Abstract
Purpose
Stereotactic ablative radiotherapy (SAbR) is a promising alternative for selected patients with renal cell carcinoma (RCC) with oligometastasis. The objective of this study was to evaluate the potential of SAbR for longitudinal control in patients with persistently oligometastatic RCC. We report the impact of SAbR on tumor control rates as well as its tolerability in systemic therapy–naïve patients with oligometastatic disease (without brain metastases) and assess the effect of SAbR on subsequent first line systemic therapy by comparison to historical controls.
Methods and Materials
We reviewed patients with metastatic RCC treated with front-line SAbR with a curative intent from 2007 to 2017 at UT Southwestern Kidney Cancer Program. We analyzed local control rates (LCR), toxicity, freedom from systemic therapy (FST), type and duration of first-line systemic therapy, and overall survival (OS). Cox regression and Kaplan-Meier analyses were used.
Results
We identified 47 patients with oligometastatic RCC treated with SAbR to 88 metastases; 11 patients had more than 1 SAbR course. The local control rate was 91.5% at 2 years with no reported grade ≥3 toxicity. With a median follow-up of 30 months (interquartile range, 13.7–40.9), median FST from first SAbR was 15.2 months (95% confidence interval [CI], 8.8–40.1). The most common systemic therapies initiated after SAbR were pazopanib (60.7%) and sunitinib (14.3%). The duration of first line systemic therapy appeared unaffected by SAbR. Improved FST was observed in patients with metachronous disease (hazard ratio, 2.67; P = .02), solitary metastasis (HR, 2.26; P = .05), and non-bone metastasis (HR, 2.21; P = .04). One-year and 2-year OS after SAbR were 93.1% (95% CI, 80.1–97.7) and 84.8% (95% CI, 69.1–92.9), respectively. Median OS was not reached.
Conclusions
SAbR is an effective and safe treatment for selected patients with oligometastatic RCC, can provide longitudinal disease control without systemic therapy for over a year, and does not appear to adversely affect the effectiveness of first-line systemic therapy once initiated. Prospective validation of these findings is being sought through a phase 2 trial.
Summary
Stereotactic ablative radiation therapy (SAbR) is an effective and safe treatment for selected patients with oligometastatic renal cell carcinoma. This front-line approach offers excellent tumor control with minimal toxicity and delays the start of systemic therapy, offering quality of life benefits. SAbR does not affect the duration of first-line systemic therapy, suggesting that the benefit of SAbR and systemic therapy may be additive.
Introduction
As the global incidence of renal cell carcinoma (RCC) increases,1 about 15% of patients exhibit distant metastases at diagnosis,2 and another 15% to 25% will develop distant metastases after nephrectomy.3,4 The standard, first-line therapy for patients with newly diagnosed metastatic RCC is systemic therapy. A problem with systemic therapy, in particular targeted therapy, is that drugs rarely eradicate all gross tumors, resistance develops requiring a change of drugs, and ultimately patients run out of effective agents. Even with recently developed immunotherapies, complete response rates are up to 10% and their toxicities may be severe. The oligometastatic paradigm, formally defined in the 1990s, suggests that some patients with a limited number of metastases may be cured with or without systemic therapy if all sites of disease are locally eradicated.5 This implies that some select patients may not harbor micrometastatic disease. Furthermore, patients with limited metastatic disease burden generally have a better prognosis.6 Even if a patient is not cured, local therapy to all metastatic sites as an initial modality may delay the onset of systemic therapy, possibly extending patient survival.
Early evidence of the benefit of aggressive local therapy comes from improved survival outcomes with metastasectomy in retrospective series,7–10 where median survival ranged from 36.5 to 142 months for patients with complete surgical metastasectomy and from 8.4 to 27 months for incomplete surgical metastasectomy. The potential of stereotactic ablative radiation therapy (SAbR) to treat oligometastatic RCC sparked worldwide interest when preclinical studies11,12 suggested that delivering high doses per fraction can overcome RCC radio-resistance to conventional fractionation.13 Multiple retrospective series14–21 and a prospective trial22 have shown local control rates >85% at 1 to 2 years for metastatic lesions from RCC and median overall survival (OS) rates of 12 to 51 months.23 However, these studies are limited by heterogeneous patient populations, use of systemic therapy before SAbR, and a primary focus on intracranial lesions.14–22
No study to date has evaluated the scope of SAbR for longitudinal disease control in patients before initiation of first-line therapy. Importantly, none has investigated whether SAbR may undermine the duration of subsequent systemic therapy, which may diminish its benefit. In this retrospective study, we assessed the effect of multiple SAbR as a tactical approach for longitudinal control of persistently oligometastatic disease in a homogenous cohort of patients with systemic–therapy-naïve, extracranial RCC.
Methods and Materials
Patients and treatments
Patients with extracranial metastatic RCC treated at our institution were reviewed using an institutional cancer registry database in an institutional review board–approved study (IRB number: 042017–028). Patients consecutively treated with SAbR at all sites of gross disease between 2007 and 2017 were included. Patients treated with conventionally fractionated radiation therapy or systemic therapy before SAbR were excluded, as were patients treated with palliative intent or brain metastasis.
For SAbR treatment planning, patients were immobilized in vacuum bags and stereotactic body frames, and they underwent computed tomography (CT) as described previously.24 Motion assessment and management strategies (eg, 4-dimensional CT, abdominal compression) were applied at the physician’s discretion. Treatment plans were optimized with multiple noncoplanar beams or volumetric arcs with intensity modulation when applicable. Patients were treated with the aid of cone beam CT image guidance. SAbR was delivered in 1 to 5 fractions, with intervals of at least 36 hours between fractions.
Outcome and statistical analysis
Patient follow-up was typically performed every 3 to 6 months using CT or magnetic resonance imaging. Freedom from systemic therapy (FST) was defined as survival without systemic therapy from the start of the first SAbR course until systemic therapy or death, whichever came first. OS and FST were defined from the start of SAbR and censored at the last available follow-up if no events occurred. Prognostic grouping was determined according to the established and externally validated Heng’s criteria.25
We used median, standard deviation, and range as descriptive statistics for continuous measures and frequencies, and percentage for categorical measures. Cox regression models and the Kaplan-Meier test were used to analyze differences in OS and FST. All statistics were assessed at the.05 significance level using SAS 9.4 (SAS Institute Inc, Cary, NC).
Results
Patient, tumor characteristics, and radiation regimens
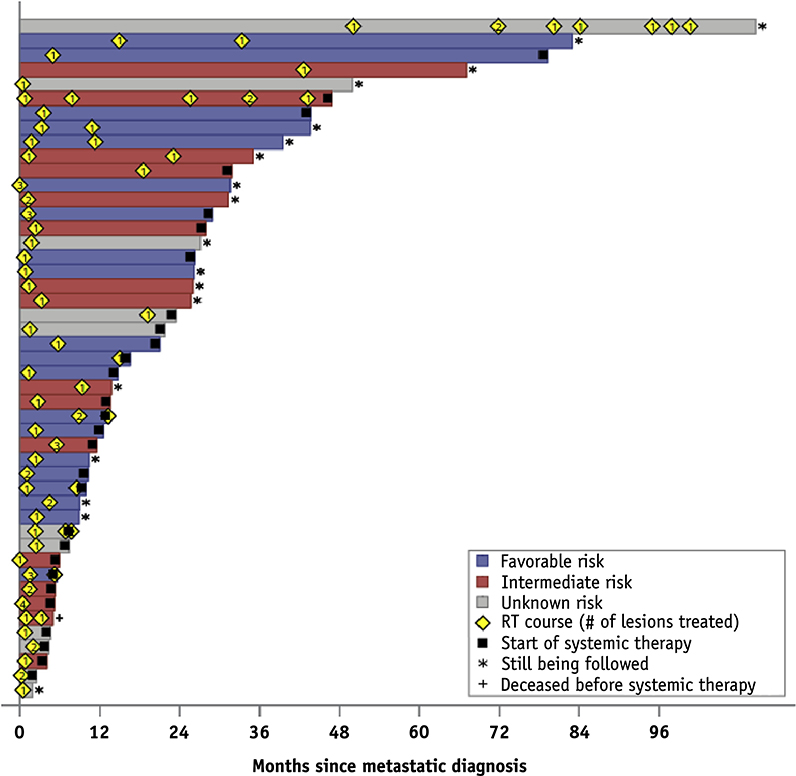
We identified 47 patients (41 patients with clear cell histology and 6 with non-clear cell histology) that were initially treated to 65 extracranial sites. Forty-five patients underwent nephrectomy at diagnosis, and the remaining 2 received other ablative therapies. Thirty-four patients (72.3%) initially presented with localized disease or locoregional disease (M0 at diagnosis) but later developed metastases. The median time from metastasis diagnosis to first SAbR was 1.7 months (Fig. 1). All patients received curative intent radiation in 1 to 5 fractions at a minimum of 5 Gy per fraction to all gross metastatic sites (Table 1).
Fig. 1.
Swimmer’s plot of months since metastatic diagnosis.
Table 1.
Patient and tumor characteristics
| Characteristics | Frequency (%) |
|---|---|
| Demographics | |
| Mean age ± SD (range) | 61.6 ± 10.1 (38–83) |
| Sex | |
| Female | 19 (40.4%) |
| Male | 28 (59.6%) |
| Race | |
| Nonwhite | 4 (8.5%) |
| White | 43 (91.5%) |
| Primary tumor | |
| Mean tumor size, cm | 7.5 ± 4.0 |
| Histology | |
| Non-clear cell renal cell carcinoma | 6 (12.8%) |
| Clear cell renal cell carcinoma | 41 (87.2%) |
| Grade | |
| 1 | 1 (2.1%) |
| 2 | 8 (17.0%) |
| 3 | 23 (48.9%) |
| 4 | 11 (23.4%) |
| N/A | 4 (8.5%) |
| pT | |
| pT1 | 15 (31.9%) |
| pT2 | 5 (10.6%) |
| pT3 | 23 (48.9%) |
| N/A | 4 (8.5%) |
| pN | |
| pN0 | 10 (21.3%) |
| pN1 | 3 (6.4%) |
| pNX | 29 (61.7%) |
| N/A | 5 (10.6%) |
| M* | |
| M0 | 34 (72.3%) |
| M1 | 11 (23.4%) |
| N/A | 2 (4.3%) |
| Risk score† | |
| 0, Favorable | 20 (42.6%) |
| 1–2, Intermediate/unfavorable | 16 (34.0%) |
| N/A | 11 (23.4%) |
| Metastasis | |
| Sum of largest diameters, cm (range) | 4.5 ± 3.5 (0.8–14.8) |
| No. of metastases treated, initial‡ | |
| 1 | 35 (74.5%) |
| 2 | 7 (14.9%) |
| 3 | 4 (8.5%) |
| 4 | 1 (2.1%) |
| No. of metastases treated, total§ | |
| 1 | 26 (55.3%) |
| 2 | 12 (25.5%) |
| 3 | 4 (8.5%) |
| ≥4 | 5 (10.6%) |
| Treatment sites‡ | |
| Bone | 28 (43.1%) |
| Lung | 10 (15.4%) |
| Liver | 6 (9.2%) |
| Soft tissue | 6 (9.2%) |
| Lymph node | 5 (7.7%) |
| Adrenal | 4 (6.2%) |
| Others | 6 (9.2%) |
| Radiation therapy‡ | |
| 22.1 ± 4.0 Gy × 1 fraction | 14 (21.5%) |
| 12.8 ± 2.2 Gy × 3 fractions | 21 (32.3%) |
| 8.0 ± 1.4 Gy × 5 fractions | 30 (46.2%) |
M stage at presentation.
Heng’s criteria measured at the start of stereotactic ablative radiotherapy (SAbR).
During initial SAbR course.
During all SAbR courses before systemic therapy.
At the time of metastatic disease diagnosis, 20 patients (42.6%) had a favorable and 16 (34.0%) had an intermediate prognosis, according to Heng’s criteria (Table 1); the remaining 11 patients (23.4%) had unknown risk prognosis. Most patients (74.5%) were treated for 1 metastasis only. The most common metastatic site was bone (43.1%). Eleven of 47 patients (23.4%) received a second course of SAbR for additional metastatic sites. Three patients (6.3%) received a third course to subsequent sites.
Local control and toxicities
The median follow-up for all patients was 30 months (range, 1.4–80.1 months). After SAbR, 4 lesions failed locally with a 2-year local control rate of 91.5%. One patient developed in-field failure of a left upper lobe lung metastasis approximately 1 year after completing 40 Gy in 5 fractions and was started on pazopanib. A second patient showed progression of a sternal lesion 9 months after completing 50 Gy in 5 fractions and was started on axitinib. One patient showed progression of a T9 vertebral body metastasis causing cord impingement approximately 2 months after 40 Gy in 5 fractions to T8, T9, and T10 vertebral bodies. The patient was scheduled to start pazopanib but died of acute respiratory failure within weeks. One patient showed failure at the margin 8 months after 33 Gy in 3 fractions to a right iliac lesion, received reirradiation of 40 Gy in 5 fractions, and was recurrence-free at the 30-month follow-up.
Radiation therapy was well tolerated, with no grade 3 or higher toxicities reported. Acute side effects included fatigue (4 patients), nausea (1 patient), abdominal cramping (1 patient), and pain at the irradiated site (1 patient). One patient developed grade 1 esophagitis that later resolved, and another patient developed grade 2 right frontal radionecrosis with left hand weakness, which became persistent. Only 1 patient experienced grade 2 radiation pneumonitis out of 8 patients who received radiation to lung metastases.
Effect of SAbR on systemic therapy
The median FST, defined as the time from the first SAbR course to the start of systemic therapy or death, was 15.2 months. Eighteen of 47 patients received no systemic therapy and were alive after SAbR at a median follow-up of 25 months. The 1 deceased patient that did not receive systemic therapy mentioned above, initially presented with bilateral renal masses and underwent partial nephrectomies, which showed mixed histology (papillary and clear cell), and developed 1 liver metastasis 8 months later that was treated with 42 Gy in 3 fractions. Shortly afterward, he developed extensive bone metastases and cord compression and died a few weeks later from sepsis, bilateral pulmonary emboli, and respiratory failure.
Among the 28 patients who initiated systemic therapy, 23 initiated because they developed multiple additional lesions. The remaining patients initiated systemic therapy because of local recurrence where reirradiation was not an option (2 patients), metastatic disease near previously irradiated sites (2 patients), or disease in the remaining kidney (1 patient) where radiation was not recommended in order to prevent treatment-related toxicities. Because of these factors, systemic therapy was started instead of radiation therapy.
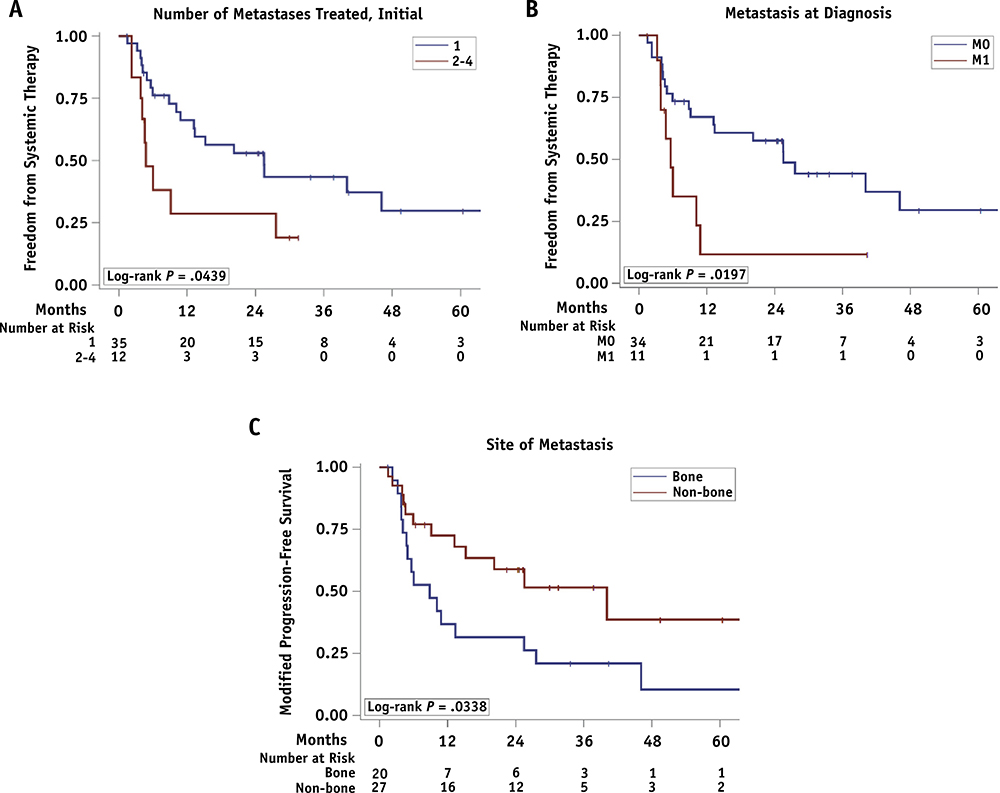
Unsurprisingly, patients with 1 lesion at the time of SAbR showed better FST than those with 2 or more lesions (25.5 vs 4.8 months; hazard ratio [HR] = 2.26; log-rank P = .04). The other factors affecting FST were metastatic disease at diagnosis (M0 vs. M1: 25.5 vs. 5.6 months; HR = 2.67; log-rank P = .02) and site of metastasis (bone vs. nonbone: 8.8 vs 40.1 months; HR = 2.21; log-rank P = .03) (Table 2 and Fig. 2).
Table 2.
Univariate FST analysis
| Median FST | 2 year FST (95% CI) | HR 95% CI | Cox P | |
|---|---|---|---|---|
| Risk group* | ||||
| Favorable | 25.5 | 54.5% (28.9–74.2) | 1 | .4053 |
| Intermediate | 13.2 | 47.4% (21.8–69.4) | 1.44 (0.61–3.43) | |
| No. of mets treated, initial† | ||||
| 1 | 25.5 | 53.0% (34.4–68.5) | 1 | .0500 |
| 2–4 | 4.8 | 28.6% (7.0–55.5) | 2.26 (1.00–5.12) | |
| No. of mets treated, total‡ | ||||
| 1 | 20.2 | 47.3% (25.9–66.0) | 1 | .9829 |
| ≥2 | 9.1 | 45.7% (23.7–65.4) | 1.01 (0.47–2.15) | |
| Histologic subtype | ||||
| Clear cell renal cell carcinoma | 20.2 | 48.9% (32.0–63.8) | 1 | .5526 |
| Non-clear cell renal cell carcinoma | 5.8 | 33.3% (4.6–67.6) | 1.38 (0.48–4.00) | |
| pT | ||||
| pT1 | 25.5 | 50.9% (23.6–72.9) | 1 | .8563 |
| pT2 | 20.2 | 40.0% (5.2–75.3) | 1.14 (0.35–3.76) | |
| pT3 | 6.0 | 42.9% (21.8–62.6) | 1.27 (0.55–2.95) | |
| M§ | ||||
| M0 | 25.5 | 57.5% (39.0–72.3) | 1 | .0248 |
| M1 | 5.6 | 11.7% (0.6–40.1) | 2.67 (1.13–6.28) | |
| Site of metastasis† | ||||
| Bone | 8.8 | 31.6% (12.9–52.2) | 2.21 (1.04–4.68) | .0387 |
| Nonbone | 40.1 | 58.9% (36.8–75.6) | 1 |
Abbreviations: CI = confidence interval; HR = hazard ratio; mets = metastases; SAbR = stereotactic ablative radiation therapy.
Heng’s criteria measured at the start of SAbR.
During initial SAbR course.
During all SAbR courses before systemic therapy.
M stage at presentation.
Fig. 2.
Freedom from systemic therapy (FST) since the start of stereotactic ablative radiotherapy (SAbR). (A) Number of metastases treated in the initial course. (B) M0 versus M1. (C) Bone versus non-bone metastasis.
The most common systemic therapies were pazopanib (60.7%), sunitinib (14.3%), and IL-2 (10.7%), with a median time to next systemic therapy of 8.8, 15.2, and 5.6 months, respectively (Table EA; available online at https://doi.org/10.1016/j.ijrobp.2019.07.023). Combining the components of overall care in this approach, disease control with SAbR followed by disease control with first-line systemic therapy ranged from 19 to 29 months depending on the first-line therapy.
Overall survival rates
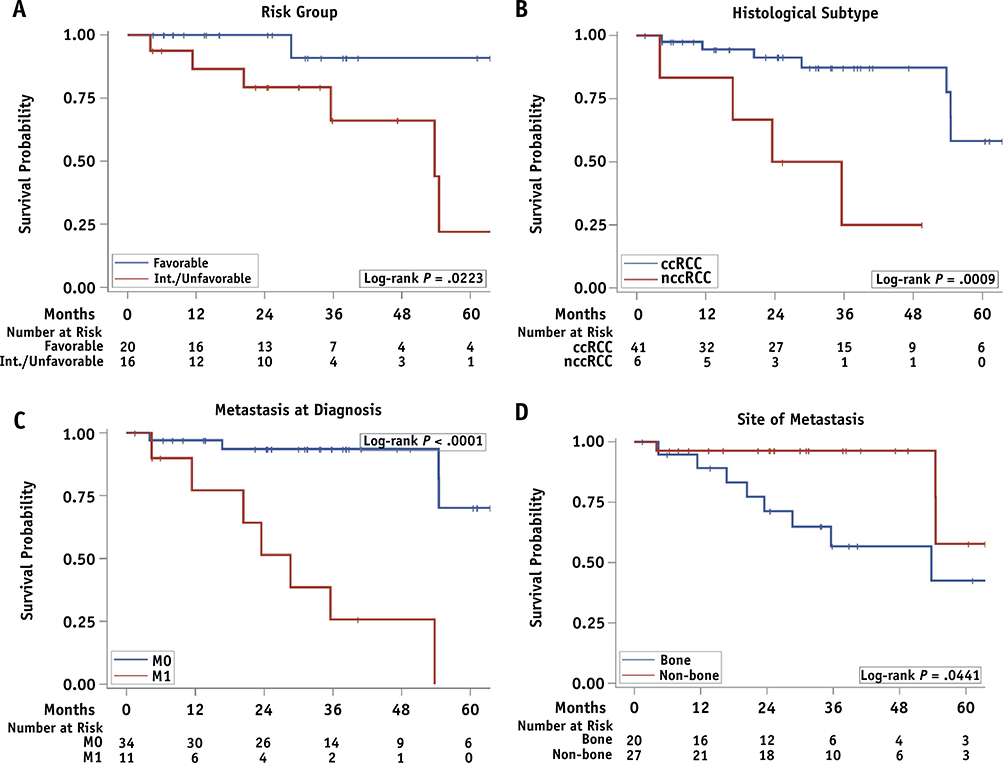
OS rates at 1 and 2 years after SAbR were 93.1% and 84.8%, respectively. Median OS was not reached. On univariate analysis, better survival was observed for patients with favorable risk (HR = 8.04, log-rank P = .02), clear cell histology (HR = 7.41, log-rank P = .0009), no metastatic disease at diagnosis (HR = 16.70, log-rank P < .0001), and non-bone metastases (HR = 3.58, log-rank P = .04) (Table 3 and Fig. 3). The number of treated metastatic lesions, pathologic T stage, and lines of systemic therapies were not associated with OS; however, the small sample sizes in subgroups may represent a source of type II errors.
Table 3.
Univariate OS analysis
| Median OS | 2 year OS (95% CI) | HR 95% CI | Cox P | |
|---|---|---|---|---|
| Risk group* | ||||
| Favorable | ∥ | 100% (#) | 1 | .0542 |
| Intermediate | 53.7 | 79.3% (48.5–92.9) | 8.04 (0.96–67.11) | |
| No. of mets treated, initial† | ||||
| 1 | ∥ | 90.2% (72.2–96.8) | 1 | .2587 |
| 2–4 | ∥ | 70.7% (33.7–89.5) | 2.05 (0.59–7.16) | |
| No. of mets treated, total‡ | ||||
| 1 | 54.5 | 96.0% (74.8–99.4) | 1 | .7198 |
| ≥2 | ∥ | 74.3% (48.8–88.4) | 1.24 (0.38–4.09) | |
| Histologic subtype | ||||
| Clear cell renal cell carcinoma | ∥ | 91.3% (75.2–97.1) | 1 | .0048 |
| Non-clear cell renal cell carcinoma | 29.6 | 50.0% (11.1–80.4) | 7.41 (1.84–29.84) | |
| pT | ||||
| pT1 | 53.7 | 93.3% (61.3–99.0) | 1 | .5200 |
| pT2 | ∥ | 100% (#) | 0.47 (0.05–4.75) | |
| pT3 | 54.5 | 72.6% (45.8–87.7) | 1.50 (0.38–5.86) | |
| M§ | ||||
| M0 | ∥ | 93.6% (76.6–98.4) | 1 | .0005 |
| M1 | 28.6 | 51.4% (16.0–78.6) | 16.70 (3.44–81.10) | |
| Site of metastasis† | ||||
| Bone | 53.7 | 71.3% (44.1–87.0) | 3.58 (0.95–13.51) | .0597 |
| Non-bone | ∥ | 96.3% (76.5–99.5) | 1 |
Abbreviations: CI = confidence interval; HR = hazard ratio; mets = metastases; OS = overall survival; SAbR = stereotactic ablative radiation therapy.
Heng’s criteria measured at the start of SAbR.
During initial SAbR course.
During all SAbR courses before systemic therapy.
M stage at presentation.
Median not reached.
No death observed before 2 years.
Fig. 3.
Kaplan-Meier overall survival (OS) curves after first stereotactic ablative radiotherapy (SAbR). (A) Favorable versus intermediate/unfavorable prognostic groups. (B) Clear cell renal cell carcinoma (RCC) versus non-clear cell RCC. (C) M0 versus M1. (D) Bone versus nonbone metastasis.
Discussion
Standard management of metastatic RCC is systemic therapy, but SAbR may be a promising strategy for select patients with limited disease burden. To our knowledge, this is the largest study to report the outcome of curative-intent SAbR on treatment-naïve, oligometastatic extracranial RCC. Furthermore, we present an innovative paradigm wherein SAbR was used repetitively for longitudinal disease control in patients who remained oligometastatic and could be effectively treated. Several retrospective studies and 1 prospective study have analyzed the outcomes of radiation therapy for patients with metastatic RCC (Table EB; available online at https://doi.org/10.1016/j.ijrobp.2019.07.023). Despite the heterogeneous patient population, often including intracranial lesions, primary tumors, palliative-intent radiation therapy, and systemic therapy use, these studies reported substantive local control rates, suggesting that SAbR is an effective local therapy for metastatic RCC, especially for patients with oligometastatic disease. A systematic review of radiation therapy to extracranial metastatic RCC lesions reported 1-year local control of 89% and <5% grade 3 and 4 toxicities.23 Although limited by short follow-up, our study showed excellent local control rates (91.5% with only 4 failures). In addition, the treatment was well tolerated with no grade 3 or higher adverse effects.
In this patient population, OS rates at 1 and 2 years after SAbR were 93.1% and 84.8%, respectively, which are comparable to those reported in the metastasectomy series.7–10 Before the era of targeted therapy, complete metastasectomy was shown to be effective in treating patients with multiple metastatic lesions,7 with 5-year survival in the 40% to 50% range. The success of metastasectomy is highest with solitary metastatic lesions, which agrees with our findings here but is relatively independent of the lesion’s location.26
In our series, most patients (74.5%) had a single lesion at initial diagnosis. The choice of SAbR for these patients often depended on multiple factors, including time to development of metastases, surgical candidacy, and patient preference. Among those who had no metastatic disease at presentation, the median time to any metastasis was 2.2 years and median time to 1 metastasis was 2.1 years. Because of the short time to metastases development, these patients may be regarded at high risk for the development of additional metastases, and less morbid SAbR may be preferable over surgery. In addition, about 40.0% of patients had bone metastases that were either unresectable or presented challenges for surgery.
Although we cannot determine whether SAbR alters the disease course for all patients, it appears to have potential as a definitive treatment for selected patients. Eighteen of 47 patients (38.2%) in this cohort received no systemic therapy after SAbR at a median follow-up of 30 months. For the remaining patients, SAbR delayed systemic therapy, with an overall median time to systemic therapy of 15.2 months. Without a comparison arm, we cannot ascertain whether all or part of this delay would prolong survival. However, we speculate that such a delay could improve patients’ quality of life, especially because most systemic therapies adversely affect patient quality of life.
It may be argued that delaying systemic therapy could allow undetectable micrometastases to progress diminishing the activity of subsequent systemic therapy because of a larger burden of disease. To attempt to evaluate this, we measured the duration of first-line systemic therapy for the 2 most common drugs used in our cohort, pazopanib and sunitinib. Their duration after initiation in our study was 8.8 months and 15.2 months, respectively. Although no historical data on upfront systemic therapy alone for our highly selected cohort are available, these times accord with reported median progression-free survival for treatment-naïve patients with clear cell RCC treated with pazopanib (8.4 months) and sunitinib (9.5 months) up front,27 suggesting that primary SAbR may not adversely affect subsequent systemic therapy. With combined local and first-line systemic therapy, time to second-line therapy ranged from 22 months for SAbR/pazopanib to 29 months for SAbR/sunitinib, comparing favorably with drug-alone experiences. Although only prospective trials can provide confirmation, these findings suggest a hope that SAbR may provide survival benefit and preserve quality of life without negatively affecting progression-free survival on subsequent lines of therapy. An ongoing prospective phase 2 trial at UT Southwestern is currently evaluating this notion (NCT 02956798).
This study has several limitations. First, it is retrospective. Second, it reports results from a single institution that has extensive experience, making the findings difficult to generalize. Third, follow-up is relatively short, and long-term control rates are unknown. This is important considering some patients can live for >5 years with newer systemic therapies. Finally, and most importantly, the study represents a select cohort of patients whom the care provider team deemed suitable for SAbR.
Ultimately, the goal is to direct treatment to the particular patient’s specific disease biology and comorbidities. As Rini and colleagues recently showed in a prospective phase 2 clinical trial,28 some patients may not need immediate therapy and are followed with active surveillance. Another group of patients, those with a long disease-free interval and 1 (or few) resectable lesion(s), may be treated by surgery. However, a group of patients with limited disease and a short disease-free interval (as in this cohort) may be at high risk for developing other metastases. Such patients may benefit from a less invasive local therapy than surgery, such as SAbR. Other patients may present with oligometastases that are not surgically accessible, or they may not be optimal surgical candidates. Our data suggest that for a subgroup of patients with RCC, SAbR is feasible, safe, and effective, and it likely does not constitute a detriment to subsequent systemic therapy.
Therapy for metastatic kidney cancer continues to evolve. Although much of this experience was collected before the advent of immune checkpoint inhibitors, primary SAbR may also be considered in light of newer, potentially curative systemic therapies, such as ipilimumab/nivolumab combinations. This treatment is associated with overall response rates of 40%, including 10% complete responses.29 Recent updates from this trial show progression-free survival rates of 28% at 2.5 years.30 Nevertheless, there may still be a role for primary SAbR even in the era of potentially curative systemic therapies because of serious life-threatening toxicities that occur in 1% to 3% of patients; a significantly higher percentage of acute, though typically manageable, toxicities such as pneumonitis, colitis, or hepatitis; and other toxicities that can undermine patient’s quality of life long term, such as rheumatological conditions or hypophysitis requiring long-term corticosteroid supplementation.
Conclusions
This study suggests that SAbR is a reasonable approach for select patients with oligometastatic RCC, offering excellent local control and potentially preserving quality of life without affecting future systemic therapy. Future studies will evaluate the role of SAbR prospectively, refining the patient population that benefits from this approach versus metastasectomy, active surveillance, or upfront systemic therapy.
Supplementary Material
Acknowledgment
The authors acknowledge Dr Jonathan Feinberg for the scientific editing of the manuscript.
James Brugarolas, Kevin Courtney, Vitaly Margulis, and Alana Christie were funded by P50CA196516. Raquibul Hannan was funded by American Cancer Society RSG-16-004-01-CCE.
Footnotes
Disclosures: none
Supplementary material for this article can be found at https://doi.org/10.1016/j.ijrobp.2019.07.023.
References
- 1.Znaor A, Lortet-Tieulent J, Laversanne M, Jemal A, Bray F. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol 2015;67:519–530. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- 3.Rabinovitch RA, Zelefsky MJ, Gaynor JJ, Fuks Z. Patterns of failure following surgical resection of renal cell carcinoma: Implications for adjuvant local and systemic therapy. J Clin Oncol 1994;12:206–212. [DOI] [PubMed] [Google Scholar]
- 4.Kim SP, Weight CJ, Leibovich BC, et al. Outcomes and clinicopathologic variables associated with late recurrence after nephrectomy for localized renal cell carcinoma. Urology 2011;78:1101–1106. [DOI] [PubMed] [Google Scholar]
- 5.Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995; 13:8–10. [DOI] [PubMed] [Google Scholar]
- 6.Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol 1999;17:2530–2540. [DOI] [PubMed] [Google Scholar]
- 7.Alt AL, Boorjian SA, Lohse CM, Costello BA, Leibovich BC, Blute ML. Survival after complete surgical resection of multiple metastases from renal cell carcinoma. Cancer 2011;117:2873–2882. [DOI] [PubMed] [Google Scholar]
- 8.van der Poel HG, Roukema JA, Horenblas S, van Geel AN, Debruyne FM. Metastasectomy in renal cell carcinoma: A multicenter retrospective analysis. Eur Urol 1999;35:197–203. [DOI] [PubMed] [Google Scholar]
- 9.Naito S, Kinoshita H, Kondo T, et al. Prognostic factors of patients with metastatic renal cell carcinoma with removed metastases: A multicenter study of 556 patients. Urology 2013;82:846–851. [DOI] [PubMed] [Google Scholar]
- 10.Zaid HB, Parker WP, Safdar NS, et al. Outcomes following complete surgical metastasectomy for patients with metastatic renal cell carcinoma: A systematic review and meta-analysis. J Urol 2017;197:44–49. [DOI] [PubMed] [Google Scholar]
- 11.Walsh L, Stanfield JL, Cho LC, et al. Efficacy of ablative high-doseper-fraction radiation for implanted human renal cell cancer in a nude mouse model. Eur Urol 2006;50:795–800; discussion 800. [DOI] [PubMed] [Google Scholar]
- 12.Ning S, Trisler K, Wessels BW, Knox SJ. Radiobiologic studies of radioimmunotherapy and external beam radiotherapy in vitro and in vivo in human renal cell carcinoma xenografts. Cancer 1997;80(12 Suppl):2519–2528. [DOI] [PubMed] [Google Scholar]
- 13.Deschavanne PJ, Fertil B. A review of human cell radiosensitivity in vitro. Int J Radiat Oncol Biol Phys 1996;34:251–266. [DOI] [PubMed] [Google Scholar]
- 14.Franzese C, Franceschini D, Di Brina L, et al. Role of Stereotactic body radiation therapy in the management of oligometastatic renal cell carcinoma. J Urol 2019;201:70–75. [DOI] [PubMed] [Google Scholar]
- 15.Meyer E, Pasquier D, Bernadou G, et al. Stereotactic radiation therapy in the strategy of treatment of metastatic renal cell carcinoma: A study of the Getug group. Eur J Cancer 2018;98:38–47. [DOI] [PubMed] [Google Scholar]
- 16.Ranck MC, Golden DW, Corbin KS, et al. Stereotactic body radiotherapy for the treatment of oligometastatic renal cell carcinoma. Am J Clin Oncol 2013;36:589–595. [DOI] [PubMed] [Google Scholar]
- 17.Stinauer MA, Kavanagh BD, Schefter TE, et al. Stereotactic body radiation therapy for melanoma and renal cell carcinoma: Impact of single fraction equivalent dose on local control. Radiat Oncol 2011;6:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stenman M, Sinclair G, Paavola P, Wersall P, Harmenberg U, Lindskog M. Overall survival after stereotactic radiotherapy or surgical metastasectomy in oligometastatic renal cell carcinoma patients treated at two Swedish centres 2005–2014. Radiother Oncol 2018;127: 501–506. [DOI] [PubMed] [Google Scholar]
- 19.Teh B, Bloch C, Galli-Guevara M, et al. The treatment of primary and metastatic renal cell carcinoma (RCC) with image guided stereotactic body radiation therapy (SBRT). Biomed Imaging Interv J 2007;3:e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wersall PJ, Blomgren H, Lax I, et al. Extracranial stereotactic radiotherapy for primary and metastatic renal cell carcinoma. Radiother Oncol: J Eur Soc Ther Radiol Oncol 2005;77:88–95. [DOI] [PubMed] [Google Scholar]
- 21.Zelefsky MJ, Greco C, Motzer R, et al. Tumor control outcomes after hypofractionated and single-dose stereotactic image guided intensity modulated radiotherapy for extracranial metastases from renal cell carcinoma. Int J Radiat Oncol Biol Phys 2012;82:1744–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Svedman C, Sandstrom P, Pisa P, et al. A prospective phase II trial of using extracranial stereotactic radiotherapy in primary and metastatic renal cell carcinoma. Acta Oncol 2006;45:870–875. [DOI] [PubMed] [Google Scholar]
- 23.Kothari G, Foroudi F, Gill S, Corcoran NM, Siva S. Outcomes of stereotactic radiotherapy for cranial and extracranial metastatic renal cell carcinoma: A systematic review. Acta Oncol 2015;54:148–157. [DOI] [PubMed] [Google Scholar]
- 24.Iyengar P, Kavanagh BD, Wardak Z, et al. Phase II trial of stereotactic body radiation therapy combined with erlotinib for patients with limited but progressive metastatic non-small-cell lung cancer. J Clin Oncol 2014;32:3824–3830. [DOI] [PubMed] [Google Scholar]
- 25.Heng DY, Xie W, Regan MM, et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: A populationbased study. Lancet Oncol 2013;14:141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jakubowski CD, Vertosick EA, Untch BR, et al. Complete metastasectomy for renal cell carcinoma: Comparison of five solid organ sites. J Surg Oncol 2016;114:375–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molina AM, Motzer RJ, Heng DY. Systemic treatment options for untreated patients with metastatic clear cell renal cancer. Semin Oncol 2013;40:436–443. [DOI] [PubMed] [Google Scholar]
- 28.Rini BI, Dorff TB, Elson P, et al. Active surveillance in metastatic renal-cell carcinoma: A prospective, phase 2 trial. Lancet Oncol 2016; 17:1317–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 2018;378:1277–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tannir NM, Frontera OA, Hammers HJ, et al. Thirty-month follow-up of the phase III CheckMate 214 trial of first-line nivolumab + ipilimumab (N + I) or sunitinib (S) in patients (pts) with advanced renal cell carcinoma (aRCC). J Clin Oncol 2019;37(Suppl 7S). abstr 547. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.