells never lost Ecad expression or Ecad expression is perturbed minimally (e.g. in pEMT). This section will focus on evidence that demonstrates a pro-metastatic role for Ecad and the existence of MET [37] across three key cancers – breast, ovarian, and pancreas.
Breast Cancer
Certain subtypes of breast cancer are an intriguing exception to the “Ecad-as-tumor-suppressor” model. In particular, invasive ductal carcinoma has been shown to express Ecad at metastatic sites. One study [38] showed that of 23 paired primary tumor-metastasis samples in patients with invasive ductal breast cancer, all metastases expressed Ecad, 10 of which had higher Ecad expression at the metastatic site compared to the primary site. This observation was reproduced in other studies [39,40].
Inflammatory breast cancer (IBC), which can be either ductal or lobular, has been also shown to express Ecad virtually uniformly at primary and metastatic sites. In one cohort of IBC tissues, all 20 patient samples (18 invasive ductal carcinoma and 2 invasive lobular carcinoma) expressed Ecad at the primary site and at all tumor emboli found in lymphatics [41]. These findings are supported by another IBC tissue cohort [42]. In short, IBC is remarkable for its propensity to form microemboli that enter the dermal lymphatic system [43]. To place this in the context of metastasis, microemboli are thought to be the direct result of CTCs from primary tumors, obstructing capillaries and forming metastatic tumors. This suggests that intravasation, the entry of tumor cells into the lymphovascular system, is accelerated. Using a novel transplantation model created specifically to model IBC [44], Ecad antibody injections caused the dissolution of the metastases observed in transplanted mice. Additionally, the metastatic properties of IBC lines could be abrogated through transfection with a dominant-negative Ecad. Therefore, these tumor microemboli are dependent upon functional Ecad.
The regulation of Ecad may be driven by environmental factors that control Ecad promoter methylation. Primary human breast cancers exhibit heterogeneous Ecad promoter methylation early in their development [45]. Methylation increases during invasion but decreases in the formation of spheroid cultures, suggesting that environmental cues determine epigenetic patterns regulating epithelial-mesenchymal phenotypes, a mechanism that would support the decreased primary tumor Ecad expression and increased metastatic tumor Ecad expression in the human tissue studies.
Functional evidence that MET is affected by environmental cues was observed in spontaneous lung metastasis models that illustrated the role of the pre-metastatic niche in modulating EMT-MET plasticity [46]. In brief, myeloid cells in the pre-metastatic lung deposit the ECM component versican, which induces MET by attenuating SMAD2 levels and promoting cell proliferation. In addition to myeloid cells, fibroblasts have been implicated in fostering a MET-promoting environment at the metastatic site. Using the same spontaneous lung metastasis model as Gao et al. [46], del Pozo Martin et al. [47] demonstrated that disseminated metastatic cells that express AXL, a receptor related to the EMT phenotype, activate lung fibroblasts through THBS2. These activated fibroblasts, in turn, inhibit EMT in the disseminated cancer cells. The MET-like cancer cells demonstrate a decrease in TGF-β signaling mediated through SMAD2–3. Consistent with the findings by Gao et al. [46], these MET-like cells show higher proliferation levels, suggesting a possible reason why MET fosters metastatic growth (Figure 2) [47]. Remarkably, forced AXL expression leads to a significant inhibition of metastatic outgrowth in a tail vein injection model[47]. Evidence that environmental cues induce MET has also been reported in breast cancer metastasis to the bone [48]. Esposito et al. demonstrated the necessity of E-selectin for bone metastases, and elucidated a non-canonical form of MET. Under these experimental conditions, Ecad expression is maintained, although EMT-TFs expression are largely unperturbed [48].
Ovarian Cancer
The ovarian surface epithelium, from which most ovarian cancers derive, is normally Ecad- [49], but it shows a remarkable upregulation of Ecad during ovarian cancer progression [49–52]. This acquisition of Ecad at the primary tumor site in ovarian cancers is not reversed during omental metastasis [53], and ovarian cancer cells in pleural and peritoneal effusions show Ecadhigh levels [54]. Functionally, Ecad transduction into normal ovarian epithelial lines is associated with the production of the tumor antigen CA-125 [55], and, much like in the case of IBC, the use of Ecad antibody results in dissociation of spheroid ovarian cancer cell clusters with presumed effects on their ability to survive in effusions [56]. The phenomenon of early-MET is not specific to ovarian cancer, and has been demonstrated in small cell lung cancer in which MET is thought to occur in peripheral circulation prior to metastatic colonization [57].
Pancreatic Cancer
Pancreatic cancer metastasis has also been linked to MET. Using a lineage labeling approach to track dissemination in a spontaneous metastatic model, CTCs were observed to express EpCAM [58]. Additionally, consistent with data on MET correlating with metastasis size in breast cancer [59], this same PDAC model demonstrated that the epithelial properties of metastatic liver lesions increased in proportion to their size [60]. Specifically, epithelial markers such as Ecad and Claudin-7 increased as disseminated cells evolved from isolated tumor cells to micrometastatic and macrometastatic clusters. Conversely, mesenchymal markers, such as FSP1 and ZEB1, were decreased. Human tissue staining confirmed the FSP1 pattern, with micrometastatic lesions having the highest FSP1 expression compared to both the primary tumor and the macrometastatic lesions. This suggests that the larger the liver metastasis becomes, the more it recapitulates the primary tumor’s epithelial properties.
Functional studies with the EMT-TF Prrx1 have corroborated the notion of MET in PDAC. Specifically, dissemination is modulated by isoform-switching between a MET-promoting isoform, Prrx1a, and an EMT-promoting isoform, Prrx1b [61]. Activation of Prrx1a (i.e., activation of MET) or suppression of Prrx1b (i.e., suppression of EMT) in orthotopic transplantation models promoted metastatic outgrowth in the liver.
It has been observed that CTCs display a mixture of Ecadlow and Ecadhigh states, but the metastases are overwhelmingly Ecadhigh [17]. Interestingly, metastatic lesions that are greater than three cells are entirely Ecad+. Aiello et al. demonstrated a similar pattern in liver metastases emerging from pancreatic cancer (Figure 2) [60]. One possibility is that EMT-MET may be in equilibrium during circulation, but MET becomes predominant at the metastatic site.
An important counter-model to EMT is “clustered migration”, or “collective invasion” [62]. The concept of collective invasion espouses the premise that tumor cells retain their epithelial features but co-opt cancer associated fibroblasts (CAFs) to collectively invade [63]. Thus, invasive tumor cells do not need to undergo EMT [64]. It is possible these clusters of cells enter circulation with a greater proclivity for metastasis. Budding of small clusters of well-differentiated cells may be a harbinger for worse outcomes [65,66]. However, it is also possible that the leading edge of the invasive front comprises cells that do undergo EMT and those in the bulk of the cluster do not.
In summary, a substantial amount of research investigating breast, ovarian and pancreatic cancers suggests the importance of MET [67]. There still remains much to be done in terms of understanding the dynamics between a cell undergoing EMT versus MET (or an intermediate state). Technological advances, such as cell tracking and single cell sequencing, will help pave the way for studying the mechanisms of plasticity necessary for these transitions.
Necessity of MET
While there is a wealth of data demonstrating that cells re-establish epithelial characteristics, such as membranous Ecad expression as described above, there is less evidence that this process is essential for metastasis. The degree to which cells must shift along the mesenchymal-to-epithelial spectrum must also be considered. Are these epithelial characteristics a driver of the metastatic colonization and outgrowth, or is it a passenger effect of changes originating from stemness? Does the metastatic destination or the site of the primary tumor matter?
To determine which potential transcriptional factors may function as drivers of metastatic colonization, Dykxhoorn et al. used four isogenic murine breast cancer lines, each exhibiting different metastatic ability [68]. Following implantation into the mammary fat pad, the group compared the one cell line able to form macroscopic metastases to the other three lines. They found that miR-200 was expressed significantly higher in this particular cell line, along with the expected decrease in Zeb2 expression and enhanced Ecad expression. They further underscored the importance of miR-200 in colonization by overexpressing it in a cell line capable of invading metastatic tissues but failing to colonize. Upon miR-200 overexpression, this cell line acquired epithelial properties (including decreased Zeb2 expression, decreased Snai1 expression, and increased Cdh1 expression). Importantly, miR-200 overexpressed cells were now significantly more likely to form metastases, specifically in the lung. The authors concluded that some tumors are able to colonize distant sites through MET [68].
Tsai et al. provided evidence for the requirement of MET, which they refer to as “reversible EMT”, in the context of squamous cell carcinoma (SCC) [69]. They employed a mouse model whereby Twist1 expression is regulated in a reversible manner. Upon doxycycline treatment, either orally or topically, papillomas form, invaginate into the skin and form SCCs. Induction of Twist1 promoted distant metastases in 86% of the mice treated topically and 23% of the mice treated orally. Twist1 induction activates EMT and promotes tumor cell intravasation (69). Additionally, EMT promotes tumor cell extravasation. Yet, loss of Twist1 is necessary for tumor cells to proliferate (Ki-67+) and form macrometastases. Because the expression of other EMT-TFs are not described, it is possible that the EMT pathway is Twist-independent at the metastatic site, but it is more likely that the reversion of EMT, namely MET, is required (Figure 2). These findings suggest that suppression of the initiating EMT signal is required for the formation of macrometastases [69].
Padmanaban et al. used the luminal MMTV-PyMT invasive ductal carcinoma model with a Cre-inducible deletion of Cdh1 [64]. Primary tumor organoids from these mouse models were infected with Cre, and then embedded in collagen I. Compared to controls, Cdh1 knockout organoids had increased invasion and dissemination, but showed lower migratory persistence and displacement. In vivo transplanted organoids without Cdh1 were smaller and the number of macrometastases was significantly reduced (by 15-fold) compared to control tumors [64]. In addition, mice with Ecad- tumors had fewer CTCs by 7.5-fold compared to control Ecad+ tumors. These results were recapitulated in multiple breast cancer models. Interestingly, forced Ecad loss did not significantly affect expression of canonical EMT transcripts, suggesting that Ecad loss affects multiple steps of metastasis without forcing a completely epithelial or mesenchymal transition. Additionally, Ecad loss forces the cells into a TGFβ-dependent ROS-mediated apoptosis. These results point to the necessity of Ecad for effective migration and survival of the metastatic cells, and they highlight a pro-metastatic role of Ecad and MET [64].
Recent work from our group is revealing in the context of metastatic PDAC. We demonstrated that p120-catenin (ctn) loss in the KPCY PDAC model is sufficient to shift the cells’ identity from epithelial to mesenchymal, with the expected loss of Ecad. Upon p120-ctn/Ecad loss, the number of metastases significantly increases, along with a dramatic change from predominantly liver metastases to predominantly lung metastases (Figure 2) [70]. p120-ctn re-expression in cells without p120ctn or Ecad is sufficient to restore liver tropism. Interestingly, liver metastases in human PDAC patients are more epithelial relative to primary tumors. This might suggest that, in the context of PDAC, MET and Ecad expression are essential for metastasis to specific sites such as the liver but not to the lung [70].
Somarelli et al. used three different mouse models of prostate cancer, two of which metastasize in a MET-independent manner and one model which metastasizes in a MET-dependent manner [71]. To study MET in vivo, the group developed a fluorescent-based system whereby epithelial-specific skipping of exon IIIc from fibroblast growth factor receptor 2 (FGFR2) results in an in-frame Cre gene and protein expression. Using a CMV-Lox-DsRed-Stop-Lox-eGFP, Ds-Red expression is specifically confined to mesenchymal cells and eGFP expression marks the epithelial cells. In the prostate cancer cell lines AT3 and DU145, MET was shown to be a rare event. Next, the group replaced the split Cre gene with a “suicide reporter” expressing the A chain of diphtheria toxin, such that cells that undergo MET undergo cell death (Table 1). AT3 cells grew out after selection of the vector, whereas a third prostate cancer cell line, DT, was unable to grow. Upon tail vein injection of the AT3 cells, lung metastases formed with confirmed expression of the MET-suicide gene. Similar results were obtained from tail-vein injections with DU145 cells, again suggesting that MET is not required for metastasis to the lungs, at least in the context of these two prostate cancer lines [71]. Context is very likely important in terms of MET necessity. Ecad is essential for lung metastasis in a breast cancer cell line [64], whereas a pancreatic cancer cell line with Ecad loss demonstrates organotropism for the lung [70].
Concluding Remarks
We have focused in this review article on the in vivo evidence of EMT and MET as well as some of the evidence that is either opposed or lacking. Divergent findings do not represent an indictment of the arguments, but underscore three pivotal points. First, the presence of EMT and/or MET is very much context dependent with cell type and tissue type are of paramount importance. Second, as much as possible, research and conclusions therein require in vivo evidence, especially using lineage tracing of cells from their origin to their ultimate destination. Lastly, current technologies that revolve around (but not limited to) single cell genomics, multiplex immunofluorescence, and live imaging will be of great utility for assessing temporal and spatial cellular plasticity and dynamics (see Outstanding Questions). Ultimately, how we translate past, current and evolving knowledge into translational targeted therapeutics will be the next, exciting step in the field of cancer cell plasticity (Box 2).
OUTSTANDING QUESTIONS.
What factors determine the necessity of EMT?
What factors determine the necessity of MET?
Are there conditions in which EMT or MET is dispensable?
At what stage(s) within the metastatic cascade, does cellular plasticity become prominent?
How can cells along the EMT-MET spectrum be targeted in a clinically-relevant manner?
Box 2: Clinical Implications.
Given the variability with which EMT and MET are deemed essential and pro-metastatic, it becomes unclear if and when in disease progression therapies targeting these pathways should be clinically administered [76]. In addition, it is important to consider the impact that FDA-approved therapies (not directly made to target EMT-MET) have on cellular plasticity.
Recently, Soundararajan et al. delve into the role that EMT has in preventing the efficacy of immunotherapy [77]. It has been shown that tumor cell PD-L1 expression (important for tumor cell immune evasion) is upregulated by EMT pathways, namely the miR-200/ZEB1 signaling axis [78]. In addition, EMT contributes to the recruitment of tumor-associated-macrophages, furthering the immunosuppressive effects of this pathway [79]. In the context of improving immunotherapy efficacy, inhibition of EMT may be an effective strategy. Additionally, an active clinical trial in Chronic Lymphocytic Leukemia patients uses an AXL kinase inhibitor, TP-0903, which has been shown previously to inhibit EMT (http://www.clinicaltrials.gov/ct2/show/NCT03572634). In fact, as of 2019, there have been over 50 clinical trials related to targeting EMT, indirectly or directly [72,73, http://clinicaltrials.gov/] while much less has emerged to target MET.
Given the previous evidence that MET is required for metastatic outgrowth, at least in some contexts [64], it is somewhat surprising that more attention has not been paid to MET inhibition. For example, in clinical situations where the tumor has already metastasized, treatment with an EMT-inhibiting agent (potentially promoting MET) might be deleterious to the primary or circulating cancer cells, while simultaneously benefiting the metastatic cells. An ideal, albeit technically challenging, therapeutic strategy might involve targeting the primary tumor and circulating tumor cells with EMT inhibitors, while selectively targeting metastatic tumors with a MET inhibitor. Future advances in detection of micro-metastasis and targeted drug-delivery have the potential to make this kind of strategy feasible. In the meantime, a more thorough understanding of how cells at different ends of the EMT-MET spectrum respond to current therapeutics is needed.
HIGHLIGHTS.
Cellular plasticity plays an important role in tumor progression, metastasis and chemoresistance.
Cells may shift along the EMT-MET spectrum, such that EMT and MET may not be dichotomous fates as evidenced by partial EMT.
The requirements of EMT and MET in primary tumorigenesis and metastasis are context dependent and require rigorous evaluation of the model systems employed.
ACKNOWLEDGEMENTS
This work was supported by the following funding sources: NCI P30 CA013696, NIH R01 DK060694 (AKR), American Cancer Society RP-10-033-01-CCE (AKR), NIH F30 CA180601 (BB and AKR), American Cancer Society - Fairfield County Comedy Against Cancer Postdoctoral Fellowship, PF-19-227-01-CMS (AMC) and the American Gastroenterology Association Research Scholar Award (JRP). Figures were made with BioRender.com. Due to space limitations, we apologize for our inability to cite all relevant references, including review articles and editorials.
Glossary
- AXL (AXL receptor tyrosine kinase)
cell surface receptor, part of the TAM kinase family
- CTCs (Circulating Tumor Cell)s
cancer cells that travel from a primary tumor to a metastatic site by way of the vasculature or lymphatic system
- Cdh1 (Cadherin-1)
the name of the gene that produces E-Cadherin. Cdh1 is a member of the cadherin super family and is also known as CD324/
- E-Cad (E-Cadherin)
the most well-studied member of the cadherin family. It binds p120-catenin and beta-catenin at the cellular membrane to establish cell-to-cell adhesion
- ECM (Extracellular Matrix)
a network of macromolecules in the extracellular space, consisting of collagen, glycoprotein, and enzymes
- EMT (epithelial-to-mesenchymal transition)
a complex cellular pathway in which epithelial cells lose cell-to-cell adhesion (characterized by membranous E-Cad loss) and gain mesenchymal characteristics (characterized by increased N-Cadherin expression and migratory capabilities). EMT is known to occur during mammalian development, wound healing, and cancer metastasis
- EpCAM (Epithelial cell adhesion molecule)
a glycoprotein on the cellular membrane involved in cell-to-cell adhesion and multiple cell signaling pathways within epithelial cells. It is also known as CD326, TACSTD1 and 17–1A antigen
- Fsp1 (Fibroblast-specific protein 1)
a cytoplasmic calcium-binding protein primarily expressed in fibroblasts. As a member of the S100 superfamily, it is also known as S100A4.
- Her2 (Human epidermal growth factor receptor 2)
a human epidermal growth factor receptor family member, widely known as an oncogene important in breast cancer progression. It is also known as ERBB2, CD340 and neu
- Kras (Kirsten Rat Sarcoma virus)
a GTPase oncogene in the RAS/MAPK pathway, frequently mutated and/or overexpressed in cancers (notably lung, colorectal and pancreatic cancer)
- MET (mesenchymal-to-epithelial transition)
a cellular pathway by which mesenchymal cells decrease motility and establish epithelial characteristics (signified by increased expression of E-Cad and increased cellular polarity).
- miR-200 (microRNA 200)
a family of microRNAs (including miR-200a, miR-200b, miR-200c, miR-141 and miR-429). Members of miR-200 are highly expressed in epithelial cells and have been shown to repress the EMT pathway
- Neoplastic stage
a term that means the benign or malignant growth of cells, the formation of a tumor. This state is typically characterized by accumulated genetic damage and disorganized, persistent growth
- P53 (tumor protein p53)
a tumor suppressor that regulates cell cycle and is frequently mutated in cancer
- Pdx1 (Pancreatic and duodenal homeobox 1)
a transcription factor required for pancreatic development and is used to identify pancreatic ductal cells
- Preneoplastic stage
a cellular state preceding neoplasia typically characterized by hypertrophy, hyperplasia, metaplasia and/or dysplasia
- Pten (Phosphatase and tensin homolog)
a tyrosine phosphatase and tumor suppressor involved in cell cycle regulation
- ROS (reactive oxygen species)
an unstable molecule or free radical that, in over-abundance, causes DNA, RNA and protein damage to cells
- Secreted factors
factors can include cytokines, structural components of the ECM, receptors, enzymes, and other chemical factors. For example, TGF-beta can be secreted by macrophages during the initiation of EMT
- Slug
the gene that encodes Snai2/Snail2, a zinc finger transcription factor involved in the promotion of EMT
- Smad2 (Mothers against decapentaplegic homolog 2)
an intracellular signaling transducer and transcriptional co-factor activated by TGF-beta signaling. In cooperation with SMAD4, SMAD2 binds to TRE elements and activates transcription
- Snail
the gene that encodes Snai1/Snail1, a zinc finger transcription factor involved in the promotion of EMT
- Sox4 (SRY-Box Transcription Factor 4)
a member of the SOX family of transcription factors important for regulating expression of genes involved in embryonic development and cell differentiation
- TGF-beta (Transforming growth factor beta)
a secrete cytokine and member of the transforming growth factor superfamily. Upon binding with its receptor, it can affect pathways such as EMT, differentiation, proliferation and apoptosis
- Twist1/2 (Twist-related protein 1/2)
Twist1/2 are basic helix-loop-helix transcription factors. Cells undergoing EMT demonstrate increased expression of Twist1/2
- Vimentin
a type III intermediate filament structural protein, characteristic of mesenchymal cells
- Zeb1/2 (Zinc finger E-box-binding homeobox 1/2)
Zeb1 and Zeb2 are paralogs belonging to the Zeb family of transcription factors. They are known to promote EMT
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Mills JC et al. (2019) Nomenclature for cellular plasticity: are the terms as plastic as the cells themselves? EMBO J. 38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta PB et al. (2019) Phenotypic Plasticity: Driver of Cancer Initiation, Progression, and Therapy Resistance. Cell Stem Cell, 24, 65–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polyak K and Weinberg RA (2009) Transitions between epithelial and mesenchymal states: Acquisition of malignant and stem cell traits. Nature Reviews Cancer, 9, 265–273 [DOI] [PubMed] [Google Scholar]
- 4.Dongre A and Weinberg RA (2019) New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 20, 69–84 [DOI] [PubMed] [Google Scholar]
- 5.Pastushenko I and Blanpain C (2019) EMT Transition States during Tumor Progression and Metastasis. Trends in Cell Biology, 29, 212–226 [DOI] [PubMed] [Google Scholar]
- 6.Brabletz T et al. (2018) EMT in cancer. Nature Reviews Cancer, 18, 128–134 [DOI] [PubMed] [Google Scholar]
- 7.Jolly MK et al. (2017) EMT and MET: necessary or permissive for metastasis? Molecular Oncology, 11, 755–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santamaria PG et al. (2017) EMT: Present and future in clinical oncology. Molecular Oncology, 11, 718–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Derynck R and Weinberg RA (2019) EMT and Cancer: More Than Meets the Eye. Developmental Cell, 49, 313–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kröger C et al. (2019) Acquisition of a hybrid E/M state is essential for tumorigenicity of basal breast cancer cells. Proc. Natl. Acad. Sci. U. S. A. 116, 7353–7362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panchy N et al. (2020) Integrative Transcriptomic Analysis Reveals a Multiphasic Epithelial–Mesenchymal Spectrum in Cancer and Non-tumorigenic Cells. Front. Oncol. 9, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aiello NM and Kang Y (2019) Context-dependent EMT programs in cancer metastasis. J. Exp. Med. 216, 1016–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grigore A et al. (2016) Tumor Budding: The Name is EMT. Partial EMT. J. Clin. Med. 5, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saitoh M (2018) Involvement of partial EMT in cancer progression. J. Biochem. 164, 257–264 [DOI] [PubMed] [Google Scholar]
- 15.Ledford H (2011) Cancer theory faces doubts. Nature 472, 273–273 [DOI] [PubMed] [Google Scholar]
- 16.Stemmler MP et al. (2019) Non-redundant functions of EMT transcription factors. Nature Cell Biology, 21, 102–112 [DOI] [PubMed] [Google Scholar]
- 17.Beerling E et al. (2016) Plasticity between Epithelial and Mesenchymal States Unlinks EMT from Metastasis-Enhancing Stem Cell Capacity. Cell Rep. 14, 2281–2288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bornes L et al. (2019) Fsp1-Mediated Lineage Tracing Fails to Detect the Majority of Disseminating Cells Undergoing EMT. CellReports 29, 2565–2569.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harper KL et al. (2016) Mechanism of early dissemination and metastasis in Her2+ mammary cancer. Nature 540, 588–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moody SE et al. (2005) The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell 8, 197–209 [DOI] [PubMed] [Google Scholar]
- 21.Ye X et al. (2015) Distinct EMT programs control normal mammary stem cells and tumour-initiating cells. Nature 525, 256–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rhim AD et al. (2012) EMT and dissemination precede pancreatic tumor formation. Cell 148, 349–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aiello NM et al. (2018) EMT Subtype Influences Epithelial Plasticity and Mode of Cell Migration. Dev. Cell 45, 681–695.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruscetti M et al. (2015) Tracking and functional characterization of epithelial-mesenchymal transition and mesenchymal tumor cells during prostate cancer metastasis. Cancer Res. 75, 2749–2759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chanrion M et al. (2014) Concomitant Notch activation and p53 deletion trigger epithelial-to-mesenchymal transition and metastasis in mouse gut. Nat. Commun. 5, 5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng X et al. (2015) Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 527, 525–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krebs AM et al. (2017) The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Nat. Cell Biol. 19, 518–529 [DOI] [PubMed] [Google Scholar]
- 28.Wellner U et al. (2009) The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat. Cell Biol. 11, 1487–95 [DOI] [PubMed] [Google Scholar]
- 29.Fischer KR et al. (2015) Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 527, 472–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korpal M et al. (2011) Direct targeting of Sec23a by miR-200s influences cancer cell secretome and promotes metastatic colonization. Nat. Med. 17, 1101–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aiello NM et al. (2017) Upholding a role for EMT in pancreatic cancer metastasis. Nature 547, E7–E8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ye X et al. (2017) Upholding a role for EMT in breast cancer metastasis. Nature 547, E1–E6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okada H et al. (1997) Early role of Fsp1 in epithelial-mesenchymal transformation. Am. J. Physiol. Physiol. 273, F563–F574 [DOI] [PubMed] [Google Scholar]
- 34.Österreicher CH et al. (2011) Fibroblast-specific protein 1 identifies an inflammatory subpopulation of macrophages in the liver. Proc. Natl. Acad. Sci. U. S. A. 108, 308–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xue C et al. (2003) The gatekeeper effect of epithelial-mesenchymal transition regulates the frequency of breast cancer metastasis. Cancer Res. 63, 3386–3394 [PubMed] [Google Scholar]
- 36.Fischer KR et al. (2017) Fischer et al. reply. Nature 547, E1–E628682326 [Google Scholar]
- 37.Rodriguez FJ et al. (2012) E-cadherin’s dark side: Possible role in tumor progression. Biochim. Biophys. Acta - Rev. Cancer 1826, 23–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kowalski PJ et al. (2003) E-cadherin expression in primary carcinomas of the breast and its distant metastases. Breast Cancer Res. 5, R217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bukholm IK et al. (2000) Re-expression of E-cadherin, alpha-catenin and beta-catenin, but not of gamma-catenin, in metastatic tissue from breast cancer patients. J. Pathol. 190, 15–19 [DOI] [PubMed] [Google Scholar]
- 40.Jeschke U et al. (2007) Expression of E-cadherin in human ductal breast cancer carcinoma in situ, invasive carcinomas, their lymph node metastases, their distant metastases, carcinomas with recurrence and in recurrence. Anticancer Res. 27, 1969–74 [PubMed] [Google Scholar]
- 41.Kleer CG et al. (2001) Persistent E-Cadherin Expression in Inflammatory Breast Cancer. Mod. Pathol. 14, 458–464 [DOI] [PubMed] [Google Scholar]
- 42.Colpaert CG et al. (2003) Inflammatory breast cancer shows angiogenesis with high endothelial proliferation rate and strong E-cadherin expression. Br. J. Cancer 88, 718–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gong Y (2008) Pathologic aspects of inflammatory breast cancer: part 2. Biologic insights into its aggressive phenotype. Semin. Oncol. 35, 33–40 [DOI] [PubMed] [Google Scholar]
- 44.Tomlinson JS et al. (2001) An intact overexpressed E-cadherin/alpha,beta-catenin axis characterizes the lymphovascular emboli of inflammatory breast carcinoma. Cancer Res. 61, 5231–41 [PubMed] [Google Scholar]
- 45.Graff JR et al. (2000) Methylation patterns of the E-cadherin 5’ CpG island are unstable and reflect the dynamic, heterogeneous loss of E-cadherin expression during metastatic progression. J. Biol. Chem. 275, 2727–32 [DOI] [PubMed] [Google Scholar]
- 46.Gao D et al. (2012) Myeloid Progenitor Cells in the Premetastatic Lung Promote Metastases by Inducing Mesenchymal to Epithelial Transition. Cancer Res. 72, 1384–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.del Pozo Martin Y et al. (2015) Mesenchymal Cancer Cell-Stroma Crosstalk Promotes Niche Activation, Epithelial Reversion, and Metastatic Colonization. Cell Rep. 13, 2456–2469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Esposito M et al. (2019) Bone vascular niche E-selectin induces mesenchymal–epithelial transition and Wnt activation in cancer cells to promote bone metastasis. Nat. Cell Biol. 21, 627–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sundfeldt K et al. (1997) E-cadherin expression in human epithelial ovarian cancer and normal ovary. Int. J. Cancer 74, 275–280 [DOI] [PubMed] [Google Scholar]
- 50.Daraï E et al. (1997) Expression of cadherins in benign, borderline, and malignant ovarian epithelial tumors: A clinicopathologic study of 60 cases. Hum. Pathol. 28, 922–928 [DOI] [PubMed] [Google Scholar]
- 51.Soler AP et al. (1997) Expression of E-cadherin and N-cadherin in surface epithelial-stromal tumors of the ovary distinguishes mucinous from serous and endometrioid tumors. Hum. Pathol. 28, 734–739 [DOI] [PubMed] [Google Scholar]
- 52.Inoue M et al. (1992) Expression of E-cadherin in normal, benign, and malignant tissues of female genital organs. Am. J. Clin. Pathol. 98, 76–80 [DOI] [PubMed] [Google Scholar]
- 53.Köbel M et al. (2011) Biomarker Expression in Pelvic High-grade Serous Carcinoma. Int. J. Gynecol. Pathol. 30, 366–371 [DOI] [PubMed] [Google Scholar]
- 54.Davidson B et al. (2000) E-cadherin and alpha-, beta-, and gamma-catenin protein expression is up-regulated in ovarian carcinoma cells in serous effusions. J. Pathol. 192, 460–469 [DOI] [PubMed] [Google Scholar]
- 55.Auersperg N et al. (1999) E-cadherin induces mesenchymal-to-epithelial transition in human ovarian surface epithelium. Proc. Natl. Acad. Sci. U. S. A. 96, 6249–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elloul S et al. (2010) Mesenchymal-to-epithelial transition determinants as characteristics of ovarian carcinoma effusions. Clin. Exp. Metastasis 27, 161–172 [DOI] [PubMed] [Google Scholar]
- 57.Hamilton G and Rath B (2017) Mesenchymal-epithelial transition and circulating tumor cells in small cell lung cancer In Advances in Experimental Medicine and Biology 994 pp. 229–245, Springer New York LLC; [DOI] [PubMed] [Google Scholar]
- 58.Rhim AD et al. (2012) EMT and Dissemination Precede Pancreatic Tumor Formation. Cell 148, 349–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bonnomet A et al. (2012) A dynamic in vivo model of epithelial-to-mesenchymal transitions in circulating tumor cells and metastases of breast cancer. Oncogene 31, 3741–3753 [DOI] [PubMed] [Google Scholar]
- 60.Aiello NM et al. (2016) Metastatic progression is associated with dynamic changes in the local microenvironment. Nat. Commun. 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takano S et al. (2016) Prrx1 isoform switching regulates pancreatic cancer invasion and metastatic colonization. Genes Dev. 30, 233–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Friedl P et al. (2012) Classifying collective cancer cell invasion. Nature Cell Biology, 14, 777–783 [DOI] [PubMed] [Google Scholar]
- 63.Ilina O et al. (2018) Intravital microscopy of collective invasion plasticity in breast cancer. DMM Dis. Model. Mech. 11, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Padmanaban V et al. (2019) E-cadherin is required for metastasis in multiple models of breast cancer. Nature, 573, 439–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maheswaran S and Haber DA (2015) Cell fate: Transition loses its invasive edge. Nature, 527, 452–453 [DOI] [PubMed] [Google Scholar]
- 66.Aceto N et al. (2015) En Route to Metastasis: Circulating Tumor Cell Clusters and Epithelial-to-Mesenchymal Transition. DOI: 10.1016/j.trecan.2015.07.006 [DOI] [PubMed] [Google Scholar]
- 67.Hugo H et al. (2007) Epithelial - Mesenchymal and mesenchymal - Epithelial transitions in carcinoma progression. Journal of Cellular Physiology, 213, 374–383 [DOI] [PubMed] [Google Scholar]
- 68.Dykxhoorn DM et al. (2009) miR-200 enhances mouse breast cancer cell colonization to form distant metastases. PLoS One 4, e7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tsai JH et al. (2012) Spatiotemporal Regulation of Epithelial-Mesenchymal Transition Is Essential for Squamous Cell Carcinoma Metastasis. Cancer Cell 22, 725–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reichert M et al. (2018) Regulation of Epithelial Plasticity Determines Metastatic Organotropism in Pancreatic Cancer. Dev. Cell 45, 696–711.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Somarelli JA et al. (2016) Distinct routes to metastasis: Plasticity-dependent and plasticity-independent pathways. Oncogene 35, 4302–4311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chase DR et al. (1984) Keratin in epithelioid sarcoma. An immunohistochemical study. Am. J. Surg. Pathol. 8, 435–441 [DOI] [PubMed] [Google Scholar]
- 73.Marck VL Van and Bracke ME (2013) Epithelial-Mesenchymal Transitions in Human Cancer. at <https://www.ncbi.nlm.nih.gov/books/NBK6362/>
- 74.Pastushenko I et al. (2018) Identification of the tumour transition states occurring during EMT. Nature 556, [DOI] [PubMed] [Google Scholar]
- 75.Nieto MA et al. (2016) EMT: 2016. Cell, 166, 21–45 [DOI] [PubMed] [Google Scholar]
- 76.Williams ED et al. (2019) Controversies around epithelial–mesenchymal plasticity in cancer metastasis. Nat. Rev. Cancer 19, 716–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Soundararajan R et al. (2019) Targeting the interplay between epithelial-to-mesenchymal- transition and the immune system for effective immunotherapy. Cancers, 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen L et al. (2014) Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat. Commun. 5, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Low-Marchelli JM et al. (2013) Twist1 induces CCL2 and recruits macrophages to promote angiogenesis. Cancer Res. 73, 662–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Voon DC et al. (2017) The EMT spectrum and therapeutic opportunities. Molecular Oncology, 11, 878–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang XG et al. (2019) Current Advance of Therapeutic Agents in Clinical Trials Potentially Targeting Tumor Plasticity. Frontiers in Oncology, 9, 887–887 [DOI] [PMC free article] [PubMed] [Google Scholar]