Abstract
Purpose of review
This review seeks to evaluate the impact of environmental exposures on the menstrual cycle length detailing timing of exposure on pathophysiology.
Recent findings
Recent literature has examined the relationship between menstrual cycle length and environmental exposures including air pollutants, parabens, and polybrominated biphenyls.
Summary
Research is limited but suggest importance of further research in evaluating environmental exposures and menstrual cycle length.
Keywords: air pollution, endocrine disruptors, environment, menstrual cycle
INTRODUCTION
Menstrual cycle length (MCL) is on average 28 days, with the thought that the luteal phase is preserved to be approximately 14 days long after ovulation [1]. Variability in menstrual cycle length is considered to be because of the time required to recruit a dominant follicle (follicular phase). In young healthy women with robust ovarian reserve, including egg donors, the follicular phase may be up to 19 days in length because of robust ovarian reserve [2]. MCL may be affected by environmental exposures and endocrine disrupting chemicals (EDCs), such as, air pollution, bisphenol A (BPA), phthalates, polychlorinated biphenyls, dichlorodiphenyltrichloroethane (DDT), diethylstilbestrol (DES), and perfluorooctanoic acid (PFOA) [3].
The Endocrine Society Statement on EDCs describes the impact of various chemicals on ovarian follicular development, cells and hormone production. They report different impacts by chemicals based on the phase of development of a follicle. For example, animal studies suggest that exposure to BPA accelerates depletion of primordial follicles [4]. Other animal studies similarly suggest negative impact on granulosa cell and the developing follicle within the early follicular phase [5,6]. In addition to impacts on the developing follicle, EDCs impact steroidogenesis and can interfere with the production of estradiol and progesterone. Human studies of patients undergoing IVF consistently demonstrate a negative impact of BPA on peak estradiol levels during ovarian stimulation [7–9]. Animal studies confirm that this correlation can be directly linked to higher concentrations of BPA exposure and its inhibition of Cyp11a1 and Star expression [10].
In this review, we aim to discuss the current literature available regarding the impact of environmental toxicants on the human menstrual cycle. We also propose a conceptual model to understand the potential pathophysiology of environmental toxicants on MCL variability.
Box 1.
no caption available
METHODS
To present a narrative review, we performed a literature search in PubMed between June 2018 and June 2020, to examine existing literature surrounding the impact of environmental exposures on normal menstrual cycles estrogen and progesterone balance. The date range for inclusion was extended from 2016 to 2020 because of limited results from 2018 to 2020. For this discussion, we selected specific outcomes to include in the search, based on clinical experience and knowledge of the existing literature with a specific focus on estrogen and progesterone levels in menstruating women.
The final search input for environmental exposure-eligible studies was as follows: using PubMed search engine with restrictions for human studies within the last 5 years and the following MESH terms: (’menstrual cycle’ [MeSH Terms]) AND (’endocrine disruptors’ [MeSH Terms]); (((’estradiol’ [MeSH Terms])); (’progesterone’ [MeSH Terms])) AND (’toxins, biological/poisoning’ [MeSH Terms]); (’air pollutants’ [MeSH Terms]) AND (menstrual cycle [MeSH Terms]); ((((menstrual cycle, follicular phase [MeSH Terms]) OR (menstrual cycle, luteal phase [MeSH Terms])) OR (menstrual cycle, proliferative phase [MeSH Terms])) OR (menstrual cycle, secretory phase [MeSH Terms])) AND (chemicals, endocrine disrupting [MeSH Terms]); ((((menstrual cycle, follicular phase [MeSH Terms]) OR (menstrual cycle, luteal phase [MeSH Terms])) OR (menstrual cycle, proliferative phase [MeSH Terms])) OR (menstrual cycle, secretory phase [MeSH Terms])) AND (chemicals, endocrine disrupting [MeSH Terms]); (((((((((estrogens [MeSH Terms]) OR (folliculogenesis [MeSH Terms])) OR (reproduction [MeSH Terms])) OR (ovarian function test [MeSH Terms])) OR (ovarian follicle [MeSH Terms])) OR (’ovarian reserve’ [MeSH Terms])) OR (’menstrual cycle’ [MeSH Terms])) OR (’ovulation’ [MeSH Terms]))) AND (environmental toxic substances).
This yielded 11 articles with an additional three from outside the search query. Articles were excluded if they were not available in English or not primary investigations. Articles were also excluded if they did not study human species, were in vitro or did not include impact on menstrual cycle and/or estrogen or progesterone hormone levels. This yielded a final article count of six articles. Search strategies are mapped according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) template (Liverati et al., 2009) and included as Fig. 1.
FIGURE 1.
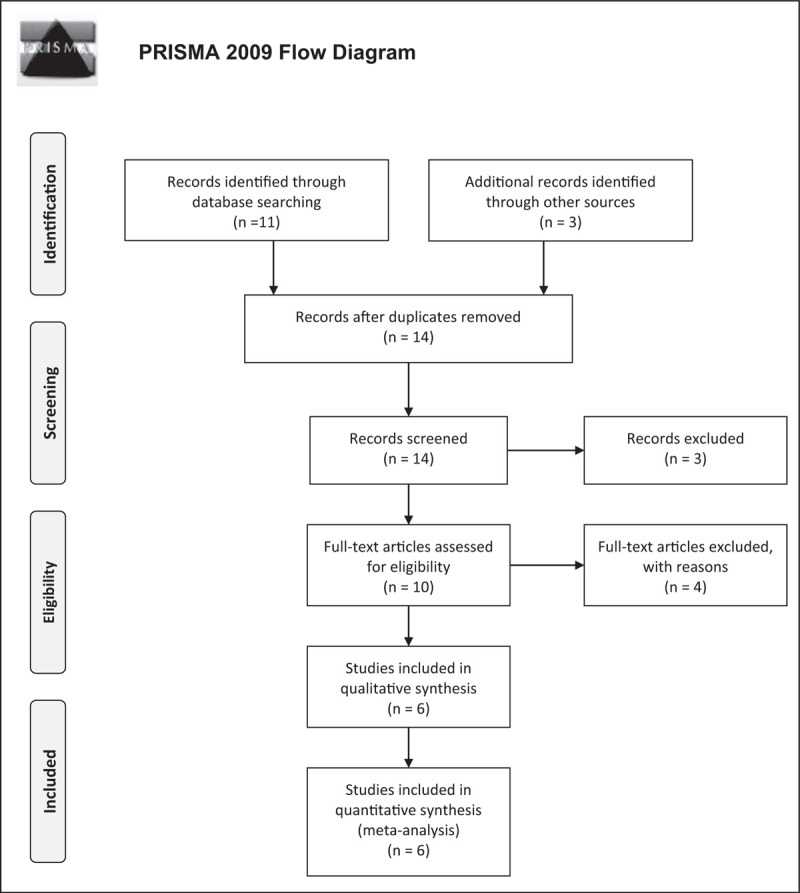
PRISMA flow diagram of included studies in the final analysis.
RESULTS BY YEAR
The following will summarize each of the resulting six studies from our investigation. Given the limited number of studies for review, we will describe each study separately in ascending chronologic order.
2016
A review published in 2016 by Ziv-Gal et al. provides evidence summarizing studies from 2007 until 2016 suggesting the BPA may be associated with infertility in women. This review itself was excluded from our final synthesis but it included a single study investigating the impact of environmental toxins on the human normal menstrual cycle that we included as additional research identified through another source (Fig. 3). Jukic et al. measured urinary BPA in pooled urine throughout women's menstrual cycle and documented follicular phase length. This study did not show a correlation with follicular phase length but it did show an association with shorter luteal phase for women with higher BPA urinary concentrations; monocarboxyoctyl phthalates [second tertile vs. first tertile: −0.5 days; 95% confidence interval (CI) −0.9 to −0.1, third vs. first: −0.4 days (95% CI −0.8 to 0.01), P = 0.04]; bisphenol A results: [second vs. first: −0.8 days (95% CI −1.2 to −0.4), third vs. first: −0.4 days (95% CI −0.8 to 0.02), P = 0.001] [11].
FIGURE 3.
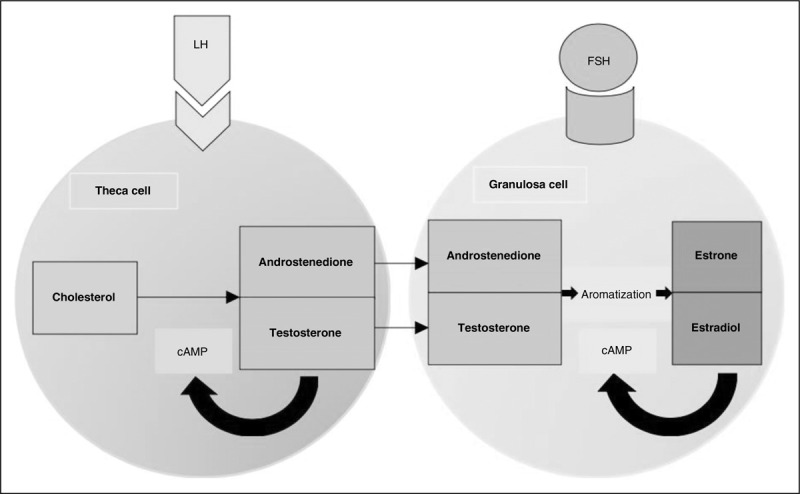
Illustration of theca cell and granulosa cell representing the two-cell theory.
Nishihama et al. investigated the impact of parabens with estrogen and cycle length. Parabens are commonly found as preservatives in products, such as cosmetics, soft drinks, frozen dairy products, and flavoring syrups in a variety of alkyl ester forms. The basis of their study is from animal and in-vitro studies that had demonstrated an estrogenic activity of parabens. Their study included 128 women and measured menstrual cycle length by days of vaginal bleeding reported and urinary estrogen equivalent total parabens. Of the parabens measured, polypropylene had the highest average concentrations; 21.7% methyl paraben, 12.3% ethyl paraben, 46.1% propyl paraben and 19.9% butyl paraben. They found that women with higher urinary paraben concentrations were more likely to have shorter menstrual cycle lengths; total paraben concentrations (odds ratio = 0.73, 95% CI 0.56–0.96) and butyl paraben concentrations (odds ratio = 0.83, 95% CI 0.70–0.99) [12].
2017
Merklinger-Gruchala et al. investigated whether air pollution can affect the length of the menstrual cycle overall and for each of its phases (follicular and luteal). This is a cross-sectional study using municipal ecological monitoring data to assess air pollution during monitored menstrual cycles. They studied 133 women and found that air pollution negatively affected the length of the luteal phase. More specifically, luteal phase shortening resulted from elevated fossil fuel combustion; luteal phase length decreased by 0.32 days when adjusted for age, menarche age, alcohol intake, caffeine intake, smoking status, usually menstrual cycle length and PC other than estimated (beta coefficient = −0.32; 95% CI (−0.60 to −0.04), P = 0.02). However, the follicular phase and overall menstrual cycle length was not impacted in the same manner. Carbon dioxide and nitric oxide emissions were also evaluated and did not have an impact on the menstrual cycle length [13].
2018
Mahalingaiah et al. correlated menstrual irregularities with air pollution. Air pollution was measured as total suspended particulate (TSP) around where adolescents were living and correlated with menstrual survey results. A cross-sectional study analysis of 34 832 women was performed. The study concluded that exposure to TSP in air during adolescence was associated with an increased odds of menstrual irregularity; (95% CI) of 1.08 (1.03–1.14) for moderate irregularity, 1.08 (1.02–1.15) for persistent irregularity and 1.10 (0.98–1.25) for persistent with androgen excess irregularity phenotype [14▪]. This study highlights the importance of temporal exposure to endocrine disrupting chemicals, such as air pollution, and its impact on the normal menstrual cycle.
2019
Howards et al. researched the impact of exposure to polybrominated biphenyls (PBBs) on the menstrual cycle. This study analyzed 70 menstruating women exposed to PBBs through consumption of contaminated farm products as children in Michigan. They monitored PBB exposure and correlated this with urinary hormone levels of FSH, estrone-3-glucuronide (E13G) and pregnanediol-3-glucuronide (Pd3G). They found a correlation between higher PBB levels and lower estrogen levels [PBB < 1.0 ppb mean (96% CI) E13G 55 (46–63) vs. 38 (31–45) for PBB > 3.0 ppb]. Higher exposure to PBB was also correlated with lower Pd3G (PBB < 1.0 ppb mean Pd3G 13 (11–16) vs. 11 (9.3–13) for PBB > 3.0 ppb). The overall cycle lengths did increase with increasing exposure to PBBs but this was not statistically significant (mean cycle length in days at PBB < = 1.0 vs 1.0–3.0 pbb vs. >3.0 ppb (95% CI) 27 (25–29) vs. 28 (27–30) vs. 29 (27–30) [15▪].
2020
Researchers continued to investigate the potential impacts of air pollution on menstrual cycles. Giorgis-Allemand et al. studied 184 noncontracepting women and their urinary hormonal markers. Urinary samples were tested for progesterone metabolite to estimate the duration of the follicular and luteal phases. Atmospheric pollution estimated from a dispersion model and exposures were estimated within various time frames from onset of menses. They found that with higher levels of nitrogen dioxide (NO2) and particulate matter with an aerodynamic diameter smaller than 2.5 μm (PM2.5) exposures for the 30-day exposure window before cycle was associated with longer follicular phases of the menstrual cycle; ‘increased by 0.7 day (95% confidence interval, CI 0.2--1.3) for each increase by 10 μg/m3 in NO2 concentration averaged over the 30 days before the cycle and by 1.6 day (95% CI 0.3--2.9) for each increase by 10 μg/m3 in PM10’. They did not find an association with air pollutants and duration of the luteal phase [16▪].
DISCUSSION
There is limited recent investigation into the direct impact of environmental toxins on the human menstrual cycle. The few above reviewed studies highlight that this is an important area of investigation given the reports of altered MCL with certain environmental exposures. In general, the above studies suggest an association with paraben exposure and shortened luteal phase [11–13,17]. Additionally, air TSP and NO2 exposures may play a role in increasing the overall menstrual cycle length or at least leading to increased menstrual irregularity, though more research is warranted given other studies showing no association [14▪,16▪].
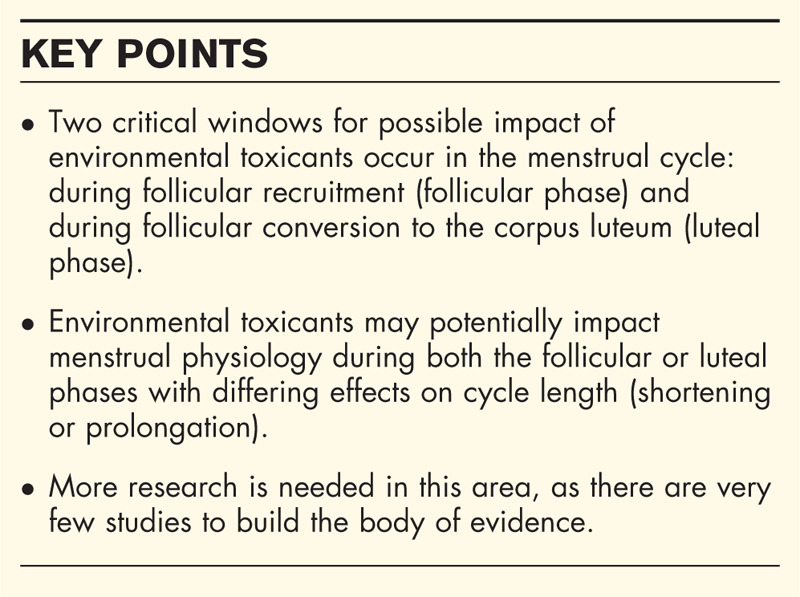
We propose two critical time windows when acute exposure to environmental toxicants may have an impact on normal menstrual cycle function are during follicular recruitment and the luteal phase (Fig. 2). The windows of opportunity for these impacts on the normal menstrual cycle are during follicular recruitment, which can occur in the late luteal phase or early follicular phase, and during the luteal phase impacting the functioning of the corpus luteum.
FIGURE 2.
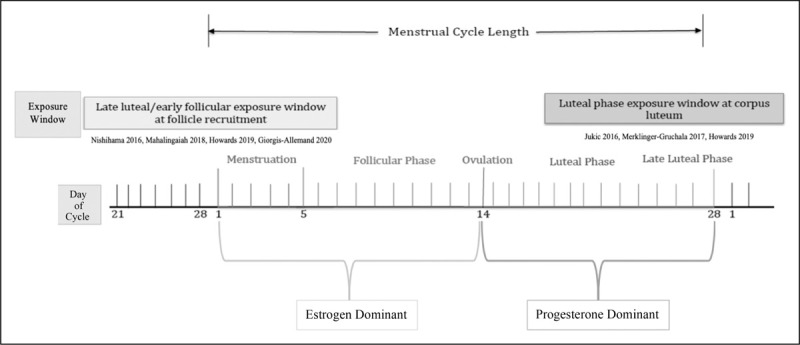
Environmental exposure window and the menstrual cycle.
Four of the studies included in this review describe potential outcomes of environmental toxicants in the follicular phase exposure window (Fig. 2). Follicular recruitment occurs during early follicular phase, and in the very late luteal phase for some women, particularly those that have diminished ovarian reserve or older in age. The variability in a normal menstrual cycle length is dependent on the time to recruit a dominant follicle for ovulation. This typically takes about 14 days but can have a wide range of variability depending on the dominant follicle [18]. A dominant follicle is established in the mid follicular phase and is able to trigger the next steps towards ovulation once it is able to achieve an estradiol concentration of at least 200 pg/ml and sustain this for about 50 h [19]. Increasing levels of estradiol lead to morphological changes in the follicle creating an enlarged follicle (greater than 15 mm in diameter) with shift of granulosa cells from squamous to cuboidal. The estradiol produced by the growing follicle is created by aromatization of androgens from the theca cells. FSH and estradiol work in a synergistic manner stimulating proliferation of the follicle's granulosa cells [18,20]. A successful dominant follicle is one that creates a high-estrogen micro-environment.
The selected dominant follicle then undergoes ovulation and the transition to the progesterone dominant phase of the menstrual cycle begins. During ovulation, the follicle's theca cells begin to express genes for the luteinizing hormone (LH) receptor and the enzyme, P450 side-chain cleavage (P450scc) and 3B-hydroxysteroid dehydrogenase. [21]. Ovarian steroidogenesis at this time point depends on LH [22]. It is important to recognize that the human follicle has two important cell types: granulosa cells and theca cells. These two cell types work together in the ‘two-cell theory’ to develop and ovulate a mature oocyte (Fig. 3). The theca cells are responsible for intake of cholesterol and creation of androgens. The granulosa cell takes in the androstenedione synthesized in the theca cell to produce estradiol and create the aforementioned estrogen-dependent micro-environment. Granulosa cells rely on theca cells to convert cholesterol to androgens as they lack the rate-limiting enzyme for this conversion. The high estrogen micro-environment created by the dominant follicle reaches an estrogen level of about 2 × 10−10 mol/l for approximately 36 h triggering the activation of the ovulation and the luteal phase [3]. Furthermore, estrogens exist in three main form endogenously in the human body: 17b-estradiol, estrone, estriol. It is estimated that hormones are produced endogenously within the human body at a magnitude of 10−11--10−9 molar compared with 10−5--10−3 of other structures/nonhormonal in the system [3,23].
In the follicular phase window, endocrine disruption leading to alterations in MCL may occur at the level of the follicle during rapid growth of the granulosa and theca cells and at the level of receptor-mediated estrogen signaling at any level of the hypothalamic--pituitary--ovarian axis. During the time of follicular recruitment, the rapidly dividing granulosa and theca cells would be most vulnerable to exposure. Mechanisms of potential toxicant effect may include reduced cholesterol uptake in theca cells or alterations in aromatase activity or granulosa cell 0function to produce estradiol. These may impact the required high estradiol micro-environment for development of a dominant follicle and ultimately ovulation.
The second exposure window to consider is the luteal phase. The luteal phase exposure window is characterized by the rapid restructuring of the follicle to the corpus luteum, which consists of theca-lutein cells. These cells are critical for production of progesterone and support of the endometrial lining and pregnancy until the placenta is able to take over progesterone production at 8--10 weeks’ gestation or until menstruation. Three of the included studies in this review addressed this important exposure period (Fig. 2). This window of time is the progesterone-dominant phase that depends on the corpus luteum. After ovulation, the oocyte undergoes meiosis, granulosa cells luteinize to produce progesterone [24]. Levels of progesterone increase from 1 to 2 ng/ml during ovulation to 10–35 ng/ml during the luteal phase [19]. Luteinized granulosa cells express an increased number of P450c17, StAR and 3-B-hydroxysteroid dehydrogenase in order to markedly increase production of progesterone [25,26]. The corpus luteum hormonal environment acts to stabilize the endometrial lining for implantation of a pregnancy. The prime embryo implantation window is 6--10 days after ovulation [3]. If pregnancy does not occur, then the corpus luteum degenerates and a steady decline of progesterone and estrogen levels leads to endometrial shedding, menses, and the cycle begins again.
The corpus luteum has a programmed lifespan unless rescued by bHCG from an implanted pregnancy. The mechanism by which degeneration of the corpus luteum occurs is not known. However, the luteal phase of the menstrual cycle remains relatively well preserved in the normal menstrual cycle lasting typically close to 14 days. EDCs and environmental toxicants may increase the rate of degradation of the corpus luteum leading to a shorter luteal phase. This may decrease the cycle-specific chance of conception as the functioning corpus luteum induces a supportive endometrial environment for implantation [19].
Limitations
There were limited existing published research on the impact of environmental factors on and MCL. Additional limitations include the difficulty in comparison across studies given variable terminology for environmental toxicants and EDCs. In attempts to focus this review we eliminated notable work reporting on central nervous system control of menstrual cycles involving the hypothalamic--pituitary--ovarian axis and adrenal gland.
CONCLUSION
We conceptualized the menstrual cycle with two critical windows within which EDCs and environmental toxicants may have detrimental impacts: follicular recruitment for a dominant follicle in an estrogen-dominant micro-environment and a corpus luteum's production of progesterone during the luteal phase. Overall, few studies have investigated the overarching impacts of environmental toxicants on the human menstrual cycles. To date, studies have focused on animal models or disorders related to the human menstrual cycle (i.e. polycystic ovarian syndrome, infertility). The limited data suggest that there may be cycle length effects (shortening/prolongation) but additional research is required for conclusive evidence.
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Hugh S, Taylor LP, Emre Seli. Speroff's clinical gynecologic endocrinology and infertility. 9th edPhiladelphia, PA: Wolters Kluwer; 2020. [Google Scholar]
- 2.Vassena R, Vidal R, Coll O, Vernaeve V. Menstrual cycle length in reproductive age women is an indicator of oocyte quality and a candidate marker of ovarian reserve. Eur J Obstet Gynecol Reprod Biol 2014; 177:130–134. [DOI] [PubMed] [Google Scholar]
- 3.Borgert CJ, Matthews JC, Baker SP. Human-relevant potency threshold (HRPT) for ERalpha agonism. Arch Toxicol 2018; 92:1685–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jung NK, Park JY, Park JH, et al. Attenuation of cell cycle progression by 2,3,7,8-tetrachlorodibenzo-p-dioxin eliciting ovulatory blockade in gonadotropin-primed immature rats. Endocr J 2010; 57:863–871. [DOI] [PubMed] [Google Scholar]
- 5.Gore AC, Chappell VA, Fenton SE, et al. EDC-2: The Endocrine Society's second scientific statement on endocrine-disrupting chemicals. Endocr Rev 2015; 36:E1–E150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peretz J, Craig ZR, Flaws JA. Bisphenol A inhibits follicle growth and induces atresia in cultured mouse antral follicles independently of the genomic estrogenic pathway. Biol Reprod 2012; 87:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petro EM, Leroy JL, Covaci A, et al. Endocrine-disrupting chemicals in human follicular fluid impair in vitro oocyte developmental competence. Hum Reprod 2012; 27:1025–1033. [DOI] [PubMed] [Google Scholar]
- 8.Mok-Lin E, Ehrlich S, Williams PL, et al. Urinary bisphenol A concentrations and ovarian response among women undergoing IVF. Int J Androl 2010; 33:385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bloom MS, Kim D, Vom Saal FS, et al. Bisphenol A exposure reduces the estradiol response to gonadotropin stimulation during in vitro fertilization. Fertil Steril 2011; 96:672–677. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peretz J, Flaws JA. Bisphenol A down-regulates rate-limiting Cyp11a1 to acutely inhibit steroidogenesis in cultured mouse antral follicles. Toxicol Appl Pharmacol 2013; 271:249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jukic AM, Calafat AM, McConnaughey DR, et al. Urinary concentrations of phthalate metabolites and bisphenol A and associations with follicular-phase length, luteal-phase length, fecundability, and early pregnancy loss. Environ Health Perspect 2016; 124:321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishihama Y, Yoshinaga J, Iida A, et al. Association between paraben exposure and menstrual cycle in female university students in Japan. Reprod Toxicol 2016; 63:107–113. [DOI] [PubMed] [Google Scholar]
- 13.Merklinger-Gruchala A, Jasienska G, Kapiszewska M. Effect of air pollution on menstrual cycle length-a prognostic factor of women's reproductive health. Int J Environ Res Public Health 2017; 14:816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14▪.Mahalingaiah S, Missmer SE, Cheng JJ, et al. Perimenarchal air pollution exposure and menstrual disorders. Hum Reprod 2018; 33:512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study highlights the association between exposure to total suspended particulate matter in the air and increased odds of menstrual irregulaties.
- 15▪.Howards PP, Terrell ML, Jacobson MH, et al. Polybrominated biphenyl exposure and menstrual cycle function. Epidemiology 2019; 30:687–694. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study analyzes the effect of exposure to polybrominated biphenyl exposure (PBB) on the menstrual cycle and found that high or medium exposure to PBB lowered estrogen levels.
- 16▪.Giorgis-Allemand L, Thalabard JC, Rosetta L, et al. Can atmospheric pollutants influence menstrual cycle function? Environ Pollut 2020; 257:113605. [DOI] [PubMed] [Google Scholar]; This article describes the increase in the length of the follicular phase of the menstrual cycle that occurs in participants that are exposed to higher levels of NO2 and particulate matter of aerodynamic diameter smaller than 2.5 μm.
- 17.Ziv-Gal A, Flaws JA. Evidence for bisphenol A-induced female infertility: a review (2007–2016). Fertil Steril 2016; 106:827–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Treloar AE, Boynton RE, Behn BG, Brown BW. Variation of the human menstrual cycle through reproductive life. Int J Fertil 1967; 12:77–126. [PubMed] [Google Scholar]
- 19.Fritz MA, Speroff L. Clinical gynecologic endocrinology and infertility. 8th ed.Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2011. [Google Scholar]
- 20.Sherman BM, Korenman SG. Hormonal characteristics of the human menstrual cycle throughout reproductive life. J Clin Invest 1975; 55:699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaffin CL, Dissen GA, Stouffer RL. Hormonal regulation of steroidogenic enzyme expression in granulosa cells during the peri-ovulatory interval in monkeys. Mol Hum Reprod 2000; 6:11–18. [DOI] [PubMed] [Google Scholar]
- 22.Richards JS. Hormonal control of gene expression in the ovary. Endocr Rev 1994; 15:725–751. [DOI] [PubMed] [Google Scholar]
- 23.Borgert CJ, Baker SP, Matthews JC. Potency matters: thresholds govern endocrine activity. Regul Toxicol Pharmacol 2013; 67:83–88. [DOI] [PubMed] [Google Scholar]
- 24.Stocco C, Telleria C, Gibori G. The molecular control of corpus luteum formation, function, and regression. Endocr Rev 2007; 28:117–149. [DOI] [PubMed] [Google Scholar]
- 25.Ravindranath N, Little-Ihrig L, Benyo DF, Zeleznik AJ. Role of luteinizing hormone in the expression of cholesterol side-chain cleavage cytochrome P450 and 3 beta-hydroxysteroid dehydrogenase, delta 5–4 isomerase messenger ribonucleic acids in the primate corpus luteum. Endocrinology 1992; 131:2065–2070. [DOI] [PubMed] [Google Scholar]
- 26.Devoto L, Kohen P, Gonzalez RR, et al. Expression of steroidogenic acute regulatory protein in the human corpus luteum throughout the luteal phase. J Clin Endocrinol Metab 2001; 86:5633–5639. [DOI] [PubMed] [Google Scholar]


