Abstract
Background
Although infection with Trypanosoma cruzi is thought to be lifelong, less than half of those infected develop cardiomyopathy, suggesting greater parasite control or even clearance. Antibody levels appear to correlate with T. cruzi (antigen) load. We test the association between a downwards antibody trajectory, PCR positivity and ECG alterations in untreated individuals with Chagas disease.
Methodology/Principal findings
This is a retrospective cohort of T. cruzi seropositive blood donors. Paired blood samples (index donation and follow-up) were tested using the VITROS Immunodiagnostic Products Anti-T.cruzi (Chagas) assay (Ortho Clinical Diagnostics, Raritan NJ) and PCR performed on the follow-up sample. A 12-lead resting ECG was performed. Significant antibody decline was defined as a reduction of > 1 signal-to-cutoff (S/CO) unit on the VITROS assay. Follow-up S/CO of < 4 was defined as borderline/low. 276 untreated seropositive blood donors were included. The median (IQR) follow-up was 12.7 years (8.5–16.9). 56 (22.1%) subjects had a significant antibody decline and 35 (12.7%) had a low/borderline follow-up result. PCR positivity was lower in the falling (26.8% vs 52.8%, p = 0.001) and low/borderline (17.1% vs 51.9%, p < 0.001) antibody groups, as was the rate of ECG abnormalities. Falling and low/borderline antibody groups were predominantly composed of individuals with negative PCR and normal ECG findings: 64% and 71%, respectively.
Conclusions/Significance
Low and falling antibody levels define a phenotype of possible spontaneous parasite clearance.
Author summary
Infection with the single-celled parasite Trypanosoma cruzi (Chagas disease) is thought to be lifelong. However, only a third of infected people develop Chagas cardiomyopathy–the main disease manifestation. This may reflect the different extent to which individuals control the parasite, with some potentially clearing it entirely. In chronically infected immunocompetent patients, a marker of parasite burden is the quantity of antibody against T. cruzi in the blood: more parasite, more immune stimulation, more antibody. In this study we show how antibody levels change over many years in a cohort of untreated patients with Chagas disease. We find that among individuals with falling or low/borderline antibody levels there was a lower rate of parasite detection in the blood and a lower rate of cardiomyopathy. 60% of subjects with falling antibody levels had no evidence of active disease, twice as many as among patients with other antibody trajectories (stable or rising). Our findings support an account of the natural history of Chagas disease in which a proportion of those infected achieve a greater control of the parasite, with some individuals potentially clearing it completely.
Introduction
Chagas disease (CD)–due to infection by Trypanosoma cruzi–is a neglected tropical disease. It affects 6–8 million people worldwide [1] and is responsible for 50,000 deaths annually [2,3]. Vectoral transmission is by contact with the feces of the infected triatomine bug, with approximately 70 million people living in areas at risk of exposure [4]. Acute infection is characterized by a mild illness in which the parasite is readily detected in the blood. This gives way to chronic tissue sequestration, particularly affecting the myocardium. However, the development of clinically apparent cardiomyopathy is variable, with most individuals remaining asymptomatic for life [5]. The mechanisms underlying this process remain poorly understood.
One hurdle to understanding the natural history Chagasic cardiomyopathy is a lack of reliable biomarkers of parasite persistence. Parasitaemia in the chronic phase is both low-grade and intermittent, and consequently diagnosis is based on serology [6,7]. When asymptomatic individuals are systematically screened–such as is the case for blood donors–a third of those identified as seropositive have low antibody levels or discordant test results[8]. These borderline cases show spatial clustering [9] and shared risk factors [8,10,11] with unequivocal seropositive cases. This suggests there was a true exposure to T. cruzi, as opposed to a simple false-positive result.
One explanation for these findings is that a proportion of infected individuals may achieve control of the parasite or, in some cases, completely clear it [12–14]. This would reduce, or completely remove, the antigenic stimulus, leading to slow seroreversion. Indeed, antibody levels have been shown to correlate with peripheral parasitaemia in a cross-sectional blood donor cohort [15]. In that same cohort, antibody trend overtime was measured in a subset of donors with more than one sample time point. The inclusion criteria (positive results on three relatively low-sensitivity assays) led to a selection bias for donors with high antibody levels at index donation. Six donors had a significant decline in antibody level and all were PCR-negative. As such, further data are needed to properly assess the existence of spontaneous seroreversion and its association with parasitaemia. Furthermore, the relationship between antibody level and clinically apparent disease–namely Chagasic cardiomyopathy–has not been reported.
Herein we characterize the trajectory of antibody levels over a number of years in a group of untreated Brazilian seropositive blood donors. We test the hypothesis that individuals with a downwards antibody trajectory have controlled (or potentially cleared) the parasite–as indicated by a negative polymerase chain reaction (PCR) and absence of typical electrocardiographic abnormalities.
Methods
Study design
This is a retrospective cohort of T. cruzi seropositive blood donors identified in routine blood donation screening at the Fundação Pró-Sangue blood bank in São Paulo, Brazil. Blood donations were made between 1996 and 2015. The screening tests varied over this time period due to the availability and cost (see S1 Table). Eligible seropositive donors were identified in institutional records and invited to attend an enrolment appointment in 2017–2018. At this appointment participants answered a questionnaire about Chagas exposure and cardiovascular risk factors. Those that reported previous benznidazole treatment were excluded. At this time a blood sample was taken for T. cruzi polymerase chain reaction (PCR) and serology testing. All participants underwent a standard resting 12-lead electrocardiogram (ECG).
VITROS Immunodiagnostic Products Anti-T.cruzi (Chagas) assay
Original blood donation samples were retrieved from storage and an aliquot taken for processing. The donation samples (1996–2015) and the samples provided at cohort enrolment (2017–2018) were sent to the Vitalant Research Institute (San Francisco, CA) for analysis using the VITROS Immunodiagnostic Products Anti-T.cruzi (Chagas) assay (Ortho Clinical Diagnostics, Raritan NJ). The protocol was performed according to the manufacturer’s instructions. The test is an enzyme-linked immunosorbent assay (ELISA) that utilizes MicroWells coated with a whole cell lysate containing T.cruzi antigens as the solid phase with chemiluminescent detection. Results are reported as the ratio of signal-to-cutoff (S/CO) which is a function of the amount of anti-T. cruzi antibody present in the test sample. The sensitivity and specificity are 97.7% and 100%, respectively [16].
We categorized the VITROS Anti-T. cruzi test results as low- and high-positive using a S/CO value of four as the threshold. This value was shown to be the nadir of a bimodal distribution of VITROS Anti-T. cruzi results in an endemic area of Argentina [8]. As such, it may distinguish patients with resolving or progressive disease trajectories. Furthermore, the difference between S/CO at follow-up and donation was calculated such that negative values indicate a falling S/CO. A decrease in S/CO of greater than one unit was used to define a subpopulation with significantly decreasing antibodies [15].
Polymerase chain reaction (PCR)
Blood samples collected at the study enrollment visit (2017–2018) were tested using a target-capture (TC) real-time (RT) PCR assay, as previously described [17]. Capture of T. cruzi was performed using magnetic beads coated with the three 20-mer capture oligonucleotides:
TCZ 1 CGAGCTCTTGCCCACACGGGAAAAAAAAAAAAAAAAAAAAAAAAAA;
TCZ 2 CCTCCAAGCAGCGGATAGTTCAGGAAAAAAAAAAAAAAAAAAAAAA AAAA and; TCZ 3 TGCTGCASTCGGCTGATCGTTTTCGAAAAAAAAAAAAAAAAAAAAA AAAAAA.
The captured DNA targets were eluted from the magnetic beads and amplified on Applied Biosystems 7500. Briefly, 25uL of DNA was added to 50uL of BSRI PCR mix. The PCR conditions were 10min at 95°C, followed by 45 cycles of 30 sec at 95°C, 30 sec at 64°C, 45 sec at 72°C. After completion of thermal cycling a dissociation step was performed, and the melting curve was analyzed. Product dissociations with one or two peaks at 80–82°C were considered positive if the cycle threshold (CT) was less than 45 cycles. Eight replicate assays were performed and the result was considered positive if at least two replicates produced a specific products based on dissociation analysis.
Electrocardiogram
ECGs were analyzed using an automated system with manual overreading and classification according to the Minnesota code. Major and minor electrocardiographic alterations were defined as previously described [18]. The final ECG classification was “major” if the ECG had any major alterations, “minor” if it had only minor alterations, and “normal” if it had neither minor nor major alterations. Furthermore, a subset of ECG findings was defined as typical of Chagas disease [19] following the 2nd Brazilian consensus on Chagas disease (2015) [20]–see S2 Table.
Statistical analysis
The exposures of interest were the change in VITROS S/CO between donation and follow-up timepoints, and the absolute S/CO value at both these time points separately. The outcome variables were PCR status and ECG alterations at follow-up. We treated the outcome variables in the analysis as follows. Firstly, PCR status was considered in isolation: positive versus negative. Secondly, the ECG was analysed in isolation, considering three different aspects: the presence versus absence of individual ECG alterations; the final ECG classification (major, minor, or normal); and the total number of typical Chagas disease alterations per trace (0, 1, or 2+). Finally, we made a joint PCR and ECG endpoint consisting of participants with both a negative PCR and an ECG free of major alterations (i.e. normal or minor alterations only). This joint endpoint provides the strongest evidence of disease inactivity, as neither parasitaemia nor cardiac involvement were identified.
Univariate analyses comparing PCR and ECG variables between categorical antibody groups were performed with the Chi-squared or Fisher exact test. Furthermore, the continuous S/CO distributions (donation, follow-up, and follow-up–donation) were compared according to the PCR/ECG joint endpoint with the Wilcoxon rank sum test.
Finally, we sought to assess the independent association between antibody level (donation, follow-up and follow-up–donation) and the joint PCR/ECG endpoint. We built multiple logistic regression models with the joint PCR/ECG end point as the dependent variable. We tested age, sex, comorbidities and smoking status as potential confounding variables in the models. Variables were retained in the model if they altered the odds ratio (OR) associated with antibody level by > 5%.
Ethics statement
The study protocol was submitted and approved by the local Ethics committee at Hospital of Clinics–Fundacão Pró-Sangue and CAPESPQ, University of São Paulo (CEP FMUSP 1.604.712). All participants provided written informed consent before enrolment.
Results
Cohort characteristics
The initial cohort consisted of 279 untreated seropositive blood donors. Three false-positive cases (non-reactive VITROS Anti-T. cruzi results at both time points and negative PCR at follow-up) were excluded, resulting in 276 cases for analysis. Twenty-three donation samples were insufficient to run the VITROS Anti-T. cruzi assay. Therefore, analyses involving only the follow-up sample consisted of 276 participants and analyses involving the donation sample consisted of 253 participants. Subject characteristics are presented in Table 1.
Table 1. Characteristics of 276 T. cruzi-seropositive blood donors responding to the follow-up questionnaire (2017–2018).
| Subject characteristics | Full cohort N = 276 n (%) or median (IQR) |
|---|---|
| Age (years) | |
| < 40 | 15 (5.5) |
| 40–49 | 56 (20.4) |
| 50–59 | 96 (35.0) |
| 60–69 | 82 (29.9) |
| > = 70 | 25 (9.1) |
| Sex | |
| Male | 133 (48.2) |
| Female | 143 (51.8) |
| Country of origin | |
| Brazil | 274 (99.3) |
| Argentina | 1 (<1) |
| Bolivia | 1 (<1) |
| Self-reported skin color | |
| White | 128 (46.4) |
| Black | 39 (14.1) |
| Mixed (pardo) | 98 (35.5) |
| Other | 11 (4.0) |
| Educational level | |
| No schooling | 10 (3.6) |
| Incomplete primary schooling | 102 (37.0) |
| Complete primary schooling | 76 (27.5) |
| Complete secondary schooling | 67 (24.3) |
| College, technical or above | 21 (7.6) |
| Comorbidities | |
| Diabetes | 34 (12.6) |
| Renal disease | 12 (4.5) |
| Stroke | 7 (2.5) |
| Myocardial infarction | 9 (3.3) |
| Hypertension | 103 (38.0) |
| High cholesterol | 94 (36.6) |
| Smoking status | |
| Current smoker | 24 (8.7) |
| Ex-smoker | 108 (39.1) |
| Never smoker | 144 (52.2) |
| Follow-up* (years) | 12.7 (8.5–16.9) |
* time (in years) between the original donation date and follow-up visit. PCR–polymerase chain reaction; ECG–electrocardiogram; IQR–interquartile range. Missing data: 6 diabetes; 8 renal disease; 1 stroke; 1 myocardial infarction; 5 hypertension; 19 high cholesterol
VITROS Immunodiagnostic Products Anti-T.cruzi (Chagas) assay
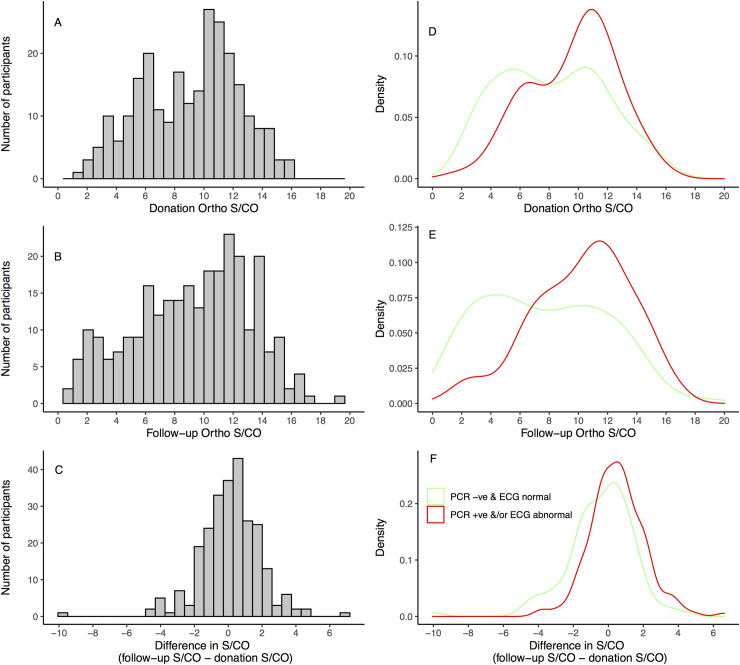
The median (IQR) time period between blood donation and the follow-up appointment was 12.7 years (8.5–16.9). The distributions of S/CO values at donation and follow-up are presented in Fig 1A and Fig 1B. There is a bimodal distribution of ELISA S/CO values in the donation samples. At follow-up the S/CO values are more widely spread and may also represent the superimposition of two distinct distributions, although this is less clear. The change in S/CO between the two time points is shown in Fig 1C and follows a roughly normal distribution.
Fig 1.
Histograms of the VITROS Anti-T. cruzi signal-to-cutoff (S/CO) values among participating blood donors at donation (A) and follow-up (B), and the distribution of the difference in S/CO between the two time points (C). Smoothed kernel density plots of the distributions of donation (D), follow-up (E) and the change in S/CO (F) stratified according to PCR and ECG result (PCR -ve & ECG normal versus PCR+ve &/or ECG abnormal). In panel D, the median [IQR] S/CO in the PCR +ve &/or ECG abnormal donors is 10.2 [7.2 to 11.7], and among PCR-ve & ECG normal donors is 8.2 [5.3 to 10.9], p = 0.001 (Wilcoxon rank sum). In Panel E, comparing the same groups, the median [IQR] values are 10.6 [7.9 to 12.6] versus 7.0 [4.2 to 11.1], respectively, p <0.001 (Wilcoxon rank sum). In panel F, the median [IQR] values are respectively 0.46 [-0.4 to 1.4] and -0.2 [-1.4 to 0.76], p<0.001 (Wilcoxon rank sum).
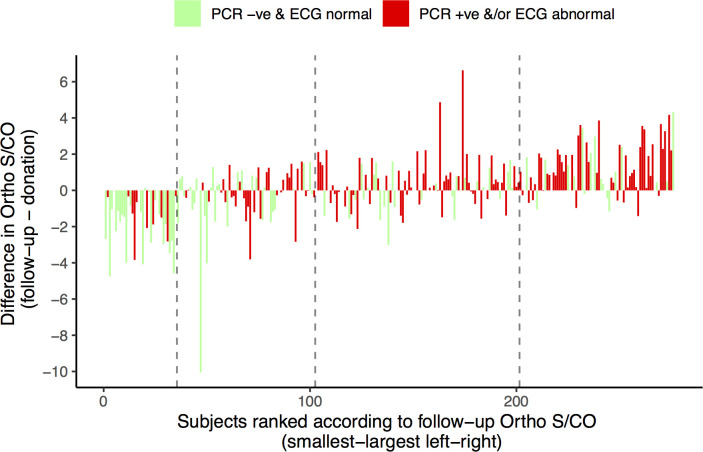
Fig 2 presents the individual changes in S/CO between donation and follow-up for all 253 subjects with both timepoints available. Subjects are ordered according to their follow-up S/CO value. It is apparent from this figure that donors with lower S/CO values at follow-up in general underwent a decline in S/CO compared to their donation sample. By contrast, those with higher follow-up S/CO values in general underwent an increase in S/CO compared to the donation value.
Fig 2. Waterfall plot of the change in S/CO between donation and follow-up visits for 253 subjects with results available for both time points.
Subjects with negative PCR and an ECG free of major alterations (PCR -ve & ECG normal) are represented with green bars, whereas subjects with a positive PCR and/or ECG with a major alterations (PCR +ve &/or ECG abnormal) are shown in red. Subjects are ranked (horizontal axis) according to the final VITROS Anti-T. cruzi ELISA S/CO, such that subjects with the lowest S/CO values at follow-up are shown on the far left, and those with the highest follow-up S/CO values are shown on the far right. Vertical dashed lines mark the rank positions corresponding to S/CO values of 4, 8 and 12 (left to right)–e.g. subjects to the left of the first dashed in had follow-up S/CO values < 4.
Fifty-six (22.1%) subjects had a significant (> 1 S/CO unit) decline in ELISA reactivity and two participants became seronegative (S/CO < 1) at follow-up. The first fell from a S/CO of 1.2 to 0.78 over the course of 5 years. The second fell from a S/CO of 3.3 to 0.56 over 17 years. The S/CO was unchanged (varied by < 1 S/CO between donation and follow-up) in 126 (50%) donors and 71 (28%) underwent an increase of >1 S/CO unit (see S3 Table). Thirty-five (12.7%) donors had a follow-up S/CO of < 4 and 241 (87.3%) of ≥ 4; on the donation sample 19 (7.5%) and 234 (92.5) were < 4 or ≥ 4 respectively. Demographic characteristics and cardiovascular risk factors were similar across the groups defined by ELISA S/CO (S4 Table).
Univariate associations between ELISA S/CO, PCR result and ECG alterations
One hundred and thirty-one (47.5% of 276) subjects had a positive PCR result on the follow-up sample. In donors with S/CO values that fell by more than one S/CO unit, the PCR positivity rate was lower than the rest of the cohort (26.8% vs 52.8%—see Table 2). Further stratifying subjects into stable antibody level (increase/decrease < 1 S/CO) and increasing antibody levels (increase > 1 S/CO) the proportion with positive PCR was similar: 52.4% and 53.5%, respectively (see S3 Table). There was a lower PCR positivity among the donors with low (<4) compared to high (≥ 4) S/CO values on both follow-up and donation samples (Table 2).
Table 2. PCR result and ECG findings at the follow-up visit among T. cruzi-seropositive blood donors.
| Disease parameters | Reduction in S/CO > 1 n = 56 | Reduction of S/CO < 1 or increase n = 197 | p-value | Follow-up S/CO < 4 n = 35 | Follow-up S/CO ≥ 4 n = 241 | p-value | Donation S/CO < 4 n = 19 | Donation S/CO ≥ 4 n = 234 | p-value |
|---|---|---|---|---|---|---|---|---|---|
| PCR result | |||||||||
| Positive | 15 (26.8) | 104 (52.8) | 6 (17.1) | 125 (51.9) | 3 (15.8) | 116 (49.6) | |||
| Negative | 41 (73.2) | 93 (47.2) | 0.001 | 29 (82.9) | 116 (48.1) | <0.001 | 16 (84.2) | 118 (50.4) | 0.009 |
| Final ECG classification, n (%) | |||||||||
| Major | 11 (19.6) | 76 (38.6) | 4 (11.4) | 94 (39.0) | 1 (5.3) | 86 (36.8) | |||
| Minor | 29 (51.8) | 82 (41.6) | 19 (54.3) | 101 (41.9) | 12 (63.2) | 99 (42.3) | |||
| Normal | 16 (28.6) | 39 (19.7) | 0.028 | 12 (34.3) | 46 (19.1) | 0.004 | 6 (31.6) | 49 (20.9) | 0.010 |
| Typical changes, n (%) | |||||||||
| 0 | 46 (82.1) | 122 (61.9) | 31 (88.6) | 149 (61.8) | 18 (94.7) | 150 (64.1) | |||
| 1 | 6 (10.7) | 57 (28.9) | 3 (8.6) | 67 (27.8) | 0 (0) | 63 (26.9) | |||
| 2+ | 4 (7.1) | 18 (9.1) | 0.010 | 1 (2.9) | 25 (10.4) | 0.006 | 1 (5.3) | 21 (9.0) | 0.009 |
| Joint PCR/ECG endpoint, n(%) | |||||||||
| PCR-ve & ECG normal | 36 (64.3) | 62 (31.5) | 25 (71.4) | 80 (33.2) | 15 (78.9) | 83 (35.5) | |||
| PCR+ve &/or ECG abnormal | 20 (35.7) | 135 (68.5) | <0.001 | 10 (28.6) | 161 (66.8) | <0.001 | 4 (21.1) | 151 (64.5) | <0.001 |
PCR–polymerase chain reaction; ECG–electrocardiogram. Final ECG classification: major if at least one major alteration, minor if only minor alterations present, normal if neither minor nor major alterations present. Typical ECG changes refer to alterations typical of Chagas cardiomyopathy following the 2nd Brazilian consensus on Chagas disease. P-values are calculated by Chi-squared test or Wilcoxon rank sum, as appropriate.
The proportion of donors with individual major and typical Chagas disease ECG alterations is shown according to antibody level in S2 Table. Of note, right bundle branch block (RBBB) was half as frequent in donors with falling antibody levels compared to those with stable or increasing levels (10.7% vs 20.3%, p = 0.14). This difference was more pronounced in donors with low (<4) versus high (≥ 4) S/CO levels: 2.9% (S/CO < 4) versus 19.9% (S/CO ≥ 4) with RBBB (p = 0.026), and 0.0% (S/CO < 4) versus 19.7% (S/CO ≥ 4) with RBBB (p = 0.029), at follow-up and donation timepoints, respectively. The proportion of ECGs classified as having a major alteration, and the number of typical Chagas alterations per trace, were lower in the decreasing antibody group, as well as the S/CO < 4 groups (Table 2). Donors with increasing antibody levels (increase > 1 S/CO) had the highest number of typical alterations per trace (S3 Table).
Considering the joint PCR/ECG end point, donors with negative PCR and normal ECG were more frequent in the falling antibody group, as well as the S/CO <4 groups at donation and follow-up (Table 2). We observe bimodal distributions of S/CO values at donation and follow-up in the PCR-ve & ECG normal group (Fig 1D and 1E). Fig 2 shows both the change in S/CO and the follow-up value for all participants. Notably, the PCR-ve and ECG normal cases cluster among donors with low and falling antibody levels.
Multivariate associations between ELISA S/CO and the joint PCR/ECG endpoint
We assessed the effect of age, sex, comorbidities (hypertension and diabetes) and smoking status as potential confounding variables for the relationship between antibody level and the joint PCR and ECG endpoint. Table 3 shows the results of the univariable logistic regression models for these variables. Building three separate multivariable models we find that none of the potential confounders altered the relationship between baseline S/CO, follow-up S/CO, or the change in S/CO and the joint PCR/ECG status. As such, only univariable association are reported.
Table 3. Results of univariable logistic regression models predicting joint PCR and ECG status at follow-up.
| OR(95%CI) | p-value | |
|---|---|---|
| Age (years) | 0.98 (0.96 to 1.01) | 0.156 |
| Sex (male) | 0.82 (0.50 to 1.35) | 0.437 |
| Hypertension | 1.10 (0.66 to 1.84) | 0.710 |
| Diabetes | 1.63 (0.78 to 3.36) | 0.189 |
| Smoking status (current or previous) | 0.58 (0.35 to 0.95) | 0.033 |
| Donation S/CO <4 | 6.89 (2.31 to 24.2) | 0.001 |
| Follow-up S/CO <4 | 4.85 (2.25 to 11.1) | <0.001 |
| Decline >1 S/CO | 5.40 (2.13 to 15.5) | 0.001 |
Odds ratios (OR) greater than 1 indicate variables associated with joint negative PCR and normal ECG status at follow-up
Discussion
We report a retrospective cohort of T. cruzi seropositive blood donors with longitudinal measurement of antibody levels over a median of 12 years follow-up. We have shown that the global humoral response to T. cruzi antigens–as measured by a highly-sensitive anti-T. cruzi ELISA–correlates with PCR positivity and electrocardiographic alterations. In particular, untreated individuals with spontaneously falling serology presented a lower proportion of positive PCR results and cardiomyopathy compared to those with other antibody trajectories. This is a novel finding, to the best of our knowledge.
The ECG alterations reported in this study are non-specific and highly prevalent in older adults without Chagas disease [22]. Therefore, a meaningful comparison group would be similarly aged seronegative blood donors. Ribeiro et al (2013) compared ECG findings in 500 seropositive and 500 seronegative blood donors in São Paulo and Montes Claros, Brazil [21]. The definition of major ECG alterations was broader than in the present study, including premature ventricular beats. Despite this, the overall prevalence of major alterations in the seropositive wing was lower (26%) than in our cohort (35%). This may reflect the older age (median 56 vs 48 years) and higher prevalence of hypertension (38% vs 23%) and diabetes (12% vs 5%) in our study. However, the prevalence of major alterations among seronegative donors was comparable to low-antibody participants (< 4 S/CO at follow-up) in our cohort: 9% and 11%, respectively.
Of the electrocardiographic abnormalities we evaluated, RBBB was most strongly associated with serology. In a recent systematic review of population-based studies (community surveys and blood donors) comparing healthy seropositive and seronegative individuals, RBBB was the most specific finding for Chagas disease [23]. The pooled prevalence of RBBB among T. cruzi infected individuals was 27%, compared to 5% among controls. In our cohort, the prevalence among low-antibody individuals was similar (3%), and in-line with seronegative arms in two comparable Brazilian cohorts [21,22].
Development of Chagasic cardiomyopathy is thought to be a direct consequence of parasite persistence within cardiac tissue [24], and parasitaemia, as detected by positive PCR, has been associated with the presence of typical ECG alterations among treated [25] and untreated [26] patients. Taken together, the observation that electrocardiographic findings among low-antibody individuals are similar to comparable seronegative populations supports the notion of spontaneous parasite clearance. The association between a negative antibody trend and ECG changes–albeit less pronounced than the absolute antibody level–also supports this hypothesis.
A number of other lines of evidence suggest that the intensity of the humoral response to T. cruzi infection is a function of ongoing parasite (antigenic) load. Long-term follow-up of patients treated with benznidazole has documented reductions in antibody levels, and occasional complete seroreversion, as measured by conventional [27] and multiplex serology [17,28–30]. Indeed, the consensus definition of treatment success is seroreversion on two of three conventional serologic tests. There are case reports of complete spontaneous seroreversion with clearly documented evidence of initial infection in patients with no apparent clinical disease [12,14]. However, in contrast to these lines of argument, rare case reports exist (for example [31]) of typical Chagasic ECG changes in patients with complete treatment-induced seroreversion, implying Chagas cardiomyopathy can develop following apparent treatment success. The significance of these findings is unclear as conduction abnormalities can develop during acute infection and may have preceded treatment initiation. Furthermore, as mentioned above, although ECG findings may be typical of Chagas disease (e.g. RBBB), they also occur in the setting of other cardiomyopathies, such as those due to hypertension and ischaemic heart disease.
In addition to these observations, we have previously shown [15], and now confirmed, that antibody levels measured at a single timepoint correlate with PCR positivity in patients with chronic CD. Furthermore, in this cohort 71% of subjects with low antibody levels (<4 S/CO) at follow-up had both a negative PCR and a normal ECG–both established proxies of parasite persistence. These findings suggest that, as a single measurement, a quantitative serology may be more informative than PCR, given the relatively stability of serology in the short- to medium-term and the issues of false-negatives with PCR.
In a sample of blood donors from the Argentinian Chaco province (20% T. cruzi seroprevalence), the distribution of antibody levels, as measured by a number of different assays, was clearly bimodal [8]. Although our sample size is small, restricting the conclusions that can be drawn, we do demonstrate apparently bimodal distributions of ELISA S/CO both at the time of donation and among jointly PCR and ECG negative subjects at both timepoints (Fig 1). PCR is frequently false-negative, whereas a positive result is unequivocal evidence of parasite persistence (excluding lab errors such as sample contamination). The ECG can also be normal in the context of parasite persistence–traditionally referred to as the indeterminate form of Chagas disease.
Therefore, the bimodal distribution of antibody levels in this PCR-ve and ECG normal group may be interpreted as follows. The population of donors in the low antibody peak represent individuals that have substantially reduced (or possibly completely cleared) the parasite. Their risk of developing ECG alterations would be the same as the non-Chagas population and PCR would be expected to be consistently negative on repeat testing. By contrast, the high antibody peak represents individuals with ongoing parasite load but false-negative PCR and normal ECG (as yet). This group might be expected to develop ECG alterations at a higher rate than the age-matched non-Chagas population and to have positive PCR if serial samples were taken.
Limitations
The VITROS Anti-T. cruzi assay is semiquantitative. This means the S/CO value only approximately reflects the amount of anti-T. cruzi antibody in a given sample. Because this source of measurement error is unlikely to be related to the outcomes–i.e. it is a non-differential measurement bias–it will have biased towards the null hypothesis. Furthermore, in calculating the difference between donation and follow-up samples their measurement errors were combined. Therefore, the change overtime in S/CO is inherently noisier than a measurement at a single timepoint. This may partly explain the association between follow-up S/CO and ECG/PCR being more pronounced than the association with the change in S/CO overtime.
Furthermore, because the Vitrios Anti-T cruzi assay is based on whole-parasite antigens, we could not look for more refined associations with specific antibodies. For example, recent work in Bolivia and Peru has shown an association between seropositivity against a lineage specific (TcII/TcIV/TcVI) epitope and severity of cardiac disease [32], with similar results in Brazil [33]. This is a detail that needs to be further explored and could not be addressed in the present cohort.
The median age of this cohort was 56 years. Therefore, initial infection with T. cruzi was likely a distant event for most participants. We can infer this for two reasons. Firstly, São Paulo does not have active T. cruzi transmission and most participants were born in countryside endemic regions, having subsequently moved to São Paulo. Secondly, effective vectoral control over the last 30 years has greatly reduced the number of incident cases [11], with a clear cohort effect. This means that only individuals that remained seropositive into their 4th to 7th decade were included. As such, people achieving spontaneous parasite clearance early in the disease course–shortly following infection–and completely seroreverting by a younger age, would not have entered this study. In removing this group, our sampling frame may have resulted in a systematic underestimation of the rate of seroreversion.
Another limitation of this cohort was the storage of frozen donation samples for many years. This process will have artificially reduced the measured S/CO on these historical samples. Thus, the magnitude of seroreductions will have been systematically underestimated and any increase in antibody levels will have been overestimated. However, the alternative approach–comparing assay results performed many years apart–introduces variations in assay manufacture and protocol. This is probably an even more significant source of error.
Conclusions
Our results, if further confirmed, suggest that quantitative serology based on whole cell lysate is an informative marker of parasite persistence and disease status in Chagas disease. The association between spontaneously falling antibody level, negative PCR and normal ECG support the notion of spontaneous clearance, but further evidence is needed. For example, we are evaluating more refined assays for characterization of antibody levels to specific T cruzi antigens using selected recombinant antigens in Luminex and protein array technologies. These assays will be evaluated on this and multiple other cohorts of untreated and treated clinical cases. These include longitudinal samples from younger seropositive donors from the Argentinian Chaco with PCR and clinical data. These ongoing studies, using the Ortho VITROS assay and more refined quantitative T cruzi antibody technologies, should provide further insights into the relationships between antibody dynamics and parasite persistence and clinical implications of spontaneous and treatment induced control or eradication of T cruzi infection.
Supporting information
(DOCX)
X indicates that an ECG finding belongs to either the group of major or Chagas-typical findings, or both. Ψ RBBB + LAHB is included in the count of RBBB
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We acknowledge Prof. Rick L. Tarleton for helpful discussion of the manuscript.
Data Availability
The dataset underlying this article has been deposited at the following directory https://figshare.com/articles/dataset/Declining_antibody_levels_to_Trypanosoma_cruzi_correlate_with_polymerase_chain_reaction_positivity_and_electrocardiographic_changes_in_a_retrospective_cohort_of_untreated_Brazilian_blood_donors/13077968.
Funding Statement
This work was supported by the National Institutes of Health [1R01AI125738]. LB recieves a scholarship from the Fundação Faculdade de Medicina (FFM) through the Insituto de Medicina Tropical da Faculdade de Medicina da Universidade de São Paulo. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Schofield CJ, Jannin J, Salvatella R. The future of Chagas disease control. Trends Parasitol. 2006;22: 583–588. 10.1016/j.pt.2006.09.011 [DOI] [PubMed] [Google Scholar]
- 2.Engels D, Savioli L. Reconsidering the underestimated burden caused by neglected tropical diseases. Trends Parasitol. 2006;22: 363–366. 10.1016/j.pt.2006.06.004 [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Weekly Epidemiology Report. 2015;90(06): 33–44. [PubMed] [Google Scholar]
- 4.Pérez-Molina J, Molina I. Chagas disease. Lancet. 2018;391: 82–94. 10.1016/S0140-6736(17)31612-4 [DOI] [PubMed] [Google Scholar]
- 5.Nunes MCP, Beaton A, Acquatella H, Bern C, Bolger AF, Echeverría LE, et al. Chagas Cardiomyopathy: An Update of Current Clinical Knowledge and Management: A Scientific Statement From the American Heart Association. Circulation. 2018;138 10.1161/CIR.0000000000000599 [DOI] [PubMed] [Google Scholar]
- 6.Otani MM, Vinelli E, Kirchhoff LV, del Pozo A, Sands A, Vercauteren G, et al. WHO comparative evaluation of serologic assays for Chagas disease. Transfusion. 2009;49: 1076–1082. 10.1111/j.1537-2995.2009.02107.x [DOI] [PubMed] [Google Scholar]
- 7.Pan American Health Organization. Guidelines for the diagnosis and treatment of Chagas disease. Washington, DC: PAHO; 2019. [Google Scholar]
- 8.Remesar M, Sabino EC, del Pozo A, Mayer A, Busch MP, Custer B. Bimodal distribution of Trypanosoma cruzi antibody levels in blood donors from a highly endemic area of Argentina: what is the significance of low-reactive samples? Transfusion. 2015;55: 2499–2504. 10.1111/trf.13180 [DOI] [PubMed] [Google Scholar]
- 9.Levy MZ, Bowman NM, Kawai V, Plotkin JB, Waller LA, Cabrera L, et al. Spatial Patterns in Discordant Diagnostic Test Results for Chagas Disease: Links to Transmission Hotspots. Clin Infect Dis. 2009;48: 1104–1106. 10.1086/597464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salles NA, Sabino EC, Cliquet MG, Eluf-Neto J, Mayer A, Almeida-Neto C, et al. Risk of exposure to Chagas’ disease among seroreactive Brazilian blood donors. Transfusion. 1996;36: 969–973. 10.1046/j.1537-2995.1996.36111297091740.x [DOI] [PubMed] [Google Scholar]
- 11.Sabino EC, Salles NA, Sarr M, Barreto AM, Oikawa M, Oliveira CD, et al. Enhanced classification of Chagas serologic results and epidemiologic characteristics of seropositive donors at three large blood centers in Brazil. Transfusion. 2010;50: 2628–2637. 10.1111/j.1537-2995.2010.02756.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Francolino SS, Antunes AF, Talice R, Rosa R, Selanikio J, Rezende JM de, et al. New evidence of spontaneous cure in human Chagas’ disease. Rev Soc Bras Med Trop. 2003;36: 103–107. 10.1590/s0037-86822003000100014 [DOI] [PubMed] [Google Scholar]
- 13.Bertocchi GL, Vigliano CA, Lococo BG, Petti MA, Viotti RJ. Clinical characteristics and outcome of 107 adult patients with chronic Chagas disease and parasitological cure criteria. Trans R Soc Trop Med Hyg. 2013;107: 372–376. 10.1093/trstmh/trt029 [DOI] [PubMed] [Google Scholar]
- 14.Dias JCP, Dias E, M. Filho O, Vitelli-Avelar D, Correia D, Lages E, et al. Further evidence of spontaneous cure in human Chagas disease. Rev Soc Bras Med Trop. 2008;41: 505–506. 10.1590/s0037-86822008000500014 [DOI] [PubMed] [Google Scholar]
- 15.Sabino EC, Lee T-H, Montalvo L, Nguyen ML, Leiby DA, Carrick DM, et al. Antibody levels correlate with detection of Trypanosoma cruzi DNA by sensitive polymerase chain reaction assays in seropositive blood donors and possible resolution of infection over time. Transfusion. 2013;53: 1257–1265. 10.1111/j.1537-2995.2012.03902.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tobler LH, Contestable P, Pitina L, Groth H, Shaffer S, Blackburn GR, et al. Evaluation of a new enzyme-linked immunosorbent assay for detection of Chagas antibody in US blood donors. Transfusion. 2007;47: 90–96. 10.1111/j.1537-2995.2007.01068.x [DOI] [PubMed] [Google Scholar]
- 17.Zrein M, Granjon E, Gueyffier L, Caillaudeau J, Liehl P, Pottel H, et al. A novel antibody surrogate biomarker to monitor parasite persistence in Trypanosoma cruzi-infected patients. PLoS Negl Trop Dis. 2018;12: e0006226 10.1371/journal.pntd.0006226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denes P, Larson J, Lloyd-Jones D, Prineas R, Greenland P. Major and Minor ECG Abnormalities in Asymptomatic Women and Risk of Cardiovascular Events and Mortality. JAMA. 2007;297: 978 10.1001/jama.297.9.978 [DOI] [PubMed] [Google Scholar]
- 19.Brito B, Pinto-Filho M, Cardoso C, Oliveira C, Ferreira A, de Oliveira L, et al. Association between typical electrocardiographic abnormalities and NT-proBNP elevation in a large cohort of patients with Chagas disease from endemic area. J Electrocardiol. 2018;51: 1039–1043. 10.1016/j.jelectrocard.2018.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dias JCP, Ramos AN Jr., Gontijo ED, Luquetti A, Shikanai-Yasuda MA, Coura JR, et al. 2nd Brazilian Consensus on Chagas Disease, 2015. Rev Soc Bras Med Trop. 2016;49: 3–60. 10.1590/0037-8682-0505-2016 [DOI] [PubMed] [Google Scholar]
- 21.Ribeiro AL, Sabino EC, Marcolino MS, Salemi VMC, Ianni BM, Fernandes F, et al. Electrocardiographic Abnormalities in Trypanosoma cruzi Seropositive and Seronegative Former Blood Donors. PLoS Negl Trop Dis. 2013;7: e2078 10.1371/journal.pntd.0002078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ribeiro ALP, Marcolino MS, Prineas RJ, Lima-Costa MF. Electrocardiographic Abnormalities in Elderly Chagas Disease Patients: 10-Year Follow-Up of the Bambuí Cohort Study of Aging. JAHA. 2014;3 10.1161/JAHA.113.000632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rojas LZ, Glisic M, Pletsch-Borba L, Echeverría LE, Bramer WM, Bano A, et al. Electrocardiographic abnormalities in Chagas disease in the general population: A systematic review and meta-analysis. PLoS Negl Trop Dis. 2018;12: e0006567 10.1371/journal.pntd.0006567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tarleton RL, Zhang L. Chagas Disease Etiology: Autoimmunity or Parasite Persistence? Parasitology Today. 1999;15: 94–99. 10.1016/s0169-4758(99)01398-8 [DOI] [PubMed] [Google Scholar]
- 25.Cardoso CS, Ribeiro ALP, Oliveira CDL, Oliveira LC, Ferreira AM, Bierrenbach AL, et al. Beneficial effects of benznidazole in Chagas disease: NIH SaMi-Trop cohort study. PLoS Negl Trop Dis. 2018;12: e0006814 10.1371/journal.pntd.0006814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sabino EC, Ribeiro AL, Lee TH, Oliveira CL, Carneiro-Proietti AB, Antunes AP, et al. Detection of Trypanosoma cruzi DNA in blood by PCR is associated with Chagas cardiomyopathy and disease severity. Eur J Heart Fail. 2015;17: 416–423. 10.1002/ejhf.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Viotti R, Vigliano C, Lococo B, Bertocchi G, Petti M, Alvarez MG, et al. Long-Term Cardiac Outcomes of Treating Chronic Chagas Disease with Benznidazole versus No Treatment: A Nonrandomized Trial. Ann Intern Med. 2006;144: 724 10.7326/0003-4819-144-10-200605160-00006 [DOI] [PubMed] [Google Scholar]
- 28.Laucella SA, Mazliah DP, Bertocchi G, Alvarez MG, Cooley G, Viotti R, et al. Changes in Trypanosoma cruzi–Specific Immune Responses after Treatment: Surrogate Markers of Treatment Efficacy. Clin Infect Dis. 2009;49: 1675–1684. 10.1086/648072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alvarez MG, Bertocchi GL, Cooley G, Albareda MC, Viotti R, Perez-Mazliah DE, et al. Treatment Success in Trypanosoma cruzi Infection Is Predicted by Early Changes in Serially Monitored Parasite-Specific T and B Cell Responses. PLoS Negl Trop Dis. 2016;10: e0004657 10.1371/journal.pntd.0004657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Viotti R, Vigliano C, Álvarez MG, Lococo B, Petti M, Bertocchi G, et al. Impact of Aetiological Treatment on Conventional and Multiplex Serology in Chronic Chagas Disease. PLoS Negl Trop Dis. 2011;5: e1314 10.1371/journal.pntd.0001314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernández ML, Hernández Y, Scollo K, Esteva MI, Riarte AR, Prado NG. Chagas cardiomyopathy associated with serological cure after trypanocidal treatment during childhood. Rev Soc Bras Med Trop. 2018;51: 557–559. 10.1590/0037-8682-0364-2017 [DOI] [PubMed] [Google Scholar]
- 32.Bhattacharyya T, Messenger LA, Bern C, Mertens P, Gilleman Q, Zeippen N, et al. Severity of Chagasic Cardiomyopathy Is Associated With Response to a Novel Rapid Diagnostic Test for Trypanosoma cruzi TcII/V/VI. Clin Infect Dis. 2018;67: 519–524. 10.1093/cid/ciy121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhattacharyya T, Falconar AK, Luquetti AO, Costales JA, Grijalva MJ, Lewis MD, et al. Development of Peptide-Based Lineage-Specific Serology for Chronic Chagas Disease: Geographical and Clinical Distribution of Epitope Recognition. PLoS Negl Trop Dis. 2014;8: e2892 10.1371/journal.pntd.0002892 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
X indicates that an ECG finding belongs to either the group of major or Chagas-typical findings, or both. Ψ RBBB + LAHB is included in the count of RBBB
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
The dataset underlying this article has been deposited at the following directory https://figshare.com/articles/dataset/Declining_antibody_levels_to_Trypanosoma_cruzi_correlate_with_polymerase_chain_reaction_positivity_and_electrocardiographic_changes_in_a_retrospective_cohort_of_untreated_Brazilian_blood_donors/13077968.