Abstract
Background
Due to COVID-19 and high demand for respirators, some healthcare professionals have been using the Halyard H600 fabric as an alternative to N95 respirators without testing the filtration efficiency of the fabric with established scientific methods. The purpose of this study was to assess the efficiency of the Halyard H600 as a respirator filtering material as compared to the NIOSH-certified N95 and P100 filters, and determine if H600 is a good alternative for respiratory protection for healthcare professionals during the COVID-19 pandemic.
Methods
Three filter types (Halyard H600, N95, and P100) were challenged with salt particles inside an exposure chamber at a flow rate of 43 LPM and relative humidity of 40 ± 2%. N95 and P100 respirator filters were tested initially to establish the validity of the chamber, followed by the Halyard H600 fabric. Particle penetration was measured using an aerosol spectrometer. The filtration efficiency was calculated for different particle sizes by measuring the particle number concentration upstream and downstream of the filter. The pressure drop across the filter materials was measured using a manometer.
Results
The efficiency of the P100 for particles ≥250 nm was 100%. The N95 efficiency was 97 ± 1% at 275 nm, 99 ± 0% at 324 nm, and 100% for larger particles. The Halyard H600 fabric had a variable efficiency with an average of 62 ± 28% at 275 nm, 89 ± 8% at 324 nm, and 100% efficiency for particles >450 nm. The pressure drop values for P100 and N95 were 32 and 8 mmH2O, respectively. The Halyard H600 fabric resistance increased dramatically from 30 mmH2O at the start of the exposure to 65 mmH2O after 16-minutes of exposure.
Conclusion
The high variability in filter efficiency for particles ≤324 nm and the increased fabric breathing resistance demonstrate that the Halyard H600 has an inferior performance and is not a good substitute for N95 and P100. Thus, the use of the Halyard H600 fabric for respiratory protection is not recommended.
Key Words: COVID-19, SARS-CoV-2, Respirator efficiency, Particle efficiency, N95 respirator
The global pandemic caused by the SARS-CoV-2 virus, the etiologic agent for COVID-19, has produced a surge in the number of patients in hospitals, the increasing use of personal protective equipment (PPE), and the overload of the health care system in the United States and worldwide.1 COVID-19 is an airborne infectious disease that may be spread when an infected person coughs, sneezes or talks, and can be spread within 6 feet of contact with an infected person.2 Healthcare workers are at greater risk of contracting the virus due to their unremitting contact with COVID-19 patients.3, 4, 5 Due to the circumstances, respirators are used by all healthcare workers in hospitals to avoid contracting the virus. This has led to high demand and low supply in respirators that include the N95 filtering facepiece respirator that has been previously used during a pandemic.6 , 7 Scarcity of N95 respirators, has led to health care workers reusing their respirators for several days to several weeks. As a result, health care professionals have been seeking alternative filter materials for respiratory protection, and this has sparked a similar interest in verifying the efficacy of different products, even those that were not originally manufactured for respiratory protection.
The National Institute for Occupational Safety and Health (NIOSH) is responsible for establishing standards for respiratory protective equipment and testing procedures for particulate filters.8 , 9 NIOSH has classified three respirator types, N, R, and P, that have been approved for respiratory protection against non-oil-based particulates, some oil-based particulates, and oil-based particulates, respectively. Each type is designated with three levels of particulate collection efficiencies that are at least 99.97%, 99%, and 95% efficient. The N95 is commonly used by professionals for respiratory protection due to low-cost and high protection efficiency, especially for non–oil-based exposure. For certification purpose, the N95 respirators are tested with polydisperse NaCl aerosol using NIOSH test conditions to ensure filtration efficiency is ≥95%.10 The N95 respirator has been evaluated in multiple laboratory studies and was found to collect particulates at 100% efficiency, except for particles between 10 and 100 nm where the efficiency drops to 95%.11, 12, 13 Airborne viruses are intracellular microorganisms that can be found in droplets or attached to other particles and measure under a high-powered microscope between 20 and 300 nm in size.14 Harnish et al15 tested N95 respirators from different manufacturers and concluded that some N95 respirators could capture the H1N1 influenza virus with an efficiency as high as 98%. Eninger et al16 tested the efficiency for N95 and N99 respirators for collecting MS2 bacteriophage viruses and concluded that both respirators had a similar performance, and reported that the collection efficiency was 95% at 50 nm. Therefore, N95 respirators with efficiencies ≥95% provide expected levels of protection against viruses when used in the context of a complete respiratory protection program including proper selection and fit testing.
Respirator efficiency for particulate matter is evaluated for different particle sizes using an airtight exposure chamber that allows the flow of particulates through the filter media at a certain flow rate.17 The respirator is challenged for particle efficiency with a specific aerosol type: sodium chloride (salt) for N-type respirators, and dioctylphthalate for R- and P-type respirators.10 The test particles are generated at an aerodynamic mass median diameter of 300 nm (0.3 µm), which is assumed to be the most penetrating particle size. The flow rate of the system of 85 ± 4 liters per minute (LPM) should be used for a single respirator, and the double respirators are tested at a flow of 42.5 ± 2 LPM through each respirator.8 The pressure drop across the respirator should have a pressure drop (breathing resistance) of ≤35 mm H2O at 85 LPM.18 For the N-type respirators, the salt particles must be neutralized and kept at a low relative humidity of 30 ± 10%. The efficiency is calculated by measuring the particle mass concentration upstream and downstream of the respirator. The N95 respirators showed ≥95% efficiency in several studies. 13 , 19, 20, 21, 22
Alternative non-NIOSH certified respirators and fabrics as filter materials have been tested for filtration efficiency and compared with N95 respirator performance.23 Noncertified surgical masks have been tested in several studies and have been proven to be far less superior for particle collection compared to N95 respirators.24, 25, 26, 27 Testing alternative fabric material for respiratory protection is not a new concept and has been explored during the past decade due to other pandemics.28 , 29 Rengasamy et al30 tested towels and scarves made from different fabrics and found that the efficiency varied from 9% to 98% for different particle sizes and concluded that these fabrics provide marginal protection against viruses. The use of large numbers of respirators during a pandemic such as COVID-19 creates a shortage of respirators. In response, healthcare workers seek alternative respiratory protection materials.
One material that created interest as an alternative filter material for respiratory protection is the Halyard H600, which is sterilization fabric material used in hospitals. The Halyard H600 polypropylene fabric consist of 2 layers (blue and white) of wrap that are used as a microbial barrier for protecting orthopedic and cardiovascular instruments. Recently, the Halyard H600 fabric was used to create masks at the University of Florida Health as was advertised as an innovative solution for respiratory protection against aerosols, droplets, and bacteria.31 The H600 manufacturer does not recommend the use of the material for making face masks, which is an off-label use of H600 as now stated on the company website.32 A recent study tested the Halyard H600 fabric that was sewn into a face mask by measuring the particle number concentration outside the mask and inside the breathing zone of the mask, and reported particle removal of H600 between 38% and 96%.33 However, the method used in the study is usually conducted for fit-testing respirators to assure that the wearer has an airtight facial seal, and not for assessing filtration efficiency. Finally, another study also tested the Halyard H600 for wearability, comfort, and breathability using a survey, and concluded that the fabric could be used as a suitable alternative in the absence of NIOSH-certified respirators.34 However, Lammers et al35 used the H600 to create a single- and double-layered mask and tested the masks using the TSI 8130A automated filter tester that complies with NIOSH standards for testing respirators. The tests showed that the efficiencies were 64.5% and 78.3% for a single- and double-layered mask, respectively, and concluded that the H600 should not be used as an N95 alternative. The TSI 8130A utilizes a photometer to measure the concentration of salt before and after the filter, and therefore, does not provide efficiency by particle size, but an overall penetration of the particles through the respirator.36 To date, no study has been published on the filtration efficiency by particle size of the Halyard H600 as a respirator filter and, thus, further investigation is warranted to confirm the claimed protection it provides.
The purpose of this study was to assess the filtration efficiency of the Halyard H600 fabric as a respirator filtering material as compared to the NIOSH-certified N95 and P100 filters. The main goal was to determine if Halyard H600 is a good immediate alternative for respiratory protection for healthcare professionals during the COVID-19 pandemic.
Methods
Filter materials
Three filter types were tested in this study: (1) Halyard H600 sterilization wrap (Halyard Worldwide, Inc., Alpharetta, GA); (2) N95 particulate respirator (3M model 8200, St. Paul, MN); and (3) P100 particulate filter (3M model 2091, St. Paul, MN). The filters were cut into 4-inch diameter discs (for H600 and P100) or domes (N95; Fig 1 ), and then were challenged with aerosolized salt particles inside a customized PVC exposure chamber. The N95 and P100 filters, being NIOSH-certified, were tested first to establish a baseline for the experimental setup and demonstrate the reliability of the exposure chamber. While the N95 is a standalone respirator, the P100 is used as a dual filter attached to a half or full facepiece respirator and, therefore, use half of the recommended 85-LPM flowrate8 (ie, 42.5 LPM). One sample for each filter type was used in every challenge experiment. Each filter type was tested in triplicate (n = 3). Similar to previous studies,12 , 19 the filters materials were not preconditioned before the experiment and were used out of the box.
Fig. 1.
Four-inch Diameter Test Filters: (A) Halyard H600, (B) N95, (C) P100.
Exposure chamber
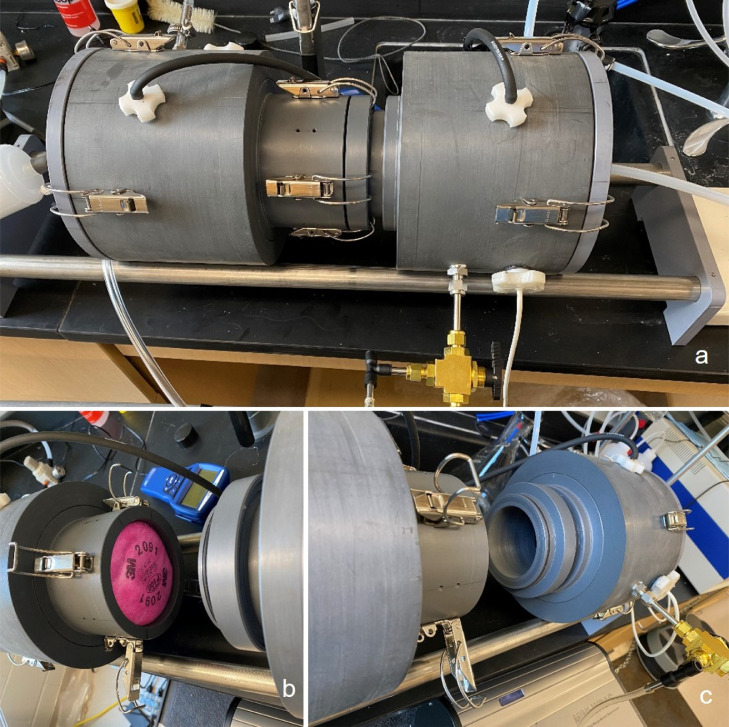
A customized 17-inch long PVC exposure chamber was used to challenge the test filters (Fig 2 ). The chamber has a 6-inch internal diameter on both ends, which was designed to fit 2 pitot tubes used to measure pressure drop upstream and downstream of the filter. The chamber opens from the middle to allow placement of the filter and has a 4-inch internal diameter on one side designed to hold the filter samples in place during testing. On the other side, the chamber has a 0.5” thick tube that presses down on the filter, creates a flow diameter of 3” through the filter, and prevents leakage. The aerosol upstream and downstream of the filter was sampled in the chamber using a 2-way valve connected to the aerosol sensor. The chamber was equipped with 4 clamps in the middle to secure the filter, in addition to 2 clamps (IRWIN QUICK-GRIP, Huntersville, NC) to create an airtight chamber.
Fig. 2.
Customized PVC Exposure Chamber for Challenging the Filter Samples: (A) closed chamber; (B) left side of the chamber holding the test filter; (C) right side of the chamber that secures the filter.
Filtration efficiency test
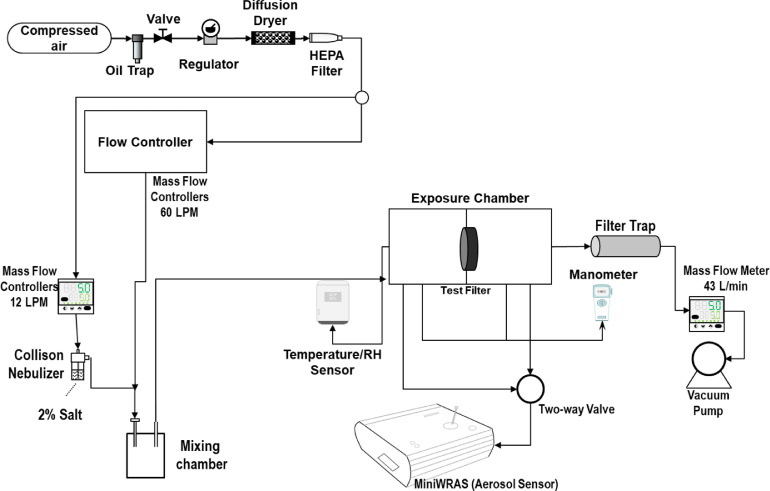
The experimental setup for the particle filtration efficiency test is shown in Figure 3 . The methodology developed for this work was adapted from the NIOSH certification method for testing particulate respirators.8 Particle-free air from a 3-stage desiccant dryer was used to supply a Miller-Nelson Model HCS-501-100 instrument (Assay Technology, Livermore, CA) that was used to control the flow and temperature of the dilution air. The desiccant dryer also supplied particle-free air to a mass flow controller (MFC, MCH-50SLPM, Alicat, AZ) that controls the flow to a 6-jet Collison nebulizer (CH Technologies, NJ) that contained a 2% salt solution. The air temperature on the Miller-Nelson was set at its maximum of 35°C to dry the generated salt particles from the Collison nebulizer. The mass flow rate for the Miller-Nelson was set at 60 LPM, and the aerosol salt generation was set at 12 LPM using the MFC. The heated dilution air and salt aerosol were delivered to a mixing chamber before entering the exposure chamber. A vacuum pump (101Q, Gast, MI) operating at 43 LPM and monitored with a mass flow meter (Sierra, Montgomery, CA) was used to pull the diluted salt aerosol from the chamber and through a HEPA filter particle trap. The temperature and relative humidity were monitored upstream of the filter using a HOBO Model U14-002 data logger (Onset Computer Corp., Pocasset, MA). The pressure drop (in mmH2O) across the filter was monitored using a DP-Calc Model 5825 micromanometer (TSI Inc., Shoreview, MN). A Portable Wide Range Aerosol Spectrometer (MiniWras 1371, GRIMM, Ainring, Germany) was used to measure the aerosol size distribution upstream and downstream of the filter. The MiniWras 1371 can capture the whole range of particle size distribution, where it measures 41 bin sizes between 10 nm and 35 µm in real-time. The MiniWras 1371 contains 2 technologies to sample air at different particle sizes. Particles between 10 nm and lower than 250 nm are sampled using a corona discharge, and particles larger than 250 are sampled using optical science.
Fig. 3.
Experimental setup for filtration efficiency test.
Particle efficiency calculation
The aerosol number size distribution, particle count per unit volume of air, was measured upstream and downstream of the filter using the MiniWRAS. The penetration and efficiency were then calculated for each particle size using the following equations:
| (1) |
| (2) |
where the and are the number concentration (#/cm3) measured for the downstream and upstream concentrations, respectively.
The duration of each test was 6 minutes, where the aerosol size distribution was measured for three minutes upstream of the filter, followed by 3 minutes downstream of the filter. The three measurements were averaged to obtain 1 measurement upstream and downstream of the filter. The experiments were conducted 3 times for each filter, and the average and standard deviation were calculated for each particle size.
Exposure system limitations
The efficiency test for N95 respirator and P100 filter was not done with dried aerosol and was also not charge neutralized. The use of non-neutralized aerosols will overestimate the filter efficiency. Therefore, efficiencies reported in this work cannot be compared to NIOSH filtration efficiencies. In addition, there were no means available to heat the diluted air above 35°C, due to the Miller-Nelson limitations. Considering that the MiniWras 1371 is designed to receive a low flow for sampling and that the current closed exposure system had a high flow and high pressure, which prevented the MiniWras 1371 from operating, the researchers were forced to lower the flow in the system to 43 LPM by using a T-connector while sampling air with the MiniWRAS 1371 on 1 side and open to the atmosphere on the other side. In addition, the corona discharge used by the MiniWras 1371 was sensitive to the aerosol generated during the experiment, and only particles larger than 250 nm were measured for this study.
Pressure drop test
The pressure drop across the test filter was measured in a closed system at 43 LPM before and after six minutes at the end of the experiment using a DP-Calc Model 5825 micromanometer (TSI Inc., Shoreview, MN). In addition, the pressure drop for the H600 was measured after 16 minutes, which equates to 10 minutes of additional salt exposure time after the end of the initial 6-minute experiment. The pressure drop experiment was used as an indication of the filter and fabric loading during the experiments.
Results
Particle filtration efficiency
The average relative humidity maintained during the experiments was 40 ± 2%. The mass concentration of the salt aerosol during the experiment was 2.0 ± 0.1 mg/m3, and the mass median diameter was 330 nm as measured by the MiniWRAS 1371.
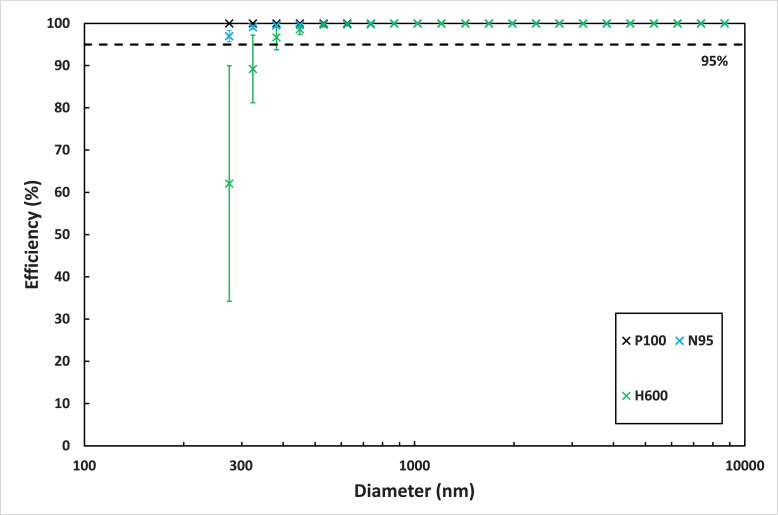
The efficiency test results for particles larger than 250 nm are shown in Figure 4 . The y-axis error bars represent the standard deviation for the three trials. The P100 efficiency at all particle sizes were 100%. The N95 efficiency was 98 ± 1% at 275 nm, 99 ± 0% at 324 nm and 100% for larger particle sizes. The Halyard H600 efficiency was 62 ± 28% at 275 nm, 89 ± 8% at 324 nm, and 100% for particles larger than 450 nm.
Fig. 4.
Filtration efficiency at different particles sizes for the P100, N95, and H600.
Pressure drop
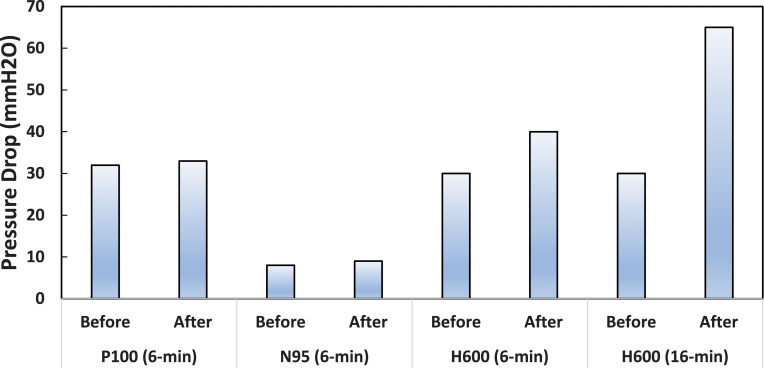
The pressure drop before and after the experiments are shown in Figure 5 . The N95 had the lowest pressure drop at 8 mmH2O, and the P100 had a pressure drop of 32 mmH2O. Both the P100 and N95 pressure drop increased by 1 mmH2O after the 6-minute experiment at 33 and 9 mmH2O, respectively. The Halyard H600 pressure drop increased dramatically from 30 mmH2O for new fabric, to 40 mmH2O and 65 mH2O after 6-minutes and 16-minutes, respectively.
Fig 5.
Pressure drop measurements before and after the 6-min and/or 16-min experiment for the P100, N95, and H600.
Discussion
The P100 results were within the standard of 99.97% efficiency based on NIOSH recommendations. The P100 results were similar to a previous study that tested the P100 with neutralized and dried salt aerosol at 85 LPM.20 The N95 efficiency was similar to previous studies that tested the N95 with salt aerosol. Eninger et al37 challenged the N95 with salt aerosol at 85 LPM and measured the efficiency at 1.6% for particles between 100 and 500 nm. In addition, the same study measured the pressure drop at 30 LPM and 85 LPM and found the resistance at 2.7 mmH2O and 7.75 mmH2O, respectively. However, in contrast to the present study, Eninger et al37 did not cut the N95 to a 4-inch diameter size but used the full size of the N95 respirator. By reducing the filter size, we reduced the surface area and consequently increased the pressure drop. Another study tested three full uncut N95 respirators manufactured by 3M at a flow rate of 85 LPM and measured the resistance between 12 and 22 mmH2O.22
The Halyard H600 efficiency was less than 95% and has an inferior performance compared to N95, particularly for the removal of particulates at 275 nm size (62% vs 98% efficiency). With the drastically decreasing trend in efficiency for H600 as the particulate size decreased, starting at around 320 nm, it is expected that its efficiency will be much lower for ultrafine particles (<100 nm). In particular for particles less than 60 nm, where studies have shown that the lowest efficiency is at the most penetrating particle size (MPPS) of ∼50 nm, and then efficiency increases for particles smaller than the MPPS.13 SARS-CoV-2 is approximately 60-140 nm in diameter38 and most of the fine and ultrafine droplets containing the virus may significantly penetrate the H600 material. The claim for the H600’s superior efficiency as a respirator filter may have been a misinterpretation of the material's bacterial filtration efficiency (BFE) stated to be from 98.9% to 99.9%, as indicated in the company website.32 Rengasamy et al39 showed that the filtration efficiency test method used for NIOSH certification of N95 respirators (ie, NIOSH NaCl method) is more conservative than the BFE method required by the Food and Drug Administration. Thus, the H600’s BFE of 99.9% cannot be compared to the filtration efficiency (≥95%) of N95′s measured using charge neutralized NaCl aerosol because the efficiency test method used for the N95 is much more stringent than the method used for the H600. The NIOSH particulate filter efficiency test, as described in the introduction, is the gold standard for testing respirators and should not be replaced with the BFE test. Comparing the methods in terms of particle size, the NIOSH NaCl aerosol test method uses neutralized ∼0.3 µm (300 nm) size particles, while the BFE method uses unneutralized ∼3.0 µm (3000 nm) size particles containing Staphylococcus aureus bacteria.39 Moreover, the high variability (as demonstrated by the standard deviation) for the H600 efficiency for particles smaller than 300 nm shows the inconsistency in the quality of the product. The H600 was not manufactured as a particulate filter for respiratory protection, so the inconsistency for such a purpose was not surprising. This study confirms the tests accomplished by using the TSI 8130A automated filter tester, even though this study utilized the blue and white fabrics as a dual-layer compared to the previous study that only utilized the blue fabric as a single and double layer.35
The breathing resistance across the H600 increased dramatically from 30 mmH2O before testing to 65 mmH2O after 16-minutes, which renders the H600 as inefficient for respiratory protection. In this study, the maximum particulate concentration generated for the experiments was below the standard concentration of 200 mg/m3 used in the NIOSH respirator testing,40 and yet the pressure drop across the H600 was negatively affected over a short period of particulate exposure. These results are consistent with the observations of local health professionals who exhibited difficulty breathing after short periods of wearing hand-made masks created from the H600 fabric. It is important that respirators have the lowest breathing resistance possible since increasing inhalation and exhalation resistances across respirators was shown to decrease worker performance.41
This study was initiated for the immediate testing of the Halyard H600 as an alternative for the N95 respirator to protect healthcare professionals in a local hospital, considering the dilemma on shortage of N95 supplies. The researchers undertook this project with limited funding and utilized the limited laboratory equipment to compare the Halyard H600 particle collection efficiency against the NIOSH-certified N95 and P100 respirators. The authors recognize the limitations of this study and, thus, future work should address such limitations by improving the exposure setup, which includes adding a neutralizer after generating the salt particles, adding an air diluter to the aerosol sensor to measure size distribution for particles smaller than 250 nm and increasing the temperature of the diluted air above 35°C that would provide a consistent aerosol relative humidity below 40%. However, despite such study limitations, the study findings were obtained using previously published and reliable methods and are still important to be published to increase awareness on the capabilities of the Halyard H600 as a respirator filter.
Based on the results of the current study, we conclude that the Halyard H600 is not recommended as a particulate filter for use in respiratory protection because its efficiency was less than 95% and performance was inferior compared to the N95. In addition, the H600 exhibited increased breathing resistance over time during filter loading. The use of Halyard H600 for creating respirators or face masks as a substitute for N95 will not guarantee protection of the wearers, particularly healthcare providers working in areas affected by COVID-19.
Footnotes
Conflicts of interest: None to report.
References
- 1.Emanuel E.J., Persad G., Upshur R., et al. fair allocation of scarce medical resources in the time of Covid-19. New Eng J Med. 2020;382:2049–2055. doi: 10.1056/NEJMsb2005114. [DOI] [PubMed] [Google Scholar]
- 2.CDC . 2020. Center for Disease Control and Prevention, Prevent Getting Sick.https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/index.html Accessed from the web in May 2020. [Google Scholar]
- 3.Ran L., Chen X., Wang Y., Wu W., Zhang L., Tan X. Risk factors of healthcare workers with corona virus disease 2019: a retrospective cohort study in a designated hospital of Wuhan in China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong J., Goh Q.Y., Tan Z., et al. Preparing for a COVID-19 pandemic: a review of operating room outbreak response measures in a large tertiary hospital in Singapore. Can J Anesth. 2020;67:732–745. doi: 10.1007/s12630-020-01620-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ng K., Poon B.H., Kiat Puar T.H., et al. COVID-19 and the risk to health care workers: a case report. Ann Int Med. 2020;172:766–767. doi: 10.7326/L20-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beckman S., Materna B., Goldmacher S., et al. Evaluation of respiratory protection programs and practices in California hospitals during the 2009-2010 H1N1 influenza pandemic. Am J Infect Control. 2013;41:1024–1031. doi: 10.1016/j.ajic.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lancet T. COVID-19: protecting health-care workers. Lancet. 2020;395:922. doi: 10.1016/S0140-6736(20)30644-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.NIOSH National Institute for Occupational Safety and Health, 42 CFR Part 84 Respiratory Protective Devices; Final Rules and Notice. Federal Register. 1995;60 [Google Scholar]
- 9.DHHS., Department of health and human services. 'Code of Federal Regulations (42 CFR 84.179) [cited 2020 April 20]; Non-powered air-purifying particulate respirators; filter identification. Available from: https://www.govinfo.gov/content/pkg/CFR-2012-title42-vol1/pdf/CFR-2012-title42-vol1-sec84-179.pdf. 2012.
- 10.NIOSH., National institute for occupational safety and health 'Code of Federal Regulation, 42 CFR Part 84 respiratory protective devices [Accessed from the web in May 2020: https://www.cdc.gov/niosh/npptl/topics/respirators/pt84abs2.html]'. 1997.
- 11.Balazy A., Toivola M., Reponen T., Podgórski A., Zimmer A., Grinshpun S.A. Manikin-based performance evaluation of N95 filtering-facepiece respirators challenged with nanoparticles. Ann Occup Hyg. 2005;50:259–269. doi: 10.1093/annhyg/mei058. [DOI] [PubMed] [Google Scholar]
- 12.Eshbaugh J.P., Gardner P.D., Richardson A.W., Hofacre K.C. N95 and P100 respirator filter efficiency under high constant and cyclic flow. J Occup Environ Hyg. 2008;6:52–61. doi: 10.1080/15459620802558196. [DOI] [PubMed] [Google Scholar]
- 13.Rengasamy S., BerryAnn R., Szalajda J. Nanoparticle filtration performance of filtering facepiece respirators and canister/cartridge filters. J Occup Environ Hygiene. 2013;10:519–525. doi: 10.1080/15459624.2013.818229. [DOI] [PubMed] [Google Scholar]
- 14.Hinds W.C. 2nd ed. Wiley-Interscience; New York: 1999. Aerosol Technology: Properties, Behavior, and Measurement of Airborne Particles. [Google Scholar]
- 15.Harnish D.A., Heimbuch B.K., Husband M., et al. Challenge of N95 filtering facepiece respirators with viable H1N1 influenza aerosols. Infect Control Hosp Epidemiol. 2013;34:494–499. doi: 10.1086/670225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eninger R.M., Honda T., Adhikari A., Heinonen-Tanski H., Reponen T., Grinshpun S.A. Filter performance of N99 and N95 facepiece respirators against viruses and ultrafine particles. Ann Occup Hyg. 2008;52:385–396. doi: 10.1093/annhyg/men019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fardi B., Liu B.Y. Performance of disposable respirators. Part Part Syst Charact. 1991;8:308–314. [Google Scholar]
- 18.CFR . 2013. Code of Federal Regulations (42 CFR 84.180) [cited 2020 April 20]; Airflow resistance tests.http://www.gpo.gov/fdsys/pkg/CFR-2007-title42-vol1/pdf/CFR-2007-title42-vol1-sec84-180.pdf Available from: [Google Scholar]
- 19.Martin S.B., Moyer E.S. Electrostatic respirator filter media: filter efficiency and most penetrating particle size effects. Appl Occup Environ Hyg. 2000;15:609–617. doi: 10.1080/10473220050075617. [DOI] [PubMed] [Google Scholar]
- 20.Rengasamy S., Miller A., Vo E., Eimer B.C. Filter performance degradation of electrostatic N95 and P100 filtering facepiece respirators by dioctyl phthalate aerosol loading. J Eng Fibers Fabrics. 2013;8:62–69. [Google Scholar]
- 21.Bałazy A., Toivola M., Adhikari A., Sivasubramani S.K., Reponen T., Grinshpun S.A. Do N95 respirators provide 95% protection level against airborne viruses, and how adequate are surgical masks? Am J Infect Control. 2006;34:51–57. doi: 10.1016/j.ajic.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 22.Viscusi D.J., Bergman M., Sinkule E., Shaffer R.E. Evaluation of the filtration performance of 21 N95 filtering face piece respirators after prolonged storage. Am J Infect Control. 2009;37:381–386. doi: 10.1016/j.ajic.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konda A., Prakash A., Moss G.A., Schmoldt M., Grant G.D., Guha S. Aerosol filtration efficiency of common fabrics used in respiratory cloth masks. ACS Nano. 2020;14:6339–6347. doi: 10.1021/acsnano.0c03252. [DOI] [PubMed] [Google Scholar]
- 24.Qian Y., Willeke K., Grinshpun S.A., Donnelly J., Coffey C.C. Performance of N95 respirators: filtration efficiency for airborne microbial and inert particles. Am Ind Hyg Assoc J. 1998;59:128–132. doi: 10.1080/15428119891010389. [DOI] [PubMed] [Google Scholar]
- 25.Grinshpun S.A., Haruta H., Eninger R.M., Reponen T., McKay R.T., Lee S.-A. Performance of an N95 filtering facepiece particulate respirator and a surgical mask during human breathing: two pathways for particle penetration. J Occup Environ Hyg. 2009;6:593–603. doi: 10.1080/15459620903120086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rengasamy S., Eimer B.C. N95-companion measurement of Cout/Cin ratios for two N95 filtering facepiece respirators and one surgical mask. J Occup Environ Hyg. 2013;10:527–532. doi: 10.1080/15459624.2013.818224. [DOI] [PubMed] [Google Scholar]
- 27.MacIntyre C.R., Seale H., Dung T.C., et al. A cluster randomised trial of cloth masks compared with medical masks in healthcare workers. BMJ Open. 2015;5 doi: 10.1136/bmjopen-2014-006577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davies A., Thompson K.-A., Giri K., Kafatos G., Walker J., Bennett A. Testing the efficacy of homemade masks: would they protect in an influenza pandemic? Disaster Med Public Health Prep. 2013;7:413–418. doi: 10.1017/dmp.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Sande M., Teunis P., Sabel R. Professional and home-made face masks reduce exposure to respiratory infections among the general population. PloS one. 2008;3 doi: 10.1371/journal.pone.0002618. e2618-e2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rengasamy S., Eimer B., Shaffer R.E. Simple respiratory protection—evaluation of the filtration performance of cloth masks and common fabric materials against 20–1000 nm size particles. Ann Occup Hyg. 2010;54:789–798. doi: 10.1093/annhyg/meq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.UFHEALTH . 2020. UF Health Anesthesiology Team Devises Respirator Mask Made From Existing Hospital Materials.https://ufhealth.org/news/2020/uf-health-anesthesiology-team-devises-respirator-mask-made-existing-hospital-materials#:∼:text=UF%20Health%20workers%20are%20crafting,water%2C%20bacteria%20and%20other%20particles Accessed from the web in August 2020: [Google Scholar]
- 32.2020. HalyardHealth, HALYARD* ONE-STEP* Sterilization Wrap.https://products.halyardhealth.com/surgical-solutions/sterilization-solutions/sterilization-wraps/halyard-kimguard-one-step-sterilization-wrap.html Accessed from the web in May 2020: [Google Scholar]
- 33.Mueller, A. V.; Fernandez, L. A., Assessment of fabric masks as alternatives to standard surgical masks in terms of particle filtration efficiency. medRxiv 2020, 2020.04.17.20069567. [DOI] [PMC free article] [PubMed]
- 34.Woolverton C.J., Ferdig R.E., Snyder A., Reed J., Dodson T., Thomas S. Repurposing surgical wrap textiles for use as protective masks during pandemic response. Appl Biosafety. 2020;0 doi: 10.1177/1535676020925958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lammers D.T., Jones I.F., Marenco C.W., et al. Safety code blue! Assessing the use of blue surgical sterilization wrap for homemade respirator masks during the COVID-19 crisis. Am JInfect Control. 2020;000:1–2. doi: 10.1016/j.ajic.2020.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.TSI . 2020. AUTOMATED FILTER TESTER 8130A.https://tsi.com/products/filter-testers/automated-filter-tester-8130a/ Accessed from the web in August 2020. [Google Scholar]
- 37.Eninger R.M., Honda T., Reponen T., McKay R., Grinshpun S.A. What does respirator certification tell us about filtration of ultrafine particles? J Occup Environ Hyg. 2008;5:286–295. doi: 10.1080/15459620801960153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China, 2019. New Eng J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rengasamy S., Shaffer R., Williams B., Smit S. A comparison of facemask and respirator filtration test methods. J Occup Environ Hyg. 2017;14:92–103. doi: 10.1080/15459624.2016.1225157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.FederalRegister, Approval Tests and Standards for AirPurifying Particulate Respirators [Accessed from the wen on August 2020: https://www.govinfo.gov/content/pkg/FR-2020-04-14/pdf/FR-2020-04-14.pdf]. 2020, 85 (72), 20598.
- 41.Caretti D.M., Coyne K., Johnson A., Scott W., Koh F. Performance when breathing through different respirator inhalation and exhalation resistances during hard work. J Occup Environ Hyg. 2006;3:214–224. doi: 10.1080/15459620600601677. [DOI] [PubMed] [Google Scholar]