Abstract
Background
The effect of favipiravir on the QTc interval during the treatment of Coronavirus Disease 2019 (COVID-19) patients is unclear. Thus, the current study objective was to evaluate any change in the QTc interval in patients who were hospitalized due to COVID-19 receiving favipiravir treatment.
Method
Patients hospitalized with COVID-19 were assessed in this single-center retrospective study. 189 patients, whose diagnosis was confirmed using real-time PCR, were included in the study. The patients were divided into three groups: those using hydroxychloroquine (Group 1, n = 66), hydroxychloroquine plus favipiravir (Group 2, n = 66), and favipiravir only (Group 3, n = 57). The QTc interval was measured before treatment (QTc-B) and 48 h after (i.e., the median) starting treatment (QTc-AT).
Results
The median age was 53 (39–66 IQR) and 97 (51%) of patients were female. The median QTc(Bazett)-change was 7 ms (p = 0.028) and 12 ms (p < 0.001) and in Group 1 and 2, respectively. In Group 3, the median QTc(Bazett)-change was observed as −3 ms and was not statistically significant (p = 0.247). In multivariable analysis, while there was a significant relationship between QTc-AT(Bazett) and hydroxychloroquine (β coefficient = 2687, 95%CI 2599–16,976, p = 0,008), there was no significant relationship with favipiravir (β coefficient = 0,180, 95% CI -6435-7724, p = 0,858). Similarly, there was a significant relationship between the QTc-AT interval calculated using the Fredericia formula and hydroxychloroquine (β coefficient = 2120, 95% CI 0,514–14,398, p = 0,035), but not with favipiravir (β coefficient = 0,111, 95% CI -6450- 7221, p = 0,911).
Conclusion
In the ECG recordings received in the following days after the treatment was started in COVID-19 patients, there was a significant prolongation in the QTc interval with hydroxychloroquine, but there was no significant change with favipiravir.
Keywords: COVID-19, Favipiravir, QTc interval
Introduction
Coronavirus Disease 2019 (COVID-19), first reported in Wuhan, China, on December 31, 2019, rapidly spread throughout the world within a few months and was declared as a pandemic by the World Health Organization. COVID-19 is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), [1] and although it causes pneumonia primarily, it also affects other organs and tissues. SARS-CoV-2 can cause myocardial injury, and depending on the main disease effects and the medications used as treatment, it can lead to prolongation of the corrected QT (QTc) interval and cardiac arrhythmias. [[2], [3], [4]] Drugs such as azithromycin and hydroxychloroquine are used to treat COVID-19; however, they have been demonstrated to cause fatal arrhythmias, such as Torsades de pointes, through prolongation of the QTc interval. [[4], [5], [6]]
Favipiravir is an inhibitor of the RNA-dependent RNA polymerase of many RNA viruses, and it has been shown to be effective in the treatment of influenza and, to some extent, Ebola virus disease (EVD) [[7], [8], [9]]. Favipiravir, which is also administered to COVID-19 patients, has a safe side-effect profile, but there are inadequate data on its effect on the QTc interval. [10]. Significant QT prolongation due to favipiravir was demonstrated in a patient with EVD in a previous case study. [11] Favipiravir was not shown to have a detectable effect on QT intervals in a study performed by Kumagai et al. on healthy adults that used moxifloxacin as a positive control to enable high-powered statistical analysis. [12] To the best of our knowledge, the effect of favipiravir on the QTc interval in COVID-19 patients has not been evaluated in any study to date. Thus, the current study objective was to investigate any change in the QTc interval in patients hospitalized due to COVID-19 receiving favipiravir therapy.
Method
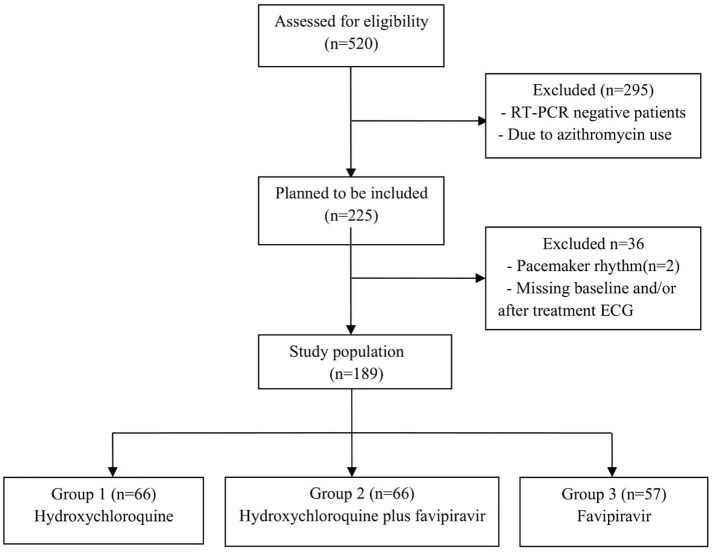
COVID-19 adult patients, hospitalized at the University of Health Sciences Diyarbakır Gazi Yaşargil Training and Research Hospital between April 15 and May 31, 2020, were the focus of this single-center, retrospective study. A COVID-19 diagnosis constituted a positive finding from a real-time reverse-transcriptase (RT) polymerase chain reaction (PCR) assay of nasal and pharyngeal swab specimens. Patients who had negative RT-PCR results, received azithromycin therapy, had a pacemaker rhythm and no electrocardiogram (ECG) recordings were excluded from the study. 189 patients were included in the study. The patients were divided into three groups: those on hydroxychloroquine (Group 1), hydroxychloroquine plus favipiravir (Group 2), and favipiravir only (Group 3). A Flow-chart of patient selection was given in Fig. 1 .
Fig. 1.
Flow-chart of patient selection.
The demographic, clinical characteristics, and laboratory parameters of the participants during their hospitalization were obtained from the hospital's electronic medical records.
Hydroxychloroquine and/or favipiravir therapy was initiated by infectious disease specialists and pulmonologists according to the clinical, laboratory, and radiological findings of the patients. Hydroxychloroquine was given orally at 400 mg BID for the first day (loading dose) followed by 200 mg BID for 4 days. Favipiravir was given orally at 1600 mg BID for the first day (loading dose) followed by 600 mg BID for 4 days.
Myocardial injury was defined as the presence of at least one cardiac troponin value above the 99th percentile upper reference limit. Two or more systemic inflammatory response syndrome (SIRS) criteria were significantly associated with QTc prolongation in a previous study that evaluated patients treated with medication that prolonged the QT interval [4]. In the current study, four SIRS criteria were evaluated; I. tachypnea (respiratory rate > 20 breaths/min), II. tachycardia (heart rate > 90 beats/min), III. fever or hypothermia (temperature of >38 °C or < 36 °C), and IV. leukocytosis, leukopenia, or bandemia (white blood cell count of >12,000/mm3, < 4000/mm3, or bandemia of ≥10%). A SIRS diagnosis was based on evidence of two or more of these criteria.
Approximately 50% of calcium in serum is bound to proteins, principally albumin. Albumin is a “negative” acute-phase reactant. Albumin levels decrease due to infection, and, in turn, are accompanied by low calcium levels. Therefore, the corrected calcium level was calculated using the following equation: corrected calcium (mg/dL) = total calcium (mg/dL) + 0.08 × (40 - albumin [g/L]) [13].
Electrocardiographic analysis
Standard 12‑lead electrocardiogram (ECG) images were obtained at 25 mm/s paper speed and 10 mm/mV calibration during emergency department admission (QTc-B) and again after a median of 48 h (at least 24 h) after starting treatment (QTc-AT). Electrocardiography was conducted using Nihon Kohden's Cardiofax® M electrocardiogram; the images were transferred to a computer via a scanner and analyzed using ImageJ® digital image processing software. The electrocardiographic analysis was performed by two experienced cardiologists who were blinded to the patient data. The opinion of a third cardiologist was sought in instances where consensus was lacking. Lead II was used for the analysis; failing that, lead V5 was utilized. When both leads II and V5 were deemed unsuitable, one of the remaining leads was chosen. The QT interval was measured from the beginning of the QRS complex to T wave termination and averaged over 3–5 beats. If a U wave followed the T wave, the T wave offset was measured as the nadir between the T and U waves. Bazzet's formula can overestimate the QTc interval at high heart rates. Therefore, the QT intervals were corrected for the effects of heart rate using both a Bazzet's formula (QTc(Bazett) (QTc = QT/[R - R]1/2) and the Fridericia formula (QTc(Fre) (QTc = QT/[R - R]1/3). The Bogossian formula [14] was used to calculate the modified QT interval: QTm = QT - 48.5% x QRS duration for patients with QRS of >120 ms.
The study was approved by the Turkish Ministry of Health and the local institutional ethics committee. The study protocol conformed to the Declaration of Helsinki.
Statistics
The continuous variables were presented as a median interquartile range (IQR) (25–75%) owing to their non-normal distribution. The categorical variables were expressed as percentages. The Wilcoxon signed-rank test was used to assess any change in the QTc after treatment. The chi-square test was employed for inter-group comparisons. Continuous variables were compared between Groups 1, 2, and 3 using the ANOVA or Kruskal–Wallis H tests as appropriate. Multivariable linear regression analysis was utilized to evaluate the relationship between the clinical parameters, medications, laboratory parameters (age, gender, potassium levels, calcium levels, SIRS, congestive heart failure, myocardial injury, loop diuretics, beta-blockers, favipiravir, and hydroxychloroquine), and QTc-AT.Two separate models were constructed with QTc-AT, calculated by the formula of Bazett's and Fredericia formula. In all the statistical analyses, a p-value of <0.050 was considered to be statistically significant. The data were analyzed using Statistical Package for Social Science® 24.0.
Results
In total, 189 COVİD-19 patients, diagnosed using RT-PCR, were included in the current study. The median age of the participants was 53 years (39–66 IQR). Ninety-seven (51%) of the patients were female. The participants were divided into three groups: those using hydroxychloroquine (Group 1, n = 66), hydroxychloroquine plus favipiravir (Group 2, n = 66), and favipiravir only (Group 3, n = 57). Compared to the other groups, the lymphocyte, albumin, and calcium levels were lower in Group 2. Conversely, age, C-reactive protein, d-dimer, pro-calcitonin, the proportion of patients admitted to the ICU, the number of patients with ≥2 SIRS criteria, and total hospital stay duration were higher in Group 2 than those of Groups 1 and 3. The baseline characteristics, and the clinical, laboratory, and electrocardiographic findings of the patients are presented in Table 1 .
Table 1.
Baseline characteristics, clinical, laboratory, and electrocardiographic findings of patients.
| Total (n = 189) | Group I (n = 66) | Group II (n = 66) | Group III (n = 57) | P value | |
|---|---|---|---|---|---|
| Age, years | 53 (40–67) | 43 (29–59) | 58 (49–70) | 50 (40–67) | <0,001 |
| Female gender, n (%) | 97 (51%) | 32 (48%) | 32 (48%) | 33 (58%) | 0,494 |
| Hypertension, n (%) | 51 (27%) | 11 (17%) | 23 (35%) | 17 (30%) | 0,047 |
| Diabetes mellitus, n (%) | 38 (20%) | 9 (14%) | 15 (%23) | 14 (%25) | 0,243 |
| Smoking, n (%) | 59 (30%) | 18 (27%) | 23 (35%) | 18 (31%) | 0,640 |
| Congestive heart failure, n (%) | 4 (2%) | 1 (0,5%) | 1 (0,5%) | 2 (1%) | 0,683 |
| Coronary artery disease, n (%) | 19 (10%) | 5 (3%) | 7 (4%) | 7 (12%) | 0,670 |
| Chronic respiratory disease, n (%) | 14 (7%) | 4 (6%) | 8 (12%) | 2 (3%) | 0,169 |
| Myocardial injury, n (%) | 9 (5%) | 2 (%3) | 5 (%8) | 2 (%3) | 0,396 |
| ≥2 SIRS criteria, n (%) | 57 (30%) | 13 (20%) | 29 (44%) | 15 (26%) | 0,008 |
| Radiographic finding of pneumonia, n(%) | 180 (95%) | 57 (86%) | 66 (100%) | 57 (100%) | <0,001 |
| Length of hospital stay, days | 7 (5–10) | 6 (5–8) | 8 (7–12) | 6 (5–10) | <0,001 |
| Intensive care unit admission, n (%) | 26 (14%) | 2 (3%) | 18 (27%) | 6 (10%) | <0,001 |
| Loop diuretic use, n (%) | 5 (3%) | 1 (2%) | 2 (3%) | 2 (4%) | 0,767 |
| Temperature, °C | 37 (36,7-37,6) | 37 (36,6-37,4) | 37,3 (36,7–38) | 37,1 (36,7-37,6) | 0,042 |
| Systolic blood pressure, mmHg | 110 (105–120) | 110 (100−120) | 120 (110–126) | 112 (108–120) | 0,023 |
| Diastolic blood pressure, mmHg | 70 (69–79) | 70 (69–77) | 70 (69–77) | 70 (69–80) | 0,590 |
| White blood cell, 103/uL | 6,13 (4,60–8,00) | 6,40 (4,79-8,57) | 6,29 (4,61-8,40) | 5,63 (4,52-7,03) | 0,262 |
| Neutrophil, 103/uL | 4,11 (3,05-5,60) | 3,96 (2,73-5,48) | 4,55 (3,19-6,21) | 3,71 (2,91-5,23) | 0,094 |
| Lymphocyte, 103/uL | 1,31 (0,93-1,76) | 1,48 (1,22−2,01) | 1,1 (0,78-1,51) | 1,30 (0,92-1,73) | <0,001 |
| Hemoglobin, g/dL | 13,6 (12,7-14,8) | 13,7 (12,6-14,9) | 13,7 (12,8-14,6) | 13,4 (12,6-14,8) | 0,919 |
| C-reactive protein, mg/L | 29 (4–68) | 4,8 (2–25) | 58 (31−102) | 29 (6–53) | <0,001 |
| Pro-calsitonin, ng/mL | 0,07 (0,04-0,11) | 0,04 (0,03-0,06) | 0,09 (0,06-0,19) | 0,08 (0,05-0,11) | <0,001 |
| D-dimer, ng/mL | 186 (122–328) | 155 (95–258) | 240 (165–484) | 190 (120−312) | <0,001 |
| Creatinine, mg/dl | 0,81 (0,71-1,03) | 0,76 (0,67-0,90) | 0,94 (0,76-1,14) | 0,81 (0,71-1,16) | 0,001 |
| Albumin, g/L | 38 (35–42) | 42 (39–46) | 37 (34–39) | 37 (34–40) | <0,001 |
| Total calcium, mg/dL | 8,60 (8,15-8,90) | 8,90 (8,50–8,91) | 8,30 (8,0-8,60) | 8,6 (8–9,05) | <0,001 |
| Corrected calcium, mg/dL | 8,62 (8,36-8,93) | 8,61 (8,39-8,83) | 8,53 (8,25-8,86) | 8,72 (8,49-9,19) | 0,016 |
| Potassium baseline, mmol/L | 4,03 (3,78-4,38) | 4,00 (3,82-4,28) | 4,00 (3,78-4,42) | 4,11 (3,71-4,35) | 0,987 |
| Heart rate baseline, beat/min | 88 (78–98) | 89 (76–100) | 88 (78–98) | 90 (79–98) | 0,773 |
| Heart rate at 3rd day, beat/min | 81 (74–89) | 80 (70–87) | 84 (75–92) | 80 (73–88) | 0,068 |
| QRS duration baseline, ms | 90 (84–100) | 92 (84–100) | 93 (84–100) | 88 (82–96) | 0,074 |
| QRS duration after treatment, ms | 92 (86–102) | 96 (86–104) | 94 (86–103) | 90 (84–100) | 0,365 |
| QT interval baseline, ms | 360 (340–377) | 355 (333–377) | 362 (344–385) | 357 (340–373) | 0,344 |
| QT interval after treatment, ms | 376 (360–400) | 380 (362–402) | 380 (365–394) | 370 (354–392) | 0,260 |
| Delta QT, ms | 20 (4–64) | 23 (10–37) | 16 (3–35) | 17 (2−32) | 0,297 |
| QTc(Bazett) interval baseline, ms | 430 (415–450) | 424 (413–446) | 431 (420–449) | 433 (419–452) | 0,068 |
| QTc(Bazett) interval after treatment, ms | 438 (421–456) | 429 (414–458) | 448 (431–464) | 434 (417–446) | 0,002 |
| Delta QTc(Bazett), ms | 5 (−8–19) | 7 (−9–21) | 12 (1–25) | -3 (−12–7) | <0,001 |
| QTc(Fre) interval baseline, ms | 405 (392–423) | 404 (388–419) | 411 (397–430) | 405 (392–418) | 0,115 |
| QTc(Fre) interval after treatment, ms | 418 (401–431) | 419 (395–431) | 423 (410–440) | 408 (400–425) | 0,011 |
| Delta QTc(Fre), ms | 10 (−4–23) | 10 (−4–22) | 14 (−1–26) | 3 (−7–14) | 0,030 |
Baseline characteristics, clinical, laboratory and electrocardiographic findings of patients Continuous variables are presented given as median (interquartile range) and categorical variables were expressed as number (%). SIRS, Systemic Inflammatory Response Syndrome; Fre, Fredericia.
The median QT-B was 360 ms (340–377 IQR), and the median QT-AT was 376 ms (360–400 IQR). A significant prolongation was observed in the QT interval in all three groups after treatment (Group 1, p < 0,001; Group 2, p < 0,001; Group 3, p 〈0,001). The median QTc-B (Bazett) was 430 ms (415–450 IQR), and the median QTc-AT(Bazett) was 438 ms (421–456 IQR). Significant QTc(Bazett) interval prolongation (5 ms) was observed after the start of treatment (−8,19 IQR, p ≤ 0.001). The median change in QTc(Bazett) was 7 ms (−9, 21 IQR, p = 0.028) and 12 ms (IQR of 1–25, p ≤ 0.001) in Groups 1 and 2, respectively. In Group 3, a change of −3 ms((−12, 7 IQR) was observed in the QTc(Bazett); however, this finding was without statistical significance (p = 0.247). The median QTc-B(Fre) was 405 ms (392–423 IQR) and the median QTc-AT(Fre) was 418 ms (401–431 IQR). Similar to QTc(Bazett), a significant prolongation was observed in Groups 1 and 2 in QTc(Fre), while the change in Group 3 was not statistically significant (respectively p < 0,001, p < 0,001, p = 0,075).
A significant relationship was observed between the QTc-AT and hydroxychloroquine using multivariable linear regression analysis (β coefficient = 2687, 95% CI 2599–16,976, p = 0,008); however, the relationship between QTc-AT and favipiravir was non-significant (β coefficient = 0,180, 95% CI-6435–7724, p = 0,858) (Table 2 , Model I). Similarly, there was a significant relationship between the QTc-AT interval calculated using the Fredericia formula and hydroxychloroquine (β coefficient = 2120, 95% CI 0,514–14,398, p = 0,035), but not with favipiravir (β coefficient = 0,111, 95% CI -6450- 7221, p = 0,911) (Table 2, Model II).
Table 2.
Multivariable linear regression analysis between the QTc-AT (Bazett and Fredericia) and clinical variables.
| Model 1 (Bazett) |
Model 2 (Fredericia) |
|||||
|---|---|---|---|---|---|---|
| β-coefficient | CI 95% | P value | β-coefficient | CI 95% | P value | |
| Age (year) | 3047 | 0,092 - 0,428 | 0,003 | 2190 | 0,018–0,346 | 0,030 |
| Gender (female) | 2465 | 1401 - 12,654 | 0,015 | 1609 | -1006–9877 | 0,109 |
| QTc-B | 10,455 | 0,503 - 0,737 | <0,001 | 9764 | 0,486–0,732 | <0,001 |
| Potassium | -2009 | −13,067 - −0,117 | 0,046 | −1828 | −12,046–0,461 | 0,069 |
| SIRS criteria ≥2 | 2189 | 0,730 - 14,107 | 0,030 | 2268 | 0,934–13,483 | 0,025 |
| Hydroxychloroquine | 2687 | 2599 - 16,976 | 0,008 | 2120 | 0,514–14,398 | 0,035 |
| Favipiravir | 0,180 | −6435 - 7724 | 0,858 | 0,111 | −6450–7221 | 0,911 |
| Congestive heart failure | −0,860 | −30,961–12,163 | 0,391 | 0,128 | −19,495–22,194 | 0,898 |
| Myocardial injury | 0,676 | −8622–17,598 | 0,500 | −0,701 | −17,252–8207 | 0,484 |
| Loop diuretic | −0,403 | −23,927–15,079 | 0,688 | −0,064 | −19,828–18,589 | 0,949 |
| Beta-blocker | 1119 | −4167–15,079 | 0,265 | 0,155 | −8520–9969 | 0,877 |
| Calcium | −1597 | −10,811–1141 | 0,112 | −0,843 | −8265–3319 | 0,401 |
QTc-B: QTC baseline; CI: Confidence Interval; QTcAT: QTc after treatment; SIRS: Systemic Inflammatory Response Syndrome.
In six patients, a QTc-AT(Bazett) intervals exceeding 500 ms or a change in the QTc intervals of >60 ms were observed, and hydroxychloroquine was discontinued in four of these patients. Four of these patients were in Group 1 and two were in Group 2. Torsades de pointes were not observed, and there were no arrhythmic deaths; however, 11 of the patients died from respiratory failure and/or sepsis in the ICU follow-up.
Discussion
A key finding in the current study was that significant QTc prolongation was observed in COVID-19 patients approximately two days after they received hydroxychloroquine and hydroxychloroquine plus favipiravir. However, this did not apply to patients who were given favipiravir only. In addition, a significant relationship was demonstrated between hydroxychloroquine and the QTc-AT interval using multivariable linear regression analysis; however, a significant relationship between the QTc-AT interval and favipiravir was not observed.
COVID-19 causes pneumonia primarily, but it can also cause cardiac injury. Although myocardial injury directly relates to viral infection, it can occur due to severe acute respiratory infection that causes hypoxia. [15] QTc prolongation and cardiac arrhythmias are the results of both cardiac damage and the drugs used in the treatment. [[4], [5], [6]] Hydroxychloroquine, which is widely used in many countries in the treatment of COVID-19, prolongs ventricular repolarization (the QT interval) and sometimes induces Torsades de pointes by blocking the potassium channels. [16] In the current study, the QTc interval was significantly prolonged in the patients in Groups 1 and 2 after hydroxychloroquine treatment. Tisdal et al. showed that parameters, such as age, gender, sepsis, myocardial infarction, heart failure, baseline QTc interval, potassium levels, number of drugs prolonging the QTc interval, and loop diuretic use, were predictors of QTc prolongation in patients using medication that caused QTc prolongation. [17] Again, in a recent study in COVID-19 patients, SIRS criteria ≥2 was observed among the parameters predicting QTc prolongation significantly. [4] In the present research, a significant relationship was identified between age, gender, QTc-B, potassium levels, SIRS criteria ≥2, hydroxychloroquine, and QTc-AT(Bazett) using multivariable linear regression analysis. Similarly, there was a significant relationship between the QTc-AT interval calculated using the Fredericia formula and hydroxychloroquine.
Favipiravir is an antiviral agent that selectively and potently inhibits the RNA-dependent RNA polymerase of RNA viruses. [18] Favipiravir, which is used to counter RNA viruses, such as EVD and influenza, is a broad-spectrum drug and is used in COVID-19 treatment, having been approved for use in COVID-19 treatment in March 2020 in China [19]. Currently, favipiravir, widely used in many countries, has a safe profile, but concerns remain about its effects on the QTc interval. [10] QTc prolongation was observed on the 9th day of treatment in a patient who was previously using favipiravir for Ebolavirus infection. [11] In this patient, the loading dose given was 6000 mg and 2400 mg daily thereafter. His admission potassium levels were 3.5 mmol/L, and ionized calcium was determined to be 1.08 mmol/L when QTc prolongation was evident. However, favipiravir was administered to this patient at a much higher dose than that used to treat COVID-19 patients. QTc interval prolongation may be due to sepsis and electrolyte abnormalities, as well as higher doses and longer use of favipiravir. Similarly, prolongation of the QTc interval was observed in some patients in Group 3 (only favipiravir) in the present study and it was determined that this prolongation was not due to favipiravir using multivariate analysis. A small-scale study (56 people) was conducted in Japan to evaluate the effect of favipiravir on the QTc interval in healthy adults. The patients were divided into four groups and were given 1200 mg favipiravir, 2400 mg favipiravir, moxifloxacin, and placebo, respectively. Significant QTc interval prolongation was not identified on ECG images, obtained three and six hours after the favipiravir was administered. [12] This study did not provide sufficient information about the effects of the treatment dose on the QTc interval and changes in the QTc interval in subsequent days since the loading dose was lower than that used for COVID-19 patients, and the ECG findings were obtained after three and six hours. In the current study, a significant change in the QTc(Bazett) and QTc(Fre) interval was not observed on the ECG images taken 48 h (median) after favipiravir treatment in Group 3 (respectively p = 0.247, p = 0,075). Favipiravir was also not shown to have a significant relationship with QTc-AT(Bazett) and QTc-AT(Fre) using multiple regression analysis (respectively p = 0,858, p = 0.911).
Despite the small number of patients in the current study, the use of favipiravir alone, or in combination with hydroxychloroquine at the dose recommended for the treatment of COVID-19 patients, appeared to be safe in terms of QTc prolongation. Large-scale prospective studies are warranted to clarify concerns about this issue.
Conclusion
In the ECG recordings received in the following days after the treatment was started in COVID-19 patients, there was a significant prolongation in the QTc interval with hydroxychloroquine, but there was no significant change with favipiravir.
Funding
No funding.
Declaration of Competing Interest
None.
References
- 1.WHO Novel coronavirus (2019-nCoV) situation Report-1. 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200121-sitrep-1-2019-ncov.pdf?sfvrsn=20a99c10_4 Available:
- 2.Mitrani R.D., Dabas N., Goldberger J.J. COVID-19 cardiac injury: implications for long-term surveillance and outcomes in survivors. Heart Rhythm. 2020;17(11):1984–1990. doi: 10.1016/j.hrthm.2020.06.026. published online ahead of print, 2020 Jun 26. (S1547-5271(20)30625-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kang Y., Chen T., Mui D. Cardiovascular manifestations and treatment considerations in covid-19. Heart. 2020;106(15):1132–1141. doi: 10.1136/heartjnl-2020-317056. published online ahead of print, 2020 Apr 30. (heartjnl-2020-317056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mercuro N.J., Yen C.F., Shim D.J. Risk of QT Interval Prolongation Associated With Use of Hydroxychloroquine With or Without Concomitant Azithromycin Among Hospitalized Patients Testing Positive for Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020;5(9):1036–1041. doi: 10.1001/jamacardio.2020.1834. published online ahead of print, 2020 May 1. e201834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization The cardiotoxicity of antimalarials: Malaria Policy Advisory Committee Meeting. 2017. https://www.who.int/malaria/mpac/mpac-mar2017-erg-cardiotoxicity-report-session2 Published March 24. Accessed April 21, 2020.
- 6.Ray W.A., Murray K.T., Hall K. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366(20):1881–1890. doi: 10.1056/NEJMoa1003833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du Y.X., Chen X.P. Favipiravir: pharmacokinetics and concerns about clinical trials for 2019-nCoV infection. Clinica Pharmacology & Therapeutics. 2020 doi: 10.1002/cpt.1844. [DOI] [PubMed] [Google Scholar]
- 8.Sissoko D., Laouenan C., Folkesson E. Experimental treatment with favipiravir for Ebola virus disease (the JIKI trial): a historically controlled, single arm proof-of-concept trial in Guinea. PLoS Med. 2016;13 doi: 10.1371/journal.pmed.1001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bai C.Q., Mu J.S., Kargbo D. Clinical and virological characteristics of ebola virus disease patients treated with Favipiravir (T-705)-Sierra Leone, 2014. Clin Infect Dis. 2016;63:1288–1294. doi: 10.1093/cid/ciw571. [DOI] [PubMed] [Google Scholar]
- 10.Pilkington V., Pepperrell T., Hill A. A review of the safety of favipiravir - a potential treatment in the COVID-19 pandemic? J Virus Erad. 2020;6(2):45–51. doi: 10.1016/S2055-6640(20)30016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chinello P., Petrosillo N., Pittalis S. QTc interval prolongation during favipiravir therapy in an Ebolavirus-infected patient. PLoS Negl TropDis. 2017;11(12) doi: 10.1371/journal.pntd.0006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumagai Y., Murakawa Y., Hasunuma T. Lack of effect of favipiravir, a novel antiviral agent, on QT interval in healthy Japanese adults. Int J Clin Pharmacol Ther. 2015;53(10) doi: 10.5414/CP202388. [DOI] [PubMed] [Google Scholar]
- 13.Payne R.B., Little A.J., Williams R.B. Interpretation of serum calcium in patients with abnormal serum proteins. Br Med J. 1973;4(5893):643–646. doi: 10.1136/bmj.4.5893.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bogossian H., Frommeyer G., Ninios I. New formula for evaluation of the QT interval in patients with left bundle branch block. Heart Rhythm. 2014;11:2273–2277. doi: 10.1016/j.hrthm.2014.08.026. [DOI] [PubMed] [Google Scholar]
- 15.Fan Y., Guo T., Lu Z. Myocardial injury in COVID-19-can we successfully target inflammation?-Reply. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.2572. published online ahead of print, 2020 Jul 15. [DOI] [PubMed] [Google Scholar]
- 16.Mazzanti A., Briani M., Kukavica D. Association of Hydroxychloroquine with QTc Interval in Patients with COVID-19. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.048476. published online ahead of print, 2020 Jun 5. [DOI] [PubMed] [Google Scholar]
- 17.Tisdale J.E., Jayes H.A., Kingery J.R. Development and validation of a risk score to predict QT interval prolongation in hospitalized patients. Circ Cardiovasc Qual Outcomes. 2013;6:479–487. doi: 10.1161/CIRCOUTCOMES.113.000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Şimşek Yavuz S., Ünal S. Antiviral treatment of COVID-19. Turk J Med Sci. 2020;50(SI-1):611–619. doi: 10.3906/sag-2004-145. Published 2020 Apr 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.XinhuaNet Favipiravir shows good clinical efficacy in treating COVID-19: official. (2020-03-17) From. http://www.xinhuanet.com/english/2020-03/17/c_138888226.htm