Abstract
Despite aggression being detrimental to children’s physical health, mental health and social development, the dispositional and neurological antecedents of aggression in the child are poorly understood. Here we examined the relationship between trait aggression as measured by Buss and Warren’s Aggression Questionnaire and personality traits measured with Big Five Questionnaire for Children in 77 primary-school children and recorded resting-state brain activity (fractional amplitude of low-frequency fluctuations [fALFF]) and resting-state functional connectivity (rsFC) using functional magnetic resonance imaging. The present results showed that trait aggression was negatively correlated with agreeableness and positively correlated with neuroticism. The brain analyses showed that children with a higher propensity for aggression had a lower fALFF mainly in the left superior temporal gyrus, right parahippocampal gyrus and left supramarginal gyrus. Physical and total aggressions were negatively associated with rsFC between the right parahippocampal gyrus and the right putamen. Further analysis revealed that this rsFC could moderate the influence of neuroticism on total aggression. Moreover, the results suggest the presence of a sex difference in the neurodevelopmental mechanisms underlying aggression in middle childhood. Overall, our findings indicate that aggressive children have lower agreeableness and higher neuroticism, and the underlying neural systems are mainly implicated in social judgment and empathy.
Keywords: middle children, aggression, personality trait, empathy, social cognition
Introduction
Aggression generally means actions intended to cause physical or psychological harm to another individual—an innate and evolutionarily conserved adaptation in social organisms for the protection of food, mating partners, progeny and territory (Anderson, 2012). Although aggression can yield competitive advantages, most forms of aggression in contemporary humans are maladaptive and produce a variety of negative social, physical and emotional consequences for perpetrators, victims and their families (Quanty, 2010; Beck et al., 2013; Dolenc et al., 2015; Coccaro et al., 2016).
Middle childhood is a period of dramatic advances in social, biological and cognitive functioning with significant developmental changes (Beauchaine, 2015; Woltering et al., 2016). It is also a critical time in the developmental trajectory of aggression (Piquero et al., 2012). Aggressive behaviors such as verbal aggression, interpersonal hostility and physical fighting are prevalent during childhood (Séguin and Zelazo, 2005). Arsenio and Lemerise (2001) indicated that aggressive children usually lack emotional and empathic responsiveness toward their victims. Studies on the association between empathy and aggression have shown that boys (i.e. 9–14 years of age) exhibit higher levels of aggression and lower empathy than do girls (Mayberry and Espelage, 2007; Malti et al., 2009; Rieffe et al., 2016). Moreover, to avoid sanctions by adults against overt aggression (i.e. direct threatening or physical hitting), children will adopt more subtle forms of aggression. For example, indirect and hostile behaviors such as rumor spreading or social exclusion are often used to harm others by threatening the peer relationship. Previous studies also suggest that boys and girls may differ in the subtypes of aggression that they utilize. Evidence shows that boys are more likely to use physical and overt aggression whereas girls tend to use relational aggression (Cote et al., 2007; Fite et al., 2014). However, some studies find no sex difference in aggression (Archer, 2004; Card et al., 2008). Obviously, these behaviors seriously affect children’s physical health, mental health, academic progress and social adaptation (Heilbron and Prinstein, 2008; Wang et al., 2009). Although aggressive behaviors tend to decrease with age, a certain number of children retain cognitive and behavioral patterns of aggression that continue through the teen years and into adulthood. Investigation of the dispositional and neurological underpinnings of aggression is likely to enhance our understanding of this maladaptive process and guide the formulation of effective prevention and intervention programs (Horton et al., 2014; Thomson and Centifanti, 2017).
Differences in aggression in children are generally attributed to individual characteristics (e.g. neurobiological substrates, personality) and environmental influences (e.g. parenting style, exposure to violent media). Personality traits comprise the relatively stable pattern of an individual’s typical emotions, attitudes and behaviors and have been considered the key predictive variables of aggression (Anderson and Huesmann, 2003; Jensen-Campbell et al., 2007; Anderson, 2012; Brees et al., 2014). Neuroticism, characterized as being easily upset and emotionally unstable, is positively related to anger and aggressive behaviors (Sharpe and Desai, 2001; Brees et al., 2014). Agreeableness, described as being good-natured, altruistic, trustful, sympathetic, and cooperative, is negatively related to self and peer-reported aggression and violence (Heaven, 1996; Gleason et al., 2004). Evidence from research on early and middle children has shown that high neuroticism and low agreeableness are closely associated with a propensity for aggression, including the subtypes of anger, relational and physical aggression and hostility (Goldberg, 2001; Shiner and Caspi, 2003). Gleason et al. (2004) linked the personality dimensions to aggression and found that agreeableness was negatively associated with both indirect and direct aggression in late childhood. Moreover, personality traits have been shown to moderate the associations between environmental influences and externalizing behaviors represented by aggressive behaviors (Yeh et al., 2011; Smack et al., 2015). Smack et al. (2015) indicate that in the context of negative parenting, children with high neuroticism and low agreeableness show higher rates of externalizing problem behaviors than do their low-neuroticism counterparts.
In addition to exploring personality traits, uncovering neural correlates is of great significance for understanding the occurrence and development of aggression. The neural basis of aggression has been investigated extensively both in humans and in non-human species (Nelson and Trainor, 2007). Functional and structural neuroimaging studies show that the biological mechanisms underlying aggressive behavior involve various regions and multiple neural pathways distributed in cortical and subcortical brain structures (Raine and Yang, 2006). The brain regions most important in executing and regulating aggression are the frontal and prefrontal areas, which functionally support executive and affective control (Kateri et al., 2010; Florian et al., 2011; Mckenna et al., 2017), empathy (Stuss et al., 2001) and emotional regulation (Bufkin and Luttrell, 2005; Hedy et al., 2008; Koenigsberg et al., 2010). Another region important for aggression is the temporal cortex, which was found to be reliably activated in emotional processing and regulation (Allison et al., 2000; Marie-Hélène and Tomás, 2006), in empathy (Carrington and Bailey, 2009), and in social cognition (Koenigsberg et al., 2011). Aggression is also associated with dysregulation of a cortico-limbic network (Davidson et al., 2000; Siever, 2015; Klasen et al., 2019). Specifically, the functioning of the prefrontal cortex-amygdala regulatory system seems to be central to successfully restraining impulsive aggression (Hoptman et al., 2010a). Aggression generally appears to involve many brain areas and cognitive functions, but these results are mainly derived from adolescent and adult samples. The present research aims to explore the relative importance of brain regions in regulating aggression among children.
Theoretical and empirical research suggests that the development of aggression is influenced by different factors at different growth stages. The Dual Systems Model (Steinberg, 2008) posits an imbalance between the development of the cognitive control system and that of the socioemotional system. Cognitive control depends on the maturity of the frontal and prefrontal cortices, and the development of these areas is slow, continuing into late adolescence (Casey et al., 2012; Duckworth and Steinberg, 2015; Meisel et al., 2019). Neuroimaging studies also indicate that children are more prone to interference and have greater difficulty in inhibiting inappropriate responses than do adults (Casey et al., 2000; Bunge et al., 2002). This deficient functioning of inhibitory control in children is mainly due to the immaturity of the frontal regions (Nitin et al., 2004; Casey et al., 2008; Laurence, 2010). Compared with the slowly developing cognitive control system, the socioemotional system, involving primary sensory cortices and the limbic subcortical regions, develops earlier and regulates impulsive behavior during early development (Mills et al., 2014; Shulman et al., 2016; Meisel et al., 2019). The superior temporal gyrus (STG) and supramarginal gyrus (SMG) of the socioemotional system are specifically implicated in social cognition and empathy (Gallagher and Frith, 2004; Koenigsberg et al., 2010; Grecucci et al., 2013; Göttlich et al., 2017) and have been found to be associated with aggressive impulsive behavior in children. Based on the Dual Systems Model and the development of aggression, we inferred that in the group of children, the neural correlates of the socioemotional system might play a relatively dominant role in establishing a propensity for aggression.
Despite the wealth of neuroimaging studies on aggression in adults and adolescents, to our knowledge, few studies have addressed the neural substrates of aggression in children, especially with the technique of resting-state functional magnetic resonance imaging (rsfMRI). Previous research has used mostly task-related functional magnetic resonance imaging (fMRI) designs to investigate the neurobiological substrates of individual aggression. However, based on the existing evidence, stable individual differences in personality are more clearly manifested in the overall brain structure and function. Thus, task-free designs may be advantageous for examining the brain bases of aggression (Telzer et al., 2018; Water et al., 2019). The rsfMRI is a reliable and frequently used task-free method of detecting intrinsic brain activity (Romero-Martínez et al., 2019). The two most popular measures of rsfMRI are: (i) the fractional amplitude of low-frequency fluctuations (fALFF), which reflects the regional properties of spontaneous brain activity and (ii) the resting-state functional connectivity (rsFC), which reflects the synchronization and functional connections between brain regions within specific neural circuits. Research has shown that the physiological noise irrelevant to brain activity found by the original ALFF approach is successfully suppressed with the fALFF approach, suggesting that this measure has greater sensitivity and specificity in detecting spontaneous brain activity (Zou et al., 2008; Zuo et al., 2010). Previous studies have used the fALFF and rsFC to detect a variety of neuropsychiatric disorders (Han et al., 2011; Hoptman et al., 2010b) and to uncover the neural mechanisms underlying human cognition, social behavior and personality in healthy populations (Cox et al., 2011; Kong et al., 2015; Wang et al., 2017). In light of the above findings, the present study was designed to investigate the neural mechanisms underlying aggression based on both the regional brain activations indexed by fALFF and the functional connectivities between brain regions indexed by rsFC.
In the present study, we examined, in primary-school children, the relationships between personality traits and aggression, and the intrinsic brain activity and functional connectivity associated with aggression. We hypothesized that in children, aggression is negatively associated with agreeableness and positively associated with neuroticism, and initially expected that the neural correlates of aggression would be found mostly in the temporal and limbic areas. We also investigated the relationships among personality, brain mechanisms and trait aggression in this population. Considering that previous research supports a role for sex differences in aggression, we also examined behavior and imaging outcomes by sex.
Methods
Participants
One hundred thirteen primary-school students (age ran- ge 9–12 years, mean age = 10.04 ± 0.9 years, 49 girls) participated in this study. All participants were from Chaoyang Primary School in Chongqing, China. All participants were right-handed, and none reported a history of psychiatric or neurological illnesses. From the 113 participants in the neuroimaging protocol, 36 were excluded because of missing data (n = 9) or excessive head motion (n = 27). Excessive head motion was defined as a maximal displacement > 2.5 mm or a maximal rotation > 2.5 degrees throughout the course of the scan, according to recent influential methodological rsFC study (Allen et al., 2011; Liao et al., 2018). These exclusions yielded a final sample of 77 participants (mean age = 10.17 ± 0.95 years, 42 girls). The independent-sample t-test was used to assess the effect of attrition (0 = excluded, 1 = remained), and no significant differences were found neither in aggression types nor in personality traits between those remained and those excluded (the Supplementary File). All participants and their parents signed the informed consent document prior to the experiment and received an honorarium at the end of the study. Ethical approval of this study was granted by the Ethics Committee of the Southwest University, and all procedures were in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki).
Trait aggression
The 34-item form of Buss and Warren’s Aggression Questionnaire (BWAQ) was administered to evaluate participants’ trait aggression (Buss and Perry, 1992; Maxwell, 2007). Each item was answered on a 5-point Likert scale ranging from 1 (extremely uncharacteristic of me) to 5 (extremely characteristic of me). The questionnaire measured five constructs related to aggression: physical aggression, verbal aggression, anger, hostility and indirect aggression. The Chinese version of BWAQ has been widely utilized and is reported to have good psychometric properties (Maxwell, 2008). We used this version and conducted a confirmatory factor analysis. The overall Cronbach’s α with the present sample was 0.92, and the internal consistency estimates for the 5 subscales were 0.88, 0.60, 0.71, 0.73 and 0.66, respectively.
Personality traits
Measurement of personality traits used the Big Five Questionnaire for Children (BFQ-C) (Barbaranelli et al., 2003 2008), consisting of 65 items that measure 5 personality traits, namely extraversion, agreeableness, conscientiousness, neuroticism and openness. The Chinese version of the BFQ-C has been widely used and demonstrates adequate reliability and validity (Zhou, 2015). Participants were required to rate each statement on a 3-point Likert-type scale of 1 (disagree) to 3 (agree). The overall Cronbach’s α with the present sample was 0.88, and the internal consistency estimates for extraversion, agreeableness, conscientiousness, neuroticism and openness were 0.62, 0.80, 0.72, 0.81 and 0.79, respectively, which indicated good reliability.
rsfMRI data acquisition and preprocessing
Image acquisition.
For each participant, an 8-min rsfMRI scan was acquired in a 3T Trio scanner (Siemens Medical, Erlangen, Germany). During scanning, each participant was asked to remain still and relaxed, not to open his/her eyes and not to think of anything deliberately. Foam pads and earplugs were employed to reduce head motion and scanning noise. We used a gradient echo planar imaging sequence to obtain the resting-state functional image, with the following parameters: repetition time = 2000 ms, echo time = 30 ms, slices = 33, slice thickness = 3.5 mm, resolution matrix = 64 × 64, flip angle = 90°, field of view = 224 × 224 mm2, slice gap = 1 mm and voxel size = 3.5 × 3.5 × 3.5 mm3. Each section contained 180 volumes. In addition, high-resolution T1-weighted structural images were acquired from all participants using the same scanner with a 3D spoiled gradient-recalled sequence, with the following parameters: repetition time = 2530 ms, echo time = 3.48 ms, filed of view = 256 × 256 mm2, flip angle = 7°, resolution matrix = 256 × 256, slices thickness = 1 mm and voxel size = 1 × 1 × 1 mm3. The latter images provided an anatomical reference for the functional scans.
Image data preprocessing.
The Data Processing Assistant for rsfMRI (DPARSF; Yang et al., 2016) was used to preprocess the image data in MATLAB (The Math Works, Inc., Natick, MA, USA) platform. Preprocessing was conducted in the following stages. The first six images were discarded to allow for participant familiarization and fMRI signal stabilization. The remaining 174 images were corrected for temporal shifts between slices, realigned to the middle volume and unwrapped to correct for susceptibility-by-movement interaction. Then, each fMRI images were registered to their segmented high-resolution T1-weighted anatomical images; regressing nuisance variables included six head motion parameters, white matter signal and cerebral spinal fluid signal. Next, each image was normalized to the Montreal Neurological Institute (MNI) template with a resolution voxel size of 3 × 3 × 3 mm3 and smoothing with a 4-mm full width at half maximum of Gaussian kernel. Finally, linear detrending and band-pass filtering (0.01–0.1 Hz) to discard physiological noise drift from scanner instabilities and head motion. Moreover, the frame-wise displacement (FD) was calculated as a measure of head motion and was treated as a covariate in subsequent data analysis.
Statistical analysis
fALFF-behavior correlation analysis.
According to the methods proposed by Zou et al. (2008), the time courses for each voxel were first converted to the frequency domain, and then, the square root of the power spectrum was computed and averaged across the specified frequency range (0.01–0.1 Hz) in each voxel. The fALFF was then computed as the sum of the amplitudes across a low-frequency range (0.01–0.1 Hz) divided by the sum of the amplitudes across the entire frequency range (0–0.25 Hz). Subsequently, the normalized fALFF was obtained by dividing the fALFF of each voxel by the global mean fALFF value. Calculations were conducted using DPARSF software (Yang et al., 2016).
To identify the brain regions showing spontaneous brain activity related to aggression, we employed whole-brain correlation analyses between the scores for the aggression constructs and the fALFF values at each voxel in the brain, with sex, age and mean FD as controlling covariates. To determine statistical significance, the results were corrected for multiple comparisons using the Gaussian random field (GRF) program, and the threshold was set to a corrected cluster P < 0.05 (single voxel P < 0.005, cluster size > 40 voxels). These analyses were conducted using the DPABI software toolbox (http://rfmri.org/dpabi, version 2.3) in MATLAB.
rsFC-behavior correlation analysis.
We performed rsFC-behavior correlation analyses to investigate whether the clusters identified through the fALFF-behavior analyses interacted with other regions to explain aggression in children. To do so, seed regions were created using the clusters with a significant relation to aggression. For each participant, we first averaged the time series of all voxels in each seed. We then performed correlation analyses between the mean time series in each seed and that of other voxels in the brain, obtaining participant-level correlation maps. For standardization purposes, the correlation maps were normalized to z maps using Fisher’s r-to-z transformation. In the group-level analyses, we conducted correlation analyses between the z maps and the aggression scores to detect any association between rsFC and aggression, with age and sex as controlling variables. For multiple comparisons correction, we used the GRF program with the threshold set to a corrected cluster P < 0.05 (single voxel P < 0.005, cluster size > 100 voxels). These analyses above were performed using DPABI software.
Moderation analysis.
To determine whether the personality traits of children affected aggression through resting-state brain activity or connectivity, we carried out moderation analysis using the PROCESS macro (Hayes, 2013) in SPSS 22.0. In the moderating model, we treated the fALFF and rsFC of brain regions as the moderating variables, agreeableness and neuroticism as the independent variables, and aggression as the dependent variable. The significance of the moderating effect was assessed using a bootstrapping method with 5000 iterations. If the 95% confidence interval (CI) did not contain zero, then the moderating effect was deemed significant.
Results
Behavior
Table 1 lists the descriptive statistics and correlations of six dimensions of aggression and five personality-trait constructs. As shown in the table, the correlations indicate that agreeableness and neuroticism were significantly related to aggression. Notably, agreeableness was negatively correlated with anger (P < 0.01), hostility, total aggression and indirect aggression (ps < 0.05). The neuroticism trait was positively correlated with all dimensions of aggression (ps < 0.01). In addition, conscientiousness was negatively correlated with anger (P < 0.05).
Table 1.
Descriptive statistics and inter-correlations between aggression types and personality constructs (N = 77)
| Vars | M | s.d. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| Sex | - | - | - | |||||||||||
| Ver | 12.74 | 3.42 | 0.077 | - | ||||||||||
| Phy | 13.61 | 5.69 | 0.077 | 0.376** | - | |||||||||
| Ang | 14.49 | 5.29 | 0.063 | 0.286** | 0.589** | - | ||||||||
| Hos | 16.6 | 6.18 | −0.080 | 0.376** | 0.342** | 0.589** | - | |||||||
| Ind | 11.43 | 3.83 | 0.185 | 0.392** | 0.598** | 0.548** | 0.542** | - | ||||||
| Total | 68.87 | 18.7 | 0.067 | 0.585** | 0.777** | 0.823** | 0.783** | 0.794** | - | |||||
| Ext | 31.31 | 3.83 | −0.006 | 0.068 | −0.021 | −0.004 | −0.017 | −0.07 | −0.002 | - | ||||
| Agr | 33.57 | 4.12 | 0.045 | 0.047 | −0.177 | −0.319** | −0.231* | −0.179 | −0.249* | 0.568** | - | |||
| Con | 31.97 | 4.11 | 0.031 | 0.047 | −0.148 | −0.245* | −0.174 | −0.215 | −0.208 | 0.511** | 0.681** | - | ||
| Neu | 21.91 | 4.92 | 0.086 | 0.378** | 0.388** | 0.434** | 0.426** | 0.298** | 0.513** | 0.045 | −0.242* | −0.227* | - | |
| Ope | 31.99 | 4.68 | 0.047 | 0.047 | −0.091 | −0.089 | −0.099 | −0.096 | −0.097 | 0.686** | 636** | 0.713** | −0.027 | - |
M, mean; s.d., standard deviation; Var, variables.
Ver means verbal aggression; Phy, physical aggression; Ang, anger; Hos, hostility; Ind, indirect aggression; Tot, the aggression summation of each type; Ext, extraversion; Agr, agreeableness; Con, conscientiousness; Neu, neuroticism; Ope, openness.
*P < 0.05, **P < 0.01.
Imaging
To reveal relationships between spontaneous brain activity and aggression, we correlated different types of aggression with the fALFF at each voxel in the brain. After adjusting for sex, age and FD, the results showed a network of activation primarily comprising the temporal, parietal and paralimbic areas (Table 2, Figure 1). In detail, anger was negatively related to the fALFF in the left STG (r = − 0.519, P < 0.0001), and indirect aggression was located in the SMG (r = − 0.528, P < 0.0001) extending into the post-central gyrus. The activation of physical aggression involved the parahippocampal gyrus (PHG) (r = − 0.483, P < 0.0001) extending into the temporal pole. Another cluster of physical aggression was found in the STG (r = − 0.682, P < 0.0001) extending into the insula. Verbal aggression was negatively related to activation in the left STG (r = − 0.55, P < 0.001). For total aggression, the activated region involved the STG (r = − 0.616, P < 0.0001) extending to the insula.
Table 2.
Brain regions where fALFF and rsFC were associated with different types of aggression
| MNI coordinates | |||||||
| Aggression | Region | Cluster size | BA | x | y | z | R |
| Correlation with fALFF | |||||||
| Anger | STG(L) | 45 | 22 | −54 | −12 | 6 | −0.408 |
| Indirect | SMG(L) | 83 | 40 | −51 | −24 | 15 | −0.497 |
| Physical | PHG(R) | 44 | 28 | 24 | 6 | −27 | −0.399 |
| STG(L) | 239 | 42 | −60 | −12 | 9 | −0.495 | |
| Verbal | STG(L) | 52 | 42 | −60 | −12 | 9 | −0.412 |
| Total | STG(L) | 184 | 42/22 | −48 | −24 | 12 | −0.464 |
| Correlation with rsFC | |||||||
| Physical | PHG-Putamen | 174 | 47 | 24 | 15 | 3 | −0.423 |
| Total | PHG-Putamen | 107 | 48 | 24 | 12 | 0 | −0.414 |
The threshold for significant regions was set at P < 0.05 at the cluster level, combined with P < 0.005 at the voxel level (GRF program for the regions of fALFF analyses: cluster size 40 voxels; for the regions of RSFC analyses: cluster size 100 voxels).
BA, Brodmann area; L, left; R, right.
Fig. 1.
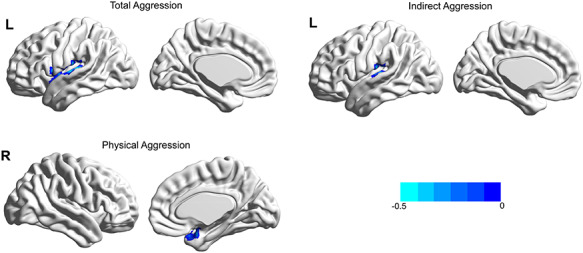
Brain region linked with various types of aggression after adjusting for age, sex and FD. Total aggression was negatively associated with the fALFF in the left STG; physical aggression was negatively associated with the fALFF in the right PHG; indirect aggression was negatively associated with the fALFF in the left SMG.
Moreover, a further FC analysis employed the activation peaks determined in the correlation results as seed regions, with connectivity changes being calculated between all pairs of seed regions (Figure 2). With the right PHG as the seed region, decreased functional connectivity with the right putamen was found to be significantly associated with physical aggression (r = − 0.376, P < 0.001) and total aggression (r = − 0.404, P < 0.0001).
Fig. 2.
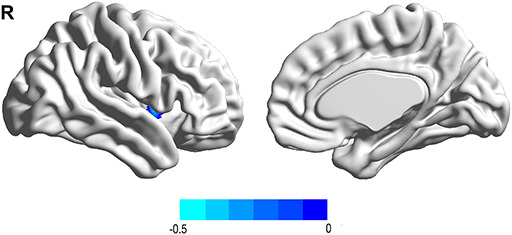
Functional connectivity linked with total aggression after adjusting for age, sex and FD. Total aggression was negatively associated with the connectivity between the right PHG and right putamen.
Moderating model analyses
Here we examined whether right PHG-right putamen functional connectivity moderated the relationship between neuroticism and aggression. After controlling for sex and age, 5000 bootstrap simulations showed a significant main effect of neuroticism (F [5, 71] = 7.17, P < 0.001) and an interaction effect of neuroticism × PHG-putamen connectivity (F [1, 71] = 6.90, P < 0.05) on total aggression. Specifically, neuroticism showed a significant positive association with total aggression (B = 0.48, SE = 0.10, t = 4.94, P < 0.01, 95% CI = [0.29, 0.68]). The neuroticism × PHG-putamen connectivity interaction was a significant negative association on total aggression (B = − 0.26, SE = 0.10, t = − 2.63, P < 0.05, 95% CI = [−0.46, − 0.06]), which revealed a moderating effect of the right PHG-right putamen connectivity on the relationship between neuroticism and aggression. We then calculated the conditional direct effect based on the moderator values to further examine the moderating effect on neuroticism. The results showed in the Figure 3, for the group with low PHG-putamen connectivity (one s.d. below the mean), a significant positive relationship between neuroticism and total aggression (B = 0.75, SE = 0.13, t = 5.58, P < 0.01, 95% CI = [0.52, 1.10]). However, for the group with high PHG-putamen connectivity (one s.d. above the mean), this relationship was not significant (B = 0.22, SE = 0.15, t = 1.50, P > 0.05, 95% CI = [−0.07, 0.51]). These results suggested that the influence of neuroticism on aggression might be moderated by the rsFC of the right PHG-right putamen, such that weaker PHG-putamen connectivity was associated with higher aggression.
Fig. 3.
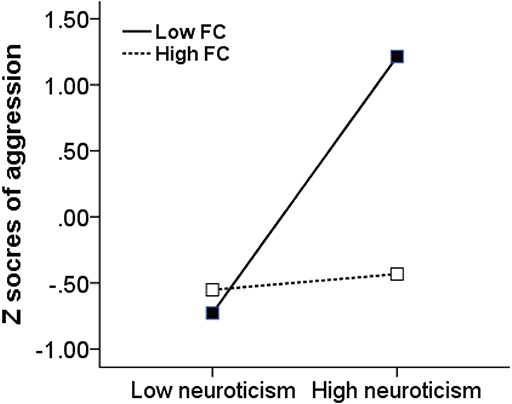
The right PHG-putamen connectivity moderates the association between neuroticism and total aggression. FC, functional connectivity of the right PHG-putamen.
Group analysis based on sex
Given the presence of sex differences in child aggression, we conducted further behavioral correlation and imaging analysis based on sex. The correlation results (Supplementary File) showed that the associations between aggression and personality traits in boys and girls were consistent with the results for the whole group. After adjusting for age and FD, the results for girls showed that indirect aggression and total aggression were negatively related to the fALFF in the SMG (r = − 0.563 and − 0.632, ps < 0.0001) (Table 3, Figure 4). Moreover, a further rsFC analysis indicated that with the SMG as the seed region, decreased functional connectivity with the left STG was significantly associated with indirect aggression (r = − 0.602 and − 0.604, ps < 0.0001) (Figure 5).
Table 3.
Brain regions where fALFF and rsFC were associated with different types of aggression in girl (N = 42)
| MNI coordinate | |||||||
| Aggression | Region | Cluster size | BA | x | y | z | R |
| Correlation with fALFF | |||||||
| Indirect | SMG(L) | 60 | 40 | −51 | −24 | 15 | −0.627 |
| Total | SMG(L) | 59 | 40 | −51 | −24 | 15 | −0.626 |
| Correlation with rsFC | |||||||
| Indirect | SMG-STG(R) | 162 | 22 | 48 | 3 | −9 | −0.612 |
| Indirect | SMG-STG(R) | 157 | 22 | 45 | 0 | −9 | −0.604 |
Notes: The threshold for significant regions was set at P < 0.05 at the cluster level, combined with P < 0.005 at the voxel level based on GRF program.
BA, Brodmann area; L, left; R, right.
Fig. 4.
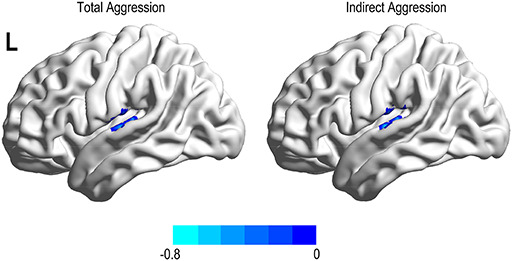
Brain region linked with aggression type after adjusting for age and FD. Total and indirect aggressions were negatively associated with the fALFF in the left SMG.
Fig. 5.
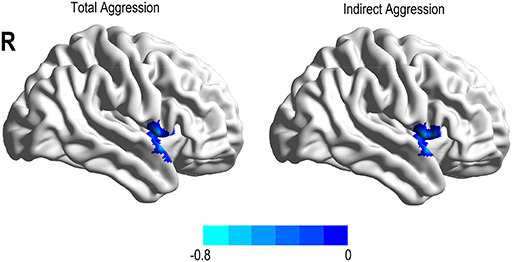
Functional connectivity linked with aggression type after adjusting for age and FD. Total and indirect aggressions were negatively associated with the connectivity between the left SMG and right STG.
The results for boys showed that physical aggression was negatively related to the fALFF in the post-central (r = 0.657, P < 0.0001) and pre-central gyrus (r = 0.682, P < 0.0001) (Table 4, Figure 6). Moreover, a further rsFC analysis indicated that with the pre-central gyrus as the seed region, decreased functional connectivity with the left angular gyrus was significantly associated with physical aggression (r = − 0.627, P < 0.0001) (Figure 7).
Table 4.
Brain regions where fALFF and rsFC were associated with different types of aggression in boy (N = 35)
| MNI coordinates | |||||||
| Aggression | Region | Cluster size | BA | x | y | z | R |
| Correlation with fALFF | |||||||
| Physical | Post-central(R) | 41 | 3 | 42 | −30 | 54 | 0.599 |
| Pre-central(L) | 66 | 4 | −39 | −27 | 66 | 0.706 | |
| Correlation with rsFC | |||||||
| Physical | Pre-central-AG(L) | 145 | 39 | −42 | −69 | 27 | −0.653 |
The threshold for significant regions was set at P < 0.05 at the cluster level, combined with P < 0.005 at the voxel level based on GRF program.
AG, angular gyrus; BA, Brodmann area; L, left; R, right.
Fig. 6.
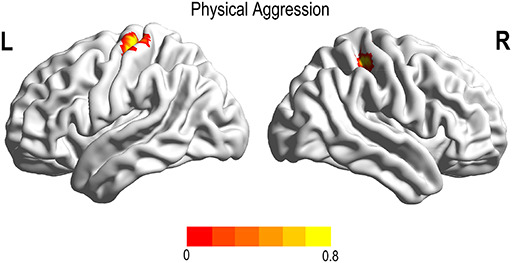
Brain region linked with aggression type after adjusting for age and FD. Physical aggression was negatively associated with the fALFF in the left pre-central gyrus and right post-central gyrus.
Fig. 7.
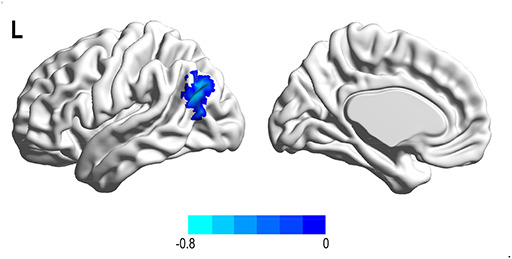
Functional connectivity linked with aggression type after adjusting for age and FD. Physical aggression was negatively associated with the connectivity between the left pre-central gyrus and left angular gyrus.
Discussion
The present study aimed to investigate the relevance of personality and neuroanatomical factors for aggression in middle children. Aggression was negatively correlated with agreeableness and positively correlated with neuroticism. The whole-brain correlation analyses showed that higher aggression was linked to lower fALFF in the left temporal lobe including the STG, temporal pole and limbic area including the right PHG, the insula and the SMG in the left parietal lobe. Moreover, we found that physical and total aggressions were negatively associated with the rsFC between the right PHG and the right putamen. Analysis of the moderation effect of brain connectivity revealed that this right PHG-right putamen connectivity could moderate the influence of neuroticism on total aggression. In addition, no sex difference was found in the preferred subtypes of aggression. Moreover, imaging results showed that in girls, a lower fALFF in the SMG and a decreased SMG-STG connectivity were associated with higher indirect aggression, whereas in boys, a lower fALFF in the pre-central gyrus and post-central gyrus and a decreased rsFC between the pre-central gyrus and angular gyrus were associated with higher physical aggression.
Existing evidence suggests that neuroticism and agreeableness are the important predictors of aggressive behavior (Sharpe and Desai, 2001; Anderson, 2012; Brees et al., 2014). Consistent with previous findings, the present results showed that trait aggression is negatively correlated with agreeableness and positively correlated with neuroticism in middle children. Specifically, children with high levels of neuroticism had high scores in all aggression subtypes (verbal, physical, anger, etc.), and children with high levels of agreeableness reported lower anger, hostility and total aggression. Research has shown that children with high agreeableness have greater perspective-taking ability and empathic concern (Melchers et al., 2016) and tend to behave communally to reduce interpersonal conflict and maintain intragroup cooperation (Graziano et al., 1996; Mooradian et al., 2011), thus showing a low level of aggression propensity. Studies also indicate that children with high neuroticism tend to fall apart under stress, show greater concern about acceptance, become flustered and disorganized and experience more negative emotion such as anxiety, fearfulness and insecurity (Goldberg, 2001; Shiner and Caspi, 2003). When encountering problems, neurotic children easily become frustrated and upset and have difficulty calming themselves. These maladjusted psychological and social functions in children with neuroticism possibly activate and aggravate aggressive manifestations.
Previous studies have revealed an anatomically widespread neurological mechanism of aggression. Historically, the temporal lobe was the second major brain area to be associated with antisocial and aggressive behavior (Raine and Yang, 2006). The present results on the resting brain activity underlying aggression in children mostly implicate the left temporal regions. Neuroimaging studies show that the temporal cortex plays an important role in linking sensory experiences to emotions, and its dysfunction might trigger impulsive responses and aggression (Bufkin and Luttrell, 2005). More specifically, Koenigsberg et al. (2011) asked subjects to apply reappraisal strategies to regulate emotions associated with both social and non-social situations. They found that pictures depicting social content activated the STG areas, which are likely involved in social cognition. Moreover, increased activity in the STG was also reported in tasks involving social judgment such as attributing mental states (Grecucci et al., 2013; Buadesrotger et al., 2016) and thinking about the intentions of others (Schultz et al., 2003; Gallagher and Frith, 2004). Previous studies also indicate that maladaptive social information processing in children aged 10 to 12 years is associated with aggressive responses and hostile attributional bias in negative social situations (Choe et al., 2015). Additionally, clinical studies have documented that aggressiveness in patients with psychiatric disorders is related to functional and structural abnormalities in the temporal cortex (Raine et al., 2010; Amen et al., 2011) and in the STG (Goldstein et al., 2009; Sun et al., 2009). Thus, deactivation of the left STG may be related to impaired social judgment in aggressive children.
The present study also implicated clusters covering the SMG, temporal pole, and insula in child propensity for aggression. Previous studies revealed the SMG to be an important hub in the perception of socio-affective stimuli (Göttlich et al., 2017), in overcoming emotional egocentricity bias in social situations (Silani et al., 2013; Klasen et al., 2018), and in functionally supporting empathy (Shamay-Tsoory, 2011; Coll et al., 2017). Heberlein et al. (2004) found that the SMG is involved in observing gestures and in evaluating the emotions conveyed by socially significant interactions. This indicated that the SMG might play a role complementary to that of the mirror neuron system by providing access to the meaning of observed actions (Grosbras and Paus, 2005). Moreover, dysfunctions of the temporal–parietal junction, which encompass the SMG, have been shown to contribute to aggressive behavior (Harenski et al., 2014; Klasen et al., 2018). The engagement of the insula and the temporal pole is consistently reported in studies of the experience of emotion (Marie-Hélène and Tomás, 2006; Coccaro et al., 2011) and empathy (Carrington and Bailey, 2009; Philipp and Christina, 2009; Bernhardt and Singer, 2012).
Taken together with the STG results, regions of resting activity including the SMG, temporal pole and insula are important neurological mechanism of empathy (Frith and Tania, 2008; Overwalle and Baetens, 2009; Shamay-Tsoory, 2011). Empathy is the capacity to identify and understand another’s thoughts, intentions and emotional states and has been proposed as a potential inhibitor of aggression (Feshbach, 1975; Hoffman, 2001). Empathy can promote pro-social behavior such as helping (Warden and Mackinnon, 2003; Eggum et al., 2011) and prevent antisocial behavior such as aggression and delinquency (Miller and Eisenberg, 1988; Jolliffe and Farrington, 2004). Research on aggression in child and adolescent samples has emphasized the role of empathy (Lovett and Sheffield, 2007; Pascualsagastizabal et al., 2019). Previous studies have shown that empathy is negatively associated with aggression in childhood aged 8 to 12 years (middle childhood) and that this association remains stable through adolescence (Lovett and Sheffield, 2007; Tampke et al., 2020). Problems of dysfunctional empathy among children are closely related to disruptive behavior and conduct disorders (Minet et al., 2010; Schwenck et al., 2012; Bons et al., 2013; Georgiou et al., 2019). The regions of brain deactivation observed in the present study might be related to poorer empathy and social judgment, which may cause children to respond with aggression (Bernhardt and Singer, 2012; Chester et al., 2014).
The association of the PHG with child physical aggression that we observed is consistent with it being an important hub within the medial temporal lobe, and an important part of the limbic system (Burwell, 2000; Eichenbaum and Lipton, 2008). Neuroimaging studies have revealed that the PHG is critical for behavioral inhibition (Peters and Christian, 2010, 2011), impulse control (Yang et al., 2010) and emotional regulation (Phillips et al., 2008). Additionally, the present study found that the rsFC of the right PHG with the right putamen was associated with total aggression, and that this rsFC further moderated the relationship between neuroticism and total aggression. Basal ganglia structures such as the putamen and caudate nucleus are implicated in the regulation of both simple and complex motor acts (Aouizerate et al., 2005; Chiang-Shan Ray et al., 2008). Abnormalities in these structures are suggested to be part of the pathophysiology of antisocial behavior (Glenn and Yaling, 2012). These results suggest that the PHG and its functional connectivity with the putamen could contribute to poor impulse control leading ultimately to aggression in children.
Previous studies indicate the presence of sex differences in the development trajectory and expression preferences of aggression. To be specific, in early and middle childhood, boys are more likely to use physical and overt aggression, whereas girls tend to use indirect and relational aggression (Cote et al., 2007; Fite et al., 2014). It has been shown that girls display higher levels of empathy and lower levels of aggression compared with boys (Mayberry and Espelage, 2007; Malti et al., 2009; Rieffe et al., 2016). However, our correlation analysis did not find any significant sex differences in the preferred subtypes of aggression. This result is consistent with other studies that found no sex differences in aggressive behaviors (Archer, 2004; Card et al., 2008). The brain imaging results by sex showed that in girls, deactivation of the SMG is associated with indirect and total aggression and that decreased functional connectivity between the SMG and the STG is associated with indirect aggression. These results suggest that in girls, aggression is likely associated with deficient empathy and social cognition (Gallagher and Frith, 2004; Koenigsberg et al., 2010; Grecucci et al., 2013; Göttlich et al., 2017). In boys, physical aggression was associated with activation of the pre-central and post-central gyri, and decreased rsFC between the pre-central gyrus and the angular gyrus, suggesting that aggression in boys is associated with poor inhibition of motor action and behavior execution (Kubler et al., 2006; Cheng et al., 2008; Blickenstorfer et al., 2009). These findings suggest the presence of sex differences in the neurodevelopmental mechanism of aggression in middle childhood.
For growing children, the ability to control impulses and empathize is critical for reducing aggressive intentions and behavior. However, we did not find any significant activity in the frontal area. The frontal cortex develops more slowly than do other brain regions, a process that continues well into late adolescence (Nitin et al., 2004; Sowell et al., 2004; Best and Miller, 2010; Meisel et al., 2019). In children, the relatively mature temporal, parietal and limbic regions, which are implicated in empathy and social cognition, play a more important role in restraining aggression than does the cognitive control ability by the frontal cortex (Decety and Michalska, 2011). The Dual Systems model (Casey et al., 2008; Steinberg, 2008; Luna and Wright, 2016) posit a rapidly developing socioemotional system and a gradually developing cognitive control system during individual development. The present study provided neurological evidence that in children, high aggressiveness and aggressive behavior are associated with regional deactivations in the socioemotional system. Moreover, the behavioral inhibition in children is more associated with the limbic PHG rather than with frontal cortex. Our results suggest that for middle children, the socioemotional system possibly plays an important role in the development of a propensity for aggression. The present results indicate that aggressiveness in children might be more related to impaired empathy and social cognition, which suggest that the key to prevention and intervention for aggressive children is to cultivate their ability and skills in empathy and social understanding.
This study has several potential limitations. First, this is the first rsfMRI study to investigate the neural basis of aggression in children, and the results therefore require verification in further studies. Second, common with the most recent resting-state studies (Choe et al., 2017; Fonzo et al., 2017; Samara et al., 2017), the design did not include a control for the participants’ degree of compliance with the experimenter’s instructions on conduct in the scanner, which is especially important for child participants. It should be noted that the calculation of fALFF is closely related to the time course of brain activity, and the elimination of bad time points can greatly change the final index. Therefore, a relatively lenient head motion criterion was adopted in this study rather than scrubbing the data. Third, we used child self-report measures of aggression propensity and personality traits, which might be influenced by the development of the child’s self-concept. Future research should ideally include both child-report and parent-report measures to optimally assess aggressive behaviors in daily life. Fourth, the present study preliminarily found a moderating effect of functional connectivity on the relationship between neuroticism and aggression in children, and further studies are required to explore the mediating mechanisms among the brain, neuroticism and aggression.
Conclusion
We conducted a detailed investigation of the relevance of personality factors and neuroanatomical factors for aggression in middle children. The behavioral results indicated that aggression is negatively correlated with agreeableness and positively correlated with neuroticism. Moreover, we found multiple brain networks contributing to aggression in children. The fALFF results showed that higher aggression is linked to deactivation in the left temporal lobe, limbic area and parietal lobe, areas functionally related to empathy and social cognition. The rsFC results showed that physical and total aggressions are negatively associated with functional connectivity between the right PHG and the right putamen and that this connectivity moderates the influence of neuroticism on total aggression. The analysis based on sex indicated a possible sex difference in the neurodevelopmental mechanisms underlying aggression in middle childhood. Our findings on the specific dispositional and neurological underpinnings of aggressiveness in children could contribute to enhancing the understanding of maladaptive processes and determining effective aggression prevention and intervention programs.
Supplementary Material
Acknowledgements
We would like to thank Chaoyang Primary School for their support of this research. We would also like to thank the Editage (www.editage.cn) for English language editing.
Supplementary data
Supplementary data are available at SOCAFN online.
Funding
This study was funded by National Natural Science Foundation of China (No. 31771237) and the Fundamental Research Funds for the Central Universities (No. SWU1709106).
Compliance of ethical standard statement
This manuscript has not been published or presented elsewhere in part or in entirety and is not under consideration by another journal. We have read and understood your journal’s policies, and we believe that neither the manuscript nor the study violates any of these.
Conflict of interest
None declared.
References
- Allen E.A., Erhardt E.B., Damaraju E. et al. (2011). A baseline for the multivariate comparison of resting-state networks. Frontiers in Systems Neuroscience, 5, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison T., Puce A., Mccarthy G. (2000). Social perception from visual cues: role of the STS region. Trends in Cognitive Sciences, 4(7), 267–78. [DOI] [PubMed] [Google Scholar]
- Amen D.G., Stubblefield M., Carmicheal B., Thisted R. (2011). Brain SPECT findings and aggressiveness. Annals of Clinical Psychiatry, 8(3), 129–37. [DOI] [PubMed] [Google Scholar]
- Anderson C.A., Huesmann L.R. (2003). Human aggression: A social-cognitive view In: Hogg M.A., Cooper J., editors. The Sage Handbook of Social Psychology, London, England: Sage Publications, 259–87. [Google Scholar]
- Anderson D.J. (2012). Optogenetics, sex, and violence in the brain: implications for psychiatry. Biological Psychiatry, 71(12), 1081–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aouizerate B., Corinne M.G., Emmanuel C. et al. (2005). Deep brain stimulation for OCD and major depression. American Journal of Psychiatry, 162(11), 2192. [DOI] [PubMed] [Google Scholar]
- Archer J. (2004). Sex differences in aggression in real-world settings: A meta-analytic review. Review of General Psychology, 8(4), 291–322. [Google Scholar]
- Arsenio W.F., Lemerise E.A. (2001). Varieties of Childhood Bullying: values, Emotion Processes and Social Competence. Social Development, 10(1), 59–73. [Google Scholar]
- Barbaranelli C., Caprara G.V., Rabasca A., Pastorelli C. (2003). A questionnaire for measuring the Big Five in late childhood. Personality and Individual Differences, 34(4), 645–64. [Google Scholar]
- Barbaranelli C., Fida R., Paciello M., Giunta L.D., Caprara G.V. (2008). Assessing personality in early adolescence through self-report and other-ratings a multitrait-multimethod analysis of the BFQ-C. Personality and Individual Differences, 44(4), 876–86. [Google Scholar]
- Beauchaine T.P. (2015). Respiratory sinus arrhythmia: a transdiagnostic biomarker of emotion dysregulation and psychopathology. Current Opinion in Psychology, 3, 43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A., Heinz A.J., Heinz A. (2013). Translational clinical neuroscience perspectives on the cognitive and neurobiological mechanisms underlying alcohol-related aggression. Current Topics in Behavioral Neurosciences, 17, 443–74. [DOI] [PubMed] [Google Scholar]
- Bernhardt B.C., Singer T. (2012). The Neural Basis of Empathy. Annual Review of Neuroscience, 278(1), 20–30. [DOI] [PubMed] [Google Scholar]
- Best J.R., Miller P.H. (2010). A developmental perspective on executive function. Child Development, 81(6), 1641–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blickenstorfer A., Kleiser R., Keller T. et al. (2009). Cortical and subcortical correlates of functional electrical stimulation of wrist extensor and flexor muscles revealed by fMRI. Human Brain Mapping, 30(3), 963–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bons D., Broek E.V.D., Scheepers F., Herpers P., Rommelse N., Buitelaaar J.K. (2013). Motor, Emotional, and Cognitive Empathy in Children and Adolescents with Autism Spectrum Disorder and Conduct Disorder. Journal of Abnormal Child Psychology, 41(3), 425–43. [DOI] [PubMed] [Google Scholar]
- Brees J., Mackey J., Martinko M., Harvey P. (2014). The Mediating Role of Perceptions of Abusive Supervision in the Relationship Between Personality and Aggression. Journal of Leadership and Organizational Studies, 21(4), 403–13. [Google Scholar]
- Buadesrotger M., Engelke C., Beyer F., Keevil B.G., Brabant G., Krämer U.M. (2016). Endogenous testosterone is associated with lower amygdala reactivity to angry faces and reduced aggressive behavior in healthy young women. Scientific Reports, 6(1), 38538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bufkin J.L., Luttrell V.R. (2005). Neuroimaging studies of aggressive and violent behavior: current findings and implications for criminology and criminal justice. Trauma, Violence & Abuse, 6(2), 176–91. [DOI] [PubMed] [Google Scholar]
- Bunge S.A., Dudukovic N.M., Thomason M.E., Vaidya C.J., Gabrieli J.D.E. (2002). Immature Frontal Lobe Contributions to Cognitive Control in Children: evidence from fMRI. Neuron, 33(2), 301–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwell R.D. (2000). The parahippocampal region: corticocortical connectivity. Annals of the New York Academy of Sciences, 911(1), 25–42. [DOI] [PubMed] [Google Scholar]
- Buss A.H., Perry M. (1992). The aggression questionnaire. Journal of Personality and Social Psychology, 63(3), 452–59. [DOI] [PubMed] [Google Scholar]
- Card N.A., Stucky B.D., Sawalani G.M., Little T.D. (2008). Direct and indirect aggression during childhood and adolescence: a meta-analytic review of gender differences, intercorrelations, and relations to maladjustment. Child Development, 79(5), 1185–229. [DOI] [PubMed] [Google Scholar]
- Carrington S.J., Bailey A.J. (2009). Are there theory of mind regions in the brain? A review of the neuroimaging literature. Human Brain Mapping, 30(8), 2313–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Getz S., Galvan A. (2008). The adolescent brain. Annals of the New York Academy of Sciences, 28(1), 62–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Giedd J.N., Thomas K.M. (2000). Structural and functional brain development and its relation to cognitive development. Biological Psychology, 54(1), 241–57. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Somerville L.H., Gotlib I.H. et al. (2012). Behavioral and neural correlates of delay of gratification 40 years later. Annals of the New York Academy of Sciences, 108(36), 14998–5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Yang C., Lin C., Lee P., Decety J. (2008). The perception of pain in others suppresses somatosensory oscillations: A magnetoencephalography study. NeuroImage, 40(4), 1833–40. [DOI] [PubMed] [Google Scholar]
- Chester D.S., Eisenberger N.I., Pond R.S., Richman S.B., Bushman B.J., DeWall C.N. (2014). The interactive effect of social pain and executive functioning on aggression: an fMRI experiment. Social Cognitive and Affective Neuroscience, 9(5), 699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang-Shan Ray L., Peisi Y., Rajita S., Tien-Wen L. (2008). Subcortical processes of motor response inhibition during a stop signal task. Neuroimage, 41(4), 1352–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe A.S., Nebel M.B., Barber A.D. et al. (2017). Comparing test-retest reliability of dynamic functional connectivity methods. Neuroimage, 158, 155–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe D.E., Shaw D.S., Forbes E.E. (2015). Maladaptive social information processing in childhood predicts young men’s atypical amygdala reactivity to threat. Journal of Child Psychology and Psychiatry, 56(5), 549–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccaro E.F., Chandra Sekhar S., Yanowitch R.N., Luan K. (2011). Corticolimbic function in impulsive aggressive behavior. Biological Psychiatry, 69(12), 1153–59. [DOI] [PubMed] [Google Scholar]
- Coccaro E.F., Fridberg D.J., Fanning J.R., Grant J.E., King A.C., Lee R. (2016). Substance use disorders: relationship with intermittent explosive disorder and with aggression, anger, and impulsivity. Journal of Psychiatric Research, 81, 127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll M.P., Tremblay M.P.B., Jackson P.L. (2017). The effect of tDCS over the right temporo-parietal junction on pain empathy. Neuropsychologia, 100, 110–19. [DOI] [PubMed] [Google Scholar]
- Cote S.M., Vaillancourt T., Barker E.D., Nagin D.S., Tremblay R.E. (2007). The joint development of physical and indirect aggression: predictors of continuity and change during childhood. Development and Psychopathology, 19(1), 37–55. [DOI] [PubMed] [Google Scholar]
- Cox C.L., Uddin L.Q., Di Martino A., Castellanos F.X., Milham M.P., Kelly C. (2011). The balance between feeling and knowing: affective and cognitive empathy are reflected in the brain’s intrinsic functional dynamics. Social Cognitive and Affective Neuroscience, 7(6), 727–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R.J., Putnam K.M., Larson C.L. (2000). Dysfunction in the neural circuitry of emotion regulation—a possible prelude to violence. Science, 289(5479), 591–94. [DOI] [PubMed] [Google Scholar]
- Decety J., Michalska K.J. (2011). How Children Develop Empathy: the Contribution of Developmental Affective Neuroscience In: Decety J., editor. Empathy: from Bench to Bedside, Cambridge, MA: MIT Press, 167–90. [Google Scholar]
- Dolenc B., Dernovšek M.Z., Sprah L., Tavcar R., Perugi G., Akiskal H.S. (2015). Relationship between affective temperaments and aggression in euthymic patients with bipolar mood disorder and major depressive disorder. Journal of Affective Disorders, 174, 13–18. [DOI] [PubMed] [Google Scholar]
- Duckworth A.L., Steinberg L. (2015). Unpacking Self‐Control. Child Development Perspectives, 9(1), 32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggum N.D., Eisenberg N., Kao K. et al. (2011). Emotion understanding, theory of mind, and prosocial orientation: relations over time in early childhood. The Journal of Positive Psychology, 6(1), 4–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H., Lipton P.A. (2008). Towards a functional organization of the medial temporal lobe memory system: role of the parahippocampal and medial entorhinal cortical areas. Hippocampus, 18(12), 1314–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feshbach N.D. (1975). Empathy in Children: some Theoretical and Empirical Considerations. Counseling Psychologist, 5(2), 25–30. [Google Scholar]
- Fite P.J., Rubens S.L., Preddy T.M., Raine A., Pardini D.A. (2014). Reactive/proactive aggression and the development of internalizing problems in males: the moderating effect of parent and peer relationships. Aggressive Behavior, 40(1), 69–78. [DOI] [PubMed] [Google Scholar]
- Florian L., Peter K., Leila H. et al. (2011). City living and urban upbringing affect neural social stress processing in humans. Nature, 474(7352), 498. [DOI] [PubMed] [Google Scholar]
- Fonzo G.A., Goodkind M.S., Oathes D.J. et al. (2017). Selective Effects of Psychotherapy on Frontopolar Cortical Function in PTSD. American Journal of Psychiatry, 174(12), 1175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith C.D., Tania S. (2008). The role of social cognition in decision making. Philosophical Transactions of the Royal Society of London, 363(1511), 3875–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher H.L., Frith C.D. (2004). Dissociable neural pathways for the perception and recognition of expressive and instrumental gestures. Neuropsychologia, 42(13), 1725–36. [DOI] [PubMed] [Google Scholar]
- Georgiou G., Demetriou C.A., Fanti K.A. (2019). Distinct Empathy Profiles in Callous Unemotional and Autistic Traits: investigating Unique and Interactive Associations with Affective and Cognitive Empathy. Journal of Abnormal Child Psychology, 47(11), 1863–73. [DOI] [PubMed] [Google Scholar]
- Gleason K.A., Jensen‐Campbell L.A., South Richardson D. (2004). Agreeableness as a predictor of aggression in adolescence. Aggressive Behavior, 30(1), 43–61. [Google Scholar]
- Glenn A.L., Yaling Y. (2012). The potential role of the striatum in antisocial behavior and psychopathy. Biological Psychiatry, 72(10), 817–22. [DOI] [PubMed] [Google Scholar]
- Goldberg L.R. (2001). Analyses of Digman’s Child-personality Data: derivation of Big-Five Factor Scores From Each of Six Samples. Journal of Personality, 69(5), 709–43. [DOI] [PubMed] [Google Scholar]
- Goldstein K.E., Hazlett E.A., New A.S. et al. (2009). Smaller superior temporal gyrus volume specificity in schizotypal personality disorder. Schizophrenia Research, 112(1), 14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göttlich M., Ye Z., Rodriguez-Fornells A., Münte T.F., Krämer U.M. (2017). Viewing socio-affective stimuli increases connectivity within an extended default mode network. Neuroimage, 148(148), 8–19. [DOI] [PubMed] [Google Scholar]
- Graziano W.G., Jensen-Campbell L.A., Hair E.C. (1996). Perceiving interpersonal conflict and reacting to it: the case for agreeableness. Journal of Personality and Social Psychology, 70(4), 820–35. [DOI] [PubMed] [Google Scholar]
- Grecucci A., Giorgetta C., Bonini N., Sanfey A.G. (2013). Reappraising social emotions: the role of inferior frontal gyrus, temporo-parietal junction and insula in interpersonal emotion regulation. Frontiers in Human Neuroscience, 7(7), 523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosbras M.-H., Paus T. (2005). Brain networks involved in viewing angry hands or faces. Cerebral Cortex, 16(8), 1087–96. [DOI] [PubMed] [Google Scholar]
- Han Y., Wang J., Zhao Z. (2011). Frequency-dependent changes in the amplitude of low-frequency fluctuations in amnestic mild cognitive impairment: a resting-state fMRI study. NeuroImage, 55(1), 287–95. [DOI] [PubMed] [Google Scholar]
- Harenski C.L., Edwards B.G., Harenski K.A., Kiehl K.A. (2014). Neural correlates of moral and non-moral emotion in female psychopathy. Frontiers in Human Neuroscience, 8(8), 741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes A.F. (2013). Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach In: Journal of Educational Measurement, New York, NY: The Guilford Press, 335–37. [Google Scholar]
- Heaven P.C.L. (1996). Personality and self-reported delinquency: analysis of the “Big Five” personality dimensions. Personality and Individual Differences, 20(1), 47–54. [Google Scholar]
- Heberlein A.S., Ralph A., Daniel T., Hanna D. (2004). Cortical regions for judgments of emotions and personality traits from point-light walkers. Journal of Cognitive Neuroscience, 16(7), 1143–58. [DOI] [PubMed] [Google Scholar]
- Hedy K., Lisa Feldman B., Josh J., Eliza B.M., Kristen L., Wager T.D. (2008). Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage, 42(2), 998–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbron N., Prinstein M.J. (2008). A review and reconceptualization of social aggression: adaptive and maladaptive correlates. Clinical Child and Family Psychology Review, 11(4), 176–217. [DOI] [PubMed] [Google Scholar]
- Hoffman M.L. (2001). Empathy and Moral Development: implications for Caring and Justice. Journal of the American Academy of Child and Adolescent Psychiatry, 40(5), 614–15. [Google Scholar]
- Hoptman M.J., Debra D.A., Dean C. et al. (2010a). Amygdalofrontal functional disconnectivity and aggression in schizophrenia. Schizophrenia Bulletin, 36(5), 1020–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoptman M.J., Zuo X., Butler P.D. et al. (2010b). Amplitude of low-frequency oscillations in schizophrenia: A resting state fMRI study. Schizophrenia Research, 117(1), 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton M.K., Margolis A.E., Cheuk T., Robert W. (2014). Neuroimaging is a novel tool to understand the impact of environmental chemicals on neurodevelopment. Current Opinion in Pediatrics, 26(2), 230–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen-Campbell L.A., Knack J.M., Waldrip A.M., Campbell S.D. (2007). Do Big Five personality traits associated with self-control influence the regulation of anger and aggression?. Journal of Research in Personality, 41(2), 403–24. [Google Scholar]
- Jolliffe D., Farrington D.P. (2004). Empathy and offending: A systematic review and meta-analysis. Aggression and Violent Behavior, 9(5), 441–76. [Google Scholar]
- Kateri M.R., Brent H., Sita C., Gabrieli J.D.E., Gross J.J., Ochsner K.N. (2010). The neural bases of distraction and reappraisal. Journal of Cognitive Neuroscience, 22(2), 248–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasen M., Wolf D., Eisner P.D. et al. (2019). Serotonergic contributions to human brain aggression networks. Frontiers in Neuroscience, 13, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasen M., Wolf D., Eisner P.D. et al. (2018). Neural networks underlying trait aggression depend on MAOA gene alleles. Brain Structure & Function, 223(2), 873–81. [DOI] [PubMed] [Google Scholar]
- Koenigsberg H.W., Jin F., Ochsner K.N. et al. (2010). Neural correlates of using distancing to regulate emotional responses to social situations. Neuropsychologia, 48(6), 1813–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsberg H.W., Jin F., Ochsner K.N. et al. (2011). Neural correlates of using distancing to regulate emotional responses to social situations. Neuropsychologia, 48(6), 1813–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong F., Hu S., Wang X., Song Y., Liu J. (2015). Neural correlates of the happy life: the amplitude of spontaneous low frequency fluctuations predicts subjective well-being. NeuroImage, 107, 136–45. [DOI] [PubMed] [Google Scholar]
- Kubler A., Dixon V., Garavan H. (2006). Automaticity and Reestablishment of Executive Control—An fMRI Study. Journal of Cognitive Neuroscience, 18(8), 1331–42. [DOI] [PubMed] [Google Scholar]
- Laurence S. (2010). A dual systems model of adolescent risk-taking. Developmental Psychobiology, 52(3), 216–24. [DOI] [PubMed] [Google Scholar]
- Liao W., Li J., Duan X., Cui Q., Chen H., Chen H. (2018). Static and dynamic connectomics differentiate between depressed patients with and without suicidal ideation. Human Brain Mapping, 39(10), 4105–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett B.J., Sheffield R.A. (2007). Affective empathy deficits in aggressive children and adolescents: A critical review. Clinical Psychology Review, 27(1), 1–13. [DOI] [PubMed] [Google Scholar]
- Luna B., Wright C. (2016). Adolescent brain development: implications for the juvenile criminal justice system In: Heilbrun K., DeMatteo D., Goldstein N.E.S., editors. APA Handbook of Psychology and Juvenile Justice, Washington, DC: American Psychological Association, 91–116. [Google Scholar]
- Malti T., Gummerum M., Keller M., Buchmann M. (2009). Children’s Moral Motivation, Sympathy, and Prosocial Behavior. Child Development, 80(2), 442–60. [DOI] [PubMed] [Google Scholar]
- Marie-Hélène G., Tomás P. (2006). Brain networks involved in viewing angry hands or faces. Cerebral Cortex, 16(8), 1087–96. [DOI] [PubMed] [Google Scholar]
- Maxwell J.P. (2007). Development and preliminary validation of a Chinese version of the Buss–Perry Aggression Questionnaire in a population of Hong Kong Chinese. Journal of Personality Assessment, 88(3), 284–94. [DOI] [PubMed] [Google Scholar]
- Maxwell J.P. (2008). Psychometric properties of a Chinese version of the Buss–Warren Aggression Questionnaire. Personality and Individual Differences, 44(4), 943–53. [Google Scholar]
- Mayberry M.L., Espelage D.L. (2007). Associations Among Empathy, Social Competence, & Reactive/Proactive Aggression Subtypes. Journal of Youth and Adolescence, 36(6), 787–98. [Google Scholar]
- Mckenna R., Rushe T., Woodcock K.A. (2017). Informing the Structure of Executive Function in Children: A Meta-Analysis of Functional Neuroimaging Data. Frontiers in Human Neuroscience, 11, 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisel S.N., Fosco W.D., Hawk L.W., Colder C.R. (2019). Mind the gap: A review and recommendations for statistically evaluating Dual Systems models of adolescent risk behavior. Developmental Cognitive Neuroscience, 39, 100681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchers M.C., Li M., Haas B.W., Reuter M., Bischoff L., Montag C. (2016). Similar Personality Patterns Are Associated with Empathy in Four Different Countries. Frontiers in Psychology, 7, 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P.A., Eisenberg N. (1988). The relation of empathy to aggressive and externalizing/antisocial behavior. Psychological Bulletin, 103(3), 324. [DOI] [PubMed] [Google Scholar]
- Mills K.L., Goddings A.-L., Clasen L.S., Giedd J.N., Blakemore S.J. (2014). The developmental mismatch in structural brain maturation during adolescence. Developmental Neuroscience, 36(3-4), 147–60. [DOI] [PubMed] [Google Scholar]
- Minet D.W., Christine G.D.W., Anton V.B. (2010). Empathy dysfunction in children and adolescents with disruptive behavior disorders. European Journal of Pharmacology, 626(1), 97–103. [DOI] [PubMed] [Google Scholar]
- Mooradian T.A., Mark D., Kurt M. (2011). Dispositional empathy and the hierarchical structure of personality. American Journal of Psychology, 124(1), 99–109. [DOI] [PubMed] [Google Scholar]
- Nelson R., Trainor B. (2007). Neural mechanisms of aggression. Nature Reviews. Neuroscience, 8(7), 536–46. [DOI] [PubMed] [Google Scholar]
- Nitin G., Giedd J.N., Leslie L. et al. (2004). Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America, 101(21), 8174–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overwalle F.V., Baetens K. (2009). Understanding others’ actions and goals by mirror and mentalizing systems: A meta-analysis. Neuroimage, 48(3), 564–84. [DOI] [PubMed] [Google Scholar]
- Pascualsagastizabal E., Puerto N.D., Cardas J., Sanchezmartin J.R., Vergara A.I., Azurmendi A. (2019). Testosterone and cortisol modulate the effects of empathy on aggression in children. Psychoneuroendocrinology, 103, 118–24. [DOI] [PubMed] [Google Scholar]
- Peters J., Christian B. (2010). Episodic future thinking reduces reward delay discounting through an enhancement of prefrontal-mediotemporal interactions. Neuron, 66(1), 138–48. [DOI] [PubMed] [Google Scholar]
- Peters J., Christian B. (2011). The neural mechanisms of inter-temporal decision-making: understanding variability. Trends in Cognitive Sciences, 15(5), 227–39. [DOI] [PubMed] [Google Scholar]
- Philipp S., Christina S. (2009). Neuroimaging of aggressive and violent behaviour in children and adolescents. Frontiers in Behavioral Neuroscience, 3(1), 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M.L., Ladouceur C.D., Drevets W.C. (2008). A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular Psychiatry, 13(9), 829–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piquero A.R., Carriaga M.L., Diamond B., Kazemian L., Farrington D.P. (2012). Stability in aggression revisited. Aggression and Violent Behavior, 17(4), 365–72. [Google Scholar]
- Quanty M.B. (2010). Aggression: its causes, consequences, and control. Aggressive Behavior, 20(6), 464–66. [Google Scholar]
- Raine A., Park S., Lencz T. et al. (2010). Reduced right hemisphere activation in severely abused violent offenders during a working memory task: an fMRI study. Aggressive Behavior, 27(2), 111–29. [Google Scholar]
- Raine A., Yang Y. (2006). Neural foundations to moral reasoning and antisocial behavior. Social Cognitive and Affective Neuroscience, 1(3), 203–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieffe C., Broekhof E., Kouwenberg M., Faber J., Tsutsui M.M., Guroglu B. (2016). Disentangling proactive and reactive aggression in children using self-report. European Journal of Developmental Psychology, 13(4), 439–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Martínez Á., González M., Lila M. et al. (2019). The brain resting-state functional connectivity underlying violence proneness: is it a reliable marker for neurocriminology? A systematic review. Behavioral Sciences, 9(1), 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Séguin J.R., Zelazo P.D. (2005). Executive function in early physical aggression In: Tremblay R.E., Hartup W.W., Archer J., editors. Developmental Origins of Aggression, New York: Guilford, 307–29. [Google Scholar]
- Samara Z., Evers E.A.T., Goulas A. et al. (2017). Human orbital and anterior medial prefrontal cortex: intrinsic connectivity parcellation and functional organization. Brain Structure & Function, 222(7), 2941–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz R.T., Grelotti D.J., Ami K. et al. (2003). The role of the fusiform face area in social cognition: implications for the pathobiology of autism. Philosophical Transactions of the Royal Society B, 358(1430), 415–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenck C., Mergenthaler J., Keller K. et al. (2012). Empathy in Children with Autism and Conduct Disorder: group-Specific Profiles and Developmental Aspects. Journal of Child Psychology and Psychiatry, 53(6), 651–59. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory S.G. (2011). The neural bases for empathy. Neuroscientist A Review Journal Bringing Neurobiology Neurology and Psychiatry, 17(1), 18. [DOI] [PubMed] [Google Scholar]
- Sharpe J.P., Desai S. (2001). The Revised Neo Personality Inventory and the MMPI-2 Psychopathology Five in the prediction of aggression. Personality and Individual Differences, 31(4), 505–18. [Google Scholar]
- Shiner R., Caspi A. (2003). Personality differences in childhood and adolescence: measurement, development, and consequences. Journal of Child Psychology and Psychiatry, 44(1), 2–32. [DOI] [PubMed] [Google Scholar]
- Shulman E.P., Harden K.P., Chein J.M., Steinberg L. (2016). The Development of Impulse Control and Sensation‐Seeking in Adolescence: independent or Interdependent Processes?. Journal of Research on Adolescence, 26(1), 37–44. [Google Scholar]
- Siever L.J. (2015). Neurobiology of aggression and violence. American Journal of Psychiatry, 20(3), 254–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silani G., Lamm C., Ruff C.C., Singer T. (2013). Right supramarginal gyrus is crucial to overcome emotional egocentricity bias in social judgments. Journal of Neuroscience, 33(39), 15466–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smack A.J., Kushner S.C., Tackett J.L. (2015). Child Personality Moderates Associations Between Parenting and Relational and Physical Aggression. Journal of Aggression, Maltreatment & Trauma, 24(7), 845–62. [Google Scholar]
- Sowell E.R., Thompson P.M., Toga A.W. (2004). Mapping changes in the human cortex throughout the span of life. Neuroscientist, 10(4), 372–92. [DOI] [PubMed] [Google Scholar]
- Steinberg L. (2008). A social neuroscience perspective on adolescent risk-taking. Developmental Review, 28(1), 78–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss D.T., Gallup G.G., Alexander M.P. (2001). The frontal lobes are necessary for ‘theory of mind’. Brain, 124(2), 279–86. [DOI] [PubMed] [Google Scholar]
- Sun J., Maller J.J., Lanting G., Fitzgerald P.B. (2009). Superior temporal gyrus volume change in schizophrenia: a review on region of interest volumetric studies. Brain Research Reviews, 61(1), 14–32. [DOI] [PubMed] [Google Scholar]
- Tampke E.C., Fite P.J., Cooley J.L. (2020). Bidirectional associations between affective empathy and proactive and reactive aggression. Aggressive Behavior, 46(4), 317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer E.H., Mccormick E.M., Peters S., Cosme D., Pfeifer J.H., Duijvenvoorde A.C.K.V. (2018). Methodological considerations for developmental longitudinal fMRI research. Developmental Cognitive Neuroscience, 33, S1878929317301950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson N.D., Centifanti L.C.M. (2017). Proactive and Reactive Aggression Subgroups in Typically Developing Children: the Role of Executive Functioning, Psychophysiology, and Psychopathy. Child Psychiatry and Human Development, 49(2), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., RJ I., Nansel T.R. (2009). School bullying among adolescents in the United States: physical, verbal, relational, and cyber. Journal of Adolescent Health, 45(4), 368–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Zhou M., Chen T. et al. (2017). Grit and the brain: spontaneous activity of the dorsomedial prefrontal cortex mediates the relationship between the trait grit and academic performance. Social Cognitive and Affective Neuroscience, 12(3), 452–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden D., Mackinnon S. (2003). Prosocial children, bullies and victims: an investigation of their sociometric status, empathy and social problem‐solving strategies. British Journal of Developmental Psychology, 21(3), 367–85. [Google Scholar]
- Water E.D., Curtin P., Zilverstand A., Sjodin A., Horton M.K. (2019). A preliminary study on prenatal polybrominated diphenyl ether serum concentrations and intrinsic functional network organization and executive functioning in childhood. Journal of Child Psychology and Psychiatry, 60(9), 1010–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woltering S., Lishak V., Hodgson N., Granic I., Zelazo P.D. (2016). Executive function in children with externalizing and comorbid internalizing behavior problems. Journal of Child Psychology and Psychiatry, 57(1), 30–38. [DOI] [PubMed] [Google Scholar]
- Yang Y., Cao S., Shields G.S., Teng Z., Liu Y. (2016). The relationships between rumination and core executive functions: A meta-analysis. Depression and Anxiety, 34(1), 37–50. [DOI] [PubMed] [Google Scholar]
- Yang Y., Raine A., Han C.B., Schug R.A., Toga A.W., Narr K.L. (2010). Reduced hippocampal and parahippocampal volumes in murderers with schizophrenia. Psychiatry Research, 182(1), 9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh M.T., Chen P., Raine A., Baker L.A., Jacobson K.C. (2011). Child psychopathic traits moderate relationships between parental affect and child aggression. Journal of the American Academy of Child and Adolescent Psychiatry, 50(10), 1054–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M. (2015). Moderating effect of self-determination in the relationship between Big Five personality and academic performance. Personality and Individual Differences, 86, 385–89. [Google Scholar]
- Zou Q.H., Zhu C.Z., Yang Y. et al. (2008). An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. Journal of Neuroscience Methods, 172(1), 137–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo X.N., Di Martino A., Kelly C. et al. (2010). The oscillating brain: complex and reliable. Neuroimage, 49(2), 1432–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


