Abstract
Cardiac complications, including clinically suspected myocarditis, have been described in novel coronavirus disease 2019. Here, we review current data on suspected myocarditis in the course of severe acute respiratory syndrome novel coronavirus-2 (SARS-CoV-2) infection. Hypothetical mechanisms to explain the pathogenesis of troponin release in patients with novel coronavirus disease 2019 include direct virus-induced myocardial injury (ie, viral myocarditis), systemic hyperinflammatory response (ie, cytokine storm), hypoxemia, downregulation of angiotensin-converting enzyme 2, systemic virus-induced endothelialitis, and type 1 and type 2 myocardial infarction. To date, despite the fact that millions of SARS-CoV-2 infections have been diagnosed worldwide, there is no definitive proof that SARS-CoV-2 is a novel cardiotropic virus causing direct cardiomyocyte damage. Diagnosis of viral myocarditis should be based on the molecular assessment of endomyocardial biopsy or autopsy by polymerase chain reaction or in-situ hybridization. Blood, sputum, or nasal and throat swab virology testing are insufficient and do not correlate with the myocardial involvement of a given pathogen. Data from endomyocardial biopsies and autopsies in clinically suspected SARS-CoV-2 myocarditis are scarce. Overall, current clinical epidemiologic data do not support the hypothesis that viral myocarditis is caused by SARS-CoV-2, or that it is common. More endomyocardial biopsy and autopsy data are also needed for a better understanding of pathogenesis of clinically suspected myocarditis in the course of SARS-CoV-2 infection, which may include virus-negative immune-mediated or already established subclinical autoimmune forms, triggered or accelerated by the hyperinflammatory state of severe novel coronavirus disease 2019.
Key Words: coronavirus, endomyocardial biopsy, heart failure, inflammatory cardiomyopathy, SARS-CoV-2
Cardiovascular complications (heart failure, myocardial infarction, arrhythmias, myocardial injury with troponin release) in the course of the novel coronavirus disease 2019 (COVID-19) have been widely reported.1 Case reports have suggested that myocarditis may be another cardiac complication of severe acute respiratory syndrome novel coronavirus 2 (SARS-CoV-2) infection. Although millions of SARS-CoV-2 infections have been diagnosed worldwide, clinically suspected myocarditis seems to be relatively uncommon. The diagnosis of myocarditis is challenging due to a wide variety of clinical presentations; thus, a uniform approach to the diagnostic process should be followed.2 According to current recommendations, the definitive diagnosis of myocarditis is based on endomyocardial biopsy (EMB) or autopsy.2 However, because data on EMB or autopsy-proven myocarditis in clinically suspected SARS-CoV-2 infection are inconclusive, in this review, we summarize current data on this topic.
The European Society of Cardiology Definition of Clinically Suspected Myocarditis
Most available reports on COVID-19–related myocarditis did not follow established international definitions. According to the European Society of Cardiology (ESC) consensus paper, myocarditis should be suspected on the basis of specific clinical and noninvasive diagnostic criteria.2 According to the definition of “clinically suspected myocarditis,” one or more of the clinical presentations and one or more abnormalities from different diagnostic categories, including electrocardiogram, troponin, noninvasive cardiac imaging (echocardiography, ventriculography, or cardiac magnetic resonance), and tissue characterization by cardiac magnetic resonance are needed.2 However, a definitive diagnosis of myocarditis is based on EMB or autopsy using established histologic and immunohistochemical criteria.2
Myocarditis can be caused by many infectious and noninfectious agents and can also be the result of an autoimmune process.2 Viral genetic material is found in up to two-thirds of cases of myocarditis or dilated cardiomyopathy, and in 30% of cases multiple viral agents are identified.2 To reach an etiologic diagnosis of infectious myocarditis, molecular investigations (polymerase chain reaction or in situ hybridization) on myocardial tissue for common cardiotropic viruses should be performed.2 Molecular analysis with polymerase chain reaction for viral presence in the myocardium should require investigation of both EMB specimens and blood samples, because this technique is the gold standard to rule out the possibility of false positives.3
Incidence of Presumed COVID-19–Related Myocarditis
COVID-19 predominantly affects the elderly, often with comorbidities such as cardiovascular disease, which contrasts with the typical epidemiology of myocarditis which clusters in children and young adults. The true frequency of myocarditis complicating COVID-19 is impossible to determine. Using the search terms (“COVID-19” OR “coronavirus” OR “SARS-CoV-2” AND “myocarditis” OR “inflammatory cardiomyopathy”) (date of search May 20, 2020), we found 15 case reports and a few retrospective cohort studies reporting cardiac complications of COVID-19 (Table ).4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 Most of these reports have major limitations and none of them showed biopsy-proven SARS-CoV-2–positive myocarditis.
Table.
Clinically Suspected Myocarditis*/Myocarditis Definition According to Criteria of the ESC Position Paper in Comparison With Available in the Literature Clinical Cases of Suspected Myocarditis in the Course of COVID-192,4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18
| ESC Criteria | 15 Reports Highlighting Clinically Suspected Myocarditis in Patients With COVID-194, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 | |
|---|---|---|
| Clinical presentation | Nonspecific; mainly chest pain, palpitations or heart failure presentation | Major symptoms: fever 60%, cough 53%, dyspnea 67%, chest pain (40%), arrhythmia (33%) including recurrent ventricular tachycardia, multiple PVCs, atrial flutter, atrial fibrillation or high-degree atrioventricular block |
| Biomarkers of myocardial damage | Lack of sensitive and specific biomarkers; troponin is elevated in over one-third of patients; normal values do not exclude myocarditis | Elevated levels of troponin (87%; 13/15) and natriuretic peptides (91%; 10/11) in most patients. |
| Cardiac imaging (echocardiography/CMR) | Crucial imaging tool in initial diagnostic work-up.No specific echocardiographic changes. Possible echocardiographic abnormalities:
|
Four of 14 patients had no echocardiographic changes; the most common abnormalities were: reduced LVEF, regional wall motion abnormalities, increased wall thickness and chambers diameter; additionally, a few patients had mild pericardial effusion. |
| Tissue characterization (CMR) | The gold standard among noninvasive imaging tools.Possible CMR abnormalities:
|
CMR was performed in almost half (7/15) of the patients: edema and late gadolinium enhancement were observed in 2 and 6 patients, respectively. |
| EMB/autopsy | The diagnostic gold standard—histologic, immunologic, and immunohistochemical confirmation | EMB has only been performed in two cases, but only one met the diagnostic criteria for a virus-negative (and SARS-CoV-2 negative) myocarditis. **Several autopsy studies shown no signs of myocardial damage33,34 or myocardial injury (lymphocytic or interstitial macrophages infiltrate) without confirmed presence of SARS-CoV-2.20 |
CMR, cardiac magnetic resonance; COVID-19, novel coronavirus disease 2019; ECG, electrocardiogram; Echo, echocardiogram; EMB, endomyocardial biopsy; ESC, European Society of Cardiology; LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction; PVCs, premature ventricular contractions; SARS-CoV-2, severe acute respiratory syndrome novel coronavirus-2.
*Clinically suspected myocarditis:
if ≥1 clinical presentation and ≥1 diagnostic criteria from different categories:
if the patient is asymptomatic, ≥2 diagnostic criteria should be met,
the more criteria met, the higher the likelihood of myocarditis,
absence of angiographically significant coronary artery stenosis and other causes that could explain the syndrome.
In the Table, we review the reports in relation to the ESC 2013 criteria. In the majority of patients, the predominant clinical presentations were fever, cough and dyspnea, whereas in clinical practice most patients have mild symptoms, mainly chest pain and/or signs of mild heart failure. Although elevated level of biomarkers of necrosis and electrocardiogram changes are commonly found in myocarditis, they are not specific and cannot be used to diagnose myocarditis. In the reported COVID-19–related myocarditis cases, 5 patients had either no echocardiographic changes or did not undergo echocardiographic evaluation. Cardiac magnetic resonance, the gold standard among noninvasive imaging tools for the diagnosis of clinically suspected myocarditis,2 , 19 was performed in less than one-half of the patients.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 Several descriptions of EMB or autopsies have shown no signs of cardiomyocyte damage with SARS-CoV-2 viral particles located in cardiac endothelial cells or in cardiac infiltrating macrophages4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18; interestingly, 1 case met ESC diagnostic criteria for a biopsy-proven but virus-negative and SARS-CoV-2–negative myocarditis.5 In an international multicenter study, autopsies were performed in 21 patients with COVID-1920 and various forms of myocardial injury were observed. In the majority of cases, increased interstitial macrophages were present, which may reflect underlying diseases and/or elevated systemic levels of proinflammatory cytokines. Multifocal lymphocytic myocarditis was observed in only 3 (cases 14%). The authors emphasized several limitations of the study, including the fact that not all cases were sampled for histology in the same manner and polymerase chain reaction for virus in the myocardium was not performed. Therefore, the study does not confirm a direct link between the SARS-CoV-2 and myocarditis.20
Treatment and Outcome
In the analyzed cases, a significant clinical improvement was observed in 9 of 13 patients (69%) for whom data were available; 3 died and 1 was still connected to extracorporeal membrane oxygenation at the time of publication. Treatment included corticosteroids, intravenous immunoglobulin, antiviral treatments, or an anti–IL-6 agent (tocilizumab) alone or in combination, although these agents have no proven beneficial role in clinically suspected myocarditis.
To date, there is no evidence-based treatment for COVID-19–related clinically suspected myocarditis; as a consequence, patients should be managed according to standard COVID and heart failure treatment protocols.2
Hypothetical Mechanisms of Cardiac Injury in COVID-19
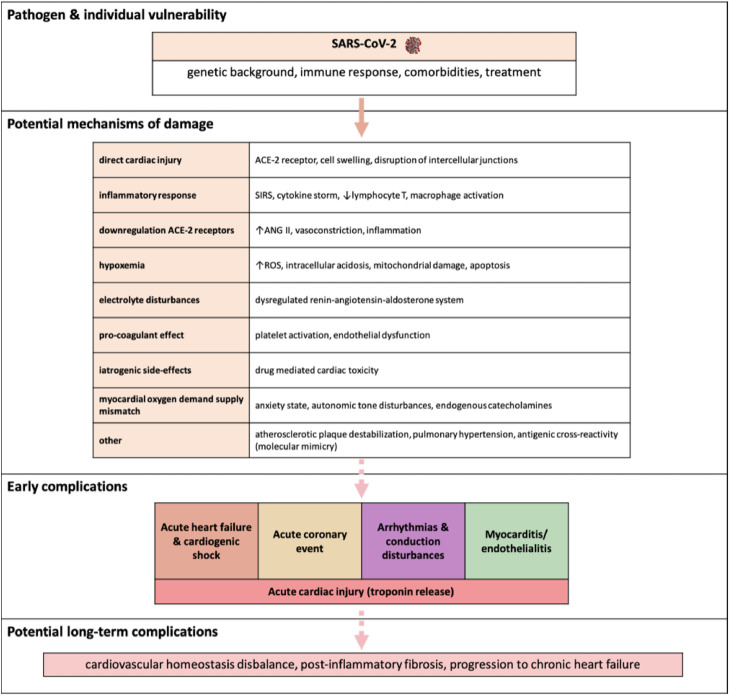
Manifestations of cardiovascular system involvement in the course of COVID-19 such as heart failure, arrhythmias, thromboembolism, and myocardial injury with increased troponins and natriuretic peptides levels have been reported.1 , 21, 22, 23 Potential mechanisms responsible for cardiotoxicity of the novel coronavirus are shown in the Figure . A pathogenic mechanism that is often mentioned in the literature to account for cardiac involvement is the high expression of the SARS-CoV-2 receptor, angiotensin-converting enzyme 2 (ACE-2), in the lungs and heart.24 However, ACE-2 upregulation may also be protective against angiotensin 2–mediated vasoconstriction and inflammatory activation. A recently performed meta-analysis showed that continuing ACE inhibitors or angiotensin 2 receptor blockers in patients with COVID-19 is safe and significantly decreases the risk of severe disease and death.25 Thus, it seems that although in principle an ACE-2–mediated effect may exist, other mechanisms resulting from COVID-19 are most likely responsible for myocardial damage.26 Another possible explanation is the increased risk of plaque instability and thrombosis owing to infection.27 Activation of the inflammatory reaction may also be responsible for increased blood coagulability.28
Fig.
The potential pathogenic mechanisms of cardiac injury among patients with SARS-CoV-2 infection. ACE-2, angiotensin-converting enzyme 2; ANG II, angiotensin II; ROS, reactive oxygen species; SARS-CoV-2, severe acute respiratory syndrome novel coronavirus-2; SIRS, systemic inflammatory response syndrome.
The recently reported endothelial cell infection (endothelitis) by SARS-CoV-2 seen in several organs, including the heart, is notable.5 , 29 The concept of not only “endotheliitis,” but rather “endothelialitis.” could be important. Endothelialitis involves not only endothelial cells infection, cell swelling, disruption of intercellular junctions, and loss of contact with the basal membrane, but also perivascular inflammation, lymphocytic infiltration, and microthrombi.30
Although the virus was detected in 1 case of cardiogenic shock, mimicking fulminant myocarditis, on EMB viral particles were shown within macrophages, but not in cardiomyocytes.4 In addition, immunohistochemistry showed low-grade myocardial inflammation, no myocyte necrosis, and no signs of vasculitis or thrombosis; interstitial fibrosis was minimal, that is, there was no histologic confirmation of active myocarditis.
It remains to be seen whether or not in selected genetically susceptible patients with COVID-19, myocyte injury, regardless of the underlying trigger (infectious or noninfectious), may initiate a secondary immunopathologic cascade progressing to chronic inflammatory cardiomyopathy via cell-mediated or autoantibody-mediated autoimmunity.2 , 31 , 32 Alternatively, an already established subclinical autoimmune myocarditis might be triggered or accelerated by the hyperinflammatory state of severe COVID-19.31 , 32 Further, various antiviral therapies tested and used in SARS-CoV-2 infection are potentially cardiotoxic; thus, drug-induced myocarditis may also occur.31 , 32
Conclusions
The hypothesis that SARS-CoV-2 causes viral myocarditis or serves as a trigger event for immune-mediated myocarditis in selected susceptible patients is possible, but should be definitively proven. So far, myocarditis is an uncommon complication in the course of SARS-CoV-2 infection and there are still no data on EMB or autopsy-proven myocarditis caused by this novel coronavirus. We strongly encourage the use of the term “myocarditis” as referring only to EMB or autopsy-proven diagnosis according to ESC criteria.2 Otherwise, there will be an inaccurate estimate of myocarditis incidence in COVID-19–based on misdiagnosis.
Acknowledgments
Conflicts of interest
None.
Availability of Data and Material (Data Transparency)
The study investigators have full access to the article datasets. External requests for data will be granted.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.cardfail.2020.11.002.
Appendix. Supplementary materials
References
- 1.Deng Q, Hu B, Zhang Y, Wang H, Zhou X, Hu W, et al. Suspected myocardial injury in patients with COVID-19: evidence from front-line clinical observation in Wuhan, China. Int J Cardiol. 2020;311:116–121. doi: 10.1016/j.ijcard.2020.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caforio ALP, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on myocardial and pericardial diseases. Eur Heart J. 2013;34:2636–2648. doi: 10.1093/eurheartj/eht210. 2648a-2648d. [DOI] [PubMed] [Google Scholar]
- 3.Sinagra G, Porcari A, Gentile P, Artico J, Fabris E, Bussani R, et al. Viral presence-guided immunomodulation in lymphocytic myocarditis: an update. Eur J Heart Fail. 2020 Jul 19 doi: 10.1002/ejhf.1969. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tavazzi G, Pellegrini C, Maurelli M, Belliato M, Sciutti F, Bottazzi A, et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22:911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sala S, Peretto G, Gramegna M, Palmisano A, Villatore A, Vignale D, et al. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J. 2020;41:1861–1862. doi: 10.1093/eurheartj/ehaa286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inciardi RM, Lupi L, Zaccone G, Italia R, Raffo M, Tomasoni D, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:819–824. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seecheran R, Narayansingh R, Giddings S, Rampaul M, Furlonge K, Abdool K, et al. Atrial arrhythmias in a patient presenting with coronavirus disease-2019 (COVID-19) infection. J Investig Med High Impact Case Rep. 2020;8 doi: 10.1177/2324709620925571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Irabien-Ortiz A, Carreras-Mora J, Sionis A, Pàmies J, Montiel J, Tauron M. Fulminant myocarditis due to COVID-19. Rev Esp Cardiol (Engl Ed) 2020;73:503–504. doi: 10.1016/j.rec.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warchoł I, Dębska-Kozłowska A, Karcz-Socha I, Książczyk M, Szymańska K, Lubiński A. Terra incognita: clinically suspected myocarditis in a patient with severe acute respiratory syndrome coronavirus 2 infection. Pol Arch Intern Med. 2020;130:446–448. doi: 10.20452/pamw.15309. [DOI] [PubMed] [Google Scholar]
- 10.Hongde H, Fenglian M, Xin W, Yuan F. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020 doi: 10.1093/eurheartj/ehaa248. Mar 16:ehaa190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devika K, Chaitra M, Rhea S. HEART BRAKE-an unusual cardiac manifestation of coronavirus disease 2019 (COVID-19) JACC Case Rep. 2020;2:1252–1255. doi: 10.1016/j.jaccas.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coyle J, Igbinomwanghia E, Sanches-Nadalez A, Danciu S, Chu C, Shah N. A recovered case of COVID-19 myocarditis and ARDS treated with corticosteroids, tocilizumab, and experimental AT-001. JACC Case Rep. 2020;2:1331–1336. doi: 10.1016/j.jaccas.2020.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim IC, Kim JY, Kim HA, Han S. COVID-19-related myocarditis in a 21-year-old female patient. Eur Heart J. 2020;41:1859. doi: 10.1093/eurheartj/ehaa288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paul JF, Charles P, Richaud C, Caussin C, Diakov C. Myocarditis revealing COVID-19 infection in a young patient. Eur Heart J Cardiovasc Imaging. 2020;21:776. doi: 10.1093/ehjci/jeaa107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doyen D, Moceri P, Ducreux D, Dellamonica J. Myocarditis in a patient with COVID-19: a cause of raised troponin and ECG changes. Lancet. 2020;395:1516. doi: 10.1016/S0140-6736(20)30912-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radbel J, Narayanan N, Bhatt PJ. Use of tocilizumab for COVID-19 infection-induced cytokine release syndrome: a cautionary case report. Chest. 2020;158:e15–e19. doi: 10.1016/j.chest.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cizgici AY, Agus HZ, Yldiz M. COVID-19 myopericarditis: it should be kept in mind in today's conditions. Am J Emerg Med. 2020;38:1547.e5–1547.e6. doi: 10.1016/j.ajem.2020.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng Yh, Liu YX, Yuan J, Wang FX, Wu WB, Li JX, et al. First case of COVID 19 complicated with fulminant myocarditis: a case report and insights. Infection. 2020;48:773–777. doi: 10.1007/s15010-020-01424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caforio ALP, Cheng C, Perazzolo Marra M, Tarantini G, Basso C, Marcolongo R, Iliceto S. How to improve therapy in myocarditis: role of cardiovascular magnetic resonance and of endomyocardial biopsy. Eur Heart J Suppl. 2019;21(Suppl B):B19–B22. doi: 10.1093/eurheartj/suz014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Basso C, Leone O, Rizzo S, De Gaspari M, van der Wal AC, Aubry MC, et al. Pathological features of COVID-19-associated myocardial injury: a multicentre cardiovascular pathology study. Eur Heart J. 2020:ehaa664. doi: 10.1093/eurheartj/ehaa664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Driggin E, Madhavan MV, Bikdeli B, Chuich T, Laracy J, Bondi-Zoccai G, et al. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID-19) pandemic. J Am Coll Cardiol. 2020;75:2353–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5:831–840. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 24.Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pranata R, Permana H, Huang I, Lim MA, Soetedjo NNM, Supriyadi R, Soeroto AY, Alkatiri AA, Firman D, Lukito AA. The use of renin angiotensin system inhibitor on mortality in patients with coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. Diabetes Metab Syndr. 2020;14:983–990. doi: 10.1016/j.dsx.2020.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozieranski K, Tyminska A, Caforio ALP. Clinically suspected myocarditis in the course of coronavirus infection. Eur Heart J. 2020;41:2118–2119. doi: 10.1093/eurheartj/ehaa353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonow RO, Fonarow GC, O'Gara PT, Yancy CW. Association of coronavirus disease 2019 (COVID-19) with myocardial injury and mortality. JAMA Cardiol. 2020;5:751–753. doi: 10.1001/jamacardio.2020.1105. [DOI] [PubMed] [Google Scholar]
- 28.Middeldorp S, Coppens M, van Haaps TF, Foppen M, Vlaar AP, Muller MC, et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ackermann M, Verleden SE, Kuehnel M, Kuehnel M, Haverich A, Welte T, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russell B, Moss C, George G, Santaolalla A, Cope A, Papa S, et al. Associations between immune-suppressive and stimulating drugs and novel COVID-19—a systematic review of current evidence. Ecancermedicalscience. 2020;14:1022. doi: 10.3332/ecancer.2020.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alijotas-Reiga J, Esteve-Valverde E, Beliznaf C, Selva-O'Callaghan A, Pardos-Gea J, Quintana A, et al. Immunomodulatory therapy for the management of severe COVID-19. Beyond the anti-viral therapy: a comprehensive review. Autoimmunity Rev. 2020;19 doi: 10.1016/j.autrev.2020.102569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barton LM, Duval EJ, Stroberg E, Ghosh S, Mukhopadhyay S. COVID-19 autopsies, Oklahoma, USA. Am J Clin Pathol. 2020;153:725–733. doi: 10.1093/ajcp/aqaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.