Abstract
Objective
To use unsupervised topic modeling to evaluate heterogeneity in sepsis treatment patterns contained within granular data of electronic health records.
Materials and Methods
A multicenter, retrospective cohort study of 29 253 hospitalized adult sepsis patients between 2010 and 2013 in Northern California. We applied an unsupervised machine learning method, Latent Dirichlet Allocation, to the orders, medications, and procedures recorded in the electronic health record within the first 24 hours of each patient’s hospitalization to uncover empiric treatment topics across the cohort and to develop computable clinical signatures for each patient based on proportions of these topics. We evaluated how these topics correlated with common sepsis treatment and outcome metrics including inpatient mortality, time to first antibiotic, and fluids given within 24 hours.
Results
Mean age was 70 ± 17 years with hospital mortality of 9.6%. We empirically identified 42 clinically recognizable treatment topics (eg, pneumonia, cellulitis, wound care, shock). Only 43.1% of hospitalizations had a single dominant topic, and a small minority (7.3%) had a single topic comprising at least 80% of their overall clinical signature. Across the entire sepsis cohort, clinical signatures were highly variable.
Discussion
Heterogeneity in sepsis is a major barrier to improving targeted treatments, yet existing approaches to characterizing clinical heterogeneity are narrowly defined. A machine learning approach captured substantial patient- and population-level heterogeneity in treatment during early sepsis hospitalization.
Conclusion
Using topic modeling based on treatment patterns may enable more precise clinical characterization in sepsis and better understanding of variability in sepsis presentation and outcomes.
Keywords: infection, machine learning, latent Dirichlet allocation, treatment heterogeneity, topic modeling
INTRODUCTION
Sepsis, the life-threatening organ dysfunction arising from a dysregulated host response to infection, is a condition with tremendous global impact.1 Sepsis affects at least 30 million patients worldwide and results in 5 million deaths each year.2 It is also a major contributor to hospital and postdischarge mortality, morbidity, and health care utilization.3–8 Survival in sepsis has steadily improved over time, owing to standardized care focused on heightening early identification and delivery of antibiotics.9–11 However, sepsis protocols are built using a “one-size-fits- all” approach and do not target specific treatments to patients with differences in underlying illness or acute presentation—except within the simplest groupings, like shock.12,13 Underlying heterogeneity in sepsis is universally cited as the major barrier to future improvements in treatment and is an issue of particular salience for a condition in which no new effective pharmacologic treatment has been identified in the past 50 years.12–15
While heterogeneity in sepsis is widely acknowledged both by researchers and clinicians, few studies have attempted to comprehensively quantify its characteristics. This gap is partly explained by the varied sources of heterogeneity in sepsis including clinical factors, genetic predisposition, host–pathogen interactions, acute disease mechanisms, immune system responses, treatment received, and temporal trajectories of disease progression.13–23 However, even within just the clinical domain, existing approaches to characterize sepsis rely on relatively narrow criteria-based or laboratory groupings.10,23–29 For example, the Systemic Inflammatory Response Syndrome criteria, which were used as a foundation for sepsis definitions in prior decades, include only 4 variables.27 A more contemporary schema, the PIRO (Predisposition, Infection, Response, Organ dysfunction) model,30 similarly uses a limited set of variables that are poorly representative of the true heterogeneity that clinicians witness in treating sepsis on a daily basis. Recent work evaluating clinical sepsis subgroups in observational and prospective clinical trial data relied on a circumscribed set of 29 vital sign, laboratory, and demographic parameters.23
Heterogeneity also impacts how sepsis care quality is measured. The past several years have seen new guidelines and mandates emerge at the state, federal, and national levels that require protocolized care in all sepsis patients within highly constrained timelines (ie, within 6, 3, or even 1 hours).1,24,31 However, these guidelines similarly fail to account for the variability in patient presentation and how these differences impact the timeliness of care. For example, antibiotic administration is measured against the same timeline whether a patient presents with obvious infectious symptoms of cough, fever, and purulent sputum or with more uncertain infectious symptoms, like diffuse abdominal pain and vomiting.13 Thus, characterizing clinical heterogeneity with greater depth is an essential first step toward understanding how to measure the adherence to and benefits of current treatment paradigms.
Clinical heterogeneity is a significant limitation to the development of new treatments and to accurately assessing sepsis quality of care and, yet, no current methods are available to quantify that heterogeneity with a computable, non–rules-based approach using comprehensive electronic health record (EHR) data. Machine learning methods have proven highly successful in empirically identifying groupings within large, complex data. In particular, a number of unsupervised learning approaches can successfully generate computable subgroups with high clinical relevance. While a diversity of methods currently exists (eg, clustering, neural network-based), prior work has shown that probabilistic topic modeling, for example, that based on the Latent Dirichlet Allocation (LDA) algorithm, can uncover relevant themes within complex EHR data.32–37 Using a library of books as a conceptual example, LDA assesses the frequency and co-occurrence of words within individual books to identify the topics represented across the entire library. Based on the words they contain, individual books can also be represented as proportions of separate topics. Importantly, these statistical approaches allow the development of a computable phenotype or subgroup that captures greater complexity of patients, rather than assigning a single label based only on simple and limited rules.
OBJECTIVE
In this study, we used an unsupervised topic modeling approach to assess treatment heterogeneity during the first 24 hours of sepsis hospitalization and to develop computable clinical signatures based on these topics that describe overall treatment patterns for each patient. By applying LDA to a heterogeneous set of EHR data from the first 24 hours of sepsis treatment, we sought to empirically describe topics early in sepsis that would reflect underlying heterogeneity in sepsis presentation based on the diversity of treatment needs. We assessed how the resulting LDA-derived topics were distributed across the entire sepsis population as well as within individual patients. Finally, we assessed how these topics impacted common quality metrics of sepsis care to evaluate how their use could impact clinical practice and quality of care.
MATERIALS AND METHODS
Overall approach and cohort
This study was approved by the Kaiser Permanente Northern California (KPNC) Institutional Review Board.
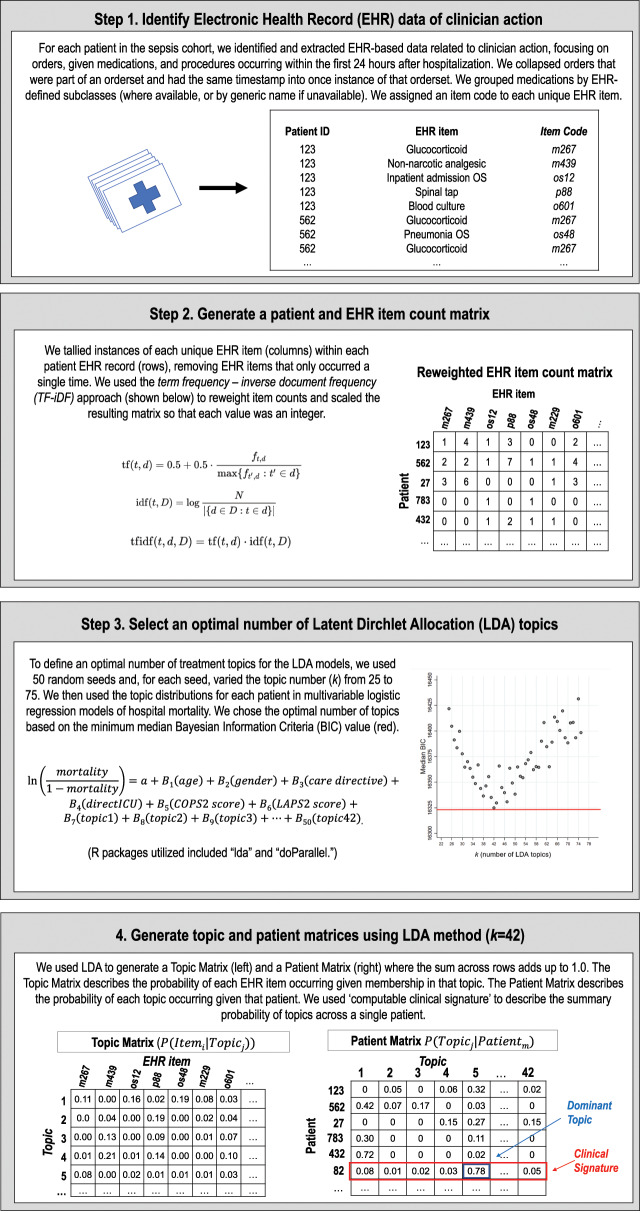
Figure 1 provides an overview of our study’s approach to characterizing early clinical treatment heterogeneity among sepsis patients by applying topic modeling to granular EHR data. Table 1 defines the terminology used throughout this article. Our cohort was drawn from 35 000 adult sepsis hospitalizations occurring within the 21 hospitals of KPNC between 2010 and 2013.38 Sepsis was defined based on the Sepsis-2 framework prevalent during that period and all patients were admitted through the emergency department and given antibiotics within 6 hours of triage. We included the first sepsis hospitalization for each patient (n = 29 253).
Figure 1.
Schematic overview of EHR data extraction and LDA implementation in hospitalized sepsis patients.
Abbreviations: EHR, electronic health record; LDA, Latent Dirichlet Allocation.
Table 1.
Terms used in describing the approach and results, and their meaning
| Term | Description |
|---|---|
| Latent Dirichlet Allocation (LDA) | Unsupervised topic modeling approach that derives topics based on the frequency and co-occurrence of EHR items in hospitalizations, allowing treatment themes within individual hospitalizations to be represented by proportions of those topics. |
| EHR Items | Granular data objects drawn from the EHR within the first 24 hours of hospitalization. These items represent treatment decisions, including orders placed, medications given, and procedures ordered. |
| Topic | The 42 latent treatment patterns derived from LDA based on the frequency and co-occurrence of EHR items across all of the hospitalizations. |
| Topic Label | Summary clinical interpretation based on post hoc consensus interpretation of the highest weighted EHR items in each topic. |
| Computable Clinical Signature | The overall treatment profile for each patient based on proportions of each of the 42 topics. |
| Dominant Topic | The topic comprising the greatest proportion of each patient’s computable clinical signature. |
Abbreviations: EHR, electronic health record; LDA, Latent Dirichlet Allocation.
EHR data items
We extracted EHR items indicating clinician actions within the first 24 hours of a sepsis hospitalization including electronic orders (n = 3 478 677), administered medications (n = 452 193), and procedures (n = 17 806; Table 2). We aggregated individual orders into order sets if they were part of the same established order set and had the same time stamp. We grouped medications by EHR subclasses (eg, glucocorticoids, glycopeptides), as classified in Epic Clarity EHR systems. We excluded any EHR item that appeared only once (n = 537), producing an EHR count matrix of 1 891 198 total and 2521 unique items. Supplementary Appendix Table 1 lists the most frequent EHR items identified.
Table 2.
Total volume of EHR data related to clinician action for patients in the sepsis cohort within the first 24 hours after ED triage. Total items represent each instance of any EHR item while unique items represent the different types of items (eg, hospital admission order set, serum potassium, glycopeptide antibiotic). The table shows the total number of items and unique items initially extracted from the EHR (left), those removed for appearing only a single time (middle italics), and those ultimately included (right) in the count matrix for LDA implementation. Of the 3 478 677 total orders drawn from the EHR, 2 305 061 were part of an established order set and were included together with other orders that were part of the same order set and had the same time stamp as 1 instance of that order set, resulting in 248 120 total order sets.
| Items initially extracted from EHR |
Items removed (appearing only once)
|
Items included in LDA count matrix |
|||
|---|---|---|---|---|---|
| EHR item type | Total | Unique | Unique | Total | Unique |
| Orders | |||||
| Individual | 1 173 616 | 1657 | 236 | 1 173 380 | 1421 |
| Order sets | 248 120 | 238 | 24 | 248 096 | 214 |
| Medications given | 452 193 | 699 | 89 | 452 104 | 610 |
| Procedures | 17 806 | 464 | 188 | 17 618 | 276 |
| Total | 1 891 735 | 3058 | 537 | 1 891 198 | 2521 |
Abbreviations: EHR, electronic health record; LDA, Latent Dirichlet Allocation.
EHR item count matrix and topics
To reduce the influence of frequently occurring EHR items found across many hospitalizations (eg, saline preparations), we applied a “term frequency-inverse document frequency” algorithm and scaled the resulting EHR item count matrix so that each value was an integer (Figure 1).39 We then used LDA to surface latent treatment topics within each patient record.32,33 The LDA implementation generates a topic matrix which represents a probability distribution of EHR items within each topic, which can be used to identify which EHR items are most associated with each treatment topic (Figure 1). It also generates a patient matrix which describes the composition of topics that describe each patient’s computable clinical signature.
Because LDA lacks prior specification about the latent topics being modeled, users must define k number of topics. To determine the optimal k, we defined k ranging from 25 to 75. We chose the optimal value of k based on the minimum median Bayesian Information Criterion (BIC) from multivariable logistic regression models with an outcome of hospital mortality based on 50 random iterations for each k.40 The models demonstrated a BIC minimum and inflection point at k = 42 (Supplementary Appendix Figure 1).
While LDA empirically surfaces latent treatment topics based on EHR items, these topics require human interpretation. Therefore, our study team applied a post hoc clinical label to each topic (ie, applying labels including pneumonia, gastrointestinal bleeding, and mechanical ventilation) based on consensus interpretation of the highest weighted items represented in the topic matrix for each topic (Supplementary Appendix Table 2). Topics with at least 4 antibiotic or microbe culture items in the top 10 highest-weighted EHR items were considered treatment for infection. We also grouped these 42 topics within 11 broader organ- or treatment-based categories (eg, respiratory, gastrointestinal, and critical care).
Assessing clinical heterogeneity within and between sepsis patients
We used the LDA output to generate sepsis clinical signatures: computable and visualizable patient-level profiles showing the proportional composition of each topic within individual patients. Within each patient’s computable clinical signature, we identified their dominant topic—the single topic which comprised the largest proportion of their signature—as well as the second largest topic to assess how often sepsis hospitalizations could be defined by a small number of main treatment topics. To demonstrate how a computable clinical signature could help identify relevant subgroups within a highly heterogeneous population, we compared visual signatures of 9 randomly selected sepsis patients with 9 of those selected by specific treatment topic co-occurrence. To visualize treatment heterogeneity between patients, we used a chord plot to visualize dominant topic co-occurrence across the entire cohort. In each plot, individual patients are represented once with a line connecting their dominant and second largest topic within their clinical signature.
Evaluating the role of heterogeneity in sepsis measures
We assessed the 42 treatment topics across 8 common measures used to characterize sepsis patients, treatments, and outcomes including (1) the time from emergency department triage to the first antibiotic;38 (2) the total volume of intravenous fluid administered within the first 24 hours41,42; (3) hospital mortality3,43; and (4) the maximum Sepsis-related Organ Failure Assessment Score (SOFA) during hospitalization7,44; (5) age; (6) acute severity of illness (based on Laboratory Acute Physiology Score, LAPS2)43,45–48; (7) chronic comorbid disease burden (Comorbidity Point Score, COPS2)43,45–48; and (8) length of stay, based on established methods. In these comparisons, patients were included only once and grouped by their dominant topic. We used scatterplots to display antibiotic timing and fluid administration amounts as well as comorbid disease burden and hospital mortality to characterize how treatment topic heterogeneity modifies commonly used outcome and quality reporting metrics.
Data are reported as number (%), mean ± standard deviation, or median (interquartile range). We conducted analyses STATA/SE 14.2 and R version 3.4.2 including packages “dplyr,”49 “lda,”50 “doParallel,”51 and “circlize.”52 The R code used in this study is included in the Supplementary Appendix.
RESULTS
Our cohort included 29 253 patients with a mean (± SD) age of 70 ± 17 years (Supplementary Appendix Table 3); hospital mortality was 9.6%. Based on Sepsis-2 strata, 10 212 (34.9%) had sepsis, 15 059 (51.5%) had severe sepsis, and 3982 (13.6%) had septic shock. The median time to antibiotics was 2.1 hours (interquartile range: 1.4–3.1).
Labeling LDA-generated treatment topics
Table 3 and Supplementary Appendix Table 2 show the most highly weighted EHR items within each of the 42 treatment topics. In most cases, topics were clinically recognizable representing specific infections or treatment needs. For example, the top 5 items of latent topic 22 were: Clostridium difficile panel; contact plus isolation; stool culture; stool white blood cell count; and metronidazole. We labeled this topic “diarrhea.” Topic 3 (“congestive heart failure”) included congestive heart failure order set, troponin I, loop diuretic, B-type natriuretic peptide, and electrocardiogram. Topic 26 (labeled “anemia”) included iron and TIBC, ferritin, vitamin B12, folic acid serum, reticulocyte count, and transferrin.
Table 3.
Top 10 items for each of 42 statistically generated treatment topics from an unsupervised machine learning approach using EHR data drawn from the first 24 hours of sepsis hospitalization. Item order is determined by item weighting from the treatment topic matrix produced using LDA. In some cases, item names have been shortened for visual clarity. Additional detail available within Supplementary Appendix Table 2. Headers represent summary clinical labels assigned following empiric algorithm topic determination as well as 11 larger categories in parentheses (italics).
| Acute coronary syndrome (Cardiovascular) | Atrial fibrillation (Cardiovascular) | Congestive heart failure (Cardiovascular) | Complex care (Complex) | End of life (Complex) | Failure to tdrive (Complex) |
|
| |||||
| Troponin I | Prothrombin time | CHF OS | Inpatient admission OS | Palliative care CS 1 | Tube feeding diet |
| ACS inpatient OS | Digoxin level | Troponin I | CBC with differential | Palliative care CS 2 | CBC with differential |
| Echocardiography | Anticoagulants coumarin | Loop diuretic | Palliative care CS 1 | Inpatient admission OS | Enteral alimentation OS |
| Electrocardiogram, 12 | Inpatient admission OS | B-type natriuretic peptide | Palliative care CS 2 | CBC with differential | Inpatient admission OS |
| Salicylate analgesics | Complete blood count | Electrocardiogram, 12 | Urinalysis without micro | Comfort care order set | Phenytoin level |
| Creatine kinase-MB | Digitalis glycosides | Inpatient admission OS | Lactic acid | IV Sodium chloride | NPO, tube feeding |
| Cardiology CS 2 | WBC differential, auto | Beta blockers | Admit to hospital | Narcotic agonists | Pneumonia OS |
| Cardiology CS 2 | Beta blockers | CBC with differential | Hospitalist initial CS | Urinalysis without micro | Urinalysis without micro |
| B-type natriuretic peptide | Lactic acid | Class IV antiarrhythmic | Blood culture | Admit to hospital | Nursing order |
|
| |||||
| Wound care (Complex) | Life support (Critical) | Mechanical ventilation (Critical) | Shock (Critical) | Critical illness (Critical) | Diabetes (Endocrine) |
|
| |||||
| Heparins | Blood culture | Prothrombin time | Urinalysis, micro only | ESBL Penicillin antibiotic | ESBL Penicillin antibiotic |
| Inpatient admission OS | Chest X-ray | Chest X-ray | Septic shock – EGDT | Mg replacement OS | Insulin sliding scale OS |
| Wound nurse CS | Arterial blood gas | Arterial blood gas | ICU admission OS 1 | K+ replacement OS | Inpatient admission OS |
| WBC differential, auto | CV sympathomimetics | Clinical restraints | Chest X-ray | Ionized calcium | CBC with differential |
| CBC with differential | Septic shock -- EGDT | Mechanical vent OS 2 | CV sympathomimetics | Phos replacement OS | Diabetes mellitus OS |
| Nursing wound care | ICU admission OS 1 | Ventilator therapy | Venous blood gas | Troponin I, POCT | Insulins - short acting |
| Glycopeptide antibiotics | Venous blood gas | Ventilation OS | Venous catheterization | Venous lactate, POCT | Urinalysis, without micro |
| Hospitalist CS 1 | ABO-Rh | Endotracheal tube | IV Sodium chloride | ICU admission OS 1 | Antipyretic non-narcotic |
| Urinalysis, micro | Clinical restraints | Anesthetic phenol | ICU admission OS 2 | Lactate, POCT | Lactic acid |
| Hospitalist CS 2 | Ventilator therapy | Benzodiazepines | CBC with differential | Critical care CS 2 | Admit to hospital |
| Urinalysis, without micro | Ventilation OS | ICU admission OS 1 | Manual differential | Critical care CS 1 | Blood culture |
|
| |||||
| Diabetic ketoacidosis (Endocrine) | Thyroid (Endocrine) | Hematologic panel (General) | Electrolyte panel (General) | Hepatic panel (General) | Severe illness (General) |
|
| |||||
| Insulin regular human | Serum albumin | Draw and hold plasma 1 | Serum magnesium | Serum AST | Troponin I, POCT |
| Ketone bodies | TSH level | Draw and hold serum | Phosphorus | Serum ALT | Lactate, POCT |
| DKA OS | Prealbumin | Draw and hold EDTA | Serum calcium | Alkaline phosphatase | Venous lactate, POCT |
| Insulins - short acting | Serum magnesium | Draw and hold serum | Chemistry panel 7 | Total bilirubin | Inpatient admission OS |
| Blood glucose, POCT | Serum calcium | Draw and hold plasma 2 | CBC with differential 2 | Serum magnesium | Venous blood gas |
| Insulin sliding scale OS | Phosphorus | Draw and hold heparin | WBC differential, auto | Serum calcium | Electrocardiogram, 12 |
| ICU admission OS 1 | Chemistry panel 7 | CBC with differential | Lactic acid | Phosphorus | WBC differential, auto |
| Intensive insulin OS | WBC differential, auto | Inpatient admission OS | CBC with differential | Chemistry panel 7 | CBC with differential |
| Chemistry panel 7 | Incentive spirometry | Manual differential | Serum albumin | Lipase | Admit to hospital |
| Ketone, serum | Inpatient dietary CS | Blood culture 2 | Serum potassium | CBC with differential | Pneumonia OS |
|
| |||||
| Abdominal pain (Gastrointestinal) | Biliary disease (Gastrointestinal) | Diarrhea (Gastrointestinal) | Liver disease (Gastrointestinal) | Gastrointestinal bleeding (Gastrointestinal) | Hepatitis (Gastrointestinal) |
|
| |||||
| CT abdomen/pelvis | Serum ALT | Clostridium difficile panel | Ammonia | ABO-Rh | Hepatitis C antibody |
| Narcotic agonists | Serum AST | Contact plus isolation | Ultrasound abdomen | Transfusion OS | Hepatitis B surface Ag |
| Gastroenterology CS 1 | Alkaline phosphatase | Stool culture | Acute renal failure OS | Antibody screen | Hepatitis B surface Ab |
| Gastroenterology CS 2 | Total bilirubin | Stool WBC | WBC differential, auto | Hemoglobin/hematocrit | Hepatitis B core antibody |
| Inpatient admission OS | Lipase | Metronidazole | Laxatives | Type and crossmatch | Hepatitis A virus IgM |
| CBC with differential | Chemistry panel 7 | Clostridium difficile toxin | Services after hours | Type and screen | HIV 1/2 antibody |
| General surgery CS 2 | CBC with differential | Inpatient admission OS | CBC with differential | Crossmatch, immediate | Ultrasound abdomen |
| General surgery CS 1 | Lactic acid | CBC with differential | Sepsis ED OS | Crossmatch, electronic | US abdomen, B-scan |
| Metronidazole | WBC differential, auto | Protozoa smear | Draw and hold pink top | Prothrombin time | Antinuclear antibody |
| Serotonin antagonist | Serum amylase | WBC differential, auto | CT, 3D rendering | GI bleed OS | Acetaminophen level |
|
| |||||
| Surgery (Gastrointestinal) | Anemia (Hematologic) | Cytopenia (Hematologic) | Coagulopatdy (Hematologic) | Cellulitis (Musculoskeletal) | Indolent infection (Musculoskeletal) |
|
| |||||
| General surgery CS 1 | Iron and TIBC | Transfusion OS | Lactate dehydrogenase | US Venous Doppler | ESR |
| General surgery CS 2 | Ferritin | ABO-Rh | Fibrinogen activity | Cellulitis OS | C-reactive protein |
| Surgery admission OS | Vitamin B12 | Antibody screen | APTT | Inpatient admission OS | Infectious disease CS 1 |
| Surgery/intra-op OS | Folic acid, serum | Neutropenic fever OS | Fibrinogen degradation | Vancomycin level, trough | Infectious disease CS 2 |
| Narcotic agonists | Reticulocyte count | WBC differential, manual | Serum ALT | CBC with differential | Orthopedics CS 2 |
| PACU/anesthesia OS | Transferrin | CBC with differential | D-dimer | Glycopeptide antibiotics | Orthopedics CS 1 |
| Transfer level of care | Occult blood specimen | Antipyretic non-narcotic | Serum AST | WBC differential, auto | Narcotic agonists |
| CT abdomen/pelvis | Protein electrophoresis | Heme-Onc CS 1 | Total bilirubin | Narcotic agonists | Vancomycin level, trough |
| Anesthetic – narcotic | TSH | Inpatient admission OS | Alkaline phosphatase | US duplex scan | Glycopeptide antibiotics |
| Anaerobic culture | Head and neck CS 1 | Heme-Onc CS 2 | Prothrombin time | Lactic acid | CBC with differential |
|
| |||||
| Osteomyelitis (Musculoskeletal) | Weakness (Neurologic) | Confusion (Neurologic) | Acute kidney injury (Renal) | Dialysis (Renal) | Pyelonephritis (Renal) |
|
| |||||
| Podiatry CS 1 | CT head, no contrast | Drug screen, urine | Sodium, urine | Hemodialysis OS | Urology CS 1 |
| Podiatry CS 2 | CT head | CT head, no contrast | Creatinine, urine | Nephrology CS 2 | Urology CS 2 |
| Radiologic exam, foot | Inpatient admission OS | CT head | Osmolality, urine | Nephrology CS 1 | Urine culture |
| Culture, miscellaneous | WBC differential, auto | Alcohol withdrawal OS | Osmolality, serum | Phosphate binders | CBC with differential |
| Gram stain | CBC with differential | Alcohol level | Sodium, serum | Vancomycin level | Gentamicin level |
| Insulin sliding scale OS | Creatine kinase | MRI brain | Ultrasound abdomen | Calcineurin inhibitors | CT abdomen/pelvis |
| Anaerobic culture | Physical therapy | Lumbar puncture OS | Eosinophils, urine | Insulin sliding scale OS | IV Gentamicin |
| Vancomycin level, trough | Urinalysis, without micro | Neurology CS 1 | Chemistry panel 7 | Diet, renal | Lactic acid |
| Glycopeptide antibiotics | Troponin I | Neurology CS 2 | Acute renal failure OS | Mycophenolate | Narcotic agonists |
|
| |||||
| Urinary tract infection (Renal) | Atypical pneumonia (Respiratory) | COPD/Astdma (Respiratory) | Non-invasive ventilation (Respiratory) | Pneumonia (Respiratory) | Viral pneumonia (Respiratory) |
|
| |||||
| Inpatient admission OS | AFB culture and smear | Pneumonia OS | BIPAP ventilation | Pneumonia OS | Pneumonia OS |
| CBC with differential | Pneumonia OS | Glucocorticoids | Arterial blood gas | Inpatient admission OS | Droplet isolation |
| Narcotic agonists | CT chest | COPD/asthma OS | Pneumonia OS | CBC with differential | Inpatient admission OS |
| WBC differential, auto | L. pneumophila Ag | Inpatient admission OS | Non-invasive ventilation | WBC differential, auto | CBC with differential |
| Antipyretic non-narcotic | M. pneumoniae IgM | Respiratory culture | Glucocorticoids | Glucocorticoids | Flu A/B, RSV PCR |
| Lactic acid | Respiratory culture | Respiratory gram stain | B-type natriuretic peptide | COPD/asthma OS | WBC differential, auto |
| Urinalysis, without micro | Respiratory gram stain | CBC with differential | ICU admission OS | Admit to hospital | Antipyretic non-narcotic |
| Serotonin antagonists | Pulmonary initial CS | Lactic acid | Chest X-ray | Blood culture | 3rd gen Cephalosporin |
| IV Sodium chloride | Head and neck CS 1 | Heme-Onc CS 2 | Prothrombin time | Blood culture 2 | Neuraminidase inhibitors |
ACS, acute coronary syndrome; CBC, complete blood count; CS, consult; CHF, congestive heart failure; CV, cardiovascular; CT, computed tomography; DKA, diabetic ketoacidosis; EGDT, early goal directed therapy; ESBL, extended-spectrum beta-lactamase; ICU, intensive care unit; IV, intravenous; K+, potassium; Mg, Magnesium; NPO, nil per os; Phos, phosphorus; POCT, point of care testing; US, ultrasound; WBC, white blood cell.
Figure 2 shows the overall occurrence of each treatment topic across the entire study cohort with the most prevalent topics attributable to “diabetes” (6.0%), “viral pneumonia” (5.4%), “pneumonia” (4.8%), and “urinary tract infection” (4.7%). Evaluating the composition of topics across the entire cohort, only 39.1% of treatments were directly for infections, while the majority of treatment was for noninfectious causes of hospitalization.
Figure 2.
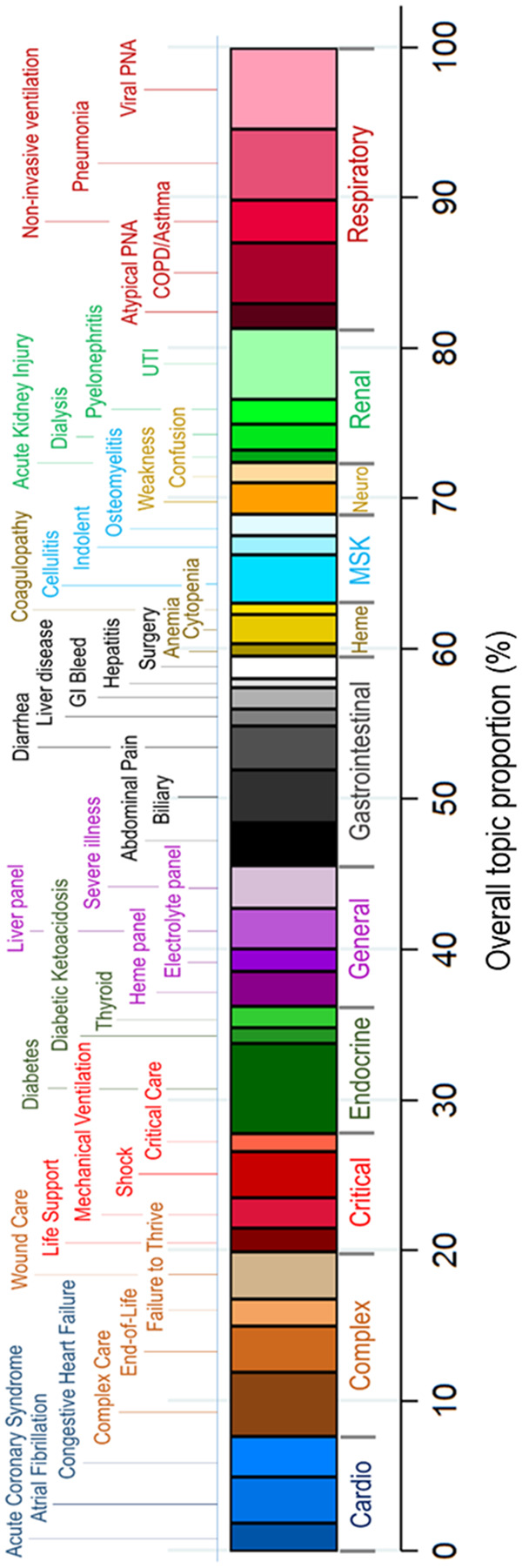
Aggregate representation of each of 42 statistically generated treatment topics based on electronic health record data, with post hoc assigned clinical labels (top) and categories (bottom and color bars). The width of each individual colored bar represents the proportion of that treatment topic within the sepsis cohort. The highest aggregate proportions are attributable to “diabetes,” “viral pneumonia” (viral PNA), “pneumonia,” and “urinary tract infection” (UTI).
Abbreviations: Cardio, cardiovascular; COPD, chronic obstructive pulmonary disease; GI, gastrointestinal; Heme, hematologic; MSK, musculoskeletal; Neuro, neurologic; PNA, pneumonia; UTI, urinary tract infection.
Computable clinical signatures and heterogeneity within sepsis patients
Clinical signatures are the proportional representation of treatment topics within individual patients and facilitate computable approaches to describing heterogeneity within each patient. In our cohort, we found that 56.9% of hospitalizations did not have a single dominant topic which accounted for more than half of their overall clinical signature, demonstrating that most sepsis patients’ treatments could not be defined only by a single label (Supplementary Appendix Figure 2). Only a small minority (7.3%) of patients had a single dominant topic that comprised >80% of their clinical signature, quantifying the clinically familiar scenario in which most sepsis patients are treated concurrently for multiple co-existing conditions.
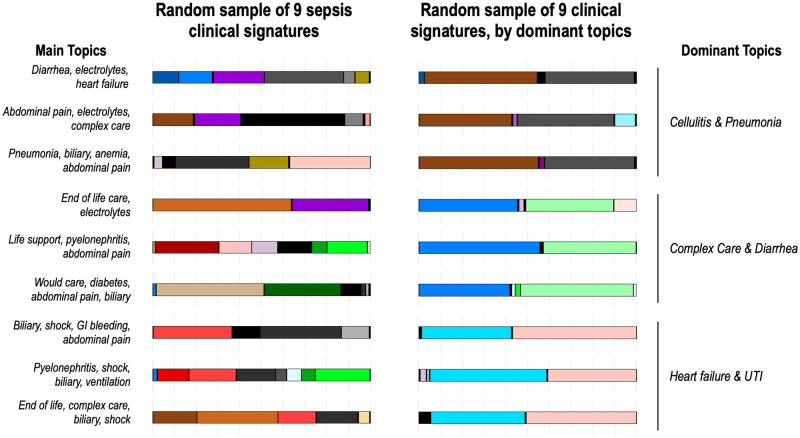
Figure 3 compares the visual representation of clinical signatures of 9 randomly assigned sepsis patients (left) with another 9 randomly chosen but based on 3 specific pairings of treatment topics (cellulitis and pneumonia; complex care and diarrhea; and heart failure and urinary tract infection) on the right. The left panel displays the heterogeneity present among randomly assigned patients with diverse combinations of treatment topics, yet all were defined as “sepsis” patients. The computable signature approach allows for sepsis patients to be defined by key dominant topics that can be used to identify similar subgroups within the overall sepsis population, as shown on the right, where signatures are similar across patients.
Figure 3.
Computable clinical signatures of individual patients based on the LDA topic modeling approach. Each bar color represents a different topic as displayed in Figure 2 and the width of the color bar represents the proportion of the clinical signature that topic composes. On the left are 9 randomly selected sepsis patients, including 3 each from sepsis (top), severe sepsis (middle), and septic shock (bottom) severity strata. On the right are 9 sepsis patients randomly chosen but based on 3 specific pairings of treatment topics (overall clinical signature comprised of at least 0.33 from both cellulitis and pneumonia; complex care and diarrhea; and heart failure and urinary tract infection).
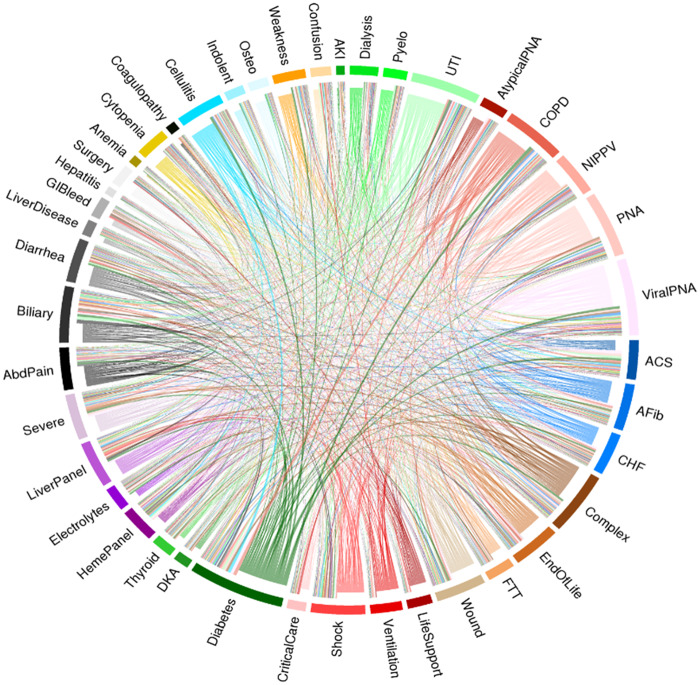
Heterogeneity in treatment was present not only within individual patients, but also across the entire sepsis population. Figure 4 displays the aggregate frequency of topic co-occurrence with each link representing a single hospitalization and exhibits the tremendous diversity in topics across the cohort. Of a total of 29 253 co-occurrence topics, even the most common ones were relatively rare, including: abdominal pain and biliary disease (n = 254, 0.9%), chronic obstructive pulmonary disease and diabetes (n = 222, 0.8%), diabetes and cellulitis (n = 217, 0.8%), and viral pneumonia with acute coronary syndrome (n = 165, 0.6%). The circle plot confirms that sepsis patients are highly diverse in their clinical signatures in a way that would not be easily characterized by a simple set of criteria.
Figure 4.
“Dominant topic” chord plot representing the co-occurrence of EHR topics within individual computable clinical signatures. The 42 topics are arrayed on the periphery with the width of each band representing the number of patients with that topic being their dominant or second topic in the clinical signature. Each line represents a single hospitalization connecting a dominant topic (bands around the periphery and lines arising from the bands of the same color) to the next topic (endpoint of the line with different color than the adjacent band). The width of the lines represents the number of hospitalizations with that same co-occurrence as the dominant and second topics in their clinical signature.
Abbreviations: AbdPain, abdominal pain; ACS, acute coronary syndrome; AFib, atrial fibrillation; AKI, acute kidney injury; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; DKA, diabetic ketoacidosis; FTT, failure to thrive; GIBleed, gastrointestinal bleeding; NIPPV, non-invasive ventilation; Osteo, osteomyelitis; PNA, pneumonia; UTI, urinary tract infection.
Evaluating treatment topics and sepsis measures
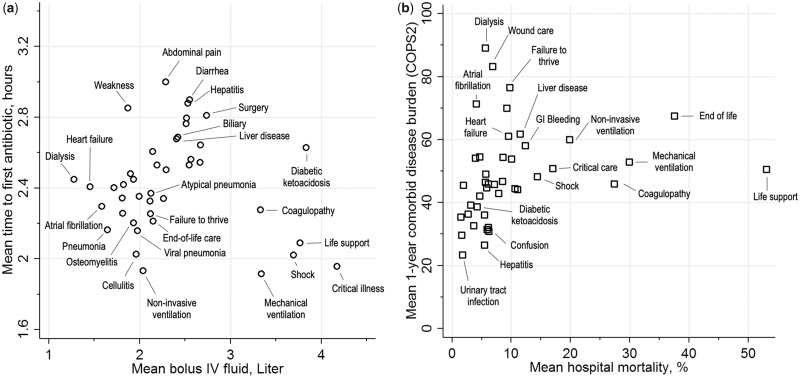
The heterogeneity revealed in the clinical signatures and circle plot also had significant impact on commonly used sepsis measures of care processes and outcomes. For example, Figure 5a displays the variation in commonly measured sepsis care processes (antibiotic timing and fluid resuscitation) across the population when patients were grouped by their dominant topics. Among an overall cohort that all received antibiotics within a very compressed emergency department timeline, the mean time to antibiotics was shorter (<2.2 hours) for conditions in which patient presentation was much more clear, including those requiring intensive care (ventilation, critical illness, shock) and with clinically obvious infections (ie, cellulitis, osteomyelitis, pneumonia). In contrast, among patients who had more uncertain presentations like weakness or abdominal pathology (abdominal pain, diarrhea, hepatitis, liver disease), the time to antibiotics was considerably longer on average.
Figure 5.
Clinical sepsis measures, stratified by dominant treatment topic of each patient’s computable clinical signatures during sepsis hospitalization. a) Mean time to first antibiotic from emergency department triage in relation to mean amount of intravenous fluid administered in the first 24 hours of hospitalization; b) Mean hospital mortality in relation to mean chronic comorbid disease burden (COPS2) score.
Similarly, large fluid volumes (>3 liters) were given to patients requiring intensive care and to other conditions that commonly require substantial fluid resuscitation (diabetic, coagulopathy). In contrast, small fluid volumes (<1.6 liters) were given to patients on dialysis or with heart failure, who are at increased risk of fluid overload, reflecting clinically familiar patterns. When antibiotic timing and fluid resuscitation were arrayed against one another, the LDA-based groupings revealed the challenge of using a “one-size-fits-all” approach to measuring adequacy in early sepsis treatment.
Figure 5b also shows considerable variability in comorbid disease burden and hospital mortality in sepsis when patients were grouped by their dominant treatment topics. Not surprisingly, patients with very high inpatient mortality (>20%) included not only those with critical illness, but also those with end-of-life care needs and coagulopathy. On the other hand, even patients with a very high presepsis burden of illness often exhibited low hospital mortality. For example, among those with substantial preexisting disease and with dominant topics of atrial fibrillation, end-stage kidney disease, or wound care, mortality was relatively low at <8%. Supplementary Appendix Table 4 and Supplementary Appendix Figure 3 similarly show wide variability in the characteristics and outcomes across the 42 topics.
DISCUSSION
In a multicenter cohort of sepsis patients treated with early antibiotics, we used machine learning to empirically identify EHR-based topics and develop computable clinical signatures to quantify the treatment heterogeneity present in early sepsis. Applying an unsupervised approach to nearly 2 million EHR items and 30 000 patients, we uncovered 42 treatment patterns or topics that were clinically recognizable and displayed the breadth and diversity of treatments used in the early part of hospitalization. Our findings highlighted the fact that, while all these patients were “septic,” their actual clinical signatures—the composition of treatment topics within a single patient—belied easy characterization by any single label. Only a minority of patients were even found to have had a single dominant topic that explained most of their hospitalization. Thus, our findings quantitatively demonstrate that singular or narrowly defined sepsis groupings fail to capture the true clinical and treatment diversity that comprises early sepsis. Similarly, when we assessed treatment topics across the entire cohort, we found tremendous heterogeneity. Further, because we were able to quantify the contribution of different topics throughout the population, we found that only 39.1% of overall treatments were definitively for infection. In sum, our study describes a computable and empiric approach to display and characterize the profound clinical heterogeneity of early sepsis treatment within individual sepsis hospitalizations and across the entire sepsis population.
While heterogeneity is universally cited as a key barrier to progress in sepsis research and treatment, to our knowledge, this is the first study that actually quantifies this treatment heterogeneity in the clinical domain and uses computable clinical signatures as a means for identifying diverse subgroups of patients.12–15 Traditional approaches to characterizing the clinical dimensions of sepsis rely on rules- or criteria-based frameworks10,24–29,53,54 and have shown value for identifying high-risk patients,19,25,44 standardizing treatment protocols,24,55,56 and enabling outcomes comparisons.10,27 However, they categorize patients across very narrow dimensions and, because of their significant limitations in capturing the diversity that is recognized clinically in sepsis, are rarely used. Rather than relying on a proscriptive approach that would require extensive clinical labeling and data curation, we sought to leverage machine learning approaches that would surface treatment subgroups without preexisting bias. We also chose to focus on clinician actions mediated through the EHR, because these digital artifacts would simultaneously capture underlying patient characteristics and clinician judgment in a way that common EHR data models might not. Finally, we chose to focus on the first 24 hours of hospitalization in order to describe sepsis heterogeneity during the most dynamic interval of inpatient care.
Our findings confirm the clinical reality that traditional approaches which rely on single labels to characterize a hospitalization—“this patient has pneumonia”—routinely fail to capture the diversity of coexisting clinical conditions present in early sepsis. Indeed, we found that for nearly half of patients with a main treatment topic of “pneumonia,” the majority of their overall clinical signature was explained by non-“pneumonia” topics. Our findings have important implications on future research in sepsis, which is currently at a crossroads when it comes to identifying clinically actionable subgroups that will be similarly responsive to treatment.12,13,22,23,57 This is of particular salience because sepsis has seen every novel therapy fail in randomized trials over the prior 5 decades. Heterogeneity is now universally identified as the major barrier to progress; however, no other methods are currently available to empirically quantify and characterize this clinical treatment diversity. Thus, even while clinicians recognize the conundrum of applying a “one-size-fits-all” treatment to highly variable patients—a commonly recounted scenario is that the same approach is taken for a young healthy patient with pneumonia as for a chronically ill elderly patient with immunosuppression and urosepsis—the lack of computable approaches means that this blunt approach to sepsis care continues to persist.12
Our findings also have important implications for current metrics that are used to assess and report quality of care in sepsis. Sepsis was recently recognized by the World Health Organization as a global health priority and is the subject of many public health awareness campaigns.1 This highly recognized status has also spurred the development of national and international standards and guidelines that use compliance with timed bundles to grade hospitals on their sepsis performance.24,31 However, as we show in this study, there is tremendous variation in the timing of antibiotics and the volume of fluid resuscitation that is attributable to the complement of coexisting clinical conditions within each patient. On average, patients with abdominal pathology received antibiotics the latest, reflecting the uncertainty of confirming infection as the reason for symptoms in these patients. Similarly, patients with conditions marked by a high risk for fluid overload—congestive heart failure and kidney disease with dialysis—received the lowest volume of resuscitation. Again, a “one-size-fits-all” approach for measuring sepsis care quality ignores the reality of underlying diversity that is revealed when computable clinical signatures can be used to quantitatively describe sepsis clinical heterogeneity.
There are several potential future applications and refinements to our approach that can facilitate improved scientific discovery and clinical treatment in sepsis. First, this method can be applied to existing randomized controlled trial or observational data to understand how patients’ clinical signatures modify their response to treatment. For example, recent landmark trials compared various protocolized treatment approaches in sepsis and found no differences in outcomes between patients.58 Quantifying the clinical signatures of individual patients has begun to show promise for revealing subgroups within the overall study population who responded differentially to protocolized care.23 We have provided our code so that our approach is easily reproducible in any EHR-based data set. Second, quantifying the clinical heterogeneity in sepsis patients can help ensure that public reporting sepsis metrics are applied to the right population. For example, the timing of antibiotic administration should account for differences in early treatment when infections are easily identifiable (eg, cellulitis, pneumonia) versus when they are more challenging (eg, abdominal symptoms, weakness). Finally, identifying clinical subgroups in real-time could help enhance medical recommender systems,33 resource allocation, and targeted care.59
It is essential to note that, in this study, we examined early sepsis heterogeneity by focusing on treatment patterns captured with clinical EHR data. However, sepsis heterogeneity arises from several sources including genetic factors, host-pathogen interactions, immune system responses, pathophysiologic disease mechanisms, and temporal trajectories of illness.12–22,25 What remains unknown is the degree to which the treatment heterogeneity we observed correlates with these other dimensions. For example, it may be that sepsis endotypes,12,23 (subgroups that capture similarity across disease mechanisms or host responses) can cluster patients together who exhibited highly disparate computable clinical signatures but would respond positively to the same treatment. What is also unknown is the extent to which the treatment heterogeneity we observed among sepsis patients is common to other inpatients. For example, is the hallmark of heterogeneity in sepsis treatment substantially greater than that present in other acute, high-impact conditions like heart failure?
The primary strength of our study was the careful use of an empiric data-driven approach to identify treatment topics and clinical signatures without specifying any preexisting categorization or criteria. We evaluated the statistically-generated topics against clinical documentation and further compared them across a set of common sepsis measures. These comparisons confirmed wide variability in treatment topics and outcomes belying the population means. Our study thus demonstrates the potential value of this approach for precisely quantifying and comparing clinical heterogeneity within and between populations using treatment topics within granular EHR data.
The main limitation of our study is that it was designed for hypothesis generation; thus, future studies are needed to confirm that these topics and computable clinical signatures reliably distinguish clinical subgroups that are responsive to differential treatments. Second, our study was conducted within a single health care system which may impact the generalizability of our findings. Third, we could not account for potential heterogeneity arising from individual clinical practice which could impact the reliability of topic generation. It is possible that some of the heterogeneity we captured actually arises from differences in practice rather than differences among sepsis patients. Fourth, while we assigned summary clinical labels to the treatment topics to improve recognition, the labels should be viewed as only approximations. Similarly, we used an empirical approach for identifying the optimal number of topics based on the findings of prior studies; however, it is possible that we captured only a local minima for BIC in our data. Finally, we limited ourselves to a single interval in hospitalization which does not fully capture the preceding and subsequent trajectory of illness. We also did not incorporate the longitudinal sequencing of EHR items.
In summary, in a multicenter cohort of sepsis patients, we applied machine learning to generate computable EHR-based clinical signatures that quantified treatment topics and, therefore, clinical heterogeneity in early sepsis care. Our findings confirmed that substantial treatment heterogeneity in sepsis manifests at both the patient- and population-level. Future research is needed to establish whether the profound heterogeneity we uncovered can drive improvements in the targeted and personalized care of sepsis patients.
FUNDING
This work was supported by The Permanente Medical Group, the Kaiser Permanente Division of Research Delivery Science Fellowship (AEF), and NIH/NIGMS R35GM128672 (VXL).
CONTRIBUTIONS
All authors have contributed substantially to the conception or design of the work or the acquisition, analysis, or interpretation of data for the work; and to drafting the work or revising it critically for important intellectual content. All authors approve of the version submitted and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Specific author contributions to project conception, analysis, interpretation, and completion are as follows: Design (AEF, JC, PK, GJE, VXL); Acquisition/analysis (AEF, JDG, BLL, VXL); Interpretation (AEF, JC, PK, GJE, VXL) Drafting/revising/final approval/agreement (all authors).
SUPPLEMENTARY MATERIAL
Supplementary material is available at Journal of the American Medical Informatics Association online.
Conflict of interest statement
None declared.
Supplementary Material
REFERENCES
- 1. Reinhart K, Daniels R, Kissoon N, Machado FR, Schachter RD, Finfer S.. Recognizing sepsis as a global health priority—a WHO resolution. N Engl J Med 2017; 3775: 414–7. [DOI] [PubMed] [Google Scholar]
- 2. Fleischmann C, Scherag A, Adhikari NK, et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med 2016; 1933: 259–72. [DOI] [PubMed] [Google Scholar]
- 3. Liu V, Escobar GJ, Greene JD, et al. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA 2014; 3121: 90–2. [DOI] [PubMed] [Google Scholar]
- 4. Prescott HC, Angus DC.. Enhancing recovery from sepsis: a review. JAMA 2018; 3191: 62–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mayr FB, Talisa VB, Balakumar V, Chang CH, Fine M, Yende S.. Proportion and cost of unplanned 30-day readmissions after sepsis compared with other medical conditions. JAMA 2017; 3175: 530–1. [DOI] [PubMed] [Google Scholar]
- 6. Prescott HC, Osterholzer JJ, Langa KM, Angus DC, Iwashyna TJ.. Late mortality after sepsis: propensity matched cohort study. BMJ 2016; 353: i2375.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schuler A, Wulf DA, Lu Y, et al. The impact of acute organ dysfunction on long-term survival in sepsis. Crit Care Med 2018; 466: 843–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Angus DC. The lingering consequences of sepsis: a hidden public health disaster? JAMA 2010; 30416: 1833–4. [DOI] [PubMed] [Google Scholar]
- 9. Stevenson EK, Rubenstein AR, Radin GT, Wiener RS, Walkey AJ.. Two decades of mortality trends among patients with severe sepsis: a comparative meta-analysis. Crit Care Med 2014; 423: 625–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rhee C, Dantes R, Epstein L, et al. Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009-2014. JAMA 2017; 31813: 1241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R.. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA 2014; 31113: 1308–16. [DOI] [PubMed] [Google Scholar]
- 12. Prescott HC, Calfee CS, Thompson BT, Angus DC, Liu VX.. Toward smarter lumping and smarter splitting: rethinking strategies for sepsis and acute respiratory distress syndrome clinical trial design. Am J Respir Crit Care Med 2016; 1942: 147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cohen J, Vincent JL, Adhikari NK, et al. Sepsis: a roadmap for future research. The Lancet. Infectious Diseases 2015; 155: 581–614. [DOI] [PubMed] [Google Scholar]
- 14. Abraham E. New definitions for sepsis and septic shock: continuing evolution but with much still to be done. JAMA 2016; 3158: 757–9. [DOI] [PubMed] [Google Scholar]
- 15. Hotchkiss RS, Sherwood ER.. Immunology getting sepsis therapy right. Science 2015; 3476227: 1201–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boomer JS, To K, Chang KC, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA 2011; 30623: 2594–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Davenport EE, Burnham KL, Radhakrishnan J, et al. Genomic landscape of the individual host response and outcomes in sepsis: a prospective cohort study. Lancet Respir Med 2016; 44: 259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wong HR, Cvijanovich NZ, Anas N, et al. Developing a clinically feasible personalized medicine approach to pediatric septic shock. Am J Respir Crit Care Med 2015; 1913: 309–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). Jama 2016; 3158: 762–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leligdowicz A, Dodek PM, Norena M, et al. Association between source of infection and hospital mortality in patients who have septic shock. Am J Respir Crit Care Med 2014; 18910: 1204–13. [DOI] [PubMed] [Google Scholar]
- 21. Sweeney TE, Shidham A, Wong HR, Khatri P.. A comprehensive time-course-based multicohort analysis of sepsis and sterile inflammation reveals a robust diagnostic gene set. Sci Transl Med 2015; 7287: 287ra71.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Angus DC, Seymour CW, Coopersmith CM, et al. A framework for the development and interpretation of different sepsis definitions and clinical criteria. Crit Care Med 2016; 443: e113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Seymour CW, Kennedy JN, Wang S, et al. Derivation, validation, and potential treatment implications of novel clinical phenotypes for sepsis. JAMA 2019. doi: 10.1001/jama.2019.5791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 2017; 433: 304–77. [DOI] [PubMed] [Google Scholar]
- 25. Shankar-Hari M, Phillips GS, Levy ML, et al. Developing a new definition and assessing new clinical criteria for septic shock: for the third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016; 3158: 775–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016; 3158: 801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kaukonen KM, Bailey M, Pilcher D, Cooper DJ, Bellomo R.. Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med 2015; 37217: 1629–38. [DOI] [PubMed] [Google Scholar]
- 28. Martin GS, Mannino DM, Eaton S, Moss M.. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 2003; 34816: 1546–54. [DOI] [PubMed] [Google Scholar]
- 29. Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR.. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001; 297: 1303–10. [DOI] [PubMed] [Google Scholar]
- 30. Rubulotta F, Marshall JC, Ramsay G, Nelson D, Levy M, Williams M.. Predisposition, insult/infection, response, and organ dysfunction: A new model for staging severe sepsis. Crit Care Med 2009; 374: 1329–35. [DOI] [PubMed] [Google Scholar]
- 31. Rhee C, Gohil S, Klompas M.. Regulatory mandates for sepsis care–reasons for caution. N Engl J Med 2014; 37018: 1673–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Blei DM, Ng AY, Jordan MI.. Latent Dirichlet allocation. Journal of Machine Learning Research 2003; 3: 993–1022. [Google Scholar]
- 33. Chen JH, Goldstein MK, Asch SM, Mackey L, Altman RB.. Predicting inpatient clinical order patterns with probabilistic topic models vs conventional order sets. Journal of the American Medical Informatics Association: JAMIA 2017; 243: 472–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang C, Blei DM. Collaborative topic modeling for recommending scientific articles. In: Proceedings of the 17th ACM SIGKDD International Conference on Knowledge Discovery and Data Mining - KDD ’11; 2011; 448 http://dl.acm.org/citation.cfm? id=2020480.
- 35. Huang Z, Dong W, Duan H.. A probabilistic topic model for clinical risk stratification from electronic health records. J Biomed Inform 2015; 58: 28–36. [DOI] [PubMed] [Google Scholar]
- 36. Lu HM, Wei CP, Hsiao FY.. Modeling healthcare data using multiple-channel latent Dirichlet allocation. J Biomed Inform 2016; 60: 210.. [DOI] [PubMed] [Google Scholar]
- 37. Liu L, Tang L, Dong W, Yao S, Zhou W.. An overview of topic modeling and its current applications in bioinformatics. Springerplus 2016; 51: 1608.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liu VX, Fielding-Singh V, Greene JD, et al. The timing of early antibiotics and hospital mortality in sepsis. Am J Respir Crit Care Med 2017; 1967: 856–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Salton G, Buckley C.. Term-weighting approaches in automatic text retrieval. Inf Process Manag 1988; 245: 512–23. [Google Scholar]
- 40. Schwarz G. Estimating the dimension of a model. Ann. Statist 1978; 62: 461–64. [Google Scholar]
- 41. Liu V, Morehouse JJ, Soule J, Whippy A, Escobar GJ.. Fluid volume, lactate values, and mortality in sepsis patients with intermediate lactate values. Ann Am Thoracic Soc 2013; 105: 466–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu VX, Morehouse JW, Marelich GP, et al. Multicenter implementation of a treatment bundle for sepsis patients with intermediate lactate values. Am J Respirat Critical Care Med 2016; 193 (11): 1264–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Escobar GJ, Greene JD, Scheirer P, Gardner MN, Draper D, Kipnis P.. Risk-adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases. Med Care 2008; 463: 232–9. [DOI] [PubMed] [Google Scholar]
- 44. Ferreira FL, Bota DP, Bross A, Melot C, Vincent JL.. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA 2001; 28614: 1754–8. [DOI] [PubMed] [Google Scholar]
- 45. Dummett BA, Adams C, Scruth E, Liu V, Guo M, Escobar GJ.. Incorporating an early detection system into routine clinical practice in two community hospitals. J Hosp Med 2016; 11 (Suppl 1): S25–S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Escobar GJ, Fireman BH, Palen TE, et al. Risk adjusting community-acquired pneumonia hospital outcomes using automated databases. Am J Manag Care 2008; 143: 158–66. [PubMed] [Google Scholar]
- 47. Escobar GJ, Gardner MN, Greene JD, Draper D, Kipnis P.. Risk-adjusting hospital mortality using a comprehensive electronic record in an integrated health care delivery system. Med Care 2013; 515: 446–53. [DOI] [PubMed] [Google Scholar]
- 48. Escobar GJ, Ragins A, Scheirer P, Liu V, Robles J, Kipnis P.. Nonelective rehospitalizations and postdischarge mortality: predictive models suitable for use in real time. Med Care 2015; 5311: 916–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wickham H, Francois R, Henry L, Müller K. dplyr: A Grammar of Data Manipulation. R package version 0.7.4. 2017. https://CRAN.R-project.org/package=dplyr. Accessed June 28, 2017.
- 50. Chang J. lda: Collapsed Gibbs Sampling Methods for Topic Models. R package version 1.4.2. 2015. https://CRAN.R-project.org/package=lda. Accessed August 15, 2017.
- 51. Calloway R Microsoft Corporation Weston S, Tenenbaum D. doParallel: Foreach Parallel Adaptor for the ‘parallel’ Package. R package version 1.0.11. 2017. https://CRAN.R-project.org/package=doParallel. Accessed January 25, 2018.
- 52. Gu Z, Gu L, Eils R, Schlesner M, Brors B.. circlize Implements and enhances circular visualization in R. Bioinformatics 2014; 3019: 2811–2. [DOI] [PubMed] [Google Scholar]
- 53. Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Critical Care Med 2003; 314: 1250–6. [DOI] [PubMed] [Google Scholar]
- 54. Iwashyna TJ, Odden A, Rohde J, et al. Identifying patients with severe sepsis using administrative claims: patient-level validation of the angus implementation of the international consensus conference definition of severe sepsis. Med Care 2012;526: e39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Seymour CW, Gesten F, Prescott HC, et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med 2017; 37623: 2235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Evans IVR, Phillips GS, Alpern ER, et al. Association between the New York sepsis care mandate and in-hospital mortality for pediatric sepsis. JAMA 2018; 3204: 358–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Marshall JC. Why have clinical trials in sepsis failed? Trends Mol Med 2014; 204: 195–203. [DOI] [PubMed] [Google Scholar]
- 58. Angus DC, Barnato AE, Bell D, et al. A systematic review and meta-analysis of early goal-directed therapy for septic shock: the ARISE, ProCESS and ProMISe Investigators. Intensive Care Med 2015; 419: 1549–60. [DOI] [PubMed] [Google Scholar]
- 59. Parikh RB, Kakad M, Bates DW.. Integrating predictive analytics into high-value care: the dawn of precision delivery. JAMA 2016; 3157: 651–2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.