Highlights
-
•
The majority of patients with stroke and COVID-19 had vascular risk factors.
-
•
Stroke and COVID-19 might be associated with severe disability and death.
-
•
There might be major disruptions in investiagtions needed for stroke.
Key Words: COVID-19, Brain, SARS-CoV-2, Stroke, Cerebrovascular accident
Abstract
Objectives
COVID-19 disproportionately affects older adults and individuals with cardiovascular co-morbidities. This report presents fifteen patients who had COVID-19 respiratory illness followed by cerebrovascular events.
Materials and Methods
A call by the Iranian Neurological Association gathered cases across the country who developed neurological symptoms attributed to hemorrhagic or ischemic stroke after a definite or probable Covid-19 respiratory illness. Definite cases were those with a typical respiratory illness, positive nasopharyngeal Covid-19 PCR test, and chest CT consistent with Covid-19 infection. Probable cases were defined by a typical respiratory illness, history of contacts with a Covid-19 case, and chest CT characteristic for Covid-19 infection.
Results
Fifteen patients (12 men and 3 women) with an age range of 38 to 93 years old (median: 65 years old) were included. Fourteen patients had a first-ever acute ischemic stroke and one patient had a subarachnoid hemorrhage. Eleven patients (73%) had previous cardiovascular comorbidities. The median time between respiratory symptoms and neurological symptoms was seven days (range 1-16 days). Stroke severity in two patients was mild (NIHSS ≤ 6), in six patients moderate (NIHSS: 7-12), and in seven patients severe (NIHSS ≥13). One patient received intravenous tissue plasminogen activator ( IV-tPA) with improved neurological symptoms. Six out of 15 patients (40%) died. All but one of those who survived had significant disability assessed by a modified ranking scale >2. The majority of patients in this case series had vascular risk factors and their stroke was associated with severe disability and death.
Conclusion
This report highlights the need for further investigation of the links between Covid-19 and cerebrovascular events.
Introduction
Coronavirus disease 2019 (COVID-19) infection has rapidly spread across the world, currently affecting more than 39 million people, leading to major societal, economical and health care system distruptions.1 Older adults and those with vascular co-morbidities are disproportionately affected.2 , 3 More than one third of patients who required intensive care unit admission had at least one underlying vascular risk factor.4 In addition to respiratory symptoms, COVID-19 infection can also lead to hematological and cardiac complications.5 , 6 ACE2 (angiotensin-converting enzyme 2) receptor, a key cell surface protein facilitating SARS-CoV-2 (the virus that causes COVID-19) entry to the cells, is found in various cells including vascular endothelium and neurons.7 Therefore, rising concerns about potential neurovascular complications of COVID-19 infection have biological plausibility.8 In this report, we present clinical data on fifteen Iranian patients who initially presented with respiratory symptoms typical for COVID-19, but subsequently developed neurological symptoms with evidence of ischemic stroke or subarachnoid hemorrhage (SAH) on imaging.
Methods
This is a case series of patients with stroke and a previous diagnosis of COVID-19 with respiratory illness from February 19, 2020 (the date first COVID-19 infection documented in Iran) to March 19, 2020. A survey was sent to all members of the Iranian Neurological Association, which is a non-profit/non-governmental organization with approximately 1400 adult neurologists as members. Neurologists were asked to report patients who developed acute cerebrovascular events after a documented COVID-19 respiratory infection. Cases were those with initial typical respiratory illness (cough and shortness of breath) associated with systemic symptoms including fever, chills or myalgia with positive COVID-19 PCR test and with characteristic findings on chest computed tomography (CT).9 Cases without PCR testing were defined by the same typical systemic and respiratory manifestations, reported contact with known COVID-19 patients and with characteristic chest CT findings which has 97% sensitivity in COVID-19 patients.10 Data on the total number of COVID-19 cases in Iran during the study period was obtained using World Health Organization situation report (https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports).
Eligible cases needed to have acute neurological symptoms following the respiratory illness. Further demographic and acute illness related data including date of initial symptoms, type of symptoms, history of close contacts with other patients with COVID-19, interval between manifestations of COVID-19 and neurological symptoms (days) were obtained for all eligible patients. Previously established clinical guidelines were applied for the diagnosis of ischemic and hemorrhagic stroke and subarachnoid hemorrhage.11 , 12 Severity of the COVID-19 was measured by Ordinal Scale for Clinical Improvement which has been proposed by a special World Health Organization (WHO) committee. This is a clinical scale which includes uninfected (0 score), ambulatory (1 and 2 scores), hospitalized mild disesase (3 and 4 scores), hospitalized severe disesase (5-7 scores), and death (8 score) (https://www.who.int/blueprint/priority-diseases/key-action/COVID-19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020.pdf)
Treating neurologists provided data on vascular risk factors and comorbidities including hypertension, diabetes, dyslipidemia, smoking, atrial fibrillation and other arrhythmias, congestive heart failure, valvular heart disease and ischemic heart disease. All patients had noninvasive cardiac investigations including electrocardiography (ECG) and transthoracic echocardiography (TTE). Due to COVID-19 outbreak and resource re-allocation across Iranian healthcare centers, transcranial Doppler (TCD), CT angiography (CTA) and/or magnetic resonance angiography (MRA) were not performed in reported cases. Patients with prior stroke, vasculitis, arterial dissection, fibromuscular dysplasia, Moymoya disease or any other established cerebrovascular diseases were not included. Modified Rankin scale (mRS) of patients in the last phone follow-up after stroke was recorded. National Institutes of Health Stroke Scale (NIHSS) at the time of stroke diagnosis was documented for all patients and categorized into mild (≤ 6), moderate (7-12) and severe stroke (≥13). This study was approved by the institutional review board (IRB) of Shiraz University of Medical Sciences. Informed consent was obtained from the patient and/or first-degree relatives.
Results
Over the inclusion time period, 15 patients out of 18407 COVID-19 cases (0.81 per 1000 cases, 95% confidence interval 1.3-0.49) were reported to have cerebrovascular events. We included 12 men and three women (median age: 65 years). They ranged from 38 to 93 years-old (median: 65 years-old) and came from all geographical regions in Iran (eight centers). Thirteen patients were from urban areas and two patients were from rural areas. Table 1 summarizes the demographic and clinical characteristics of these patients. Systemic manifestations of COVID-19 infection include fever, cough, dyspnea and myalgia. All patients were hospitalized due to COVID-19 respiratory symptoms. Interval time between systemic manifestations and neurological symptoms ranged from one to 16 days (median: 7 days). Median of severity of the COVID-19 in in ordinal scale for clinical improvement was 6. Fourteen patients had acute ischemic stroke and one patient had subarachnoid hemorrhage (Fig. 1 ). Among patients with ischemic stroke, two patients had bilateral multifocal stroke, nine had unilateral, two had striatocapsular, one had subcortical lacunar and two had brainstem strokes. Eleven patients (73%) had underlying cardiovascular risk factors. All the patients underwent TTE. None of the patients had myocarditis, endocarditis or pericarditis. Spot ECGs and in-hospital cardiac monitoring did not show evidence of atrial fibrillation or other arrhythmias for any patient. Similarly, none of our patients had deep vein thrombosis or severe hemodynamic instability at the time of stroke diagnosis. One patient (case number 10) was diagnosed with disseminated intravascular coagulation (DIC). Lymphopenia (absolute lymphocyte counts less than 1500 mm3) was seen in 12 patients (80%) at the time of stroke diagnosis. A significant pro-inflammatory state assessed by C-reactive protein (CRP) was seen in all patients who were tested (13 patients). Doppler ultrasound of cervical arteries did not show hemodynamically significant stenosis in four patients with ischemic stroke. Stroke severity in two patients (13%) was mild (NIHSS ≤ 6), in six patients (40%) was moderate (NIHSS: 7-12) and in seven patients (47%) was severe (NIHSS ≥13). One patient (case number 4) received intravenous tissue plasminogen activator (IV-tPA) with good response (initial NIHSS: 14, discharge NIHSS: 7). Six out of 15 patients (40%) died. In those who survived, significant disability (mRS>2) was seen in all but one patient. Fig. 2 illustrates acute ischemic stroke on MRI for case number 7.
Table 1.
Characteristics of 15 COVID-19 patients with subsequent acute cerebrovascular events.
| Variables | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | Patient 10 | Patient 11 | Patient 12 | Patient 13 | Patient 14 | Patient 15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Male | Male | Female | Male | Male | Male | Male | Female | Male | Male | Male | Male | Male | Male | Male |
| Age, years | 58 | 75 | 78 | 65 | 83 | 78 | 50 | 49 | 60 | 38 | 45 | 93 | 57 | 65 | 76 |
| Place of living | Urban | Urban | Urban | Urban | Urban | Urban | Urban | Urban | Urban | Rural | Urban | Urban | Urban | Urban | Rural |
| Stroke risk factors and Comorbidities | None | Diabetes Hypertension Heart failure, Pacemaker |
Diabetes Hypertension Hyperlipidemia |
Hypertension | Hypertension Cirrhosis | Hypertension smoking | None | None | Hypertension | None | Hypertension Smoking |
Hypertension | Diabetes | Diabetes ``Hypertension Hyperlipidemia |
Hypertension Smoking |
| Confirmatory covid-19 test | CT | PCR | CT | CT | CT | CT | PCR | PCR | CT | PCR | CT | CT | PCR | CT | CT |
| Severity of covid-19 according to OSCI | 5 | 8 | 5 | 4 | 6 | 6 | 4 | 8 | 8 | 8 | 8 | 3 | 8 | 3 | 5 |
| Time from covid-19 symptoms to stroke symptoms, days | 7 | 3 | 3 | 10 | 7 | 7 | 1 | 14 | 1 | 8 | 1 | 1 | 4 | 16 | 9 |
| Hematological abnormalities | None | Lymphopenia | Lymphopenia | Leukocytosis Lymphopenia |
Leukocytosis Lymphopenia | Lymphopenia | Leukocytosis | Lymphopenia | Leukocytosis Lymphopenia |
DIC | Lymphopenia | Lymphopenia | Lymphopenia | Lymphopenia | Lymphopenia |
| CRP | 3+ | 1+ | 3+ | +4 | +4 | +3 | +4 | NA | +2 | +4 | NA | +2 | +3 | +2 | +2 |
| Type of Stroke | Ischemic Unilateral |
Ischemic Unilateral |
Ischemic Unilateral |
Ischemic Unilateral |
Ischemic Unilateral |
Ischemic Unilateral |
Ischemic Bilateral Multifocal |
Ischemic Unilateral |
Subarachnoid hemorrhage | Ischemic Bilateral Multifocal |
Ischemic Unilateral |
Ischemic Unilateral |
Ischemic Unilateral |
Ischemic Unilateral |
Ischemic Unilateral |
| Brain Imaging | CT | CT | CT | CT | CT | CT | MRI | CT | CT | CT | CT | CT | CT | MRI | MRI |
| Initial severity of stroke (NIHSS) | Severe (≥13) | Severe(≥13) | Moderate (7-12) | Severe (≥13) | Severe (≥13) | Moderate (7-12) | Moderate (7-12) | Severe (≥13) | Severe (≥13) | Severe (≥13) | Moderate (7-12) | Moderate (7-12) | Severe (≥13) | Moderate (7-12) | Mild ≤ 6 |
| Discharge mRS | 5 | 6 | 5 | 3 | 4 | 4 | 3 | 6 | 6 | 6 | 6 | 5 | 6 | 3 | 2 |
| Stroke treatment | Antiplatelet Statin |
Antiplatelet Statin |
Antiplatelet, Statin |
Intravenous Thrombolytic (tpa) Antiplatelet, Statin |
Antiplatelet Statin |
Antiplatelet Statin |
Antiplatelet Statin |
Antiplatelet Statin |
Blood pressure control, Calcium channel blocker | Coagulation factors | Antiplatelet Statin |
Antiplatelet Statin |
Antiplatelet Statin |
Antiplatelet Statin |
Antiplatelet Statin |
| Outcome status | Discharged Rehab |
Death | Discharged Rehab |
Discharged Home |
Discharged Home |
Discharged Rehab |
Discharged Home |
Death | Death | Death | Death | Discharged Rehab |
Death | Discharged Home |
Discharged Home |
Abbreviations: NA: not available, mRS: modified ranking scale, PCR: polymerase chain reaction, NIHSS: National Institute of Health stroke scale, OSCI:ordinal scale for clinical improvement, OSCI: ordinal scale for clinical improvement.
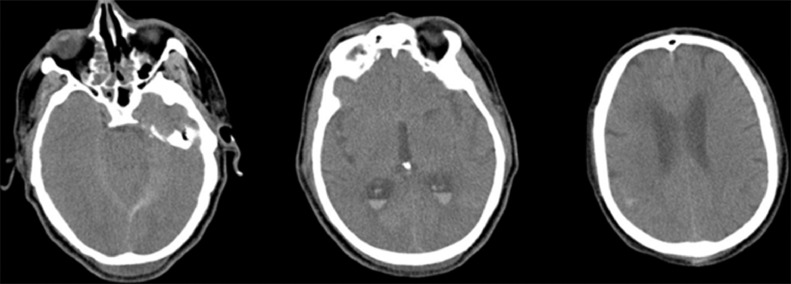
Fig. 1.
Case number 9: A 60 year-old man with hypertension referred for chest pain, headache and confusion. Chest CT scan showed characteristic findings of COVID-19 infection. Brain CT scan shows subarachnoid with intraventricular hemorrhage. CTA and MRA were not performed. The patient passed away. Autopsy was not done.
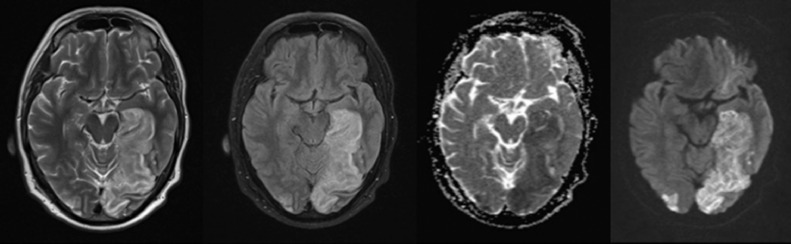
Fig. 2.
Case number 7: A 50 year-old man without any vascular risk factor developed dry cough, high grade fever and dyspnea. One day later he developed visual blurring, right hemiparesis, dysarthria and disequilibrium. MRI shows bilateral PCA strokes. Doppler ultrasound of cervical arteries and transthoracic echocardiography were unremarkable. PCR for COVID-19 was positive.
Discussion
We describe 15 cases who presented with COVID-19 and subsequently progressed to develop cerebrovascular complications manifesting as ischemic stroke and SAH. In this study, stroke was associated with a prominent risk of mortality and disability. Such data may reflect the reality of care in many parts of the world with the current pandemic and likely to underestimate the extend of cerebrovascular accidents after COVID-19 infection and missing milder cases of stroke who opted not to seek medical attention during the pandemic.
Available evidence indicate that cardiovascular comorbidities are major predictors for severe symptoms and case fatality in patients with COVID-19. Hypertension (53.8%), diabetes (42.3%), coronary heart disease (19.2%) and cerebral infarction (15.4%) are significant comorbidities in patients who died.13 In this group of patients 73% had underlying morbidities and all of them had at least one cardiovascular risk factor. While there are limited data on the link between COVID-19 and consequent stroke; multiple pathways can be considered as potential contributors to this link. An increasing number of studies show that COVID-19 can lead to cardiac complications.14 In addition, coagulation abnormalities including elevated D-dimer, fibrin/fibrinogen degradation products and fibrinogen are reported in patients with COVID-19. These hematological abnormalities were closely related to more severe clinical status.15 Recently Zhang et al presented three patients with multifocal brain infarcts with clinically significant coagulopathy and positive antiphospholipid antibodies raising a possibility that a pro-coagulant state might underlie the risk of stroke in COVID-19 patients.16 A similar concern was raised in 2003 SARS outbreak where a disproportionate number of thromboembolic complications were identified in severely ill SARS patients 17, including reports of large vessel occlusion stroke as it was recently reported for COVID-19.18 , 19 In addition to potential cardiac and hematological causes for stroke, COVID-19 patients also are at higher risk for developing hypoxic and hypotensive episodes, both of which can lead to ischemic injuries in vulnerable patients. As presented in table-1, 11 out of 15 of the patients in this series had severe COVID-19 disease according to ordinal scale for clinical improvement. Increasing data shows that severity of COVID-19 infection is related to a higher risk of cerebrovascular events in these patients. A recent study and meta-analysis by Siepmann and colleagues demonstrated that patients with severe COVID-19 course have more than four times higher risk of developing stroke.20 Patients with complicated courses of COVID-19 are more likely to have underlying cardiovascular co-morbidities and diseases which can predispose them to developing stroke.
Shortcomings of this case series need to be acknowledged. Case finding was simply done through a survey, not a systematic prospective registry. Some patients with milder strokes and COVID-19 infection might have not referred to the hospitals. Patients with COVID-19 infection and no respiratory symptoms who then had a stroke would not have been identified in current series as well. All these issues might skew the mortality rate described. Also, cardiac evaluation was limited to TTE and more sophisticated testing such as transesophageal echocardiography, cardiac MRI and long-term ECG monitoring could not be performed. In addition, the lack of detailed cerebrovascular imaging including brain and neck CTA and MRA and autopsy data was a major barrier in determining the etiology and mechanisms of stroke in our patients.
The data in this report were gathered early during the COVID-19 pandemic in the midst of outbreak in Iran which lead to major disruptions in traditional stroke care, which may have adversely impacted patient outcomes. However, the potentially worse outcomes in this setting may also represent the clinical environment in many other countries during this pandemic. It should be noted that in this study there was no control group to compare characteristics and outcomes in stroke patients with and without COVID-19 and we suggest future studies involving all patients with stroke during the pandemic.
Increasing number of patients in the world are being affected by this acute respiratory condition requiring intubation and sedation which can mask neurological symptoms and early recognition of stroke. Stroke can lead to worse clinical outcomes and long-term disabilities in these patients. In this group of patients, we observed that baseline cardiovascular co-morbidities in combination with significant inflammatory was a prominent feature and was associated with higher risk of mortality and disability. This report can potentially stimulate further research on the mechanistic underpinnings linking SARS-CoV2 infection and cerebrovascular outcomes, particularly in high-risk patient populations. Further prospective and larger studies with more complex methodologies and through ancillary investigations are critical and necessary to improving stroke care during and after this pandemic.
Declaration of Competing Interest
Nothing to report.
Acknowledgments
Author contributions
A.B., B.S.,: conception and design of the study, acquisition and analysis of data, drafting the manuscript and figures. M.M., F.A., S.K., L.P., Z.B., S.A.B., Z.H., E.M, H.M, S.P., N.R., A.S., M.S., M.R.N.,: acquisition and analysis of data, revision the manuscript and figure. R.B.S., A.B., F.S.,: conception of the study, revision of the manuscript and figures.
Acknowledgment
This work used no available grant and individuals contributed using their own time and budget.
References
- 1.Lipsitch M, Swerdlow DL, Finelli L. Defining the epidemiology of Covid-19 - studies needed. N Engl J Med. 2020;382:1194–1196. doi: 10.1056/NEJMp2002125. [DOI] [PubMed] [Google Scholar]
- 2.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to Covid-19 in Italy. JAMA. 2020;323:1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 3.Clerkin KJ, Fried JA, Raikhelkar J. Coronavirus disease 2019 (Covid-19) and cardiovascular disease. Circulation. 2020;141:1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 4.Wang D, Hu B, Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou F, Yu T, Du R. Clinical course and risk factors for mortality of adult inpatients with Covid-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi S, Qin M, Shen B. Association of cardiac injury with mortality in hospitalized patients with Covid-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou P, Yang XL, Wang XG. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of Covid-19 patients. J Med Virol. 2020;92:552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernheim A, Mei X, Huang M. Chest CT findings in coronavirus disease-19 (Covid-19): relationship to duration of infection. Radiology. 2020;295 doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (covid-19) in China: a report of 1014 cases. Radiology. 296:E32-E40. [DOI] [PMC free article] [PubMed]
- 11.Powers WJ, Rabinstein AA, Ackerson T. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2019;50:e344–e418. doi: 10.1161/STR.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 12.Steiner T, Juvela S, Unterberg A. European stroke organization guidelines for the management of intracranial aneurysms and subarachnoid haemorrhage. Cerebrovasc Dis. 2013;35:93–112. doi: 10.1159/000346087. [DOI] [PubMed] [Google Scholar]
- 13.Deng SQ, Peng HJ. Characteristics of and public health responses to the coronavirus disease 2019 outbreak in China. J Clin Med. 2020;9:E575. doi: 10.3390/jcm9020575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng YY, Ma YT, Zhang JY. Covid-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han H, Yang L, Liu R. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58:1116–1120. doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Xiao M, Zhang S. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382(17):e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lew TW, Kwek TK, Tai D. Acute respiratory distress syndrome in critically ill patients with severe acute respiratory syndrome. JAMA. 2003;290:374–380. doi: 10.1001/jama.290.3.374. [DOI] [PubMed] [Google Scholar]
- 18.Umapathi T, Kor AC, Venketasubramanian N. Large artery ischaemic stroke in severe acute respiratory syndrome (SARS) J Neurol. 2004;251:1227–1231. doi: 10.1007/s00415-004-0519-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oxley TJ, Mocco J, Majidi S. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020;382(20):e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siepmann T, Sedghi A, Simon E. Increased risk of acute stroke among patients with severe Covid-19: a multicenter study and meta-analysis. Eur J Neurol. 2020 doi: 10.1111/ene.14535. [DOI] [PubMed] [Google Scholar]