Supplemental digital content is available in the text.
Key words/Abbreviations: cardiac interoception, neurological diseases, hypertension, dimensional approaches, machine learning, linear discriminant analysis, ACC = anterior cingulate cortex, AD = Alzheimer’s disease, AAL = Automated Anatomical Labeling Atlas, bvFTD = behavioral variant frontotemporal dementia, ECG = electrocardiography, EEG = electroencephalography, HBD = heartbeat detection, HEP = heart-evoked potential, HR = heart rate, FC = functional connectivity, MS = multiple sclerosis, ST = stroke
ABSTRACT
Objective
Neurological nosology, based on categorical systems, has largely ignored dimensional aspects of neurocognitive impairments. Transdiagnostic dimensional approaches of interoception (the sensing of visceral signals) may improve the descriptions of cross-pathological symptoms at behavioral, electrophysiological, and anatomical levels. Alterations of cardiac interoception (encompassing multidimensional variables such as accuracy, learning, sensibility, and awareness) and its neural correlates (electrophysiological markers, imaging-based anatomical and functional connectivity) have been proposed as critical across disparate neurological disorders. However, no study has examined the specific impact of neural (relative to autonomic) disturbances of cardiac interoception or their differential manifestations across neurological conditions.
Methods
Here, we used a computational approach to classify and evaluate which markers of cardiac interoception (behavioral, metacognitive, electrophysiological, volumetric, or functional) offer the best discrimination between neurological conditions and cardiac (hypertensive) disease (model 1), and among neurological conditions (Alzheimer’s disease, frontotemporal dementia, multiple sclerosis, and brain stroke; model 2). In total, the study comprised 52 neurological patients (mean [standard deviation] age = 55.1 [17.3] years; 37 women), 25 cardiac patients (age = 66.2 [9.1] years; 13 women), and 72 healthy controls (age = 52.65 [17.1] years; 50 women).
Results
Cardiac interoceptive outcomes successfully classified between neurological and cardiac conditions (model 1: >80% accuracy) but not among neurological conditions (model 2: 53% accuracy). Behavioral cardiac interoceptive alterations, although present in all conditions, were powerful in differentiating between neurological and cardiac diseases. However, among neurological conditions, cardiac interoceptive deficits presented more undifferentiated and unspecific disturbances across dimensions.
Conclusions
Our result suggests a diffuse pattern of interoceptive alterations across neurological conditions, highlighting their potential role as dimensional, transdiagnostic markers.
INTRODUCTION
As attested by psychiatry, transdiagnostic dimensional approaches represent useful complements to traditional categorical systems (1–3), as they improve descriptions of cross-nosological symptoms across several levels of analysis. Now, under the guidance of neuroscience (4), this research domain criteria framework (1,2) has begun impacting on neurology (5). A promising target in this trend is the study of interoception, i.e., the sensing of visceral signals (6). In particular, cardiac interoception encompasses multidimensional variables, such as accuracy, learning, and awareness (7), with well-established neural signatures (8) that are affected in several neurological disorders, including Alzheimer’s disease (AD), multiple sclerosis (MS), behavioral variant frontotemporal dementia (bvFTD), and stroke (ST) (9,10). However, with some exceptions (10), cardiac interoceptive dimensions have not been studied transnosologically in neurological disorders. To bridge this gap, we implemented a machine learning framework to evaluate whether different markers of cardiac interoception (cutting across behavioral, electrophysiological, and neuroimaging levels) successfully discriminate among neurological disorders (AD, bvFTD, MS, ST). Moreover, we included a contrastive cardiac condition (hypertensive disease (11), with expected cardiac interception deficits but with basic neuroanatomical preservation) (12,13) to disentangle the potential role of neurological versus autonomic disruptions in cardiac interoceptive impairments.
Cardiac interoception is commonly assessed through heartbeat detection (HBD) tasks, in which participants are requested to identify their heartbeats in a given period of time through motor tracking or counting. HBD tasks are widely used because heartbeats are discrete and frequent events that can be easily, noninvasively, and objectively measured (7) and/or manipulated (14). More particularly, motor-tracking HBD tasks allow for assessing participants’ precision in following individual heartbeats, contrary to counting, where only a global estimation can be analyzed (15).
HBD tasks allow for tapping multiple cardiac interoceptive dimensions across varied levels of analyses in healthy and neurological samples (10,12,16,17). Behaviorally, this task yields measures of interoceptive accuracy (the ability to correctly monitor each heartbeat), sensibility (subjective impressions of one’s own interoceptive performance), learning (improvement of behavioral accuracy after auditory feedback), and awareness (metacognitive assessments of one’s own interoceptive skills) (10,18). Neurophysiologically, cardiac interoceptive processes are indexed by the heart-evoked potential (HEP) (19–24), a cortical marker modulated by conscious attention to heartbeats (10,13,20,25). Moreover, cardiac interoceptive processes (8,26,27) and dysfunctions (9,13) are linked to the volume and functional connectivity (FC) (28) of the bilateral insula, the anterior cingulate cortex (ACC), the middle cingulate cortex, and the somatosensory cortex. Taken together, these markers allow for comprehensive assessments of cardiac interoceptive mechanisms (29).
Such assessments may be usefully informed by comparisons among neurological disorders (10). For instance, although both bvFTD and AD patients show alterations in cardiac interoceptive accuracy and awareness alongside abnormal neural signatures (reduced HEP modulations, reduced brain volume, and aberrant FC across interoceptive regions), only AD patients show reduced cardiac interoceptive learning (10). In ST, cardiac interoceptive accuracy deficits can emerge despite spared awareness, accompanied by reduced HEP modulations and insular damage but no FC alterations (10). In MS, a more diffuse model with abnormal body signal processing, cardiac interoceptive deficits (atypical HEP, atrophy, and FC) have been observed without behavioral impairments (9) and in association with fatigue (30). In combination, these findings suggest that markers of cardiac interoceptive attention (accuracy and HEP modulations) might be transnosologically affected across neurological conditions, whereas other signatures (cardiac interoceptive learning and awareness, structural and functional neuroimaging markers) may prove more idiosyncratically affected in them.
Moreover, the specific role of brain abnormalities in interoceptive deficits can be better understood by comparing neurological and cardiac diseases. In the latter, including HTD (31–33), vascular-system alterations disrupt cardiac interoceptive dynamics in the absence of brain damage (13). Thus, the juxtaposition of neurological and cardiac samples can inform which interoceptive disturbances are more crucially related to central (neural) or peripheral (autonomic) disruptions. Considering previous findings (9,10,13), behavioral and neurophysiological (HEP) abnormalities should prove more consistent between neurological and cardiac diseases than neuroanatomical or FC disruptions of relevant brain systems.
Here we assessed different cardiac interoceptive dimensions and levels of analysis in four neurological conditions (AD, bvFTD, MS, ST) relative to a cardiac condition (11) and controls. First, participants performed a validated HBD task (34–36) with ongoing electroencephalography (EEG) recordings to measure the HEP. Also, offline structural and functional magnetic resonance imaging (MRI and fMRI) recordings were included to perform voxel-based morphometry and FC analyses. Afterward, to compare both models of cardiac interoceptive disturbance (central versus peripheral), we applied a machine learning pipeline to identify which dimensions (accuracy, sensibility, awareness) and levels (behavior, HEP, neuroimaging) afforded the most important features discriminating between neurological and cardiac disorders (model 1). Then, we repeated this analysis but only within the neurological patients, to test whether these features distinguished among relevant conditions (model 2). Considering previous findings, we expected a strong differentiation between neurological and cardiac conditions (model 1) and a more diffuse discrimination among neurological conditions (model 2). In model 1, behavioral measures would have better predictive power to discriminate groups, as the cardiac group may have a more specific alteration of the core cardiac interoceptive domain (accuracy) with no major compromise in the underlying neural mechanisms. Also, following assumptions from dimensional approaches (37), model 2 should present a less differentiated pattern across neurological conditions, with unspecific and less convergent predictive features.
The purpose of our study was to test overall classification accuracy rather than test hypotheses regarding the interoceptive profiles of individual groups. Indeed, considering that interception hinges on widespread brain structures and that various neurological conditions present multidimensional, nonspecific interoceptive deficits (37), we would not expect large differences between them.
MATERIALS AND METHODS
Participants
The study comprised 149 participants, including two pathological groups (neurological and cardiac) with a total of 77 patients and 72 healthy controls. The neurological group (n = 52; 37 female; age: mean [standard deviation], or M [SD] = 55.13 [17.43]; years of education: M [SD] = 14.71 [4.56]) was composed of 11 AD patients, diagnosed following the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association criteria (38,39); 9 bvFTD patients, diagnosed following the current revised criteria (40); 25 early-stage relapsing-remitting MS patients, all fulfilling McDonald’s criteria (41); and 7 nonhemorrhagic ST patients with frontoinsular lesions, evaluated at least 6 months post-ST. The cardiac group comprised 25 HTD patients (13 female; age: M [SD] = 66.16 [9.06] years; years of education: M [SD] = 15.40 [3.91]), diagnosed following the current revised criteria (42). For further details regarding the diagnosis, sample selection criteria, and demographics, see Supplemental Digital Content, http://links.lww.com/PSYMED/A687 (Participants’ Demography section) and Supplementary Table 1, http://links.lww.com/PSYMED/A687. Each pathological group was paired with a healthy control group matched for sex, age, and education. None of healthy participants reported a history of drug abuse, neuropsychiatric disease, cognitive impairment, or hypertension. Matching was performed for each dimension of analysis (accuracy, interoceptive sensibility and awareness, HEP, brain volume, brain connectivity) for each pathological group (neurological and cardiac), with some controls being shared by both. The exact number of data available for each dimension and each group, as well as demographics per variable can be found in Supplementary Data Table 2, http://links.lww.com/PSYMED/A687.
Data collection took place at the Institute of Cognitive and Translational Neuroscience of the National Scientific and Technical Research Council INECO. Data were collected between January 2014 and July 2017. The study was approved by the ethics committee of the Institute of Cognitive Neurology as part of an ongoing protocol (9,10,13,17,18,43). All participants provided informed consent in accordance with the Declaration of Helsinki, and the study was carried out observing the privacy rights of human participants and in line with the Recommendations for the Conduct, Reporting, Editing and Publication of Scholarly Work in Medical Journals (44).
Data Acquisition and Preprocessing
HBD Task Performance: Accuracy and Learning
Cardiac interoception was examined through a validated HBD task (10,27,34,45–47) (Figure 1A) encompassing three conditions. The interoceptive accuracy condition comprised two 2.5-minute blocks during which participants tapped a key to follow their own heartbeats without any external cues (7). This condition provides an objective measure of the individuals’ ability to track their own heartbeats (8). In a feedback control condition, participants repeated the same task while receiving auditory feedback via a stethoscope that was held by themselves with the left hand, while they tapped on the keyboard with the right one, as in previous studies (19). This condition is a good proxy to measure the participant’s reaction time to noninteroceptive signals (as it is based on an auditory cue) and thus account for (or rule out) potential slowing, gross motor impairments, or attentional deficits (10,19,48). In the learning condition, participants were instructed to follow their heartbeats again without external cues. The first and third conditions were repeated twice. The feedback block was not included in classification analyses because it is an externally controlled condition (i.e., external feedback), and previous research has shown no relevant modulations (10). Electrocardiographic (ECG) recordings were obtained during the whole task. Participants were requested to respond with their dominant hand, to keep their eyes on a fixation cross, and to avoid excessive blinking and moving while the latter remained on screen.
FIGURE 1.
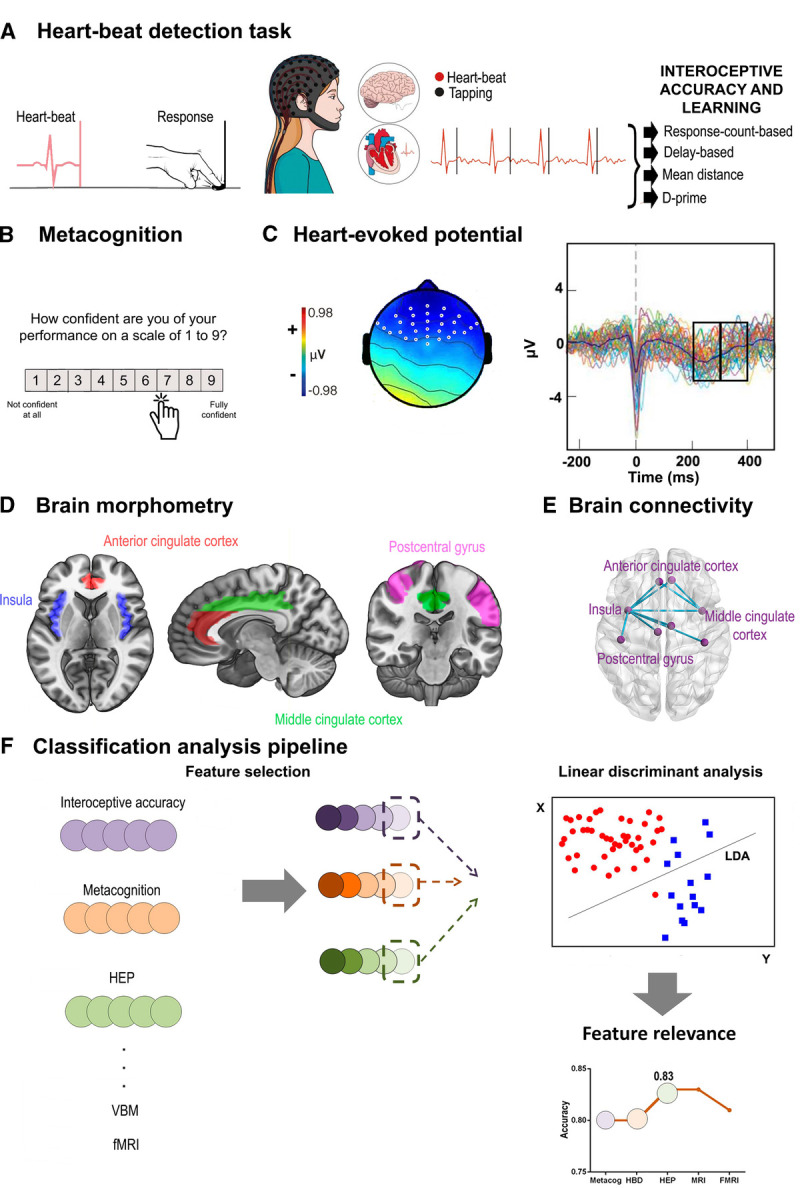
Research framework. A, In the HBD task, subjects were asked to follow their heartbeats by pressing a keyboard key. Four interoceptive accuracy indexes were calculated. B, After each block, the participants rated their confidence about their performance; this score was used to derive the confidence and metacognition indexes. C, HEP is a negativity presented between 200 and 400 milliseconds after the R-peak wave of the heartbeat. Two windows were selected: one between 200 and 300 milliseconds, and the other one between 300 and 400 milliseconds. D, Interoceptive regions of interest were selected from the brain volume analysis for the classification analysis: bilateral middle cingulate cortex (green), anterior cingulate cortex (red), postcentral gyrus (fuchsia), and insula (blue). E, Based on resting-state recordings, we calculated functional connectivity between the same areas considered for structural analysis. F, Classification linear discriminant analyses were performed with one feature selected from each level and dimension previously presented; a feature relevance analysis was then performed to determine the weight each feature had in the classification. HBD = heartbeat detection; HEP = Heart-evoked potential; fMRI = functional magnetic resonance imaging; VBM = Voxel-based morphometry; LDA = Linear discriminant analysis.
Cardiac interoceptive accuracy was assessed through different approaches to avoid method-selection bias (for further details, see Supplementary Data, data acquisition and preprocessing section, http://links.lww.com/PSYMED/A687). The first one, response-count accuracy, is derived from Schandry’s classic index (49). It was calculated as the difference between actual heartbeats (as registered by the ECG) and total motor responses (instead of counted heartbeats, as in Schandy’s formula). The second one, delay-based accuracy, computes the total of correct responses instead of total responses, therefore considering response delay. This index considers two components: correct responses, which were estimated by comparing each participant’s motor response relative to the time window of the corresponding heartbeat and the total of recorded heartbeats during each condition (10,13,16,27,47,48,50,51). To control for individual differences in heart rate (HR), each tapped response was time-locked to the ECG R-peak based on three different time windows: 750 milliseconds after the beat for an HR less than 69.76 beats/min, 600 milliseconds after the beat for an HR between 69.75 and 94.25 beats/min, and 400 milliseconds after the beat for an HR higher than 94.25 beats/min. The third cardiac interoceptive metric, mean distance accuracy, estimates the synchronization between motor responses and corresponding heartbeats (17,18). This index evaluates the synchronization between heartbeats and associated motor responses. Within each condition, each block is subdivided in overlapping windows starting at each individual heartbeat and extending for 10 seconds. Then, for each window, the absolute difference between cardiac frequency (measured as 1/mean R-R) and response frequency (1/mean interresponse intervals) is computed. The last approach, termed d-prime index, offers greater reliability by factoring in false alarms. This score is based on signal detection theory, a framework that allows for distinguishing ambiguous stimuli as signal or noise (52). In the context of the HBD, a heartbeat is considered a signal, whereas its absence represents noise. Accordingly, participants’ responses are tagged as a “yes” (when pressing the keyboard, a hit) or as a “no” (when omitting, a miss). An affirmative response can only be considered correct if it occurs in a given window time-locked to the R-wave of the preceding heartbeat (the signal). Therefore, the absence of a response outside the window is a correct rejection, whereas a response outside the window is a false alarm.
HBD: Metacognition (Interoceptive Sensibility and Awareness)
After each block of each condition, participants reported their confidence in their own performance by indicating how well they thought they performed the task on a scale from 1 to 9 (Figure 1B). Two values were then calculated. A direct measurement of confidence, called interoceptive sensibility (6,7), was obtained by calculating the average answer in each block per condition. Afterward, a corrected-by-accuracy score (interoceptive awareness) was calculated by dividing the interoceptive sensibility outcome by the mean performance accuracy for the same condition. To match all previous accuracy values, a metacognition score was calculated for each of them (10). All values were previously transformed into a percentage scale. In this way, confidence was normalized by performance and considered relative to zero (scores nearer zero mean better metacognition).
Electrophysiological Signatures (HEP)
During the HBD task, high-density EEG signals were recorded with a Biosemi Active-two 128-channel system at 1024 Hz. Also, two external electrodes were included to record ECG data. The signals were later resampled offline at 256 Hz. Data were band-pass filtered during recordings (0.1–100 Hz) and offline (0.3–50 Hz) to remove undesired frequency components. The reference was set by default to link mastoids and re-referenced offline to the average electrodes. Segments that showed eye movement contamination were removed from further analysis by using independent component analysis and a visual inspection procedure. All epochs were baseline-corrected (baseline: −300 to −200 milliseconds) (53). Epochs that showed noise signal were rejected from the analysis using a visual inspection procedure as done in previous studies (10). There were no significant differences between groups or conditions in the total number of epochs (Supplementary Table 3, http://links.lww.com/PSYMED/A687).
EEG data were segmented between −300 and 500 milliseconds to analyze the HEP. Epochs were time-locked to each peak of the R-wave during the cardiac interoceptive block. Considering previous publications, we chose two windows (200–300 and 300–400 milliseconds) to analyze the HEP signal only after the 200-millisecond mark, as previous reports suggested a possible contamination of the signal by cardiac field artifacts (54) before this time (55). These windows have been typically reported to show HEP modulations (10,13,27,47). Considering the frontal topography of the HEP (10,20,21,56) and following previous reports (13,27,51), HEP analysis was delimited to a frontal region of interest (ROI). As done previously (10,21,57), we selected three ROIs for analysis: a right frontal ROI (Biosemi C3, C4, C5, C6, C7, C9, C10, C13, C14, C15), a left frontal ROI (Biosemi C26, C27, C28, C31, C32, D3, D4, D5, D6, D7), and a centrofrontal ROI (Biosemi C11, C12, C18, C19, C20, C21, C22, C23, C24, C25; Figure 1C). We obtained the mean signal for each window in each condition for each participant.
Neuroimaging
Image acquisition and preprocessing are reported as indicated by the Organization for Human Brain Mapping (58,59). Participants were scanned in a 1.5-T Phillips Intera scanner with a standard head coil (eight channels). First, whole-brain T1-weighted anatomical three-dimensional spin echo volumes were acquired parallel to the plane connecting the anterior and posterior commissures. The following parameters were used: repetition time, 57,489 milliseconds; echo time, 53,420 milliseconds; flip angle, 8 degrees; 196 slices; matrix dimension, 256 × 240; and voxel size, 1 × 1 × 1 mm3. The sequence duration was of 7 minutes. Then, sequentially and ascendingly functional spin echo volumes, parallel to the anterior-posterior commissures, covering the whole brain, were acquired with the following parameters: repetition time, 2777 milliseconds; echo time, 50 milliseconds; flip angle, 908 degrees; 33 slices; matrix dimension, 64 × 64; voxel size in plane, 3.6 mm × 3.6 mm; slice thickness, 4 mm; and number of volumes, 209. During acquisition, participants were asked not to think about anything, to keep their eyes closed, and to avoid moving or falling asleep. Participants were discarded if they had artifacts or acquisition mistakes established through visual inspection of the data and/or excessive head motion (movements greater than 3 mm and/or rotations higher than 3°; Supplementary Table 4, http://links.lww.com/PSYMED/A687.
Structural Data
Structural brain data were preprocessed, as described in previous reports (10,45,60,61), using the Statistical Parametric Mapping software (SPM12; http://www.fil.ion.ucl.ac.uk/spm/software/spm12/). T1-weighted images were segmented in native space using the default parameters of the SPM12 (bias regularization was set to 0.001 and bias full-width at half-maximum FWHM was set to 60-mm cutoff) in white matter (WM), gray matter (GM), and cerebrospinal fluid (CSF). The sum of these three tissues was later used to estimate the total intracranial volume. Then, using the GM and WM segmented images, we ran the “DARTEL (create template)” module—with the default parameters indicated by the SPM12—to create a template that is generated from the complete data set (increasing the accuracy of interparticipant alignment) (62). To affine register, the last template from the previous step into the MNI Space, we ran the “Normalise to MNI Space” module from DARTEL Tools. This transformation was then applied to all the individual GM segmented scans to also be brought into standard space. Subsequently, all images were modulated by Jacobian determinants to correct volume changes introduced by the normalization and to avoid bias in the intensity of an area due to its expansion during warping. To favor normal data distributions for subsequent parametric analysis, an isotropic Gaussian kernel of 12-mm full-width at half-maximum was applied to all images. The size of the kernel was selected based on previous recommendations (60).
Then, to analyze key interoceptive areas, we selected six ROIs involving the bilateral regions of the insula, ACC, middle cingulate cortex, and the somatosensory cortex (8,20,26,35,63,64). Also, a mask was created with all the areas together. Coordinates for the ROIs—namely, areas 9–34 and 57–58 (Figure 1D)—were obtained from the Automated Anatomical Labeling Atlas (AAL) (65). From these ROIs, GM volume size was obtained for each area and participant.
Functional Data
To study functional resting-state data, images were first preprocessed using the Data Processing Assistant for Resting-State (DPARSF V2.3) (66), an open-access toolbox that generates automatic analysis pipelines for imaging data. This toolbox also works with the Statistical Parametric Mapping (SPM12) and the Resting-State fMRI Data Analysis Toolkit (based on default functions of the REST V.1.7 toolbox). The first five volumes of each participant were discarded before preprocessing to ensure that magnetization achieved a steady state. Afterward, using as reference the middle slice of each volume, images were slice time corrected and aligned to the first scan of the session to correct for head movement. To reduce the effect of motion and physiological artifacts (such as cardiac and respiration effects), six motion parameters, CSF, and WM signals were removed as nuisance variables. After co-registration of each participant’s structural image with the functional image, tissue segmentation of each participant’s T1 scan in native space with SPM12 was performed, obtaining CSF and WM masks for this procedure. Later, a normalization of functional images was done to the MNI space using the echo-planar imaging template from SPM (67), and then they were smoothed using an 8-mm full-width-at-half-maximum isotropic Gaussian kernel (SPM functions). Using the REST V.1.7 toolbox, data were band-pass filtered between 0.01 and 0.08 Hz given the relevance of slow frequency in the analysis of resting-state networks (68,69).
To analyze differences in pairwise connection patterns, we estimated interregional connectivity based on the connectivity strength between each pair of region associations (based on the 116-node FC network). The same key interoceptive areas extracted as features from the Voxel-based morphometry analysis were used to estimate each pair of regions of the connectivity matrix for each participant (Figure 1E). To obtain this information, we used the AAL (65) to extract mean time courses by averaging the Blood oxygenation level dependent signal of all voxels from each of the ROIs (70). Pearson correlation coefficient was used to construct a node FC network for each participant. A matrix of functional correlation was obtained.
Classification Data Analysis
To evaluate the relevance of the cardiac interoceptive dimensions in the discrimination of neurological and cardiac pathologies, we implemented a classification pipeline using the metrics of each behavioral dimension (accuracy and metacognition) and level of analysis (HEP, brain morphometry, and FC) described previously. This procedure was also performed for a model in which we compared the capacity of cardiac interoceptive features to discriminate between different subtypes of neurological conditions.
The first preprocessing step of the classification analysis consisted of eliminating outlier values within each participant, considering a threshold of ±2.5 SDs for each group and variable. This helps to avoid bias in sample variance introduced by extreme (higher or lower) values. Overall, less than 5% of data were eliminated. The exact number of data available for each variable and each group can be found in Supplementary Data Table 2, http://links.lww.com/PSYMED/A687. Concerning the age gap between the two target groups (Supplementary Data Table 1, http://links.lww.com/PSYMED/A687), all scores of all levels of analysis were weighed by the age of their respective control sample (after outliers were eliminated), as done in previous research (71). After this procedure, we obtained weighted scores for each patient for all measures, which also allowed us to compare both pathological groups in a classification analysis, as they both have different control groups (for further details, see Supplementary Data, data acquisition and preprocessing, http://links.lww.com/PSYMED/A687). To choose the best features for each level of analysis, an attribute selection analysis was performed within each one (Figure 1F). This analysis was performed with the Waikato Environment for Knowledge Analysis suite of ML software (72,73), as in previous research (74), using the “SVMAtributteSelection” function (an attribute evaluator); for further details, see Supplementary Data, http://links.lww.com/PSYMED/A687 (classification data analysis).
Two models were run for classification analysis. The first one compared the two target pathologies (neurological versus cardiac), whereas the second one looked within the neurological group to discriminate among neurological diseases (AD, MS, ST, and bvFTD). For the first analysis, our objective was to determine which feature (or combination of features) best established group membership between the neurological and hypertensive groups. For the second analysis, our aim was to analyze whether these same or different features could differentiate between specific neurological diagnoses (Supplementary Data, classification data analysis, http://links.lww.com/PSYMED/A687). For the second model, four comparisons were performed: one for each neurological pathology (AD, MS, ST, and bvFTD) in contrast with the other neurological diseases, allowing for a binary classification model. Because of the differences in group sizes, we repeated the analysis for 10 randomized subsamples of the other neurological disease, for the ST, AD, and bvFTD, and obtained mean results of all subsamples for each comparison.
Using the PredPsych package of R (75), we used the cross-validated Linear Discriminant Analysis function, to perform cross-validated Linear Discriminant Analysis (Figure 1F). A fivefold cross-validation analysis was performed for training and testing data set, with a 70% fraction of data to keep for training data. To determine whether classification accuracy was biased by the sample size difference between the pathological groups, we repeated the classification analysis 10 times with different randomized subsamples of the neurological group that were of the same size as the cardiac group (Supplementary Data, results classification, http://links.lww.com/PSYMED/A687). To clarify which dimension contributed most to both models’ classification accuracy, we performed a feature relevance analysis (Figure 1F). To do this, we repeated the classification analysis that was performed by omitting one dimension for each of the five features in the classification, as done in previous reports (76). Finally, classification was repeated again by taking out one by one the features following the merit order (Supplementary Data, Results Classification, http://links.lww.com/PSYMED/A687).
RESULTS
Feature Selection
To determine the cardiac interoceptive features that best discriminate between neurological and cardiac samples, we performed a support vector machine attribute selection analysis. From model 1 (comparison between the neurological and cardiac groups, both w-scored with age for each control group), the first rank variables obtained in each dimension were the following: a) for the HBD task, accuracy based on the mean distance value for the interoceptive accuracy condition (Figure 2IA and Supplementary Data Table 5, http://links.lww.com/PSYMED/A687); b) for the metacognitive dimension, interoceptive sensibility for the post-feedback interoceptive accuracy condition (Figure 2IB and Supplementary Data Table 6, http://links.lww.com/PSYMED/A687); c) for the HEP modulation, the central ROI in the 200- to 300-millisecond window after the R-wave peak in the interoceptive accuracy condition (Figure 2IC and Supplementary Data Table 7, http://links.lww.com/PSYMED/A687); d) for the structural data, the mean brain-total volume of all four interoceptive areas on the right hemisphere (Figure 2ID and Supplementary Data Table 8, http://links.lww.com/PSYMED/A687); and d) for FC, the correlation of connectivity between the left anterior cingulate (area 31 AAL) and the right somatosensory region (area 58 AAL; Figure 2IE and Supplementary Data Table 9, http://links.lww.com/PSYMED/A687).
FIGURE 2.
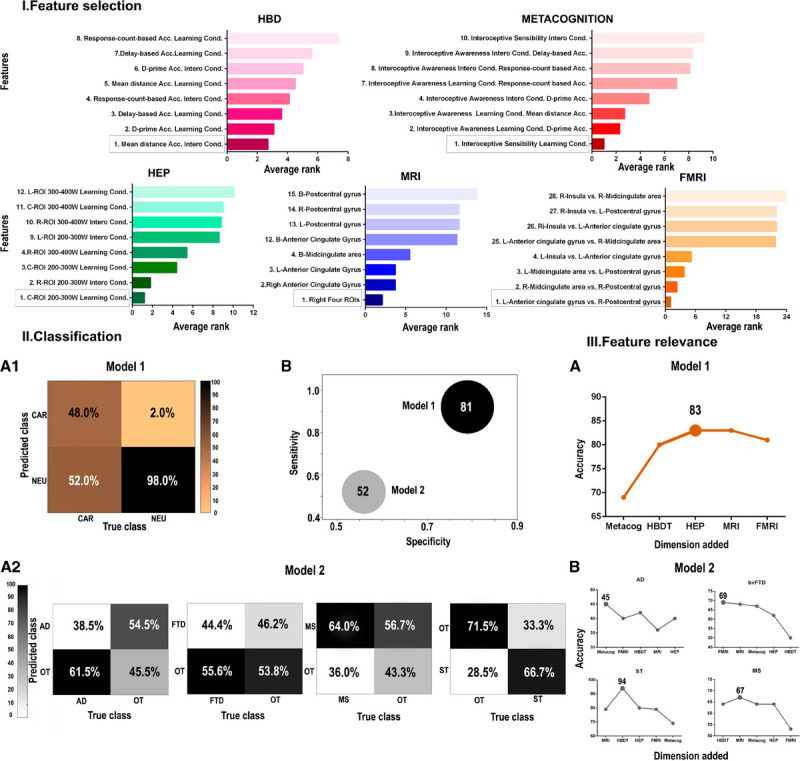
Main results. I, The average rank of features is shown for each dimension, presenting the first and the last four variables ranked. II1, Model 1: classification between the neurological and the cardiac group. II2, Model 2: classification among neurological conditions. A. Confusion matrix of each analysis. B, Accuracy, specificity, and sensitivity are represented for both analyses. Sphere size is proportional to accuracy value. II3, Feature relevance analysis for each model (A, for the comparison between the neurological and the cardiac group; B, for the comparison among neurological conditions). These figures show the model’s accuracy as each dimension is added following the order of importance of each feature. L = left; R = right; Acc = accuracy; B = bilateral; cond. = condition; AD = Alzheimer’s disease; ST = stroke; MS = multiple sclerosis; FTD = (behavioral variant) frontotemporal dementia; NEU = neurological; CAR = cardiac; OT = other neurological diseases; HDB = heartbeat detection task (accuracy); HEP = heart-evoked potential; MRI = magnetic resonance imaging; fMRI = functional magnetic resonance imaging; ROI = region of interest; W = window.
We performed additional analyses to discard potential motor and/or attentional deficits that could impact HBD outcomes in any patient group (thus biasing the model’s good discriminatory power). Specifically, we compared the performance of the neurological and cardiac groups relative to their matched controls in the control (feedback) condition of the HBD task. Results revealed nonsignificant differences (Supplementary Data, Results: Control of motor and attentional deficits in pathological samples, http://links.lww.com/PSYMED/A687).
Classification
Model 1
In the first analysis, in which we tested the classification between the neurological and cardiac groups using the most relevant features of each cardiac interoceptive dimension and level (detailed in previous section), the model yielded 0.81 accuracy, 0.92 sensitivity, and 0.79 specificity (Figure 2IIB). The confusion matrix of these results showed a better prediction for the neurological group, as shown by a 98% accuracy (Figure 2IIA1). We repeated the classification analysis to determine whether the classification accuracy was biased by the sample size difference between the pathological groups. Overall classification accuracy was the same when using different subsamples of the neurological group (Supplementary Data, Results: Classification, http://links.lww.com/PSYMED/A687). In conclusion, these results suggest that multimodal measures of cardiac interoception are different (nondimensionally shared) across cardiac and neurological conditions.
Model 2
The average classification results within the neurological conditions yielded a mean 0.53 accuracy test, a mean 0.52 sensitivity, and mean 0.56 specificity (Figure 2IIB and Supplementary Table 11, http://links.lww.com/PSYMED/A687). The confusion matrix showed a better prediction for the ST group, as shown by a 66.7% accuracy (Figure 2IIA2). In brief, neurological conditions are not well discriminated based on multimodal measures of cardiac interoception.
Feature Relevance
Model 1
For the distinction between neurological and cardiac conditions, the variable that contributed the most was metacognition, as its removal induced the higher decrease in classification accuracy (0.71). This was followed by interoceptive accuracy (0.73) and HEP (0.80). Eliminating the brain volume dimension did not change the accuracy of the model (0.81), and taking out the FC dimension increased accuracy (0.83; Figure 2IIIA and Supplementary Table 12, http://links.lww.com/PSYMED/A687). Then, we repeated the classification analyses by pruning in each step the least informative variable based on the previous results: we found that the highest accuracy was reached with the combination of metacognition, HEP, and HBD (0.83). In both cases, adding education levels to the model only decreased the ensuing accuracy (Supplementary Table 15, Feature relevance section, http://links.lww.com/PSYMED/A687). Results show that direct psychophysiological measures of cardiac interoception can differentiate between cardiac and neurological conditions.
Model 2
In the discrimination among neurological conditions, the highest predictor variable was different for each group, namely, HBD task accuracy for ST and MS, metacognition for AD, and FC for bvFTD. The lowest predictors were FC for MS, metacognition for ST, brain volume for AD, and HBD task accuracy for bvFTD (Supplementary Table 13, http://links.lww.com/PSYMED/A687). Then, we repeated the classification analyses by pruning in each step the least informative variable based on the previous results. The highest accuracy was reached with the combination of brain volume and HBD task accuracy for ST and MS. For AD and bvFTD, the highest classification accuracy was reached only with the first relevant feature for each group, metacognition for AD, and FC for bvFTD. However, the percentage of accuracy was low across comparisons (Figure 2IIIB and Supplementary Table 14, http://links.lww.com/PSYMED/A687). Thus, these cardiac interoceptive markers seem to be unspecific and undifferentiated across neurological conditions.
DISCUSSION
The goal of our study was to evaluate the relevance of interoception as a transnosological marker of neurological (relative to cardiac) conditions (model 1) and as a discriminatory marker among neurological diseases (model 2). Cardiac interoceptive outcomes were different enough to classify between groups in model 1 (80% accuracy) but not in model 2 (53% accuracy). These results suggest that multimodal cardiac interoceptive deficits would be sensitive to discriminate between central and peripheral compromise of neurovisceral pathways, but probably not to differentiate between neurological conditions.
One critical caveat of cardiac interoception is the complexity of dimensions in subsumes across behavioral and multimodal neural levels (7,29,77). Multimodal (behavioral, electrophysiological, and neuroimaging) signatures are hardly systematically reported (but see Ref. (29) for an exception). We assessed different measures (several HBD accuracy metrics, metacognition, HEP, brain volume, and FC) to evaluate their capacity to discriminate between the cardiac and neurological groups. For a) HBD behavioral results, mean distance accuracy was the best predictor, confirming previous comparisons of different indexes (29). This metric presents advantages to capture ongoing and temporally subtle adaptations across the task (18,78). Moreover, cardiac interoceptive accuracy proves more sensitive than cardiac interoceptive learning (10,13,30) across cardiac and neurological conditions. Regarding b) metacognition, the top feature was afforded by sensibility (i.e., confidence about one’s own performance (7,79,80)) of the learning condition. This is consistent with deficits in confidence of learning (10,81) and lack of insight (82–85) in neurological conditions, whereas cardiac patients show normal confidence ratings (86). As regards c) HEP, modulation during the learning condition was another selected feature, supporting the effects of learning in this event-related potential (19,21). Regarding d) brain volume, results evidenced that main right interoceptive regions ranked first, consistent with previous lateralization results (87–89). Finally, brain connectivity was betted indexed by the left ACC and the right postcentral gyrus (somatosensory regions). Although the insula is a key interoceptive hub, both the ACC and the somatosensory cortex are also critical (8,10,90), and both cardiac and neurological conditions have shown similar insular connectivity alterations (10,13). In summary, the top multifeatured ranking across different measures of cardiac interoception is consistent with previous results, paving the way for these potential markers to be studied in future multidimensional approaches.
The discrimination of cardiac versus neurological conditions (model 1) yielded a robust classification accuracy, even when both groups presented cardiac interoceptive alterations. This might imply a nondimensional disturbance triggered by different physiopathological deficits (central versus peripheral disruptions of neurovisceral pathways). Notably, direct ongoing measures of cardiac interoception (metacognition, accuracy, and HEP) were the best predictors. Insight impairments, usually linked to metacognition, are a hallmark of neurological conditions assessed here (82,84,85). Moreover, the direct measure of interoception (accuracy) is the most representative index of cardiac interoception (7,77). The HEP, another ongoing measure of cardiac interoception, followed the highest point of discrimination. The classification then declined with the inclusion of brain volume and FC. This is not unexpected because these interoceptive areas (8) are also involved in other cognitive processes and are not necessarily a direct proxy of the interoceptive performance. Also, because the ongoing measures of the cardiac interoceptive task provide the strongest classification power, those of neuroimaging hardly contribute to the classification while the others are present (see Supplementary Data, http://links.lww.com/PSYMED/A687, for an analysis of neuroimaging alone showing an improved classification than in combination with direct cardiac interoceptive measures).
Contrary to model 1, the analysis within neurological conditions (model 2) was barely superior to chance. Thus, the features observed in one condition were not different enough from those in other conditions, suggesting a more common disturbance. Moreover, subsequent analysis evidenced that different features appear in each subcomparison, suggesting that beyond a shared impairment in cardiac interoception, only a few levels of cardiac interoceptive impairments can be distinguished across conditions. These undifferentiated patterns were present even when the involved diseases have different patterns of compromise, including nonhemorrhagic focal lesions (ST), progressive local degeneration (AD and bvFTD), and diffuse alterations of GM and WM (MS). These effects were preserved when we controlled for different sample sizes. Taking this into consideration, cardiac interoceptive deficits seem to be similar and undifferentiated across disparate neurological conditions.
Some caveats from this study must be mentioned. First, we acknowledge that HBD performance in motor-tracking tasks can be affected by slowing, gross motor impairments, and/or attentional deficits. Indeed, these impairments are expected in patients, particularly those with neurological conditions involving frontal, ACC, and insular damage (10,91–94). To address these potential confounds in the models’ performance, we compared the accuracy of the neurological and healthy participants in the control (feedback) condition of the HBD task, which required them to follow an external stimulus (i.e., an auditory cue). We also compared the performance of both groups in sensitivity (confidence) ratings for the control (feedback) condition. Results of those analyses revealed nonsignificant differences between neurological patients and controls (Supplementary Data, Results: Control of motor and attentional deficits in pathological samples, http://links.lww.com/PSYMED/A687), suggesting that interoceptive deficits could hardly be explained by unspecific attentional or response/processing speed deficits. The same was true for the cardiac group.
Relatedly, systematic comparisons of performance in motor-tracking HBD tasks between neurological (9,10) or hypertensive (13) patients and healthy controls revealed no relevant modulations associated with the exteroceptive (i.e., externally guided) condition. More specifically, patients presented selective deficits in the interoceptive condition, alongside disrupted HEP modulations and anatomofunctional abnormalities in key interoceptive brain regions (10,13). Selective interoceptive differences have also been reported for psychiatric patients (27,47), suggesting that cardiac interoceptive deficits could represent a marker for diverse diseases, irrespective of motor or attentional difficulties (37).
Finally, research in healthy participants has revealed that accuracy in the interoceptive (but not the exteroceptive) condition of a motor-tracking HBD task is associated with canonical markers of interoception, including HEP negative-going; FC within insular, somatosensory, and frontal networks; and socioemotional skills (17,37). Altogether, evidence from healthy and disease supports the validity of the motor-tracking HBD task used here and the specificity of its interoceptive condition as a robust marker of cardiac interoception.
As a second caveat, possible extrapolations of present results to other interoceptive modalities must be taken with caution. Beyond cardiac sensations, interoception includes the monitoring of other internal signals, such as temperature, respiration, touch, pain, and gastric contractions (35,95,96). Although some studies suggest a direct (although modest) association between cardiac and noncardiac interoception, specifically gastric signal perception (97,98), most evidence shows uncoordinated responses across different interoceptive channels (77,99–106). Thus, more research is needed to assess whether our results are generalizable beyond the cardiac domain.
As already pointed out (106), inferences about general interoceptive ability should not be drawn from studies targeting individual domains. Even studies focusing on bivariate associations between different interoceptive modalities are insufficient to reach firm conclusions. Future research should aim at disentangling how the brain integrates multimodal visceral signals (be they correlated or not) to provide a coherent sense of the body’s dynamic physiological states (35) and how this process can be best tracked using behavioral tasks (106). The integration of multimodal signals, mainly grounded in the insular cortex (16,64), could prove critical for survival by allowing for successful allostasis, i.e., neural anticipation of physiological needs and efficient preparation of the organism to meet them according to the context (107–109).
Nonetheless, beyond cross-modal integration, cardiac interoception is itself a relevant research target given its potential as a clinical marker (95,110–112) and its relationship with cognitive functions such as emotion processing (6,8,113), empathy (20,114,115), time perception (116), memory (117,118), and decision making (119–121), all of which are frequently impaired in neurological conditions.
Finally, our study present limitations and provides novel avenues for further research. Our sample was small and inconsistent among subgroups. Still, although machine learning methods work better with larger (122,123) and equal-sized (124) groups, we replicated result when using subsampling analyses. Moreover, all data were normalized against controls and weighted by age. In addition, we also analyzed the relevance of education, which did not change the results. Nevertheless, future studies should use larger samples to confirm present results. Also, although our results support the hypothesis of dimensional and nonspecific interoceptive deficits in neurological diseases, future studies should use not only larger but also balanced samples to test specific predictions regarding the interoceptive profile of each condition.
Lastly, we included only neurological conditions as central nervous system disorders. However, psychiatric conditions are comorbid in different neurological patients (125–127) and present cardiac interoceptive deficits (27,47). Comparing interoception across cardiac, psychiatric, and neurological conditions may represent a thought-provoking approach to dimensionality. Future studies may extend the present results by adding other measures of interoceptive awareness and sensitivity (128), as well as neuroimaging from active HBD tasks (64,129).
CONCLUSIONS
Transdiagnostic dimensional approaches are quite scarce in neurology, and despite theoretical proposals (12), few empirical reports have assessed interoception across neurological conditions (10). Ours result reinforce the need to study the potential application of research domain criteria (2,86,130–132) to neurological conditions. Moreover, considering the artificial distinctions among neurology and psychiatry that have pervasively affected cognitive neuroscience (133,134), research on interoception may bring a new agenda for transnosological research. This may also allow for exploring interoceptive dimensions that are not usually evaluated in clinical neurology and for evaluating the potential to refine patient characterization and treatment.
Supplementary Material
Acknowledgments
The authors appreciate the kind disposition from all participants, especially from the patients and their caregivers.
Acknowledgments
Source of Funding and Conflicts of Interest: This work was supported by the National Scientific and Technical Research Council; FONCYT-PICT (2017-1818, 2017-1820); CONICYT/FONDAP (15150012); the Interamerican Development Bank, Programa Interdisciplinario de Investigación Experimental en Comunicación y Cognición (PIIECC), Facultad de Humanidades, USACH; Sistema General de Regalías (BPIN2018000100059), Universidad del Valle (CI 5316), Alzheimer's Assocaition GBHI ALZ UK-20-639295; and the Multi-Partner Consortium to Expand Dementia Research in Latin America (ReDLat), funded by the National Institutes of Aging of the National Institutes of Health under award number R01AG057234, an Alzheimer’s Association grant (SG-20-725707-ReDLat), the Rainwater Foundation, and the Global Brain Health Institute. The authors have no competing interests to declare, and there are not any financial and personal relationships with other people or organizations that could inappropriately influence (bias) their work.
The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health, Alzheimer’s Association, Rainwater Charitable Foundation, or Global Brain Health Institute. The sponsors have no role of the in-study design, collection, analysis, interpretation, writing, and submission of this work.
Author Contributions: S.A.: Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. S.F.: Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing, Supervision. A.M.G.: Supervision, Writing – review & editing. M.D.: Software, Writing – original draft. H.S.-G.: Software, Writing – review & editing. A.B.: Software, Writing – review & editing. A.Y.: Data curation, Investigation. M.K.H.: Data curation, Formal analysis. P.S.: Data curation, Investigation, Software. L.A.D.l.F.: Data curation, Software, Validation, Methodology. S.A.M.: Data curation, Formal analysis. I.G.C.: Investigation, Data curation, Software. M.M.C.: Investigation, Data curation. R.M.P.: Supervision, Writing – review & editing. C.S.: Resources, Writing – review & editing. L.S.: Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Visualization, Writing – original draft, Writing – review & editing. A.I.: Conceptualization, Funding acquisition Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Footnotes
S.A. and S.F. are co-first authors.
Supplemental Content
Contributor Information
Sofía Abrevaya, Email: abrevayasofia@gmail.com.
Adolfo M. García, Email: adolfomartingarcia@gmail.com.
Martin Dottori, Email: dottorimartin@gmail.com.
Hernando Santamaria-Garcia, Email: nanosanta@gmail.com.
Agustina Birba, Email: agustina.birba@gmail.com.
Adrián Yoris, Email: aeyoris@gmail.com.
Malin Katharina Hildebrandt, Email: malin.hildebrandt@googlemail.com.
Paula Salamone, Email: pausalamone@gmail.com.
Alethia De la Fuente, Email: lauralethia@gmail.com.
Sofía Alarco-Martí, Email: sofi.alarco28@gmail.com.
Indira García-Cordero, Email: indira.garciacordero@gmail.com.
Miguel Matorrel-Caro, Email: m.martorellcaro@gmail.com.
Ricardo Marcos Pautassi, Email: rpautassi@gmail.com.
Cecilia Serrano, Email: ceciliamserrano@yahoo.com.ar.
Lucas Sedeño, Email: lucas.sedeno@gmail.com.
Agustín Ibáñez, Email: agustin.ibanez@gbhi.org.
REFERENCES
- 1.Insel TR. The NIMH Research Domain Criteria (RDoC) Project: precision medicine for psychiatry. Am J Psychiatry 2014;171:395–7. [DOI] [PubMed] [Google Scholar]
- 2.Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry 2010;167:748–51. [DOI] [PubMed] [Google Scholar]
- 3.Kraemer HC, Noda A, O’Hara R. Categorical versus dimensional approaches to diagnosis: methodological challenges. J Psychiatr Res 2004;38:17–25. [DOI] [PubMed] [Google Scholar]
- 4.Brugger P, Lenggenhager B. The bodily self and its disorders: neurological, psychological and social aspects. Curr Opin Neurol 2014;27:644–52. [DOI] [PubMed] [Google Scholar]
- 5.Carter R, Ffytche DH. On visual hallucinations and cortical networks: a trans-diagnostic review. J Neurol 2015;262:1780–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garfinkel SN, Critchley HD. Interoception, emotion and brain: new insights link internal physiology to social behaviour. Commentary on: “Anterior insular cortex mediates bodily sensibility and social anxiety” by Terasawa et al. (2012). Soc Cogn Affect Neurosci 2013;8:231–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garfinkel SN, Seth AK, Barrett AB, Suzuki K, Critchley HD. Knowing your own heart: distinguishing interoceptive accuracy from interoceptive awareness. Biol Psychol 2015;104:65–74. [DOI] [PubMed] [Google Scholar]
- 8.Adolfi F, Couto B, Richter F, Decety J, Lopez J, Sigman M, Manes F, Ibanez A. Convergence of interoception, emotion, and social cognition: a twofold fMRI meta-analysis and lesion approach. Cortex 2017;88:124–42. [DOI] [PubMed] [Google Scholar]
- 9.Salamone PC, Esteves S, Sinay VJ, Garcia-Cordero I, Abrevaya S, Couto B, Adolfi F, Martorell M, Petroni A, Yoris A, Torquati K, Alifano F, Legaz A, Cassara FP, Bruno D, Kemp AH, Herrera E, Garcia AM, Ibanez A, Sedeno L. Altered neural signatures of interoception in multiple sclerosis. Hum Brain Mapp 2018;39:4743–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Cordero I, Sedeno L, de la Fuente L, Slachevsky A, Forno G, Klein F, Lillo P, Ferrari J, Rodriguez C, Bustin J, Torralva T, Baez S, Yoris A, Esteves S, Melloni M, Salamone P, Huepe D, Manes F, Garcia AM, Ibanez A. Feeling, learning from and being aware of inner states: interoceptive dimensions in neurodegeneration and stroke. Philos Trans R Soc Lond B Biol Sci 2016;371:20160006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heddaeus D, Dirmaier J, Brettschneider C, Daubmann A, Grochtdreis T, von dem Knesebeck O, Konig HH, Lowe B, Maehder K, Porzelt S, Rosenkranz M, Schafer I, Scherer M, Schulte B, Wegscheider K, Weigel A, Werner S, Zimmermann T, Harter M. Study protocol for the COMET study: a cluster-randomised, prospective, parallel-group, superiority trial to compare the effectiveness of a collaborative and stepped care model versus treatment as usual in patients with mental disorders in primary care. BMJ Open 2019;9:e032408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoris A, Garcia AM, Salamone P, Sedeno L, Garcia-Cordero I, Ibanez A. Cardiac interoception in neurological conditions and its relevance for dimensional approaches. In: Tsakiris M, Preester H, editors. The Interoceptive Mind: From Homeostasis to Awareness. London: Oxford University Press;2018. [Google Scholar]
- 13.Yoris A, Abrevaya S, Esteves S, Salamone P, Lori N, Martorell M, Legaz A, Alifano F, Petroni A, Sanchez R, Sedeno L, Garcia AM, Ibanez A. Multilevel convergence of interoceptive impairments in hypertension: new evidence of disrupted body-brain interactions. Hum Brain Mapp 2018;39:1563–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khalsa SS, Rudrauf D, Sandesara C, Olshansky B, Tranel D. Bolus isoproterenol infusions provide a reliable method for assessing interoceptive awareness. Int J Psychophysiol 2009;72:34–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brener J, Ring C. Towards a psychophysics of interoceptive processes: the measurement of heartbeat detection. Philos Trans R Soc Lond B Biol Sci 2016;371:20160015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Couto B, Adolfi F, Sedeño L, Salles A, Canales-Johnson A, Alvarez-Abut P, Garcia-Cordero I, Pietto M, Bekinschtein T, Sigman M, Manes F, Ibanez A. Disentangling interoception: insights from focal strokes affecting the perception of external and internal milieus. Front Psychol 2015;6:503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fittipaldi S, Abrevaya S, Fuente A, Pascariello GO, Hesse E, Birba A, Salamone P, Hildebrandt M, Martí SA, Pautassi RM, Huepe D, Martorell MM, Yoris A, Roca M, García AM, Sedeño L, Ibáñez A. A multidimensional and multi-feature framework for cardiac interoception. Neuroimage 2020;212:116677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de la Fuente A, Sedeno L, Vignaga SS, Ellmann C, Sonzogni S, Belluscio L, Garcia-Cordero I, Castagnaro E, Boano M, Cetkovich M, Torralva T, Canepa ET, Tagliazucchi E, Garcia AM, Ibanez A. Multimodal neurocognitive markers of interoceptive tuning in smoked cocaine. Neuropsychopharmacology 2019;44:1425–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canales-Johnson A, Silva C, Huepe D, Rivera-Rei Á, Noreika V, Garcia Mdel C, Silva W, Ciraolo C, Vaucheret E, Sedeño L, Couto B, Kargieman L, Baglivo F, Sigman M, Chennu S, Ibáñez A, Rodríguez E, Bekinschtein TA. Auditory feedback differentially modulates behavioral and neural markers of objective and subjective performance when tapping to your heartbeat. Cereb Cortex 2015;25:4490–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukushima H, Terasawa Y, Umeda S. Association between interoception and empathy: evidence from heartbeat-evoked brain potential. Int J Psychophysiol 2011;79:259–65. [DOI] [PubMed] [Google Scholar]
- 21.Pollatos OS, Schandry R. Accuracy of heartbeat perception is reflected in the amplitude of the heartbeat-evoked brain potential. Psychophysiology 2004;41:476–82. [DOI] [PubMed] [Google Scholar]
- 22.Montoya P, Schandry R, Muller A. Heartbeat evoked potentials (HEP): topography and influence of cardiac awareness and focus of attention. Electroencephalogr Clin Neurophysiol 1993;88:163–72. [DOI] [PubMed] [Google Scholar]
- 23.Pollatos O, Kirsch W, Schandry R. On the relationship between interoceptive awareness, emotional experience, and brain processes. Brain Res Cogn Brain Res 2005;25:948–62. [DOI] [PubMed] [Google Scholar]
- 24.Schandry R, Montoya P. Event-related brain potentials and the processing of cardiac activity. Biol Psychol 1996;42:75–85. [DOI] [PubMed] [Google Scholar]
- 25.Terhaar J, Viola FC, Bar KJ, Debener S. Heartbeat evoked potentials mirror altered body perception in depressed patients. Clin Neurophysiol 2012;123:1950–7. [DOI] [PubMed] [Google Scholar]
- 26.Hassanpour MS, Yan L, Wang DJ, Lapidus RC, Arevian AC, Simmons WK, Feusner JD, Khalsa SS. How the heart speaks to the brain: neural activity during cardiorespiratory interoceptive stimulation. Philos Trans R Soc Lond B Biol Sci 2016;371:20160017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoris A, Garcia AM, Traiber L, Santamaria-Garcia H, Martorell M, Alifano F, Kichic R, Moser JS, Cetkovich M, Manes F, Ibanez A, Sedeno L. The inner world of overactive monitoring: neural markers of interoception in obsessive-compulsive disorder. Psychol Med 2017;47:1957–70. [DOI] [PubMed] [Google Scholar]
- 28.Tulley RT, Appel MJ, Enos TG, Hegsted M, McCutcheon KL, Zhou J, Raggio AM, Jeffcoat R, Birkett A, Martin RJ, Keenan MJ. Comparative methodologies for measuring metabolizable energy of various types of resistant high amylose corn starch. J Agric Food Chem 2009;57:8474–9. [DOI] [PubMed] [Google Scholar]
- 29.Fittipaldi S, Abrevaya S, De La Fuente LA, Pascariello GO, Hesse E, Birba A, Salamone P, Hildebrandt M, Alarco Martí S, Pautassi R, Martorell Caro M, Yoris A, Roca M, García AM, Sedeño L, Ibáñez A. Multidimensional and multi-feature framework of cardiac interoception. Neuroimage 2019;212:116677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gonzalez Campo C, Salamone PC, Rodriguez-Arriagada N, Richter F, Herrera E, Bruno D, Pagani Cassara F, Sinay V, Garcia AM, Ibanez A, Sedeno L. Fatigue in multiple sclerosis is associated with multimodal interoceptive abnormalities [published online November 28, 2019]. Mult Scler. doi: 10.1177/1352458519888881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gray MA, Rylander K, Harrison NA, Wallin BG, Critchley HD. Following one’s heart: cardiac rhythms gate central initiation of sympathetic reflexes. J Neurosci 2009;29:1817–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gray MA, Taggart P, Sutton PM, Groves D, Holdright DR, Bradbury D, Brull D, Critchley HD. A cortical potential reflecting cardiac function. Proc Natl Acad Sci U S A 2007;104:6818–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shivkumar K, Ajijola OA, Anand I, Armour JA, Chen PS, Esler M, De Ferrari GM, Fishbein MC, Goldberger JJ, Harper RM, Joyner MJ, Khalsa SS, Kumar R, Lane R, Mahajan A, Po S, Schwartz PJ, Somers VK, Valderrabano M, Vaseghi M, Zipes DP. Clinical neurocardiology defining the value of neuroscience-based cardiovascular therapeutics. J Physiol 2016;594:3911–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Couto B, Salles A, Sedeno L, Peradejordi M, Barttfeld P, Canales-Johnson A, Dos Santos YV, Huepe D, Bekinschtein T, Sigman M, Favaloro R, Manes F, Ibanez A. The man who feels two hearts: the different pathways of interoception. Soc Cogn Affect Neurosci 2014;9:1253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 2002;3:655–66. [DOI] [PubMed] [Google Scholar]
- 36.Cameron OG, Minoshima S. Regional brain activation due to pharmacologically induced adrenergic interoceptive stimulation in humans. Psychosom Med 2002;64:851–61. [DOI] [PubMed] [Google Scholar]
- 37.Yoris A, García A, Salamone P, Sedeno L, García-Cordero I, Ibanez A. Cardiac interoception in neurological conditions and its relevance for dimensional approaches. In: Tsakaris M, Preester H, editors. The Interoceptive Basis of the Mind. Oxford: Oxford University Press; 2018:. [Google Scholar]
- 38.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging–Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011;7:263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984;34:939–44. [DOI] [PubMed] [Google Scholar]
- 40.Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, van Swieten JC, Seelaar H, Dopper EG, Onyike CU, Hillis AE, Josephs KA, Boeve BF, Kertesz A, Seeley WW, Rankin KP, Johnson JK, Gorno-Tempini ML, Rosen H, Prioleau-Latham CE, Lee A, Kipps CM, Lillo P, Piguet O, Rohrer JD, Rossor MN, Warren JD, Fox NC, Galasko D, Salmon DP, Black SE, Mesulam M, Weintraub S, Dickerson BC, Diehl-Schmid J, Pasquier F, Deramecourt V, Lebert F, Pijnenburg Y, Chow TW, Manes F, Grafman J, Cappa SF, Freedman M, Grossman M, Miller BL. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011;134(Pt 9):2456–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, Havrdova E, Hutchinson M, Kappos L, Lublin FD, Montalban X, O’Connor P, Sandberg-Wollheim M, Thompson AJ, Waubant E, Weinshenker B, Wolinsky JS. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sánchez RA, Ramos F, Giannone C, Fischer P, Masnatta L, Baglivo HP, Ramírez AJ, Hollenberg NK. Parallel renal and extremity blood supply abnormalities in nonmodulation: responses to ACE inhibition. Hypertension 2003;41:919–24. [DOI] [PubMed] [Google Scholar]
- 43.Gonzalez Campo C, Salamone PC, Rodríguez-Arriagada N, Richter F, Herrera E, Bruno D, Pagani Cassara F, Sinay V, García AM, Ibáñez A, Sedeño L. Fatigue in multiple sclerosis is associated with multimodal interoceptive abnormalities [published online November 28, 2019]. Mult Scler. doi:10.1177/1352458519888881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.International Committee of Medical Journal Editors Recommendations for the conduct, reporting, editing, and publication of scholarly work in medical journals. 2016. Available at: http://www.icmje.org/recommendations/. Accessed July 2019. [PubMed]
- 45.Melloni M, Billeke P, Baez S, Hesse E, de la Fuente L, Forno G, Birba A, Garcia-Cordero I, Serrano C, Plastino A, Slachevsky A, Huepe D, Sigman M, Manes F, Garcia AM, Sedeno L, Ibanez A. Your perspective and my benefit: multiple lesion models of self-other integration strategies during social bargaining. Brain 2016;139:3022–40. [DOI] [PubMed] [Google Scholar]
- 46.Sedeño L, Couto B, Melloni M, Canales-Johnson A, Yoris A, Baez S, Esteves S, Velásquez M, Barttfeld P, Sigman M. How do you feel when you can’t feel your body? Interoception, functional connectivity and emotional processing in depersonalization-derealization disorder. PLoS One 2014;9:e98769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoris A, Esteves S, Couto B, Melloni M, Kichic R, Cetkovich M, Favaloro R, Moser J, Manes F, Ibanez A, Sedeno L. The roles of interoceptive sensitivity and metacognitive interoception in panic. Behav Brain Funct 2015;11:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Melloni M, Sedeno L, Couto B, Reynoso M, Gelormini C, Favaloro R, Canales-Johnson A, Sigman M, Manes F, Ibanez A. Preliminary evidence about the effects of meditation on interoceptive sensitivity and social cognition. Behav Brain Funct 2013;9:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schandry R. Heart beat perception and emotional experience. Psychophysiology 1981;18:483–8. [DOI] [PubMed] [Google Scholar]
- 50.García-Cordero I, Esteves S, Mikulan EP, Hesse E, Baglivo FH, Silva W, García MDC, Vaucheret E, Ciraolo C, García HS, Adolfi F, Pietto M, Herrera E, Legaz A, Manes F, García AM, Sigman M, Bekinschtein TA, Ibáñez A, Sedeño L. Attention, in and out: scalp-level and intracranial EEG correlates of interoception and exteroception. Front Neurosci 2017;11:411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Couto B, Adolfi F, Velasquez M, Mesow M, Feinstein J, Canales-Johnson A, Mikulan E, Martinez-Pernia D, Bekinschtein T, Sigman M, Manes F, Ibanez A. Heart evoked potential triggers brain responses to natural affective scenes: a preliminary study. Auton Neurosci 2015;193:132–7. [DOI] [PubMed] [Google Scholar]
- 52.Stanislaw H, Todorov N. Calculation of signal detection theory measures. Behav Res Methods Instrum Comput 1999;31:137–49. [DOI] [PubMed] [Google Scholar]
- 53.Szczepanski SM, Crone NE, Kuperman RA, Auguste KI, Parvizi J, Knight RT. Dynamic changes in phase-amplitude coupling facilitate spatial attention control in fronto-parietal cortex. PLoS Biol 2014;12:e1001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kern M, Aertsen A, Schulze-Bonhage A, Ball T. Heart cycle–related effects on event-related potentials, spectral power changes, and connectivity patterns in the human ECoG. Neuroimage 2013;81:178–90. [DOI] [PubMed] [Google Scholar]
- 55.Park HD, Correia S, Ducorps A, Tallon-Baudry C. Spontaneous fluctuations in neural responses to heartbeats predict visual detection. Nat Neurosci 2014;17:612–8. [DOI] [PubMed] [Google Scholar]
- 56.Gray MA, Critchley HD. Interoceptive basis to craving. Neuron 2007;54:183–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pollatos O, Kirsch W, Schandry R. Brain structures involved in interoceptive awareness and cardioafferent signal processing: a dipole source localization study. Hum Brain Mapp 2005;26:54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nichols TE, Das S, Eickhoff SB, Evans AC, Glatard T, Hanke M, Kriegeskorte N, Milham MP, Poldrack RA, Poline JB, Proal E, Thirion B, Van Essen DC, White T, Yeo BT. Best practices in data analysis and sharing in neuroimaging using MRI. Nat Neurosci 2017;20:299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Poldrack RA, Baker CI, Durnez J, Gorgolewski KJ, Matthews PM, Munafo MR, Nichols TE, Poline JB, Vul E, Yarkoni T. Scanning the horizon: towards transparent and reproducible neuroimaging research. Nat Rev Neurosci 2017;18:115–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage 2000;11(6 Pt 1):805–21. [DOI] [PubMed] [Google Scholar]
- 61.Sedeno L, Piguet O, Abrevaya S, Desmaras H, Garcia-Cordero I, Baez S, Alethia de la Fuente L, Reyes P, Tu S, Moguilner S, Lori N, Landin-Romero R, Matallana D, Slachevsky A, Torralva T, Chialvo D, Kumfor F, Garcia AM, Manes F, Hodges JR, Ibanez A. Tackling variability: a multicenter study to provide a gold-standard network approach for frontotemporal dementia. Hum Brain Mapp 2017;38:3804–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage 2007;38:95–113. [DOI] [PubMed] [Google Scholar]
- 63.Sedeno L, Couto B, Garcia-Cordero I, Melloni M, Baez S, Morales Sepulveda JP, Fraiman D, Huepe D, Hurtado E, Matallana D, Kuljis R, Torralva T, Chialvo D, Sigman M, Piguet O, Manes F, Ibanez A. Brain network organization and social executive performance in frontotemporal dementia. J Int Neuropsychol Soc 2016;22:250–62. [DOI] [PubMed] [Google Scholar]
- 64.Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci 2004;7:189–95. [DOI] [PubMed] [Google Scholar]
- 65.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002;15:273–89. [DOI] [PubMed] [Google Scholar]
- 66.Chao-Gan Y, Yu-Feng Z. DPARSF: a MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front Syst Neurosci 2010;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum Brain Mapp 1999;7:254–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci 2005;102:9673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Raichle ME. A paradigm shift in functional brain imaging. J Neurosci 2009;29:12729–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Troisi JR., 2nd Sensation within the skin. ACS Chem Neurosci 2015;6:209–10. [DOI] [PubMed] [Google Scholar]
- 71.Chung J, Yoo K, Lee P, Kim CM, Roh JH, Park JE, Kim SJ, Seo SW, Shin JH, Seong JK, Jeong Y. Normalization of cortical thickness measurements across different T1 magnetic resonance imaging protocols by novel W-score standardization. Neuroimage 2017;159:224–35. [DOI] [PubMed] [Google Scholar]
- 72.Hall M, Frank E, Holmes G, Pfahringer B, Reutemann P, Witten IH. The WEKA data mining software: an update. ACM SIGKDD Explor Newsl 2009;11:10–8. [Google Scholar]
- 73.Witten IH, Frank E, Hall MA, Pal CJ. Data Mining: Practical machine learning tools and techniques. Burlington, MA: Morgan Kaufmann; 2016. [Google Scholar]
- 74.Santamaría-García H, Reyes P, García A, Baéz S, Martinez A, Santacruz JM, Slachevsky A, Sigman M, Matallana D, Ibañez A. First symptoms and neurocognitive correlates of behavioral variant frontotemporal dementia. J Alzheimers Dis 2016;54:957–70. [DOI] [PubMed] [Google Scholar]
- 75.Koul A, Becchio C, Cavallo A. PredPsych: a toolbox for predictive machine learning-based approach in experimental psychology research. Behav Res Methods 2018;50:1657–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bachli BSL, Ochab JK, Piguet O, Kumfor F, Reyes P, Torralva T, Roca M, Cardona JF, Gonzalez Campo C, Herrera E, Slachevsky A, Matallana D, Manes F, García AM, Ibáñez A, Chialvo DR. Evaluating the reliability of neurocognitive biomarkers of neurodegenerative diseases across countries: a machine learning approach. Neuroimage 2019;208:116456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Garfinkel SN, Manassei MF, Hamilton-Fletcher G, In den Bosch Y, Critchley HD, Engels M. Interoceptive dimensions across cardiac and respiratory axes. Philos Trans R Soc Lond B Biol Sci 2016;371:20160014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Werner G, Mountcastle VB. The variability of central neural activity in a sensory system, and its implications for the central reflection of sensory events. J Neurophysiol 1963;26:958–77. [DOI] [PubMed] [Google Scholar]
- 79.Critchley HD, Garfinkel SN. Interoception and emotion. Curr Opin Psychol 2017;17:7–14. [DOI] [PubMed] [Google Scholar]
- 80.Chua EF, Bliss-Moreau E. Knowing your heart and your mind: the relationships between metamemory and interoception. Conscious Cogn 2016;45:146–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lak A, Costa GM, Romberg E, Koulakov AA, Mainen ZF, Kepecs A. Orbitofrontal cortex is required for optimal waiting based on decision confidence. Neuron 2014;84:190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Harwood DG, Sultzer DL, Wheatley MV. Impaired insight in Alzheimer disease: association with cognitive deficits, psychiatric symptoms, and behavioral disturbances. Neuropsychiatry Neuropsychol Behav Neurol 2000;13:83–8. [PubMed] [Google Scholar]
- 83.Harwood DG, Sultzer DL, Feil D, Monserratt L, Freedman E, Mandelkern MA. Frontal lobe hypometabolism and impaired insight in Alzheimer disease. Am J Geriatr Psychiatry 2005;13:934–41. [DOI] [PubMed] [Google Scholar]
- 84.Horning SM, Melrose R, Sultzer D. Insight in Alzheimer’s disease and its relation to psychiatric and behavioral disturbances. Int J Geriatr Psychiatry 2014;29:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gilleen J, Greenwood K, David AS. Lack of insight and awareness in schizophrenia and neuropsychiatric disorders. In: Myoshi K, Morimura Y, Maeda K, editors. Neuropsychiatric Disorders. Tokyo, Japan: Springer; 2010:33–50. [Google Scholar]
- 86.Akram F, Giordano J. Research domain criteria as psychiatric nosology. Camb Q Healthc Ethics 2017;26:592–601. [DOI] [PubMed] [Google Scholar]
- 87.Pollatos O, Schandry R, Auer DP, Kaufmann C. Brain structures mediating cardiovascular arousal and interoceptive awareness. Brain Res 2007;1141:178–87. [DOI] [PubMed] [Google Scholar]
- 88.Leopold C, Schandry R. The heartbeat-evoked brain potential in patients suffering from diabetic neuropathy and in healthy control persons. Clin Neurophysiol 2001;112:674–82. [DOI] [PubMed] [Google Scholar]
- 89.Meyer S, Strittmatter M, Fischer C, Georg T, Schmitz B. Lateralization in autononic dysfunction in ischemic stroke involving the insular cortex. Neuroreport 2004;15:357–61. [DOI] [PubMed] [Google Scholar]
- 90.Khalsa SS, Rudrauf D, Feinstein JS, Tranel D. The pathways of interoceptive awareness. Nat Neurosci 2009;12:1494–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jezzini A, Caruana F, Stoianov I, Gallese V, Rizzolatti G. Functional organization of the insula and inner perisylvian regions. Proc Natl Acad Sci 2012;109:10077–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lansley J, Mataix-Cols D, Grau M, Radua J, Sastre-Garriga J. Localized grey matter atrophy in multiple sclerosis: a meta-analysis of voxel-based morphometry studies and associations with functional disability. Neurosci Biobehav Rev 2013;37:819–30. [DOI] [PubMed] [Google Scholar]
- 93.Foundas AL, Leonard CM, Mahoney SM, Agee OF, Heilman KM. Atrophy of the hippocampus, parietal cortex, and insula in Alzheimer’s disease: a volumetric magnetic resonance imaging study. Neuropsychiatry Neuropsychol Behav Neurol 1997;10:81–9. [PubMed] [Google Scholar]
- 94.Xie C, Bai F, Yu H, Shi Y, Yuan Y, Chen G, Li W, Chen G, Zhang Z, Li S-J. Abnormal insula functional network is associated with episodic memory decline in amnestic mild cognitive impairment. Neuroimage 2012;63:320–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Khalsa SS, Lapidus RC. Can interoception improve the pragmatic search for biomarkers in psychiatry? Front Psychiatry 2016;7:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alfonsi P, Adam F, Bouhassira D. Thermoregulation and pain perception: evidence for a homoeostatic (interoceptive) dimension of pain. Eur J Pain 2016;20:138–48. [DOI] [PubMed] [Google Scholar]
- 97.Herbert BM, Muth ER, Pollatos O, Herbert C. Interoception across modalities: on the relationship between cardiac awareness and the sensitivity for gastric functions. PLoS One. 2012;7:e36646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Whitehead WE, Drescher VM. Perception of gastric contractions and self-control of gastric motility. Psychophysiology 1980;17:552–8. [DOI] [PubMed] [Google Scholar]
- 99.Ferentzi E, Köteles F, Csala B, Drew R, Tihanyi BT, Pulay-Kottlár G, Doering BK. What makes sense in our body? Personality and sensory correlates of body awareness and somatosensory amplification. Personal Individ Differ 2017;104:75–81. [Google Scholar]
- 100.Krautwurst S, Gerlach AL, Gomille L, Hiller W, Witthöft M. Health anxiety—an indicator of higher interoceptive sensitivity? J Behav Ther Exp Psychiatry 2014;45:303–9. [DOI] [PubMed] [Google Scholar]
- 101.Krautwurst S, Gerlach AL, Witthöft M. Interoception in pathological health anxiety. J Abnorm Psychol 2016;125:1179–84. [DOI] [PubMed] [Google Scholar]
- 102.Suschinsky KD, Lalumiere ML. Is sexual concordance related to awareness of physiological states? Arch Sex Behav 2012;41:199–208. [DOI] [PubMed] [Google Scholar]
- 103.Pennebaker JW. The Psychology of Physical Symptoms. New York, NY: Springer Science & Business Media; 2012. [Google Scholar]
- 104.Steptoe A, Noll A. The perception of bodily sensations, with special reference to hypochondriasis. Behav Res Ther 1997;35:901–10. [DOI] [PubMed] [Google Scholar]
- 105.Mauss IB, Levenson RW, McCarter L, Wilhelm FH, Gross JJ. The tie that binds? Coherence among emotion experience, behavior, and physiology. Emotion 2005;5:175–90. [DOI] [PubMed] [Google Scholar]
- 106.Ferentzi E, Bogdány T, Szabolcs Z, Csala B, Horváth Á, Köteles F. Multichannel investigation of interoception: sensitivity is not a generalizable feature. Front Hum Neurosci 2018;12:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kleckner IR, Zhang J, Touroutoglou A, Chanes L, Xia C, Simmons WK, Quigley KS, Dickerson BC, Barrett LF. Evidence for a large-scale brain system supporting allostasis and interoception in humans. Nat Hum Behav 2017;1:0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schulkin J, Sterling P. Allostasis: a brain-centered, predictive mode of physiological regulation. Trends Neurosci 2019;42:740–52. [DOI] [PubMed] [Google Scholar]
- 109.Sterling P. Homeostasis vs allostasis: implications for brain function and mental disorders. JAMA Psychiat 2014;71:1192–3. [DOI] [PubMed] [Google Scholar]
- 110.Murphy J, Brewer R, Catmur C, Bird G. Interoception and psychopathology: a developmental neuroscience perspective. Dev Cogn Neurosci 2017;23:45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Khalsa SS Adolphs R Cameron OG Critchley HD Davenport PW Feinstein JS Feusner JD Garfinkel SN Lane RD Mehling WE Meuret AE Nemeroff CB Oppenheimer S Petzschner FH Pollatos O Rhudy JL Schramm LP Simmons WK Stein MB Stephan KE Van den Bergh O Van Diest I von Leupoldt A Paulus MP, Interoception Summit 2016 participants . Interoception and mental health: a roadmap. Biol Psychiatry Cogn Neurosci Neuroimaging 2018;3:501–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Murphy J, Geary H, Millgate E, Catmur C, Bird G. Direct and indirect effects of age on interoceptive accuracy and awareness across the adult lifespan. Psychon Bull Rev 2018;25:1193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Shah P, Catmur C, Bird G. From heart to mind: linking interoception, emotion, and theory of mind. Cortex 2017;93:220–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mul CL, Stagg SD, Herbelin B, Aspell JE. The feeling of me feeling for you: Interoception, alexithymia and empathy in autism. J Autism Dev Disord 2018;48:2953–67. [DOI] [PubMed] [Google Scholar]
- 115.Ernst J, Northoff G, Böker H, Seifritz E, Grimm S. Interoceptive awareness enhances neural activity during empathy. Hum Brain Mapp 2013;34:1615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Di Lernia D, Serino S, Pezzulo G, Pedroli E, Cipresso P, Riva G. Feel the time. Time perception as a function of interoceptive processing. Front Hum Neurosci 2018;12:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Garfinkel SN, Barrett AB, Minati L, Dolan RJ, Seth AK, Critchley HD. What the heart forgets: cardiac timing influences memory for words and is modulated by metacognition and interoceptive sensitivity. Psychophysiology 2013;50:505–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Werner NS, Peres I, Duschek S, Schandry R. Implicit memory for emotional words is modulated by cardiac perception. Biol Psychol 2010;85:370–6. [DOI] [PubMed] [Google Scholar]
- 119.Dunn BD, Galton HC, Morgan R, Evans D, Oliver C, Meyer M, Cusack R, Lawrence AD, Dalgleish T. Listening to your heart: how interoception shapes emotion experience and intuitive decision making. Psychol Sci 2010;21:1835–44. [DOI] [PubMed] [Google Scholar]
- 120.Furman DJ, Waugh CE, Bhattacharjee K, Thompson RJ, Gotlib IH. Interoceptive awareness, positive affect, and decision making in major depressive disorder. J Affect Disord 2013;151:780–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Werner NS, Schweitzer N, Meindl T, Duschek S, Kambeitz J, Schandry R. Interoceptive awareness moderates neural activity during decision-making. Biol Psychol 2013;94:498–506. [DOI] [PubMed] [Google Scholar]
- 122.Hubert M, Van Driessen K. Fast and robust discriminant analysis. Comput Stat Data Anal 2004;45:301–20. [Google Scholar]
- 123.Qiao Z, Zhou L, Huang JZ, editors. Effective linear discriminant analysis for high dimensional, low sample size data. Proceeding of the World Congress on Engineering; 2008 : Citeseer. Available at: http://www.iaeng.org/publication/WCE2008/WCE2008_pp1070-1075.pdf. Accessed July 2019. [Google Scholar]
- 124.Pohar M, Blas M, Turk S. Comparison of logistic regression and linear discriminant analysis: a simulation study. Metodoloski Zvezki 2004;1:143. [Google Scholar]
- 125.Nuyen J, Schellevis FG, Satariano WA, Spreeuwenberg PM, Birkner MD, van den Bos GA, Groenewegen PP. Comorbidity was associated with neurologic and psychiatric diseases: a general practice-based controlled study. J Clin Epidemiol 2006;59:1274–84. [DOI] [PubMed] [Google Scholar]
- 126.Anttila V, Bulik-Sullivan B, Finucane HK, Walters RK, Bras J, Duncan L, Escott-Price V, Falcone GJ, Gormley P, Malik R. Analysis of shared heritability in common disorders of the brain. Science 2018;360:eaap8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Marrie RA, Reingold S, Cohen J, Stuve O, Trojano M, Sorensen PS, Cutter G, Reider N. The incidence and prevalence of psychiatric disorders in multiple sclerosis: a systematic review. Mult Scler J 2015;21:305–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ferentzi E, Drew R, Köteles F. Investigating different measures of interoception. Eur Health Psychol 2016;18(S):971. [Google Scholar]
- 129.Kleint NI, Wittchen H-U, Lueken U. Probing the interoceptive network by listening to heartbeats: an fMRI study. PLoS One. 2015;10:e0133164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cuthbert BN. Research domain criteria: toward future psychiatric nosology. Asian J Psychiatr 2014;7:4–5. [DOI] [PubMed] [Google Scholar]
- 131.Clark LA, Cuthbert B, Lewis-Fernandez R, Narrow WE, Reed GM. Three approaches to understanding and classifying mental disorder: ICD-11, DSM-5, and the National Institute of Mental Health’s research domain criteria (RDoC). Psychol Sci Public Interest 2017;18:72–145. [DOI] [PubMed] [Google Scholar]
- 132.Carcone D, Ruocco AC. Six years of research on the National Institute of Mental Health’s research domain criteria (RDoC) initiative: a systematic review. Front Cell Neurosci 2017;11:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ibanez A, García AM, Esteves S, Yoris A, Muñoz E, Reynaldo L, Pietto M, Adolfi F, Manes F. Social neuroscience: undoing the schism between neurology and psychiatry. Soc Neurosci 2018;13:1–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ibanez A, Kuljis RO, Matallana D, Manes F. Bridging psychiatry and neurology through social neuroscience. World Psychiatry 2014;13:148–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


